Note: Descriptions are shown in the official language in which they were submitted.
2 ~ 7 ~
HOECHST AKTIENGESELLSCHAFT HOE 91/F 211 Dr.WE/St
Description
Salts of pyridylsulfonylurea6 as herbicides and plant
growth regulators, their preparation and their use
It has been disclo6ed that some 2-pyridylsulfonylureas
have herbicidal and plant-growth-regulating properties;
cf. EP-A-13,480(US-A-4,435,206), EP-A-272,855
(US-A-4,838,926),EP-A-84,224 (US-A-4,629,494),
US-A-4,421 550, ~P-A-103,543 (US-A-4,579,583),
US-A-4,946,494,US-A-4,487,626, EP-A-125,864
(US-A-4,723,991), WO 88/04297, EP-A-178,101
(US-A-4,756,742~.
2-Pyridylsulfonylureas having ~pecific radicals in the
3-position of the pyridyl radical have already been
proposed as herbicides and plant growth regulators in PCT
Patent Application No. PCT/EP 90/02308 (WO-91/10660).
Salts of the compounds are only mentioned in this
publication as alternative use forms of the herbicides.
It has now been found that certain agriculturally utiliz-
able salts of 2-pyridylsulfonylureas having specific
radicals in the 3-position of the pyridyl radical are
particularly suited as herbicides and growth regula$0r3.
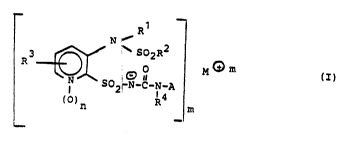
The present invention relates to compounds (salts) of the
formula (I)
Rl 1
~502-N-C-N-,I ¦
~)n R4
_ _
2 0 ~ 3 ~S
- 2 -
in which
Rl is H, (Cl-C6)alkyl which is unsubstituted or
substituted by one or more radicals Qelected
from the group comprising halogen, (C1-C4)-
alkoxy, (cl-c~)alkylthior (C,-C,)alkylsulfinyl,
~C,-C,)alkylsulfonyl, [(C~-C~)alkoxy]carbonyl
and CN, (C3-C~)alkenyl which is unsubstituted
or substituted by one or more halogen atoms,
(C3-C6)alkynyl which is unsubstituted or sub-
stituted by one or more halogen atoms, (C,-C,)-
alkylsulfonyl which is unsubstituted or sub-
stituted by one or more halogen atoms, phenyl-
sulfonyl wh~re the phenyl radical i8 unsubsti-
tuted or substituted by one or more radicals
selected from the group comprising halogen,
(C,-C,)alkyl and (C,-C,)alkoxy, (Cl-C~alkoxy or
[(C1-C4)alkyl]carbonyl, which is unsubstituted
or substituted by one or more halogen atoms,
R2 is ( Cl-C4 )alkyl which i5 unsubstituted or sub-
stituted by one or more halogen atoms, phenyl,
where the phenyl radical is unsubstituted or
substituted by one or more radicals selected
from the group co~prising halogen, (Cl-C4)alkyl
and (C,-C4)alkoxy, or is di-[(C,-C4)alkyl]amino
or
R1 and R2 together are a chain of the formula -(CH2~o~
where the chain can additionally be substituted
by 1 to 4 (C,-C3)alkyl radicals, and o is 3 or
4,
R3 i6 H, (Cl-C~)alkyl, preferably (C,-C3)alkyl,
(Cl-C3)haloalkyl, halogen, NO2, CN, ( Cl-C3 ) -
alkoxy, (Cl-C3)haloalkoxy, (C,-C3)alkylthio,
(Cl-C3)alkoxy-(C,-C3)alkyi, t(C,-C3)alkoxy]car-
bonyl, ( C,-C3 )alkylamino, di-[(Cl-C3)alkyl]-
amino, (Cl-C3)alkylsulfinyl, (Cl-C3)alkylsul-
fonyl, SO2NR~Rb or C(O)NR~Rb,
RA and Rb independently of one another are H, (C1-C3)-
