Note: Descriptions are shown in the official language in which they were submitted.
1
,c
WO 91/12354 ~~ ~ 2 ~ ~ ~ PCT/US90/04378
-1-
COMPOSITIONS AND PROCESS FOR CORROSION
INHIBITION OF FERROUS METALS
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to new and
improved corrosion inhibiting compositions, an
unexpected and new use of biodegradable corrosion
inhibitors, and to improved processes for inhibiting
corrosion of ferrous metal surfaces (susceptible to
corrosion) in the presence of an aqueous medium. More
particularly, this invention relates to corrosion
inhibiting amino acids and processes for the use of
such corrosion inhibiting amino acids effective to
inhibit corrosion of ferrous metals under use
conditions in the presence of an otherwise corrosive
aqueous medium.
Description of the Related Art
An important mechanism for protecting the
metal against corrosive deterioration is achieved
through the use of inhibitors. Unfortunately, certain
common corrosion inhibitors such as nitrogen- and
aromatic compound-containing formulations, used widely
as additives for inhibiting corrosion in aqueous
heating and cooling systems, have been found to be
hazardous to public health and to the surrounding
environment. Removal of such hazardous compounds by
precipitation or other treatments is complicated and
expensive. Other corrosion inhibitors, such as
chromatic salts have been banned from use because they
are suspected carcinogens. Consequently, it has
become desirable to examine the inhibition properties
of biologically compatible a.nd/or biodegradable
compounds. Such compounds, if nontoxic, easy to
produce in high purities, ar,~d biodegradable, can
dramatically ease the chore of removal or recycling.
Amino acids have been proposed for limited use.
WO 91/12354 PCT/US90/04378
2 0 7 2 8 81 -2-
For example, Nippon Kokoh, in Japanese
Patent J50091546-A, July 22, 1975, disclosed that
mixtures requiring both amines and amino acids or
their salts, when dissolved in water to form 20%
aqueous solutions, inhibited atmospheric corrosion of
various ferrous and non-ferrous metal sheets. The pH
of the moisture absorbed on the sheets is believed to
have been approximately 5.5 or less.
However, more extensive studies on common
l0 amino acids alone have not proven promising. For
example, in V. Hluchan et al, "Amino Acids As
Corrosion Inhibitors in Hydrochloric Acid Solutions,"
Warkstoffe and Korrosion, 39, 512-517 (1988) 22 of the
most common amino acids were investigated as
inhibitors for the corrosion of iron in 1.0 M
hydrochloric acid, at pH of about 0. Generally, those
having inhibiting characteristics at acid pH did not
demonstrate corrosion inhibition efficiencies
effective for immediate industrial use. The longer
hydrocarbon chain amino acids and those having
additional amino groups, or groups which could
increase electron density on the amino groups,
demonstrated the only tendency toward effective
corrosion inhibition.
Notably, aspartic acid, the preferred amino
acid for use in the present invention, and glutamic
acid did not come within the scope of the "tendency".
The conclusion was that such amino acids are
particularly poor inhibitors because of the single
amino group, the short carbon chain and the additional
carboxyl group.
Moreover, it is considered a drawback by
those skilled in the art to employ aspartic acid as an
inhibitor at above acid pH conditions because aspartic
acid is known to be inherently corrosive at slightly
alkaline pH conditions. See K. Ramakrishnaiah, "Role
of Some Biologically Important Compounds on the
WO 91/12354 PCT/US90/04378
_ ~p72881 ~ _3._
Corrosion of Mild Steel and Copper in Sodium Chloride
Solutions", Bulletin of Electrochemistry, ~(1), 7-10
(1986). Therein it was disclosed that aspartic acid
at a pH of 8 actually accelerated corrosion
(inhibition efficiency of -25.4%). In fact, even when
combined with an excellent corrosion inhibitor for
mild steel such as papaverine, the presence of
aspartic acid maintained the: solution's corrosiveness.
An associated prox>lem in the industry is
that fluid movement is known to increase the rate of
corrosion for ferrous metals when exposed to an
aqueous environment. Accordingly, whatever corrosive
effect which might be anticipated from amino acids
such as aspartic acid in aqueous media would be
expected to worsen, as a practical matter, if such
amino acids were present in automotive, cooling, or
heating devices where such media would be set in
motion.
Therefore, amino .acids such as aspartic
acid, although nontoxic and biodegradable, have been
avoided as corrosion inhibitors.
A process for inhibition of corrosion of
ferrous metals by using amino acids having only a
single amino group, and having an additional carboxyl
group (such as aspartic acid) under conditions wherein
such amino acids are fully ionized would represent a
surprisingly unexpected discovery while satisfying a
long-felt need in the industry. Likewise, a corrosion
inhibitor for ferrous metals which would decrease the
rate of corrosion, even under increased aqueous fluid
movement conditions, would represent a substantial
improvement in the art.
SUMMARY OF 'THE INVENTION
It is the principal object of the present
invention to provide a new and improved corrosion
inhibiting composition for ferrous metals in the
presence of an aqueous medium.
PCT/ US90/04378
2072881 -4-
It is the primary object of the present
invention to provide new and improved processes for
inhibiting the corrosion of ferrous metals in the
presence of an aqueous medium.
It is another primary object of the present
invention to provide new and improved processes for
inhibiting the corrosion of ferrous metals in the
presence of an aqueous medium under static conditions.
Still another primary object of the present
invention to provide new and improved processes for
inhibiting the corrosion of ferrous metals in the
presence of an aqueous medium under dynamic fluid
movement conditions.
It is a further object of the present
invention to provide new and improved processes for
using amino acids having a single amino group as
corrosion inhibitors for ferrous metals in the
presence of an aqueous medium.
Other and further objects of the present
invention will become apparent from the accompanying
description and claims.
It has been found that certain amino acids,
particularly aspartic acid, previously known to
accelerate corrosion of metal in mildly alkaline
aqueous media, unexpectedly function effectively as
corrosion inhibitors when fully ionized under use
conditions. Such amino acids provide a 100 to 1000
fold decrease in the corrosion rate of ferrous metals.
Surprisingly, this corrosion inhibiting effect
improves with increased fluid velocity.
BRIEF DESCRIPTION OF THE DRAWINGS
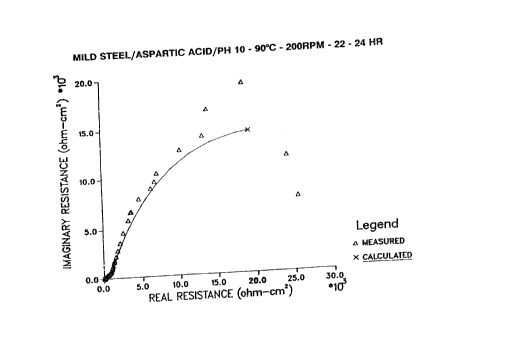
Fig. 1 shows a plot of the impedance
spectrum in real versus imaginary coordinates for a
mild steel electrode rotating at 200 rpm in an aqueous
solution at 90°C containing 1000 ppm aspartic acid at
a pH of 10.
..., WO 91/12354 2 0 7 2 8 81 ~. PCT/US90/04378
-5-
Fig. 2 shows a plot of the impedence
spectrum in real versus imaginary coordinates for a
mild steel electrode rotating at 200 rpm in an aqueous
solution at 90°C at a pH of 10 without aspartic acid,
but with conductivity adjusted with sodium sulfate.
