Note: Descriptions are shown in the official language in which they were submitted.
\-0 sl/o66~8 PCr/l!S90/06081
EXPRESSION OF BACTERIAL HEMOGLOBIN AND ENHANCEMENT
OF EXPRESSION OF CLONED AND NATIVE PRODUCTS IN
STREPTOMYCES
This is related to Serial No. 342,451, filed January 24,
1989 as PCT application No. PCT US88-03745, which is ~
continuation-in-part of Serial No. 113,014 filed
October 23, 1987 and Serial No. 151,526, filed
February 2, 1988.
TECHNICAL FIELD
This invention relates to the expression of Vltreoscilla
hemoglobin in Streptomyces sp. to enhance growth
characteristics and antibiotic yields at low or reduced
oxygen levels.
This invention relates to the use of Vitreoscilla
hemoglobin gene promoter to obtain high level expression
of cloned proteins in Streptomvces.
BACKGROUND ART
The use of an intracellular globin to enhance growth and
productivity in StreptomYces is based on severGl
observations. First, the maximum cell concentration in
Streptomyces fermentations is often limited by oxygen
availability (Tuffile and Pinho, Biotechnol. Bioena.,
XII:849, 1970). Second, we have observed that in
unicellular organisms there exists a possibly
~ 25 significant diffusional barrier between environmental
': ' ' ' . . .
. - . - :
- . :
~()91/0~62~ PCT/~S90/060~1
2r. ~
-2-
oxygen and the cytochromes where the energy-producing
reactions necessary for cell growth occur. Third, the
globins represent a family of heme-containing proteins
that reversibly ~ind oxygen and are thus able to enhance
the oxygen transfer rate to cells in multicellular
organisms. Fourth, the synthesis of many antibiotics
is reduced at low culture oxygen concentrations
tNormansell, "Antibiotic-producing Streptomyces", The
Bacteria, Queener and Day, Academic Press, N.Y., 1986).
Final~y, the expression of bacterial hemoglobin has been
shown to enhance the growth properties of the bacteria
Escherichia coli and Vitreoscilla, especially under
conditions of reduced oxygen (Khosla and Bailey, Nature,
331:633, 1988). Expression of intracellular hemoglobin
in StreptomYces may act to overcome the diffusional
barrier, especially under conditions of low external
oxygen, resulting in enhanced cell growth.
Intracellular hemoglobin may also enhance antibiotic
production per unit cell mass.
The bacteria of the genus Stre~tomvces are used for the
production of approximately 60~ of the commercially
available antibiotics (Atkinson and Mavituna,
Biochemical Enqineerinq and Biotechnoloqv Handbook,
Macmillan, England, 1987). Examples of widely-used -
antibiotic compounds produced in Streptomyces
fermentations include the spriamycins, neomycins,
tetracyclines, and streptomycins (Demain and Solomon,
Manual of Industrial Microbioloav and Biotechnoloav,
American Society for Microbiology, 1986). In addition,
many compounds produced by Stre~tomyces have
antineoplastic (the bleomycins, mithramycins, and
; daunomycins) and antihelminthic (the avermectins)
activity. Recombinant DNA technology has been used to
develop strains that overproduce or synthesize hybrid
antibiotics with novel activities (Rhodes, et al.,
Biochem. Soc. Trans., 12: 1078, 1984). Through the use
: - . .
~ - . . -
WO91/06fi~X PCT/US90/06081
_3_ 2 ~ 5
of high-productlon strains and optimize growth
protocols, the efficiency of antibiotic production can
be dramatically improved. However, low antibioticyields
remain a major problem confronting industrial antibiotic
production using Streptomvces.
Stre~tomyces are obligate aerobes that require high
levels of oxygen for optimal growth. Providing
sufficient oxygen to a high cell density culture
represents a major obstacle due to the tendency for
StreptomYces to form long filamentous strands that
results in a highly viscous culture. Viscosity
dramatically reduces the oxygen transfer rate to the
culture medium. A typical Streptomyces fermentation
begins with the growth of cells to high densities
(growth phase). There is little antibiotic production
during growth phase. The final cell densities achieved
are usually limited by the oxygen supply. After
cessation of growth, antibiotic -synthesis begins
(production phase). The antibiotic production phase is
maintained as long as possible by supplying the
appropriate nutrients. Eventually, acidic waste
products accumulate and the cells die.
Althouyh the factors regulating antibiotic production
are largely unknown, certain environmental factors
including phosphate and nitrogen concentration, carbon
source, shear effects, and oxygen concentration have
been shown to strongly influence antibiotic
productivity. For example, in batch culture,
cephalosporin production in S. clavuliqerus dropped by
a factor of three under reduced oxy~en conditions
(Yegneswaran, et al., Biotechnol. Letts., lO: 479,
1988). In addition, spectacular improvements in
nikkomycin yields in S. tendae were achieved when the
dissolved oxygen (DO) was maintained well above oxygen-
limiting conditions during the production phase
~ .. . . .
' ~
:: . . .:.
, . .. ..... . ..
~'O 91/0662~3 PCI`/VS90/061181
2 ~ 4
(Aharonowitz and Demain, Blotechnoloq: Potentials andLimitations, Springer and Verlag, Heidelberg, W.
Germany, 1986). Unfortunately, maintaining sufficiently
high DO levels in high density fermPntations is
5 technically difficult and often not economically '
feasible.
The mechanism by which reduced oxygen levels decreases
antibiotic production in Stre~tomvces is unknown. One
possibility is that lower respiration rates have a
negative regulatory effect on secondary metabolic
pathways (Vanek and Hostalek, Over~roduction of
Microbial Metabolites, Butterworth, MA, 1986). Aeration
rates have also been shown to directly affect carbon
source regulation of antibiotic synthesis (Brana, et
aI., Biotechnol. Letts., 5: 791, 1983). In addition,
oxygen regulation of hydrolytic enzymes may play a role
in antibiotic stability (Atkinson and Mavituna, ibid).