alkyl, (C3-C4)alkenyl or propargyl, or together
are -(CHz) 4-, - ( CH2 ) 5- or -CH2CH2OCH2CH2-,
` - 3 - ~ 73 ~ '3 ~
R4 is H or CH3,
n is zero or 1,
m i8 1 or 2,
A is a radical of the formula
N ~
~0~
N
5 X and Y independently of one another are H, halogen,
( Cl-C3 ) alkyl, (C1-C3)alkoxy or ( C~-C3 )alkylthio,
the abovementioned alkyl-containing radicals
being unsub~tituted or mono- or polysub~ituted
by halogen or mono- or disubstituted by
(C,-C3)alkoxy or (Cl-C3)alkylthio, furthermore a
radical of the formula NRsR6, (C3-C~)cycloalkyl,
(C2-C4)alkenyl, (C2-C4~alkynyl, ~ C3-C4 )alkenyloxy
or ( C3-C4 )alkynyloxy,
Z i~ CH or N,
15 R5 and R6 in~ependently of one another are H, (Cl-C3)-
alkyl or ( C3-C4 ) alkenyl, and
M is an atom ~elected from the group comprising
the alkali metals, alkaline earth metals or a
group of the formula Ml
R~
R7-N-R9 (M l)
R'
in which R7, R8, R9 and R10 independently of one
another are H, (C,-C,2)alkyl, (C3-C6)alkenyl,
(C3-C6)alkynyl, (C3-C8)cycloalkyl or phenyl, the
last 5 radicals mentioned in each case indepen-
dently of one another being unsub6tituted or
sub6tituted by one or more radicals ~elected
from the group comprising halogen, (Cl-C6)-
alkyl, hydroxyl, (C1-C~)alkoxy, thio, ( Cl-C4 ) -
alkylthio, [(C,-C4)alkoxy]carbonyl and
` 2~73.13P~
- -- 4 --
optionally substituted phenyl, or two of the
radicals R7 to R10 together are a saturated or
unsaturated chain of 3-7 carbon atoms, it being
possible for 1-2 carbon atoms to be replaced by
atoms selected fxom the group compri~ing 0, N
or S and for the chain to be substituted by 1-3
(Cl-C~)alkyl radicals, and the remaining two
radicals have the abovementioned meanings of
individual radicals R7 to R10.
In formula (I) and hereinafter, alkyl, alkoxy, haloalkyl,
alkylamino and alkylthio radicals and the corresponding
unsaturated and/or substituted radicals can in each case
be straight-chain or branched. Alkyl radicals, also in
composite meanings such as, inter alia, alkoxy and
haloalkyl, are, for example, methyl, ethyl, n- or
i-propyl or n-, i-, t- or 2-butyl; alkenyl and alkynyl
radicals have the meanings of the unsaturated radicals
which are possible and which correspond to the alkyl
radicals, such as 2-propenyl, 2- or 3-butenyl, 2-propynyl
or 2- or 3-butynyl. Halogen is fluorine, chlorine,
bromine or iodine; haloalkyl is alkyl which is substi-
tuted by one or more atoms selected from the halogen
group; haloalkyl i8, for example, CF3, CHF2 or CH2CF3.
Optionally substituted phenyl i5 preferably unrubstituted
phenyl or phenyl which is substituted by one or more,
preferably 1 to 3, radicals selected from the group
comprising halogen, alkyl, alkoxy, nitro, cyano, alkoxy-
carbonyl, alkamoyl, carbamoyl, mono- and dialkylamino-
carbonyl, mono- and dialkylamino, alkylsulfinyl or
al~ylsulfonyl, preferred alkyl-containing radicals being
those having 1 to 4 carbon atoms.