Fig. 3 shows a plot of the impedance
magnitude versus logarithm of frequency for the mild
steel electrode in Figs. 1 and 2.
Fig. 4 shows a plot of the phase angle
versus logarithm of the frequency for the mild steel
electrode in Figs. 1 and 2.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
Useful in the present invention are amino
acids having a single amino .group and salts thereof.
Preferably, these compounds ;have an excess of carboxyl
groups over "free" amino groups, for example, two
carboxyl groups and one amino group, although a
carboxyl group/amino group ratio of 1 is suitable.
Suitable amino acids are represented by the following --..
formula:
R3 O
R~-(-NH-(-CH-)x-C-J~ R2
0
wherein R~ represents H, H2N-CH-IC- , or H2N-(-CH2-)Y ;
3 0 CFi2COOH
RZ represents HO-,HOOC-CH-NH-, or HOOC-(-CH2-)Y NH- ;
CH2COOH
R3 represents H or -COOH ;
x and y each independently represents an
integer from 1 to :3 ; and
n represents an integer for the number of
repeating aminoacy:l units.
Illustrative of suitable compounds are
glycine, polyglycine, aspart.ic acid, polyaspartic
WO 91/12354 PCT/US90/04z~H
2072$$ -
acid, glutamic acid, polyglutamic acid, and salts
thereof. Nonlimiting suitable salts include, for
example, alkali metal, soluble alkaline earth metal,
and C~-C4 alkylamine salts.
These compounds are readily available from a
number of sources and can be manufactured either by
chemical synthesis or microbial fermentation.
These compounds tend to be ineffective as
corrosion inhibitors when in the fully protonated
cationic form, and become even worse by actually
accelerating corrosion as the pH rises from acidic to
alkaline. However it has been found that once they
become fully ionized under sufficiently alkaline use
conditions, they dramatically reverse the corrosion
rate of ferrous metals. In general, alkaline pH
values of at least about 8.9, depending upon the
temperature and the specific compound employed, are
suitable. Under such use conditions, the corrosion
rate is reduced 100 to 1000 fold compared with the
rate of corrosion of ferrous metals under comparable
pH conditions in the absence of these compounds.
The corrosion inhibitors of the present
invention may be employed (in the aqueous medium) at
concentrations as low as 100 parts per million to as
high as 5.0 weight percent and above. It is
particularly preferred to utilize the corrosion
inhibitors of the present invention at a concentration
of from about 1000 ppm to about 3.3 weight percent.
It is understood, however, that concentrations greater
than 5.0 weight percent of the corrosion inhibitors
can be utilized, if desired, so long as the higher
amounts are not detrimental to the system in which the
corrosion inhibitors are employed.
The corrosion inhibiting effect of the
compositions of the present invention can be found at
temperatures as low as room temperature or about 25°C
or below and as high as about 90°C and above.
,...., WO 91/12354
PCT/US90/04378
Although temperature is known to accelerate
the corrosion of metals, it is particularly noted that
an increase in temperature does not affect the
corrosion inhibiting properties of the present
invention beyond whatever effect temperature has on
the pH. For example, the pH of the system may
decrease by 1 unit from the 'value measured at 25°C,
compared to that measured at 90°C. The pK of the
fully ionized form of the amino acid will also
decrease with an increase in temperature. However, so
long as the temperature does not decrease the pH below
the point at which the inhibitors become fully
ionized, the compositions of the present invention
will remain effective.
In a particularly preferred embodiment, the
compositions of the present .invention are employed in
dynamic, flowing systems. Surprisingly, the corrosion
rate of ferrous metals in such systems does not
increase with increasing fluid velocity. In fact,
there tends to be a significant decrease in the
corrosion rate with an increase in fluid velocity.
Normally, in the absence of 'the compositions of the
present invention, an increase in fluid velocity from,
for example, 200 revolutions per minute (rpm) to about
1000 rpm in a rotating cylinder electrode results in
an increase in the corrosion rate of ferrous metals in
the presence of such an aqueous medium during a period
of at least 24 hours. This .increase in corrosion rate
occurs commonly for steels in water and other aqueous
systems because the reduction of oxygen is often the
rate limiting step. That is, the rate of mass
transfer of oxygen to the corroding surface increases
with increasing fluid velocity.
The pH of the aqueous medium under use
conditions for the corrosion inhibiting compositions
of the present invention may vary from about 8.9 to
WO 91/12354
PCT/US90/04'~78
20728 81 _8_
about 14, preferably from about 9.5 to about 12, as
measured at ambient or room temperatures (about 25C).
It is particularly preferred to use the compositions
of the present invention at a pH of about 10 or
greater, as measured at ambient or room temperatures.
It is understood, however, as previously noted, that
the pH will vary, depending upon the temperature at
which it is measured.
Therefore, where an aqueous medium is
inherently acidic, one preferred embodiment of this
invention is to employ a suitable amino acid,
preferably aspartic acid, in compositions comprising
an effective amount of base to raise the overall pH of
the aqueous medium to above about 9.5, most preferably
at above about 9.9-10, at which pH the amino acid
exists in the fully ionized (conjugate base) form.
The pH of the aqueous medium may be adjusted
by addition of any suitable base such as an alkali
metal hydroxide, for example, sodium hydroxide and
potassium hydroxide. Additional bases which my be
employed in this invention include alkali metal
carbonates, hydrocarbylamines, alkaline earth metal
hydroxides, and ammonium hydroxides.
The pH of a corrosive environment may be
inherently alkaline, such as, for example, aqueous
solutions in contact with lime deposits, concrete, and
fertilizer, and automotive antifreeze solutions. In
such systems, corrosion inhibition may be effected by
merely adding a suitable amino acid or salt thereof in
an amount sufficient to provide in the aqueous medium
the concentrations previously described, without
having to add extraneous bases.
It is within the scope of the present
invention that the corrosion inhibitors may also be
used in aqueous media which contain various inorganic
and/or organic materials, particularly all ingredients
or substances used by the water-treating industry, the
WO 91/12354
-- ~ ~ ~ ~ ~ ~ ', PCT/US90/04378
_g_.
automotive industry, and others such as with
antifreeze compositions, metal cleaning compositions,
and radiator flush compositions.
The effectiveness of corrosion inhibition
for metal surfaces is commonly determined by
measurement of the rate of corrosion of the subject
metal under specified conditions. Two modes of
measurement of corrosion rate were employed herein.
For convenience, these may bE: referred to as (1) the
standard metal coupon mass loss test, also referred to
as static immersion test, and (2) electrochemical
impedance technique.
In the standard metal coupon mass loss test
mode, metal coupons of known mass are immersed in an
aqueous solution whose corrosion inhibiting properties
are to be determined. The aqueous media is maintained
at a specified set of conditions for a specified
period of time. At the concllusion of the exposure
period, the coupons are removed from the aqueous
solution, cleaned in an ultrasonic bath with soap
solution, rinsed with deionized water, rinsed with
acetone, patted dry with a hint-free paper towel,
blown with a stream of nitrogen, and weighed to
determine mass loss and examined under a stereoscope
at suitable magnification to determine penetration of
the metal surface due to corrosion.
Corrosion, however,, is an electrochemical
process rather than a strictly chemical reaction.