Regardless of the mechanism, it is clear that it is
desirable to facilitate oxygen transfer to the cells to
increase antibiotic yields. Two general approaches to
increasing the oxygen transfer rate to the culture
medium include the development of improved bioreactor
designs (Normansell, ibid.) and modification of the
culture medium (Adlercreutz and Mattiason, Eur. J. A~
Microbiol. Biotechnol., 16: 165, 1982).
-- .
The effect of bacterial hemoglobin expression on qrowth
of a unicellular organism was investigated by Khosla and
Bailey (Khosla and Bailey, ibid.). The bacterial
hemoglobin was originally discovered in the obligate
aerobic bacterium, Vitreoscilla (Tyree and Webster, J.
Biol. Chem., 2S3: 6988, 1978). The hemoglobin is a
soluble, dimeric protein that combines with oxygen and
displays a spectral response to carbon monoxide binding
characteristic of eukaryotic hemoglobin~ (Wakabayashi,
et al., Nature, 332: 481, 1986). It was conjectured
.... ~, .
.
. .................... : . :
- : : ''
WO9l/~662~ PCT/~'S90/06081
~5~ 2~SJ~ 5
that the hemoglobin proteln functioned to facilitate
oxygen transfer to Vitreoscilla and thus allowed it to
propagate under oxygen-poor conditions.
The gene for the Vitreoscilla hemoglobin has been
isolated along with its native transcriptional
regulatory sequences. (Khosla and Bailey, Mol. Gen.
Genet, 214: 158, 1988). Interestingly, this gene was
expressed from its native promoter when introduced into
E. coli. Of particular interest was that expression of
hemoglobin was regulated by the culture oxygen content
- such that maximal induction occurred under microaerobic
conditions. Under fed-batch fermentation conditions,
E. coli cells expressing hemoglobin displayed
significantly higher specific growth rates and achieved
2-3 fold the final cell densities as non-expressing
strains (Khosla and Bailey, Nature, 331:633, 1988).
As mentioned previously, current progress in producing
new antimicrobial compounds has involved the
development, through recombinant DNA technology, of
Streptomvces strains that produce novel 'hybrid"
antibiotics. So far, the expression of heterologous
genes involved in secondary metabolite production has
relied on the ability of the recipient strain to
correctly recognize the transcriptional initiation
sequence (promoter) of the incoming gene. The isolation
of a universal, highly-active promoter for the
expression of cloned genes in Streptomvces would be
extremely useful, but has so far xemained elusive.
DISCLOSURE OF THE INVENTION
The present invention relates to oxygen-binding
:!
proteins, particularly hemoglobins, a recombinant-DNA
method of producing same, and to portable DNA sequences
capable of directing intracellular production of these
oxygen-binding proteins in Stre~tomyces. The present
- - ,
:. :
'
WO~1/0662~ PCT/~iS~30/06081
2~7~ 6- -
invention also relates to vectors containing these
portable DNA sequences.
One object of the present invention is to provide a
recombinant-DNA method for the production of these
oxygen-binding proteins. To facilitate the recombinant-
DNA synthesis of these oxygen-binding proteins, it is
a further object of the present invention to provide
portable DNA sequences capable of directing
intracellular production of oxygen-binding proteins in
0 Stre~tomvces. It is also an object of the present
invention to provide cloning vectors containing these
portable sequences. These vectors are capable of being
used in recombinant Stre~tomvces to enhance the growth
characteristics of organisms, and to produce useful
quantities of oxygen-binding proteins. Augmented by
intracellular synthesis of oxygen-binding proteins,
product formation can also be enhanced.
The present invention also provides novel methods and
materials for expression of cloned genes in
Streptomvces. Particularly, it related to
promoter/regulators, a recombinant-DNA method of
producing same, and to portable DNA sequences capable
of directing the translation and transcription
initiation and control of the expression of desired gene
products.
Thus, another object of the present invention is to
provide for the expression in Streptomvces of any
selected chromosomal or extrachromosomal gene or DNA
sequence through the incorporation of a
promoter/regulator DNA sequence. Such expression may
thus provide native or heterologous enzyme activities
which increase antibiotic production or which enable
synthesis of modified or novel antibiotics.
: :;
~091/Oh62X PCT/US90/060Rl
~7~ 2r 7~v~5
To achieve the objects and in accordance with the
purposes of the present invention, promoter/ regulators
are also set forth. To further achieve the objects in
the accordance with the purposes of the present
invention, as embodied and broadly described herein,
portable DNA sequences forthese promoter/regulators are
provided. Particularly preferred promoter/regulator DNA
sequences for use in the practice of the present
invention are derived from the filamentous bacterium
Vitreoscilla. Portable nucleotide sequences are
provided for these promoter/regulators. ~he portable
sequences may be either synthetic sequences or
restriction fragments ("natural" DNA sequences).
Additionally, portable DNA sequences useful in the
processes of the present invention may be synthetically
created. ~hese synthetic DNA se~uences may be prepared
by polynucleotide synthesis and sequencihg techniques
known to those of ordinary skill in the art.
Additionally, to achieve the ob~ects and in accordance
with the purposes of the present invention, a
recombinant-DNA method is disclosed which results in
manufacture by cells of the genus Streptomyces of the
instant oxygen-binding proteins using the portable DNA
sequences referred to above.
Additionally, to achieve the objects and in accordance
with the purposes of the present invention, recombinant-
DNA methods are disclosed which provide transcription
and translation of gene products by a host Streptomyces
using the portable DNA sequences referred to above.