Preferred compounds of the formula I are those in which
R3, Ra, Rb and A are as defined above and n = zero,
Rl i8 H, tC1-C4)alkyl which is unsubstituted or
substituted by one or more halogen atoms or by
a radical selected from the group comprising
~C1-C3)alkoxy, (Cl-C3)alkylthio, (Cl-C3)alkyl-
` - 5 - 2~73:~3~
sulfonyl, [~cl-c4)alkoxy]carbonyl and CN, or is
~ C3-C, ) alkenyl, ~C3-C4)alkynyl, ~C1-C,)alkyl-
sulfonyl, ~Cl-C3)alkoxy or ~Cl-C4)alkyl]car-
bonyl, and
R2 is (C1-C4)alkyl which i8 unsubstituted or sub-
stituted by l to 3 radicals selected from the
halogen group, or
R1 and R2 together are a chain of the formula -(CH2)o-
~in which o is 3 or 4.
Particularly preferred compounds of the formula ~I) are
those in which
Rl i8 hydrogen, ( C~-C4 ) alkyl or halo(C~-C~alkyl,
R2 i8 ( Cl-c3 ) alkyl or halo(C1-C~)alkyl,
R3 is H, (Cl-C3)alkyl, ( C~-C3 ) alkoxy, halogen or
(C1-C3)alkylthio,
z CH or N,
X is (C1-C3)alkyl, (C1-C3)alkoxy, (C,-C3)haloalkyl,
~C~-C3)haloalkoxy, (C1-C3)alkoxy-( C~-C3 )alkyl or
(Cl-C3)alkoxy-(Cl-C3)alkoxy, and
Y is halogen, (Cl-C3)alkyl, (Cl-C3)alkoxy or
(C1-C3)alkylthio, it being possible for the
last 3 radicals mentioned to be monosubstituted
or polysubstituted by halogen or monosubstitu-
ted or disubstituted by radicals selected from
the group comprising (C1-C3)alkoxy and
(C1-C3)alkylthio,
or a radical of the formula NR5Rs in which Rs
and Rs have the abovementioned meaning, or
(C3-C6)cycloalkyl, (C2-C,)alkynyl, (C3-C.)-
alkenyloxy or (C3-C,)alkynyloxy.
X is preferably (C1-C2)alkyl, (C1-C2)alkoxy, OCF2H, CP3
or OCH2CF3 and
Y is preferably (Cl-C2)alkyl, (Cl-C2)alkoxy, halogen or
OCF2H .
M is preferably Na, Li, R, Ca or a group of the
formula M1
- 6 - 2 ~7 ~.~3~j
NR7Re~9R'0 (M1)
in which R7, R8, R9 and Rl independently of one
another are H, (Cl-Cl2)alkyl, ( C3-c, ) alkenyl, ~ C3-C4 ) -
alkynyl, (C5-C6)cycloalkyl, [(Cl-C4)alkoxy]carbonyl-
(Cl-C4)alkyl, hydroxy-(Cl-C4)alkyl, phenyl, benzyl,
l-phenylethyl or 2-phenylethyl, or
M is preferably piperidine, pyrrolidine, morpholine or
pyridine.
The present invention furthermore relates to processes
for the preparation of the compounds of the formula (I),
which comprise reacting a compound of the formula (II)
N ~ R
R3 ~ ~ 502R2
N SO2N~-C-N-A
~)n o R4
in which A, Rl, R2, R3, R4 and n are defined as in formula
(I) as claimed in claim l, with a base of the formula
(IIIa) or (IIIb)
Mm~ (R"O )m (Illa)
(Mm~)pC032 (Illb),
in which M and m are defined as in formula (I) and
R1l is H or an aliphatic or aromatic organic radical,
preferably (C1-C,)alkyl, and p iB the number 2 in the
event that m = l and the number l in the event that
m = 2,
or~ in the event that R10 = H, reacting a compound of the
formula (II) with an amine of the formula (IV)
2~3.
- 7 -
R7
N ` (l~
R8 ~ R~
i n
which R7 to R9 are defined as in formula M1.