Electrochemical techniques, for example, the
electrochemical impedance technique, therefore,
provide a useful and convenicant indication of
corrosion rate. In the electrochemical impedance
technique, it is helpful to visualize that a corroding
metal surface is comprised o:E a large number of local
anodes and a large number of local cathodes whose
sites may actually shift or be at the same location as
the corrosion reaction ensuea. At the anodic site,
WO 91/123 PCT/US90/04378
2~ 7288"1" ~~ -1°-
the metal is being oxidized, while at the cathodic
site reduction is occurring, reduction of hydrogen
ions in acidic solutions. The magnitude of the
current, in amperes per centimeter squared (A/cmZ), at
the open circuit potential, as measured relative to a
reference electrode, is a measure of the tendency for
the respective reaction to proceed. This corrosion
current density is referred to as the "corrosion
rate". In many instances, corrosion rate is converted
l0 to "penetration rate" of corrosion, in mils per year
(mpy), or mass loss, by assuming, for example, two
electrons per ionized iron atom.
The "electrochemical impedance technique" is
applied wherein the frequency at an electrode
interface is varied, using a small voltage amplitude
wave of, for example, 5 to l0 millivolts (mV). The
response is used to estimate the corrosion rate and to
draw some conclusions about the corrosion mechanism.
Analysis of the impedance spectra provides a term
called the "polarization resistance", measured in ohm-
centimeter squared (ohm-cm2), which is inversely
proportional to the corrosion current density
(corrosion rate). Accordingly, the corrosion rate, in
accordance with Ohm's law (i = V/Rp), equals
proportionality factor (for the subject metal),
measured in volts, divided by the polarization
resistance. For example, a common proportionality
factor for carbon steels is 0.025 volts. And since
the polarization resistance is inversely proportional
to the corrosion rate, relative degrees of
polarization resistance are used to determine the
degree to which various compositions will either have
lower or higher corrosion rates. Thus, a polarization
resistance of 100 ohm-cmZ is created by a corrosion
rate that is about 100 times faster than a corrosion
rate having a polarization resistance of 10,000 ohm-
cm~. A polarization resistance of 100 ohm-cm2
WO 91/12354 1~ ~ 7 2 ~ 8 1 ~ PCT/US90/04378
-11-
represents a corrosion rate on the order of about 100
mpy, while that of 1000 ohm-cm2 represents a corrosion
rate on the order of about 10 mpy. Conversion of
polarization resistance to corrosion rate (as mpy) can
be made by assuming a proportionality constant of 25
mV and Faraday's law.
For a primer on th.e electrochemical
impedance technique, see D. C. Silverman, "Primer on
the AC Impedance Technique," in ~,lectrochemical
Techniques for Corrosion Eng~ineerinq (R. Baboian,
ed.), National Association of Corrosion Engineers,
Houston, 1986, pp. 73-79.
The following specific examples illustrating
the best currently-known method of practicing this
invention are described in oletail in order to
facilitate a clear understanding of the invention. It
should be understood, however, that the detailed
expositions of the application of the inventions,
while indicating preferred embodiments, are given by
way of illustration only and are not to be construed
as limiting the invention since various changes and
modifications within the spirit of the invention will
become apparent to those sk~.lled in the art from this
detailed description.
In the following examples, unless otherwise
specified, all parts and percentages are by weight,
all temperatures are in degrees Celsius ('C), pH was
measured at 25'C, and "mass loss" is intended to mean
"penetration rate".
EXAMPLE 1
The electrochemical impedance technique was
used to estimate corrosion :Eor two mild steel (C1018)
electrodes, labelled as Samples A and B. The
parameters and results are :shown in Table 1 and Table
2.
Steel coupons were fabricated to be used as
electrodes in a rotating cylinder electrode apparatus.
I
2~~2g~~
-12- 43-21(7746)A
The apparatus is described in detajil in D. C.
Silverman, "Rotating Cylinder Electrode for Velocity
Sensitivity Testing," in Corrosion, 40 (5), 220-
226(1984). The electrochemical impedance technique is
described in detail in D. C. Silvetrman and J. E.
Carrico, "Electrochemical Impedanbe Technique - A
Practical Tool for Corrosion Prediction,", in
Corrosion, 44(5), 280-287 (1980.
The cylindrical electrode was fabricated
from mild steel (C1018). ThE: electrode was sanded
with 600 grit silicon carbidE: paper prior to immersion
in the solution to be investigated. Also, the
solution was heated to the desired temperature of 90'C
prior to immersing the electrode. The electrode was
mounted on a cylindrical shaiEt, then immersed and set
to rotate at 200 rpm in order to guarantee turbulent
flow conditions. The water :line spas at the center of
the upper Rulon~ [graphite-impregnated
poly(tetrafluoroethylene), E.I. d~ Port de Nemours &
Company] spacer to prevent hydrodynamic and effects
from interfering with the results to insure optimal
flow and current lines.
In situ data, tabulated in Table 1 (as
Sample A) was obtained by exposing the mild steel
electrode to a sodium aspart;ate solution at a pH of l0
in the rotating cylinder apparatus. The ppm of the
sodium aspartate was approximately 1000. The
temperature was adjusted to 90'C, although the pH was
measured at 25'C.
In a similar manner, in situ data, tabulated
in Table 2, was obtained for Sample 8, except that
sodium aspartate was absent <~nd instead, the same
ionic strength was achieved using', sodium sulfate
(which has no material effect on corrosion).
Corrosion potentials were measured for the
steel electrode employed for each of Sample A and
Sample B by measuring the voltage between the steel
t :a
WO 91/12354 ~ ~ ~ ~ ~ ~ ~ PCT/US90/04378
- -1:3
electrode and a saturated calomel electrode. The
electrodes for each of Samples A jand B were rotated at
various velocities over identical exposure times. The
polarization resistances were determined as described
in Silverman and Carrico, Ib~ and were used to
estimate the corrosion rates which were converted to
the penetration rate or mass loss in mils per year
(mPY)
Impedance spectra for the steel coupon
electrodes (Samples A and B) were generated at a pH of
10 in each of the aqueous solutions employed for
Samples A and B and at 200 rpm, using the rotating
cylinder electrode apparatus. These spectra (curves)
are shown in Figs. 1, 2, 3, and 4. The agreement
between the calculated curve and the actual data
demonstrates how well the model used to obtain the
polarization resistance agrees with the actual
results. The localized nature of~the attack noted for
the static immersion test under cbmparable conditions
(in Runs 4 and 5 of Example 2, below) was absent on
the rotating cylinder electrode. This behavior
suggests that the presence of a uniform velocity field
advantageously enables the aspartic acid to inhibit
corrosion more uniformly. In addition, the increased
uniform inhibition suggests that the process is aided
by the smoother 600 grit used for the electrode, as
compared to the 120 grit finish fbr the coupons used
in the static immersion tests. The net result of the
smoother finish is that the surface topography of the
electrode was less heterogeneous than that of the
static immersion coupons. As such, more uniform
velocity and a smoother steel surface decreased the
aspartic acid concentration required to inhibit
corrosion uniformly on all parts df the surface.