.. ~
To further accomplish the objects and in further accord
with the purposes of the present invention, cloning
vectors are provided comprising at least one portable
.:
`
.
:. , ~ i-
:;- . . .
~'O91/06628 PCTt~'S90/06081
2~ 8-
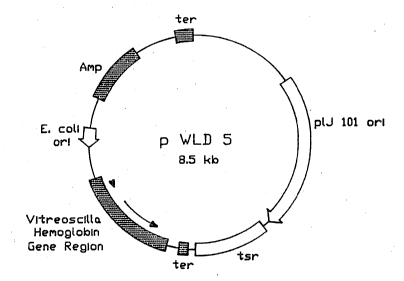
DNA sequence. In particular, plasmids pWLD5 and pWLDl0
ar~ disclosed.
It is understood that both the foregoing general
description and the following detailed description are
exemplary and explanatory only and are not restrictive
of the invention, as claimed.
The accompanying drawing, which is incorporated in and
constitutes a part of this specification, illustrates
one embodiment of the invention and, together with the
description, serves to explain the principles of the
invention.
BRIEF DESCRIPTION OF THE DRAWINGS
/ Figure l is a partial restriction map of plàsmids
pWLD l0 and pWLD 5.
BEST MODES FOR CARRYING OUT THE INV~NTION
Reference will now be made in detail to ~he presently
preferred embodiments of the invention, which, together
with the drawing and the following examples, serve to
explain the principles of the invention.
Expression of hemoglobin in Stre~tomvces serves to
enhance aerobic growth, respiration, and/or antibiotic
productivity. Thus, one objective of this invention is
metabolically improved Stre~tomyces cells which have
preferred functional characteristics in aerobic
manufacturing processes. As noted above, the present
invention relates in part to portable DNA ~equences
capable of directing intracellular production of oxygen-
binding proteins in a variety of Streptomvces species.
-,;
!'Portable DNA sequence" in this context is intended to
refer either to a synthetically produced nucleotide
sequence or to a restriction fragment of a naturally
occurring DNA sequence. For purposes of this
. , .
- - ' . ~ . '
091/06628 PCT/~S90/0608
9 zr~ S~
specification, "oxygen~binding protein" is intended to
mean a protein with a primary structure as defined by
the codons present in the deoxyribonucleic acid sequence
which directs intracellular production of the amino acid
sequence, and which may or may not include post-
translational modifications. ~t is contemplated that
such post-translational modifications include, for
example, association with a heme prosthetic group. It
is further intended that the term "oxygen-binding
protein" refers to either the form of the protein as
would be excreted from a cell or as it may be present
in the cell from which it was not excreted. Because of
the sensitivity of antibiotic synthesis in StreptomYces
to oxygen supply, it is also envisioned that the
intracellular presence of cloned hemoglobin may provide
a modified form(s) of the antibiotic molecule(s)
normally produced by the host strain of Streptomyces.
In a preferred embodiment, the portable DNA sequences
are capable of directing intracellular production of
hemoglobin. In a particularly preferred embodiment, the
portable DNA sequences are capable of directing
intracellular production of a hemoglobin biologically
equivalent to that previously isolated from the
filamentous bacterium, Vitreoscilla. By "biologically
equivalent", as used herein, it is meant that a protein,
produced using a portable DNA sequence of the present
invention, is capable of binding oxygen in the same
fashion, but not necessarily to the same degree, as the
homodimeric soluble heme protein ~subunit MW 15,775)
isolable from-Vitreoscilla.
As noted above, the present invention also relates in
part to portable DNA sequences which contain
promoter/regulators which are capable of directing
intracellular expression of endogenous or exogenous gene
products, in a variety of host cells and host
microorganisms. "Portable DNA sequence" and
:. . ' : ' ,
.
. w o 91/~662X P(~r/VS90/06081
--10--
"promoter/regulator" in this context are intended to
refer either to a synthetically produced nucleotide
sequence or to a restriction fragment of a naturally
occurring DNA sequence.
The portable DNA sequences of the present invention may
also include DNA sequences downstream from a
promoter/regulator which code for at least one foreign
protein. For purposes of this speci~icatIon, "foreign
protein" is intended to mean a protein with a primary
1~ structure as defined by the codons present in the
deoxyribonucleic acid sequence which directs
intracellular production of the corresponding amino acid
sequence, and which may or may not include post-
translational modifications. It is further intended
that the term "foreign protein" refers to either the
form of the protein as it would be excreted from a cell
or as it may be present in the cell from which it was
not excreted.
In a particularly preferred embodiment, the
promoter/regulator sontains transcription and
translation initiation and control sequences
substantially equivalent to those for directing
intracellular production of a hemoglobin protein
biologically equivalent to that previously isolated from
the filamentous bacterium, Vitreoscilla.
It is of course intended that the promoter/ regulators
of the present invention may control and initiate
transcription and translation of an unlimited number of
endogenous and/or exogenous foreign proteins. In
particular, by expressing enzymes involved in antibiotic
synthesis or modification, antibiotic productivity may
be improved and the nature of the antibiotic may be
modified.
, .. . . - , .: : . .
." , . .
, ~ .