WO 91/12354 PCT/US90/04378
20~288~ 14
SABLE 1
CORROSION OF MILD STEEL WITH 1000 PPM SODIUM ASPARTATE
(pH = 10, adjusted at 25'C)
Exposure Rotation Polarization Estimated
Time (hr) Rate (rpm) Resistance Corrosion
Sohm-cm2l Rate
LDY
0.5 200 361 32.0
4 200 4530 2.5
6 1000 13950 0.80
23 200 40160 0.29
1000 138300 0.09
47 200 92340 0.13
49 1000 2170800 0.01
50 200 1103800 0.02
20
Sample A corrosion potential is -310mV (S.C.E)
TABLE 2
25
CORROSION OF
MILD STEEL
WITH SODIUM
HYDROXIDE
(pH = 10, adjusted at 25C)
Exposure Rotation Polarization Estimated
Time (hr) Rate (rpm) Resistance Corrosion
~ ohm-cm2 ) Rate
Smpy)
0.5 200 256 45
4 200 296 39
6 1000 167 69
23 200 226 51
25 1000 144 80
47 200 245 47
49 1000 241 47
200 289 40
Sample B corrosion potential is -630 mV (S.C.E.)
"~, WO 91/12354 PCT/US90/04378
2072881 ~-15-
As Tables 1 and 2 indicate, the corrosion
potential of Sample A with sodium aspartate is -310 mV
(S.C.E.), while the corrosion potential of Sample B
without sodium aspartate is :Ear more active at -630 mV
(S.C.E.). This difference between the corrosion
potentials suggests that the sodium aspartate has a
greater tendency to oxidize lthe steel surface.
Nevertheless, the corrosion rates of the
respective samples reveal a reverse relationship to
this oxidation tendency. The magnitude of the
difference between the corro:aion rates of Sample A vs.
Sample B after identical exposure times demonstrates
that the aspartate inhibits corrosion by 100 to 1000
times. Although corrosion began at about the same
rate for both Sample A (32 mpy) and Sample B (45 mpy),
the rate quickly decreased in the presence of sodium
aspartate while it remained very constant in its
absence.
Moreover, in the absence of the aspartate,
an increase in the rotation irate or fluid velocity
resulted in an increase in corrosion rate at least up
to 24 hours into the run. A:Eter 48 hours, there was
no change, most likely because of a corrosion product
build-up on the surface. This behavior is normal for
carbon steel and water becau:~e the reduction of oxygen
is the rate limiting step. The rate of mass transfer
of oxygen to the corroding surface often determines
the corrosion rate, this rate of oxygen transfer can
be affected adversely when corrosion products build up
on the surface. However, in the presence of the
aspartate, the corrosion rate did not increase with
the increasing velocity. In fact, there was a
significant decrease in corrosion rate with increase
in rotation rate consistentl!~r throughout the runs.
The decrease in corrosion rai-~e, achieved by increasing
velocity, seems to be irreversible because even after
the rotation rate is subsequeantly reduced to 200 rpm's
WO 91/1; PCT/US90/04378
-16-
as noted from the rates in Table 1 determined at
exposure times of 46-48, 49, and 50 hours, the
corrosion rate did not return to the 200 rpm 0.13 mpy
rate that the sample had prior to increasing the fluid
velocity to 1000 rpm.
Accordingly, a sodium salt of aspartic acid,
under basic conditions, performs as a corrosion
inhibitor for ferrous metals in an unexpected fashion.
The impedance spectra themselves were
studied as a function of the rotation rate or fluid
velocity using the rotating cylinder electrode over a
48 hour period. Plots at 200 rpm and after 24 hours
are shown in Figs. 1, 2, 3, and 4.
Two peaks exist in the phase angle plots for
mild steel in contact with sodium aspartate. This is
shown in Fig. 4. Such behavior suggests two
relaxation time constants which, in turn, suggests
that either a strongly adsorbed intermediate or a
tightly adherent film is involved in the corrosion
mechanism. The high frequency peak is attributed to
the adsorbed intermediate on the film, while the low
frequency peak is related to the corrosion rate.
Accordingly, while not desiring to be bound by any
theory for corrosion mechanism or to limit the present
invention in any way, the aspartate ions are believed
to form some type of adsorbed layer on the steel
surface, even though the mechanism is not completely
understood. Further evidence of the presence of some
type of adsorbed layer on the steel surface in the
presence of aspartate ions is provided by the phase
angle plot for mild steel under comparable conditions,
but in the absence of aspartate ions. In such plot,
which is also shown in Fig. 4, there is only one peak
which suggests that only the charge transfer corrosion
reaction is occurring.
~WO 91/12354 PCT/US90/04378
-1'-~ 2 0 7~2 8 8 1 ~f
PL:~ _
Fourteen identical mild steel (C1018) coupon
specimens were sanded using 120 grit silicon carbide
paper, rinsed with deionized water, dried, and
weighed. Thereafter, the specimens were subjected to
static immersion tests. The parameters and results
are reported in Table 3, below. The specimens were
hung on glass hooks in glass jars, each containing
about 600 cm3 (or cc) of the L-aspartic acid test
solution. The solutions were prepared using deionized
water and L-aspartic acid in an amount sufficient to
provide the desired aspartic acid concentration. The
hooks were mounted through rubber stoppers which
sealed the tops of the jars. A gas sparger waterwas
introduced at the side of the stopper for aeration of
the solutions with water-saturated air from which
carbon dioxide had been removed. The jars were placed
in constant temperature baths in which the temperature
was maintained at 90°C. The coupon exposure times
were 5 to 7 days, during which time deionized water
was periodically added to the aspartic acid test
solution to compensate for water loss via evaporation
at the elevated temperatures. The pH of each solution
was adjusted at the beginning of the test by use of
sodium hydroxide and was measured at both room
temperature (RT, approximately 25°C) and at the
temperature of the test.
At the conclusion of the coupon exposure
times, the coupons were removed from the solutions,
cleaned in an ultrasonic bath with soap solution,
rinsed with deionized water, rinsed with acetone,
dried, and weighed. The coupon surfaces were examined
under a stereoscope at between lOX and 30X
magnification after exposure. Corrosion rates were
estimated in the manner previously explained by
measuring the weight change (both before and after
exposure to the aspartic acid solution) and then
WO 91/12354 PCT/US90/04378
~07288~_ -18-
calculating the penetration rate or mass loss in
either mpy or grams per hour. In those cases in which
corrosion was extremely nonuniform or localized to
certain areas on the surface, only the mass loss in
grams divided by the total exposure time in hours was
reported. The reason is that corrosion rate averaged
across the entire surface does not accurately describe
the magnitude of corrosion if corrosion occurs in very
confined areas. Nevertheless, the results in Table 3
from the static immersion test as compared to the
results in Tables 1 and 2 from the constant flowing
system, demonstrate that under fairly stagnant flow
conditions, there is an increase in the required
concentration of aspartic acid needed to accomplish an
equivalent level of corrosion inhibition.
2 0728 81 .
..-". PCT/US90/04378
WO
91/12354
- 19
-
m c a,
o, .-, o U
w .-, ..~ --~ ro
c
a ~ o a
a
b .a . m >.
o c
'' ~: ' > a ro
m "
b o a > .x a r ,~e a
r t
11 C N .x tt m U 'U
-.1 U
't:
~ptl N ~ U .~! a C
m a -a
IJ 41
m tT ..a a U ..t C ~ G1 ..a
O m ~ C1
.a
U U v O -1
-.1 t C J~ y~
a U
J~
.a ,~ or a ~ o d a -a
..a x
o a o ar at a ..t w tr o .-~ 3
a
w U 0 m at tr O 'o a a
>'r tr
c0
O B m ~ O C 41 fa 0t
N w O O
.a m O -t C ~1 ~ tT
C O t a~
I'.
01 H m -.1 0~ i.r 01 C
.x ~ s~
w C U m w m a m G1 ro N
tT m L~ l7
U
a a to O >r ~ -a 'o t U
a ~t
p ,!C 01 U Gl a t 3 C U C
y at t
Iv
i ~! U C X m ~1 U fr ' ro
C W U C
11 Iy
m . a O N a a dv it 01 m .a
U a a Yt
a t~
a
y a r~ -a " d ,, ~ m .c m m a
--
y~ ~ m w w U W m ro .C1
. 4t y U
w ~
o ,~ a o o ro x o o x
m E ro -.a
o
o, a tr CL d ~ U ,-t w
sr O ' 3
o m b s~ m w at o a o
m cn o o
y
H v w ~ O a c at a o ~ m
y c c ~ .-I~ x
ro ~
o at U a~ ro ~ +~ m m .
a ~w o ~ b
ar at
t~ 3 sr U ~ ro c a .-a U .~
s~ o w
o at .a ~ a .a w o E o ro o
U ai o ..r
a e c~
y ro c~ w ro a +r' -.a ~ t
m U . .c
a N
O t H ..a -.I .-1 t +~ +~ m
O a m t~ .-1 m
U t7 m
~
y tr d ro c d .-I o~ a ro d
m >', w a a ro d
tr ro w
~ ~~~ ow '~~c '' ''
~~oo
ro o .u c c
. O.
x cn c~ a cn v~ m ..-I z +~
s~ U m ro cn H +~
a ro m a
ro
H
a
a.
a ~ o~ o 0 0 ~o
~, M
.-, ~ .-~ ~ .-r .-,
w
m ~ N 1f1 O O~ t0 M
m y, et h m n rn o
~ N a 0 0
o
o .-t . t
:
a,' m ,,, , . . O . O I
OI O O O O
E' H
z a
O
ri ri ri
. ~ 1 . .
H i ~ n i ; o
a
0
w
a
H U U U U
U U U
w o 0 o H H O H O H O
O
H ~' a' ao~ xo~
a a a
o o o
~ ~ ~
m, ~
er mr
er ~r
O O .-I O a0 O Ca
r'1
O O 0~ N O N O
O~
O~ tD O N N ri N ~i ri
m ~ r
N
H C
H
W t EC ~ fC ~C it
t
H '
U U U U U U U
w~ ~ ~ ~ ~ ~ ~ w ~ ~ a
~ ~ ~
s
c ~
3
" a ~ ~ wa w~'' aa,
~
a.w w a .
'
m m m m m N m O m N
N N t~
R', ~ ~ ~ ~ O ~ O rt O
O O O O
1 i 1 I 1 O 1 O t O
O O O O
V a,~ a.1 a.~ a.~ ai a.1 a
0
z
a
0
Q; .1 N f'1 d' If1 t0 f~
tf1 O tf1 O If1 O tn
,-I e-1 N N f'1 f'7
WO 91/12354 PCT/US90/04'~78
- 20 -
~0728 81
w G1
-
~ m
m
p, m ro y .a y ro
'C7 O O m Tf y y U ~ C
y
O y d .-r m C ro 01 01 O
ro U
tr 0! O G1 .a G1 U w 1a ..1
.C
41 ~ m b Ir G O y C 01 H +~
y y 0!
y ro m .-1 U O G1 C ro x 'O ro
U 01
a ~ ro ~ o
~
ro c c ' E
i o s~
E
.
a c w a m c ar ai ~ i -a .,
ar
m C O C ~ O x m U y m
w d
O U 1.a ...1 y ..1 11 I ~
b U O m
t~
c.a m oy,c m ro ro ~ a~ a,ro ro
a o
p .,a y U O O ~ r-~ 'p ~ t1
f.~ y
m
W ro 01 H w y C 01 C 01 41 Cl
0 ro
H m s~ d c w ro -a .c a a a >
~s .~ ~ m
~ m o o ~ ~ w m w b m
m ~ .
c
o
W ro c x y --~ m d m s~ -a
.c m E
y d ro C U a 01 w 'O O b O a C
U b
m w m -.~ ro 3 x c .~ w o ..~
E ro -r .r
a~ .a
~ m y y ~, o m a o v ro
o
m y w ro .c ar ro c m .c y y .c
w y ,c
or
...1 U ro C y O m m m il~ U
~ G! ~ 'C ~ O
O m
o ~ W G! y G) N y .1 ro 01 U y
G~ m 01 H 01
a y 07 C~ U ro y
O O
Cl
a ~ ; ~ m c v ro ro .a .c x .c
, ~c
O s~ y c y wo y V y d 3 y
O y
H o .a . .c c sr .c O d ac
m 7C ro .a m x
a
V .~ m rn ro a o ,-~ c x ar o
x ai a~ m v c
.~
ro c -a a m ~ ro U O U Ir --a
3 m 3 .a ro
ro O
. ..~ ..a ~ .a ro ro a .-r
..a E o .a w ..,
~
A m .a m 3 w d m ro y y y y m
c c c .c ~ y
V y O 1r .1 S.~ sr ..1 y U ~ U
fr ..-1 m a U
>, ro >~ C .1 >, 01 ro ro a >~
C7. G1 b O 01 m 01
01
~ a a w s o~ y s~ > y a y w
a .o y y ~
~ v ~ b z m >
~ w > m a ~
> v > o ~ E z .
v
H
x
a
w
,o ~ ~ ~o ~o 0
w o vo .1 ~..~ c~ ~o
.-i .1 ~ ~ .-m -i .1
W ~ ~ o ~ \
M W m ~ ~ ~ ~ a
., o
H m
W ~ ~ ~ o
,. a o o o o o
a
m C7i 0 0 0 0 0 0 O
m
ro
x
w
ro
0
o o 0 0 0
H i
v~ ai
W E
a
V U U U U
H U
V [~ o H O H O H O H O U
p
W xo, H ao~ xo~ xo~ xo~
H ~~
~r ~ a ~r ~ ~ er w m
v~ v
0
er v er ~' O .1 N N .~ .~ .wo
G1. a, a, ~ ~ ,n fy . . . . . . . X
. .
O p~ O O~ O O~ .1 O r1 .-1 N
ri r1 ri r1 ri r1
1r
fi
.~ ~ '~ ~ b b
H ~ ~ ~ ~ ~ ~ EC ro
H
~ U U U U U U U 0
~
W "a ...~ ..-1 ...1 -.1 -.i -.1
c ro ~' ~ ~ ro m ~ ~ ~ ro ~ ro
3 v
a~ W W ~ f~ ~ GL 3
CL C4 f~ do
3
~ ~ ~ o ~ ~ a m
o ~ ~ c o t o o o E
U aN a a~ a~ a~ a~ a~ ro
~
.
a~
0
ro
c o
a ao o~ o ..a N ~ ~ E.,
~
w o m o
w o m
,.i ri N N c7 c'1
WO 91/12354 2 ~ T 2 8 81 ~ p~/US90/04378
-21.-
XAMP:LE 3
The procedure described in Example 2 was
employed, except that the solutions did not contain
aspartic acid and only three steel coupons were
subjected to the static immersion test. The solutions
were adjusted to have the same conductivity as those
containing aspartic acid by the addition of sodium
sulfate, thereby limiting the corrosion to that
created solely by oxygen contained in the water at the
designated pH. The results are set forth in Table 4.