WO9l/0662X ~ ~?~ PCT/~'S90/06081
A preferred portable DNA sequence for the
promoter/regulators of the present invention contains
at least a portion of the following nucleotide sequence,
which reads 5' to 3' and includes the translation
initiation sequence ATG (underlined) and some of the
nucleotide sequence of the Vitreoscilla structural gene
(also underlined):
Hin:
AAGCTTAACG GACCCTGGGG TTAAAAGTAT TTGAGTTTTG ATGTGGATTA
AGTTTTAAGA 60
GGCAATAAAG ATTATAATAA GTGCTGCTAC ACCATACTGA TGTATGGCAA
AACCATAATA 120
ATGAACTTAA GGAAGACCCT CATGTTAGAC CAGCAAACCA TTAACATCAT
CAAAGCCACT 180
GTTCCTGTAT TGAAGGAGCA TGGCGTTACC ATTACCACGA CTTTTTATAA
AAACTTGTTT . 240
GCCAAACACC CTGAAGTACG TCCTTTGTTT GATATGGGTC GCCAAGAATC
TTTGGAGCAG 300
;
CCTAAGGCTT TGGCGATGAC GGTATTGGCG GCAGCGCAAA ACATTGAAAA
TTTGCCAGCT 360
ATTTTGCCTG CGGTCAAAAA AATTGCAGTC AAACATTGTC AAGCAGGCGR
GGCAGCAGCG 420
CATTATCCGA TTGTCGGTCA AGAATTGTTG GGTGCGATTA AAGAAGTATT
GGGCGATGCC 480
GCAACCGATG ACATTTTGGA CGCGTGGGGC AAGGCTTATG GCGTGATTGC
AGATGTGTTT 540
':
. . . :
.
:~ -
: ' ~ ':' ' ' ' :
WO 91J0662~ ?~ ' 5 PCT/US90/06081
--12-- "--
ATTCAAGTGG AAGCAGATTT GTACGCTCAA GCGGTTGAAT AAAGTTTCAG
GCCGCTTTCA 600
GGACATAAAA AACGCACCAT AAGGTGGTCT TTTTACGTCT GATATTTACA
CAGCAGCAGT 660
TTGGCTGTTG GCCAAAACTT GGGACAAATA TTGCCCTGTG TAAGAGCCCG
CCGTTGCTGC 720
GACGTCTTCA GGTGTGCCTT GGCAT 745
The nucleotide bases represented by the above
abbreviations are as follows: A = Adenine, G = Guanine,
C = Cytosine, and T = Thymine.
The above sequence exhibits homology with certain
sequences which are highly conserved in a variety of
promoter/regulators. Using conventional numbering, with
the underlining showing the homology in the above
sequence to the consensus sequence, the -10 consensus
sequence or Pribnow box sequence is TATAAT(A/G). The
-35 consensus sequence is TTGAC_, and the consensus
Shine-Dalgarno sequence is AGGAGGTXXX(XX)ATG.
In a preferred embodiment, the above sequence is
operatively fused with at least a portion of a
downstream sequence of nucleotides which code for at
least a portion of the Vitreoscilla hemoglobin protein
which contains at least a portion of the following amino
acid sequence:
5 10
Met-Leu-Asp-Gln-Gln-Thr-Ile-Asn-Ile-Ile-
15 20
Lys-Ala-Thr-Val-Pro-Val-Leu-Lys-Glu-His-
25 30
Gly-Val-Thr-Ile-Thr-Thr-Thr-Phe-Tyr-Lys-
. ~:
~, . . : .. ,, , ~,
~091/0662X PCT/~'SgO/06~1
2~ ~
-13-
35 40
Asn-Leu-Phe-Ala-Lys-His-Pro-Glu-Val-Arg-
Pro-Leu-Phe-Asp-Met-Gly-Arg-Gln-Glu Ser-
S5 60
Leu-Glu-Gln-Pro-Lys-Ala-Leu-Ala-Met-Thr-
Val-Leu-Ala-Ala-Ala-Gln-Asn-Ile-Glu-Asn-
Leu-Pro-Ala-Ile-Leu-Pro-Ala-Val-Lys-Lys-
Ile-Ala-Val-Lys-His-Cys-Gln-Ala-Gly-Val-
~00
Ala-Ala-Ala-His-Tyr-Pro-Ile-Val-Gly-Gln-
105 llO
Glu-Leu-Leu-Gly-Ala-Ile-Lys-Glu-Val-Leu-
115 120
Gly-Asp-Ala-Ala-Thr-Asp-Asp-Ile-Leu-Asp-
125 130
Ala-Trp-Gly-Lys-Ala-Tyr-Gly-Val-Ile-Ala-
135 140
Asp-Val-Phe-Ile-Gln-Val-Glu-Ala-Asp-Leu-
,., j
145 150
Tyr-Ala-Gln-Ala-Val-Glu
, ,
.
- - - . : .-.,
. , : . ~ .,.:
: ' - '~:
WO91/ll662X ~..~7~ ~ PCT/US90/06081
-14-
This amino acid sequence is disclosed in Wakabayashi et
al., supra, Nature 322:483, 1986. It is presently
believed that the protein purified and prepared through
the practice of this invention will exhibit a homology
of over 80% with this sequence. The protein of this
invention has beer. observed tv enhance functioning of
a cell in low oxygen environments (Khosla and Bailey,
unpublished results).
The amino acids represented by the foregoing
abbreviations are as follows:
Amino Acid 3-Letter Symbol
Glycine Gly
Alanine Ala
Valine Val
Leucine Leu
Isoleucine Ile
Arginine Arg
Lysine Lys
.Glutamic acid Glu
Aspartic acid Asp
Glutamine Gln
Asparagine Asn
Threonine Thr
Serine Ser
Cysteine Cys
Methionine Met
Phenylalanine Phe
Tyrosine Tyr
Tryptophan Trp
Proline Pro
Histidine His
It must be borne in mind in the practice of the present
invention that the alteration of some amino acids in a
protein sequence may not affect the fundamental
properties of the protein. Therefore, it is also
. - contemplated that other portable DNA sequences, both
those capable of directing intracellular production of
identical amino acid sequences and those capable of
directing intracellular production of analogous amino
acid sequences which also possess oxygen-binding
,:
WO91/0662X ~ ~ t~ 5 PCT/VS90/06~81
-15-
actlvity, are included ~ithin the ambit of the present
invention.