WO 91/12354 PCT/US90/04378
-22-
TABLE 4
STATIC IMMERSION T~~~~°~LD STEEL WITHOUT
Run No. pH Total Mass Loss Comments
mpy -gt hrt-
1 8.0 @ RT 12.4 0.0987/119 Severe general
7.1 @ 90°C corrosion across entire
surf ace .
2 10.0 @ RT 21.4 0.1725/119 Severe general
8.7 @ 90°C corrosion across entire
surf ace .
3 12.0 @ RT 0.30 0.0024/119 Some stains which have
10.4 @ 90°C appearance of pitting
initiation sites.
Total grams per total hours exposure time.
At a pH of 8, the corrosion rate is higher
in the presence of aspartic acid than in its absence
when the results of Runs 2 and 3 from Table 3 are
compared to those of Run 1 from Table 4. This tends
to confirm that at a pH of 8 there is no beneficial
corrosion inhibition from aspartic acid; instead, it
behaves as a corrosion accelerator. The same behavior
is found at a pH of 9.5 and a concentration of 3
weight percent aspartic acid (Run 10 of Table 3).
Such behavior is attributed to the fact that at a pH
of 9.5 or less, the aspartic acid does not exist in
the completely or fully ionized (conjugate base) form.
This is clearly evidenced by an observed change in
behavior at a pH higher than 9.5, for example, at a pH
of 10 and higher, even at levels of aspartic acid as
low as 1000 ppm. Virtually all corrosion disappears
under the static test conditions of Table 3 at
concentrations of 1 weight percent at a pH of 10 (Run
9). Thus, at pH values between 8.5 and about 9.0, as
measured at 90°C, or at a pH of 10 or higher at room
temperature (approximately 25'C) aspartic acid, under
static conditions, inhibits corrosion whereas it
increases or accelerates corrosion at a lower pH.
WO 91/12354 ~ 0 7 2 8 8 1 ~A PCT/US90/04378
-2:3-
The fact that some: attack or corrosion is
noted at concentrations of 7.000 ppm (Runs 4 and 5) and
5000 ppm (Run 8) at a pH of 9.9 to 10 does not mean
that the aspartic acid does not inhibit corrosion at
those concentrations. The large areas of no attacks
strongly suggest that aspart:ic acid is indeed
inhibiting corrosion. This apparent inconsistency
results from the inability of the aspartic acid to be
distributed uniformly on the: steel coupon under the
stagnant flow or static conditions in the immersion
test runs. EXAMF~LE 4
Steel coupons were' fabricated to be used as
electrodes in the rotating cylinder electrode
apparatus described in Example 1 at three different pH
levels (8, 10, and 12) for aspartic acid solutions
containing 1000 ppm aspartic: acid. A fourth coupon
was subjected to the same procedure (for comparison
purposes) at a pH of 10, except that aspartic acid was
omitted and the solution was adjusted with sodium
sulfate to have the same conductivity as if aspartic
acid were present. Corrosion was estimated using the
electrochemical impedance technique described in
Example 1. The results are shown in Table 5.
Electrochemical impedance spectra were
generated to 0.01 hertz (hz;l after about 30 minutes to
obtain an estimate of the corrosion rate at short
exposure. Thereafter, speci~ra were generated to 0.001
hz at 200 rpm each day. In addition, spectra were
generated to 0.01 hz at 1000 rpm to obtain estimates
of the effect of fluid velocity on corrosion.
Experiments were run at pH ~~alues of 8, 10, and 12
with 1000 ppm of aspartic acid and at a pH of 10 in
the absence of aspartic acid. The amplitude of
perturbing voltage signal w,as small (5 mV) to insure
that linearity existed between perturbation and
response.
WO 91/12354 PCT/US90/04'~78
-24-
The steel electrodes were weighed both
before and after the experiment. The mass loss was
used to make an additional estimate of the corrosion
rate. Note that at a pH of 10 and especially 12, the
mass losses were affected by water seepage behind the
electrode. The polarization resistances were
estimated using the circuit analogues shown Figure 2
of Silverman and Carrico, Ibid.
The results of the rotating cylinder
electrode experiments show that under ideal conditions
of fluid velocity, aspartic acid concentrations at
least as low as 1000 ppm can decrease the corrosion
rate to the order of 0.1 to 0.5 mpy from the 50 to 100
mpy exhibited in its absence (at a pH of 10). In the
absence of aspartic acid, fluid motion increases
corrosion until the surface becomes so corroded that
the velocity profile is affected near the surface.
This dependance is expected for corrosion of mild
steel and low alloy steels in water. However, in the
presence of aspartic acid at a pH above 9.5 (measured
at room temperature), corrosion is decreased by fluid
motion.
~ ~ ~ ~ ~ ~ ~
WO 91 /12354 PCT/US90/04378
_2~~_
TABLE 5
ELECTROCHEMICAL
IMPEDANCE
RE;SULTS
FOR MILD
STEEL AT
90C
Exposure Rotation Polarization ElectrochemicalCorrosion
Time (hr) Rate (rpm) Resistance impedance Rate Mass
(ohm-cm~ Loss (mpvl
AsDartic Acid Solution - 1000 ppm
pH = 8 @ 25C
0.5 200 2T1 84
1 200 32'3 71
3-5 200 204 112 90
20-22 200 200 114
23 1000 12.8 179
24 200 1516 117
pH = 10 25C
0.5 200 -----
3-5 200 41Et0 5.5
21-23 200 137Et0 1.7
24 1000 682Ei0 0. 33
25 200 25000 0.91 2.7
55 200 395510 0.58
117-119 200 367F30 0.62
3 0 120 1000 419F30 0 . 54
DH = 12 25C
0.5 200 32280 0.71
3 5 3-5 200 352:30 0. 65 Water
22-24 200 39790 0.57 Seepage
25 1000 39800 0.5? Behind
26 200 32580 0.71 Electrode
45-47 200 1139!50 0.20 Spacer
4 0 48 1000 278000 0.080
49 200 12001)0 0.19
No As~ar~tic Acid
pH = 10 25C
45
0.5 200 2:56 89
3-5 200 2'96 77
22-24 1000 167 137
25 200 2.26 101 5?
50 26 1000 143 160
45-47 200 245 93
48 1000 241 95
49 200 288 79
WO 91/12354 PCT/US90/Oaz~B
-26-
202881
EXAMPLE 5
This Example demonstrates that a precorroded
surface can be protected by the corrosion inhibitors
of the present invention. The results shown in Table
6 were determined by exposing a steel cylinder
electrode precorroded in deionized water in the
rotating cylinder electrode apparatus described in
Example 1 with 2000 ppm of sodium sulfate (to have
l0 about the same conductivity as 1000 ppm aspartic acid
at a pH of 10) and 50 ppm of sodium chloride. In 24
hours, the electrode suffered a significant mass loss
and had a red-brown rust layer. This electrode was
placed in an aqueous solution having an aspartic acid
concentration of 5000 ppm and adjusted to a pH of
about 10 with sodium hydroxide and held under constant
rotation. The polarization resistance quickly
increased over 24 hours, indicating that the corrosion
rate decreased with exposure time. The corrosion rate
never decreased to the value of an electrode not
precorroded and exposed to 1000 ppm aspartic acid.
Compare, for example, the results shown in Table 1.
This difference indicates that the concentration was
not optimized for this particular system. Of greater
significance, however, is the observation that even
1000 ppm aspartic acid can inhibit corrosion of steel,
even precorroded steel, under the proper conditions.
""., WO 91/12354 ~ ~ ~ ~ ~ ~ ~'~ PCT/US90/04378
-27-
TABhE 6
ELECTROCHEMICAL IMPEDANCE FOR MILD STEEL IN ASPARTIC
ACID AT 90 ° C' EFF~'1'BIyFI~L~'~URB~BIDQ~ ON CORROSION
Exposure Rotation F~olarization Corrosion
Time (hr) Rate (rpm) Ptesietance Rate by Mass
(ohm-cm21 Loss (mpvl
Pre-Corroded in Water at vH = 5.75. 90°C
5-7 200 242 71
17-19 200 87 (81 mpy by
21 1000 182 impedance)
Immersed Electrode in !5000 pom Aspartic Acid
~1~H = 9.91 @ 25°C1
0.5 200 610
4-6 200 1520
19-21 200 2980
22 1000 5400 Not
24 2000 19000 Determined
42-44 200 6020
45 1000 >10000
EXAMPLE 6
Static immersion tests were conducted as
described in Example 2, except that glutamic acid,
5 glycine, and certain acids commonly used in anti
freeze formulations were employed in place of the
aspartic acid. The parameters and results are shown
in Table 7. As can be seer, glutamic acid and
glycine, respectively, show behavior and corrosion
10 inhibition similar to the aspartic acid. While
slightly more staining of t:he coupon was observed, the
mass loss was similar to that with aspartic acid at
the same pH. In addition, these results reveal that
aspartic acid behaves comparably to that of a mixture
15 of benzoic acid, sebacic acid, and octanoic acid at
90°C. Because acids such as the latter-named acids
are commonly used in anti-:Freeze formulations, the
results for aspartic acid (Runs 11 and 12 in Table 3
of Example 2) indicate that aspartic acid may be
20 employed as a suitable substitute for such acids. The
WO 91/1235 PCT/US90/04't78
207288~I
ammonium salt of aspartic acid, as can be seen from
Table 7, does not appear to function as well as the
sodium salt because the pH decreased to 7.7, a pH
below the point at which aspartic acid (as the
conjugate base) can exist in the fully ionized form.
20 7288 1
WO /12354
91
PCT/US90/04378
- 29
-
d
E ~
C C 1 0! C WO
r 'O
~ C C a ro
C
v .. x .- 'C - H
1 ~1 l O ~ x
O
m m w a al a o
U .c v
.c
0 o ar m
ro .a ro
m
~ H a ~ N
f i ~
o 1 r O~ C t
r . ~
~
o v o ~ .-1 c al a v
ro .c ro
ro
.c
a w U i C ~1 .C ~
-~1
y
U C f i~
O r O
.~ H ~ O GI ~ m m ..
C l
C
01
b a Id r-1 J.1 C: m 01
~1 U C
U
L1 l~ m 1.a m w W ..1 m p
C -.a
C
w d m a~ H a a m -.~
o ai
ro
E~ C C C E C CI ~r
.-1
m o ar a~ m a m
..~ ~
a .a
ro
~ w a 'A d 3 .o
r H m H
D
rt ~ w % b w ~ 0 ~ C 0
O 1 C
a
. 0 x ,. a ~ ~ ro
1 .,
~
1 m 'C 1r 'O 'O U .C m
La 0! >r
1~
.G
N O N w m >, ~ ~ m 1r
a O~ a O
+~
U ~ C U C O H 1r L~ m m
m ~ ~
o O 41 d1 H ro 01 N m a C
i~ m
O x 0! x m N > rt U m
x 0l ,.> ~
U 1r U b 41 7C b
U 1~ GI ~
'O
GI
~ a ~ C ~ ~ 1~
~ -.~
4~
.a
U .- .- .~ O m
1 i~ l 1 ~
i! 1~
rC ,C7 .G a C x m x b m
C ~ C 01 m
N ~0 d O -~ a w U m V .-1 H
N
a
m
o d d .~ m o a o a o ro
-a .n
o a
o
H U m v ro H ~ .a ,~ .c .-~
c m m o,
,c .a
U ro m a H o a m ~ " m a
c~ m Ts
c
w o w a~ ro a m a d v
> o H ,..,
H .,.,
m
H H H > H d cl m sr H
al H ro a
a~
m
' ' ~ v o ro
b ~ ro c
~ ~ x z v
n n s ~ v
d
~
H
x
a
n.
o,
a .
~ a, ce .i ao o~ a
w .~ ~ .-~ w .~ .-~ r,
w w ~ ~ o~ w w w
~ W n r~ u, o o .
H .
, N
0 0 o o o
0
N m o1 0 0 0 0 0 0 0
x ro
x
x
w
ro
w ~ ~
1 1
H ~ ~ 1 1 0 0 0
5
w
w
x
;~ U U U U
U U
o Ea E O E~ E-~ O E-~
O O O
a ~' a~ ao~ xo~ ac,
a
o o
~ ~
~~ gym, gym,
o ~~ ~~
N d' N ~!' O CD O O O
O~
N ~
O O~ O O~ N O N O O
OD
w O r O ri ri .-1 r1 ri ri
~ r1
N
U
H
O ~ ~ ~ ~ ~ ~ ~
. - .. . .. - .
.1 .I 1 ~ 1 .I 1
~ ..a -Va ..Va . Va
~ VI
, . -. E
E p ~ ~ ~ ao
~0 O
p a
~ O ~J 1~ ~1 i~ ~J O
O O O O
a o a a o a o a o a o a
~-1
U .~ .-i .a .a .-r .i .i .1 .-a
.1 .1 .-~ .1 e~
C9 rl C9 O -i C9 C~ C9 i C~
~ ~
O
x
c
a
a .~ N ~ ~r ~, ,a
w o w o w o In
rl r-1 N N c'1 M
2 0 7 2 8 81 ~t.~
WO PCT/US90/04378
91/12354
_ 3 ~ _
c
C
~ b U m
0l G O m O m
N
y ' '~
'
a ro
,4 m m a o
C m ~ U
b a .a a
ra Z' J~
~ ~ N
.- m a al
1 U
-.1 C
O
O
p , m ~ t~ 0
C .
-i
w
~ '~ o
b ~
b .. a ~
a -
o~
~ v o m y x m ~
b
3
~ ~
~
~
m ~ ~ m G
C .
v ~ a m .~ ~
.c
o ~
p O a .-1 ~ U Sa .~G C
O
a 1.t a U
01
U
~ ~
d U G! d
p w O~ ~ ~ O ~
a
~ ' N a
U
..-1 J.~ -.1 Ql 4
J.~ W
C1
s a ~
O C
U ~ a O . ! O
~ C 0
,.., cn z ~ w o v v~ .c:
..a
a
H
x
a
a.