It must also be borne in mind in the practice of the
present invention that the alteration of some nucleotide
bases in a DNA sequence may not affect the fundamental
- properties of the coding sequence. Therefore, it is
also contemplated that other ana ogous portable DNA
promoter/regulator sequences are included within the
ambit of the present invention.
It is contemplated that some of these analogous amino
acid sequences will be substantially homologous to
native ~itreoscilla hemoglobin while other amino acid
sequences, capable of functioning as oxygen-binding
proteins, will not exhibit substantial homology to
native Vitreoscilla hemoglobin. By "substantial
homology" as used herein, is meant a degree of homology
to native Vitreoscilla hemoglobin in excess of 50~,
preferably in excess of 80~.
Similarly, it is contemplated that some of these
analogous DNA sequences will be substantiallyhomologous
to the sequence set forth above, while other DNA
sequences, capable of functioning as the
promoter/regulator described above, will not exhibit
substantial homology to the sequence-outlined above.
As noted above, the portable DNA sequences of the
present invention may be synthetically created, by hand
or with automated apparatus. It is believed that the
means for synthetic creation of these polynucleotide
sequences are generally known to one of ordinary skill
in the art, particularly in light of the teachings
contained herein. As examples of the current state of
the art relating to polynucleotide synthesis, one is
directed to ~aniatis et al., Molecular Cloning--A
... .
- - - : . . : -
. . . ~ - ' ' ' ~ - .
:. - , , . . ~: . .. ,.,,,. : .
wo 91/06&~ PCr/l)S90/060~1
2~ ~
-16-
Laboratory Manual, Cold Spring Harbor Laboratory (1984),
and Horvath ~ ., An Auto~ated DNA Synthesizer
Em~loyina Deoxynucleoside 3'-Phospho~a~idites, Methods
in Enzymology 154:313-326, 1987, hereby incorporated by
reference.
Additionally, the portable DNA sequence may be a
fragment of a natural sequence, i.e., a fragment of a
polynucleotide which occurred in nature. In one
embodiment, the portable DNA sequence is a restriction
fragment isolated from a genomic library. In this
preferred embodiment, the genomic library is created
from the bacterium Vitreoscilla. In other alternative
embodiments, the portable DNA sequence is isolated from
other genomic and cDNA libraries.
While it is envisioned that the portable DNA sequences
of this invention may desirably be inserted directly
into the host chromosome, the present invention also
provides a series of vectors, each containing at least
one of the portable DNA sequences described herein. It
is contemplated that additional copies of the portable
DNA sequence may be included in a single vector to
increase a host cell's ability to produce large
quantities of the desired oxygen-binding protein. It
is also envisioned that other desirable DNA sequences
may also be included in the vectors of this invention.
Further, the invention may be practiced through the use
of multiple vectors, with additional copies of at least
one of the portable DNA sequences of this invention and
perhaps.other desirable DNA sequences.
In addition, the cloning vectors within the scope of the
present invention may contain supplemental nucleotide
sequences preceding or subsequent to the portable
promoter/regulator and/or DNA sequence. These
supplemental sequences are those that will not adversely
-, .
:
~VO91/06628 z~7~ ~ PCT/~59~/0608
-17-
lnterfere with transcription of ~he portable
promoter/regulator and/or any fused DNA sequence and
will, in some instances, enhance transcription,
translation, posttranslational processing, or the
ability of the primary amino acid structure of the
resultant gene product to assume an active form.
~ A preferred vector of the present invention is set forth
J in Figure l. This vector, pWLDlO, contains the
preferred nucleotide sequence which codes for the amino
acids set forth above. Plasmid pWLDlO (and pWLD5) may
- also contain supplemental nucleotide sequences such as
terminators, enhancers, attenuators and the like. For
proteins to be exported from the intracellular space,
at least one leader sequence and any other DNA sequences
necessary or preferred for appropriate transcription and
subsequent translation of the vector DNA may be included
within the scope of this invention.
In a preferred embodiment, cloning vectors containing
and capable of expressing the portable DNA sequence of
the present invention contain various operational
elements in addition to or instead of the
promoter/regulator disclosed and claimed herein. These
"operational elements" may include at least one
promoter, at least one sequence that acts as expression
regulator, and at least one terminator codon, at least
one leader sequence, and any other DNA sequences
necessary or preferred for appropriate transcription and
subsequent translation of the vector DNA.
Additional embodiments of the present invention are
envisioned as employing other known or currently
undiscovered vectors which would contain one or more of
the portable DNA sequences described herein. In
particular, it is preferred that these vectors have some
or all of the following characteristics: (l) possess a
i
U!O9l/0662~ ~ir r~ PCT/US90/06081
minimal number of host-organism sequences; (2) be stable
in the desired host; (3) be capable of being present 1n
a high copy number in the desired host; (4) possess a
regulatable promoter; and ~5) have at least one DNA
sequence coding for a selectable trait present on a
portion of the plasmid separate from that where the
portable DNA sequence will be inserted. Alteration of
vectors to meet the above criteria are easily performed
by those of ordinary skill in the art in light of the
available literature and the teachings herein. It is
to be understood that additional cloning vectors may now
exist or will be discovered which have th~ above-
identified properties and are therefore suitable for use
in the present invention and these vectors are also
contemplated as being within the scope of this
invention.
Any strain of Streptomyces which admits stable insertion
of cloned DNA can serve as a host for the practice of
this invention. Examples of Streptomyces strains which
can be transformed or transduced are:
Streptomyces lividans 66 - Hopwood, et al.,
Genetic Manipulation of Streptomvces: A Laboratory
Manual, ~he John Innes Foundation, Norwich, 1985.
Stre~tomyces coelicolor - Hopwood, et al, loc.
cit.
Streptomyces ~arvulus - Hopwood, et al., loc.
cit.
Streptomyces fradiae - Chung, J. Bacteriol.,
169: 4436, 1987.
Stretomyces ambofaciens - Matsushima and
Baltz, J. Bac_erlol., 169: 4834, 1987.
Stre~tomyces ariseofuscus - Larson and
Hershberger, J. Bacteriol., 157: 314, 1984.
Streptomyces avermitilis - MacNeil and Klapko,
35 J. Industr. Microbiol., 2:209, 1987.
:
- :-. ,
: . .:
WO 91J066~X ~ ,5 ~ PCl/~'S90/060~il
-19-
Various vector systems will be suitable for Streptomyces
species, including plasmids, and bacteriophages The
following, noninclusive list of cloning vectors is
believed to set forth vectors which can easily be
altered to meet the above criteria and are therefore
preferred for use in the present invention. Such
alterations are easily performed by those of ordinary
skill in the art in light of the available literature
and the teaching herein.
For example, the following Streptomyces plasmids have
been used as vectors:
pIJ699 - Kieser and Melton, Gene, 65:83, 1988,
pIJ702 - Katz, et al., J. Gen. Microbiol., 129: 2703,
1983),
pHJL400 - Larson and Hershberger, Plasmld, 15: 199,
1986),
pKC505 - Richardson, et al., Gene, 61:231, 1987,
pSLP124 - Bibb and Cohen, Mol. Gen. Genet., 187: 265,
1982,
pSKO2 - Brawner, et al., Gene, 40:191, 1985,
pJAS14 - Forsman and Jaurin, Mol. Gen. Genet., 210:23,
1987, and
pARCI - Horinouchi and Beppu, J. Bacteriol., 162:406,
1985.
Phages used as Stre~tomyces vectors include derivatives
of ~C31 (Hopwood, et. al., Methods Enzymol., 153:116,
1987). See, for example, phage KC515 - Rodicio, et al.,
Gene, 34:283, 1985.
Synthesis and/or isolation of necessary and desired
component parts of cloning vectors, and their assembly
is believed to be within the duties and tasks performed
by those with ordinary skill in the art and, as such,
are capable of being performed without undue
experimentation.
-
WO91/~6628 2~ PCT/-'S90/06081
-20-
In construction of the cloning vectors of the present
invention, it should additionally be not~d that multiple
copies of the promoter/regulator with any fused gene
sequences and/or of the portable DNA sequence coding for
the oxygen-binding protein and its attendantoperational
elements as necessary may be inserted into each vector.
In such an embodiment, the host organism would produce
greater amounts per vector of the cloned protein. The
number of multiple copies of the DNA sequence which may
be inserted into the vector is limited only by the
ability of the resultant vector, due to its size, to be
transferred into and replicated and expressed in an
appropriate host.
Additionally, it is preferred that the cloning vector
contain a selectable marker, such as a drug resistance
marker or oth~r marker which causes expression of a
selectable trait by the host. In a particularly
preferred embodiment of the present invention, the gene
for thiostrepton resistance is included in vector
pWLDlO. Such a drug resistance or other selectable
marker is intended in part to facilitate in the
selection of transformants. Additionally, the presence
of such a selectable marker on the cloning vector may
be of use in keeping contaminating microorganisms from
multiplying in the culture medium. In this embodiment,
such a pure culture of the transformed host organisms
would be obtained by culturing the organisms under
conditions which require the induced phenotype for
survival.
It is noted that the portable DNA sequence of the
present invention may themselves be used as a selectable
marker, in that they provide enhanced growth
characteristics in low oxygen circumstances.
.. ... ..
.. .
. , '
- .,
:. -
, ' ' ' .
.
~'091/0662X PCT/~'S90/06081
2r~ 5
-21-
The promoter/regulators of this invention are oapable
of controlling expression of proteins or, thereby, of
controlling synthesis of metabolites normally made by
a cell, or of natural or unnatural metabolites and
proteins expressed in a cell via genetic manipulation.
This would include heterologous proteins--either
intracellular or extracellular~-as well as antibiotics
and other chemicals produced by Streptomyces cells.
This invention also relates to a recombinant-DNA method
for the production of oxygen-binding proteins.
Generally, this method includes:
(a) preparing a portable DNA sequence
capable of directing a Streptomvces host
cell to produce a protein having oxygen-
binding activity;
(b) transferring the portable DNA sequence
directly into the host, or cloning the
portable DNA sequence into a vector
~ capable of being transferred into and
replicating in the host cell, such
vector containing operational elements
for the portable DNA sequence;
(c) transferring thP vector containing the
portable DNA sequence and operational
~5 elements into the host cell capable of
expressing the oxygen-binding protein;
(d) culturing the host cell under conditions
appropriate for replication and
propagation of the vector and/or
~ expression of the protein; and
, , , : ~ . .
.
. : . .
~09l/~6628 PCT/US90/0608l
2~
-22-
(e) in elther order: i
(i) harvesting p~otein, ifdesired; and
(ii) permitting the protein to assume an
active structure whereby it possesses
oxygen-binding activity.
It is envisioned that the portable DNA sequences may be
inserted directly into the host chromosome, or
alternatively may utilize a vector cloning system. The
vectors contemplated as being useful in the present
method are those described above. In a preferred
embodiment, the cloning vectors pWLDlO and pWLD5 are
used in the disclosed method.
A vector thus obtained may then be transferred into the
appropriate Streptomyces species. It is believed that
any Streptomyces species having the ability to take up
exogenous DNA and express those genes and attendant
operational elements may be chosen. Particular hosts
which may be preferable for use in this invention
include those described above. Methods for transfer of
vectors into hosts are within the ordinary skill in the
art. For ultimate expression in Streptomyces, it may
be desirable that the cloning vector be first
transferred into another microorganism such as
Escher _ ia coli, where the vector would be allowed to
replicate and, from which the vector would be obtained
and purified after amplification, and then transferred
into the Streptomyces for ultimate expression of the
oxygen-binding protein.