~o
.~ .-,
w w
m tn ~D N ~O
m
w a '~ 0
a o o
m c1 0 0 0
ro
x
x
o .-,
w a
o '
0
E, o 0
d
H
U U U
U O
w a o a o a o
~ ~ ~
E
.,
x ~ ~r ~ ~ mr ~ m
o
o e, e,
o an o r o ~ ~ r x
o ao o ao o ao a
.1 0~ r m
w
O
N O
x
U
O 'd dv ~ 'a
O
~
H ~ O U rt U m O
~
~
E do
' c o ro ~
~ ~
te
~ -. ..
a a
N ~ m
U O U 3 a .C O t7 m
y N b U
U
U ,~ .- t
O c~ 0
c~ as ~n a
o w
cr
0
x a
0
ao o~ o ~ H
,n o w o ~n
-I N N
207288
WO 91/12354 -PCT/US90/04378
'w -31-
EXAMPLE 7
Static immersion tests were conducted as
described in Example 2, except that polyaspartic acid
at concentrations from between 2000 ppm to 3.3 percent
and polyaspartyl hydroxamic acid (to show the effects
of the absence of the amino group of the amino acid)
at 90°C were employed in place of aspartic acid. The
parameters and results are shown in Table 8. The 2000
ppm concentration was chosen so that the carboxyl
concentration would be similar to that of aspartic
acid at 1000 ppm. Corrosion inhibition was found for
pH values of 9.5 and higher when measured at 25°C
(which converts to a pH of about 8.4 at 90°C). This
is very similar to the pH threshold for aspartic acid,
and would suggest that higher loading of polyaspartic
might be required for total :inhibition on all surface
sites to occur on heterogeneous surfaces, though a
significant degree of inhibiition was observed at 2000
ppm. It is anticipated, however, that under higher
fluid velocity such as that used with the rotating
electrode, the corrosion inhibition properties of
polyaspartic acid would increase.
Polyaspartyl hydro:xamic acid, which does not
contain an amino group, showed poorer inhibition at
the same concentration as aspartic acid.
WO 91/12354 - 3 -
2
PCT/US90/04378
207288
m
m
~ w ~ w
ro ro
.- . ro a ~ c
a c
x w ~ v m c b
o m m ~'
m w d O o I 4 b
x
G1 ro G
~ C ~
b
[-r . c fr .C ~
-~ ~ O
0l m
m ~o ~ b o a cr ro
~ ro
o
.a v .a ~o o -a >
~ ar
.a
o c a r~ ,~ a ...~ o m
ro x ..a
m s,
H ' .-, o ro v v ro ~
o m m
sa, ro
c E ~ o ~ x >. ,
~ d w w
.., .r o m v ro
y s~
o
v . ro ~ ar ~ E s,
w ro ro
d
sr
v i ~ ~ v ~ a
o > ~
v a
x x v.
o
H a U .-1 O O H ro c 0
m w
C
E, t~ ro --r --a a~ ~
ro d O 0 a
ro
a ~ 3 b ~ o ro a
b ~
3 . v
s c ro
r a
ar ~ ro > m g w m ~
ro
.a ' w
o
i ~
-.
a
ar
..
.~ .-~ c ro c .~
d ~ ro
c m
ro ro .a.~ a m m oro
oro v o
.c .c sr o~ x m c w
s~ ro w
m +~ d w .c s~ c o m
ro . w
c
w
a c w v d ro 0 .~
O .~ o ro
0
0
>, c~ .~ ~ ~ v ro >,
m ~
ro
c~ s~ o~ d or ro m
m m
~
> ar 3 ro a c
w m ~
-m~ E a
~
x
,,a . ~ ,
W G~ y G! o~ d ro ~
ii d . ~
m w ro
. ro , c
W E ~ ~
ro
m
m ..r > ~ ~ ro
> a d ~
v ~ m i m -.~
~ a~
~
v
v ~ ro o w~
~ o
o
W H t~1 f1 fn r1 M
a m ~ ~ ~ a
m .~ .~ .~ .,
,~ o
E~ p a ~ o ~ m o
o ~ o 0
w m
m . .o ~ 0 0 0
~ ~ o .-i o 0 0
ro
i o i i
W E ~ ~
~, E i i
E
Ei
U U U U
W 0 V a o 0 0l
E' ~ a ~
0 a
ao~ ~~ o o o
~ ~ ,
H W r1 .1 W O O t~ X
t~ ~
o,n . . . . . . v
o o ao o~ o a~
ao ao
~ ~ m
W w
H c
.
c
o o ~ ~ a ~
V ..v i .. .. . ~, ro
a . i a
H ro.C~ 4 i 4 ~ V
~
s w -. o
r ~ a
a ~ a w w ' w w a
o ~
E-~ c 3 m m ~ m ~ m ~ ~
o m
X s~
~N ~~ ~~' ~" ~ '
a a - - b~
U ~ ~ ~ ~
~ ~ ~n E
ro
v~
0
x
ro
ri N f1 ~' M y)
a o
a H
m o w o w o
H H N N M
2072881_ -
WO 91/12354 -33- 'CT/US90/04378
EXAMPLE 8
This Example demonstrates that the
compositions of the present inventions are effective
as corrosion inhibitors at relatively low
temperatures.
Static immersion teats for steel in water at
a pH of 10 with no inhibitor, 3% aspartic acid, or 3%
polyaspartic acid at 30°C were conducted as described
in Example 2. The parameters. and results are shown in
Table 9. Both the aspartic acid and the polyaspartic
acid imparted significant corrosion inhibition under
these relatively low temperature conditions (10.0 mpy
decreased to less than 0.1 mpy with no localized
corrosion).
- 3 4
-
WO 91/12354 PCT/US90/04Z78
~072$$~
m
3 d m
U
a ~ ~ rl
o G ro ro
M G1 !1
w ~ ro
Ea
+~ .c
m
U
m m
m
O ~ Qt
d
V .. .a
a
V m
m
H w3
O
V m m
.-1 m m
C ro
b ~
a
O O~ x b .~t b
U
V ro O
W
0 O
i~ m
ro a~
z ~
H ~ z y
a~ D
m ~ ~ :
m
N
cx a ~
,
o
d
m ., o
~ "
~
~n
E~
a
ro o
N
~
v O
Ea
x
w o
N
Z
O 0, r~ ~ O
o
0 o .G
r., .i
W rI
ro
0
o w ~~ ~ ~ +i
V .~ +~
o
w
~ ~ ro
a v ~ ~ m
ro i
m ~ ~ b
l:
U Z r7 a P~ et
~i
O
N
O
C
tL1 O 1n O
r-I e-I N
2072881_
WO 91/12354 -35- PCT/US90/04378
Thus, it is apparent that there has been
provided, in accordance with the present invention,
compositions and a process for inhibiting corrosion of
ferrous metals in the presence of an aqueous medium
that fully satisfy the objects and advantages set
forth hereinabove. While the invention has been
described with respect to various specific examples
and embodiments thereof, it is understood that the
invention is not limited thereto and many
alternatives, modifications, and variations will be
apparent to those skilled in the art in light of the
foregoing description. Accordingly, it is intended to
embrace all such alternatives, modifications, and
variations as fall within the spirit and broad scope
of the invention.