The host cells are cultured under conditions appropriate
for the expression of the oxygen-binding protein. These
conditions are generally specific for the host organism,
and are readily determined by one of ordinary skill in
the art.
.
:
()9l~(l662~ PCT/US90/06081
--23- 2~. s~
It is understood that application of the teachings of
the present invention to a specific problem or
environment will be within the capabilities of one
having ordinary skill in the art in light of teachings
contained hPrein. Examples of the products of the
present invention and representative processPs fortheir
isolation, use and manufacture appear below.
INDUSTRIAL APPLICABILITY
The products and processes of the present invention find
usefulness in the production of antibiotics and the
expression of any cloned proteins using Streptomyces in
laboratory and industrial applications. The invention
provides metabolically engineered cells with enhanced
growth characteristics for increasing production of
proteins, antibiotics, or other metabolites in
Streptomyces. The invention also provides a DNA
sequence that acts as a strong transcriptional
initiation sequence for the expression of cloned
proteins in Streptom~ces.
EXAMPLE 1
Expression of a Bacterial hemoglobin in Streptomyces
Enhances Cell Growth and ~xygen Uptake Rates under
Oxygen-Limited Conditions.
A plasmid was constructed for the expression of a
bacterial hemoglobin in Streptomyces. This plasmid,
pWLD5, contains the Vitreoscilla hemoglobin gene and its
native transcriptional regulatory sequences [Khosla and
Bailey (1988) Mol. Gen. Genet., 214:158] cloned into a
common Stre~tomYces plasmid, plJ699 [Keiser and Melton
(1988) Gene, 65:83]. Specifically the 1.2 kilobase
Hind III/SphI Vitreoscilla DNA fragment containing the
hemoglobin gene was first inserted into the HindIII/SphI
site of the Escherichia coli plasmid pUCl9. This
construct was then linearized with Hindiii and ligated
- , ~''': ., ~ . :
: . - . , : . -: . , ,
- ~ -
:~ . . . . .
. ~
WO91/0662X 2~ PCT/US90/06081
-24-
i~to HindIII-cut pIJ699. The resulting plasmid, pWLD5,
was stably maintained in both E. coli and Streptomyces
lividans.
S. lividans strain TK64 (pro2, str6, obtained from Dr.
S David Hopwood, John Innes Institute, Norwich,
England) was transformed with pWLD5 DNA. A single
thiostrepton-resistant colony, designated TK64:pWLD5,
was selected for further experiments. Hemoglobin
expression in TK464:pWLD5 was confirmed by Western
analysis of total cell protein. A crude cell extract was
generated by sonication and the proteins separated by
SDS-polyacrylamide gel electrophoresis. The proteins
were then electrotransferred to nitrocellulose membrane
and screened with polyclonal antiserum generated against
pure Vitreoscilla hemoglobin. A hemoglobin band of
identical molecular weight as pure hemoglobin was
detected in the cell extracts. Hemoglobin expression
appeared to be constitutive as the levels were similar
in cells sampled from any stage of growth. Expression
of functional hemoglobin was demonstrated by a carbon
monoxide difference spectrum technique [Webster and Liu
(1974) J. Biol. Chem. 249:42573.
To investigate the effect of hemoglobin expression on
cell growth and respiration, TK64:pWLD5 was compared
with the plasmid-free strain (TK64~ under two culture
conditions corresponding to high and low aeration. The
culture medium used for the experiment was as follows:
3% dextrose, 2% N-Z amine Type I, 1% yeast extract, and
1% v/v. trace elements mix (0.1% FeSO4 7H2O, 0.1%
MnSO4 7H2O, 0.0025% CuCl2 2H2O, 0.01% CaCl2 2H2O, 0.00056%
H3BO3, 0.002% ZnS04 7H2O, o.ools% (NH4)6Mo7024 4H2O). 5
ug~mL of thiostrepton was added to the TK64:pWLD5
culture. The first condition (high aeration) was a 50
mL culture volume in a 250 mL unbaffled erlenmeyer flask
shaken at 250 rpm at 300C. The second condition (low
; . .
.
~9l/~6628 PCT/~S90/06081
-25-
aeration) was a 75 culture volume in a 250 mL unbaffled
erlenmeyer flask shaken at 150 rpm at 30OC. With high
aeration, the two strains had similar maximum specific
growth rates (0.22-0.24 hl) but the plasmid-free strain
reached a higher final cell density (OD590=7.0) compared
to TK64:pWLD5 (OD590=5.0). With lower aeration, however,
TK64:pWLD5 reached a higher final cell density
(ODs90=1.95) than the plasmid-free strain (ODs90=1.25).
This represents a 56~ increase in the final cell density
in cells expressing hemoglobin under reduced aeration
conditions. The maximum specific growth rates of the two
strains were similar (0.10-O.11 hl) under reduced
aeration. Hemoglobin expression levels in the two
strains were similar throughout the experiment as
demonstrated by Western analysis.
Oxygen uptake rates (OUR's) were compared between
TK64:pWLD5 and the plasmid-free strain throughout this
experiment. Cells were removed at various times,
washed, and resuspended in fresh medium at an ODs9~ of
O.10. The ~UR's were then measured using a Yellow
Springs instruments biological oxygen monitor. The rates
were normalized to cell weights and compared throughout
the growth curve (Table 1). Although the OUR's of the
two strains were similar throughout the experiment with
high aeration (Table lA), they were consistently higher
in the hemoglobin-expressing strain with lower aeration,
especially at the later stages of growth (Table lB). For
example, at an ODs90 of approximately 0.6, the OUR for
the plasmid-free strain was 0.22 mM O2/h-g whereas the
OUR for TK64:pWLD5 was 0.29 mM O2/h-g, a difference of
32%.
This experiment indicates that Stre~tomyces cells
expressing a bacterial hemoglobin grow to significantly
higher cell densities and have higher oxygen uptake
rates than the non-expressing strain under reduced
... , , . . ~
: ' ' - ` ' ' ' '
- ~ .
,
~'0 91/06628 r PC~/~'S90/06081
. t.~.
-26-
aeration conditions. A similar plasmid, pWLD15,containing the same Vitreoscilla hemoglobin gene
(including its transcriptional regulatory sequence)
fragment as that in pWLD5, except that it was cloned
into the opposite orientation, also expresses hemoglobin
in Stre~tomyces. This latter finding is evidence that
the expression of the hemoglobin gene originates in the
inserted fragment (originating from Vitreoscilla) as
opposed to elsewhere on the StrePtomyces-based pIJ699
plasmid.
Table lA - High aeration
Strain O.D. 590 O.U.R. (mM O2/h-g)
TK64 0.5 0.32
TK64:pWLD5 0.4 0.35
15 TK64 0.9 0.32
TK64:pWLD5 0.8 0.33
TK64 5.0 0.11
TK64:pWLD5 4.6 0.12
Table lB - Low aeration
20 Strain O.D.590 O.U.R. (mM O2/h-g)
TK64 0.3 0-35
TK64:pWLD5 0.3 0.42
TK64 9.6 0.22
TX64:pWLD5 0.6 0.29
25 TK64 2.0 <0.10
TK64:pWLD5 2.0 0.27
EXAMPLE 2
Growth enhancement of hemoglobin-expressing Streptomvces
grown under two additional conditions of reduced oxygen.
The enhanced growth of hemoglobin-expressing
Streptomyces was examined under two additional
conditions of low aeration in shakeflask cultures~
Strains TK64 (no plasmld) and TK65:pWLD5 were cultured
: :
'-:
~'0 91/06628 PCltUS90/06081
2~ S
-27-
in 12.5 and 25 mL culture volumes in 250 mL flasks for
72 hours at lS0 rpm at 30C. The medium used was the
same as in Example 16. The final cell densities were
measured at OD590. in the 12.5 mL culture, TK64:pWLD5
reached a final ODs90 of 5.8 while TK64 reached an ODs90
of only 4.0, a difference of 45%. In the 25 mL culture,
TX64:pWLD5 reached a final ODs90 of 4.5, while TK64
reached an OD590 of only 3.3, a difference of 41%. This
experiment indicates that hemoglobin expression benefits
Streptomyces cell growth under two additional conditions
of reduced culture oxygen.
EXAMPLE 3
Expression of bacterial hemoglobin in Streptomyces
coelicolor.
To demonstrate that Vitreoscilla hemoglobin can be
expressed in another streptomycete, a plasmid similar
to pWLD5 was constructed by inserting BamHI-linearized
pRED2 [Khosla and Bailey (1988) Mol. Gen. Genet.
214:158] into BgIII-digested plJ699. pRED2 contains the
identical hemoglobin sequence as pWLD5 but contains an
additional 1.5 kb of non-essential DNA. The resultant
plasmid, PWLD10, was transformed into Streptomyces
coelicolor strain M145 (SCP1~ SCP2- obtained from Dr.
David Hopwood, John Innes Institute, Norwich, England)
and a single thiostrepton-resistant transformant,
designated M145:pWLD10, was selected for further
experiments.
M145:pWLD10 cells were grown in liquid culture to
exponential phace in 50 mL YEME medium (0.3~ yeast
extract, 0.5% peptone, 0.3% malt extract, 1% glucose,
34% sucrose, 5 mM MgCl2 6H2O) at 250 rpm at 30~C. A cell
extract was prepared by sonication and the proteins
separated by SDS-PAGE and screened with anti-
Vitreoscilla hemoglobin antisera. Western analysis
-
" ~ :
': ' ' ' '
~VO 91/0662X p~S~ r?~ rj PC'r/US90/06081
-28 - .
indicated that a significant level of hemoglobin of
identical molecular weight as pure Vitreoscilla
hemoglobin was present in c~ll extracts of M145:pWLD10
but not in the plasmid-free strain. This indicates that
Vitreoscilla hemoglobin is stably expressed in another
species of Strept_m~ces.
These data also indicate that the Vitreoscilla
hemoglobin promoter element functions in S. coelicolor
to express a heterologous protein. Thus, this promoter
functions in different strains of Streptomyces.
EXAMPLE 4
Expression of Bacterial Hemoglobin in Streptomyces
coelicolor Results in Higher Final Antibiotic Levels.
Antibiotic production in Streptomyces coelicolor strains
M145 and M145:pWLD10 was compared in a shake flask
culture experiment. one mL of exponential phase cells
were inoculated into 50 mL of YEME medium (5 ug/ml
thiostrepton was added to the M145:pWLD10 culture) in
250 mL unbaffled flasks. The cells were grown at 250
rpm at 30C. Ten days later the cultures were analysed
for the production of t,he pigmented antibiotic,
undecylprodigiosin. The assay was performed by mixing
equal volumes of the culture and 0.1 M NaOH followed by
a 30" sonication'(50 Watt output) on ice. The sonicate
was then filtered through a 0.2 uM nitrocellulose
membrane. The OD4~ of the filtrate, which is a measure
of undecylprodigiosin, was then determined. While the
hemoglobin-expressing strain had an OD4~ of 1.4, the non-
expressing strain had an OD4~ of only 0.6. This
indicated that greater than twice as much antibiotic is
produced in a hemoglobin-expressing strain of
Streptomyces.