The reaction is carried out for example in the presence
of solvents which are inert under the reaction con-
ditions. Suitable solvents are inorganic solvents such aswater or organic solvents, for example alcohols, such as,
for example, methanol or ethanol, halogenated hydro-
carbons such as dichloromethane, ethers such a~ tetra-
hydrofuran or dioxane, ketones such as acetone or MIBR
(methyl isobutyl ketone), amides such as DMF, nitriles
such as acetonitrile, and sulfoxides such as DMS0. The
reaction temperatures are mostly between 0C and the
boiling point of the solvent.
An analogous process with reaction condition~ is
described in W0 90/06308.
The products of the formula (I) can be isolated in
virtually quantitative yield and in good purity, in some
caser directly by filtration or, if they are readily
soluble, after removing the solvent by distillation.
~he starting materials of the formula (II) are known or
can be prepared analogously to known processes. Their
synthesis is described, for example, in DE-A-4,000,503.
Most of the bases of the formulae (IIIa) and (IIIb) are
standard reagents and in some ca~es commercially avail-
able even on an industrial scale. They are, for example,alkali metal hydroxides, alkali metal alcoholat~, alkali
metal carbonates, alkaline earth metal hydroxides,
alkaline earth metal alcoholates or alkaline earth metal
carbonates, but also quaternary ammonium hydroxides, such
as, for example, tetrabutylammonium hydroxide.
The bases of the formula (IV) comprise ammonia, primary,
2~73:l3~
-~ - 8 -
secondary and tertiary amines such as, for example,
methylamine, diethylamine, triethylamine, diethanolamine
or benzylamine, but also heterocyclic amines such as, for
example, pyridine, pyrrolidine, piperidine or morpholine.
The baseæ of the formulae ~IIIa), (IIIb) or (IV) are
employed, for example, in amounts of 0.5 to 1.5 mol,
preferably 0.9 to 1.1 mol per mole of sulfonylurea of the
formula II.
The compounds of the formula (I) according to the inven-
tion have an excellent herbicidal activity against a
broad range of economically important monocotyledon and
dicotyledon harmful plants. The active substances also
act efficiently on perennial weeds which produce shoots
from rhizomes, rootstocks or other perennial organs and
which are difficult to control. In this context, it does
not matter whether the substances are applied before
sowing, pre-emergence or post-emergence. Specifically,
examples may be mentioned of some representatives of the
monocotyledon and dicotyledon weed flora which can be
controlled by the compounds according to the invention,
without the enumeration being a restriction to certain
species.
Examples of weed species on which the active substance
acts efficiently are, from amongst the monocotyledons,
Avena, Lolium, Alopecurus, Phalaris, Echinochloa, Digi-
taria, Setaria and also Cyperus species from the annual
sector and from amongst the perennial species Agropyron,
Cynodon, Imperata and Sorghum, and also perennial Cyperus
species.
In the case of the dicotyledon weed species, the range of
action extends to species such as, for example, Galium,
Viola, Veronica, Lamium, Stellaria, Amaranthus, Sinapis,
Ipomoea, Matricaria, Abutilon and Sida from amongst the
annuals, and Convolvulus, Cir~ium, Rumex and Artemisia in
the case of the perennial weeds.
`~ ~
9 ~ ' 3.~
The active substances according to the invention equally
effect outstanding control of weeds which occur under the
specific conditions of rice growing, such as, for ex-
ample, Sagittaria, Alisma, Eleocharis, Scirpus and
S Cyperus.
If the compounds according to the invention are applied
to the soil surface before germination, then the weed
seedlings are either prevented completely from emerging,
or the weeds grow until they have reached the cotyledon
stage but then their growth stops, and, eventually, after
three to four weeks have elapsed, they die completely.
If the active substances are applied post-emergence on
the green parts of the plants, growth likewise stops
drastically a very short time after the treatment and the
weed plants remain at the growth stage of the point of
time of application, or they die completely after a
certain time, so that in this manner competition by the
weeds, which i8 harmful to the crop plants, is eliminated
at a very early point in time and in a sustained manner.
Even though the compounds according to the invention have
an excellent herbicidal activity against monocotyledon
and dicotyledon weeds, crop plants of economically
important crops, Quch as, for example, wheat, barley,
rye, rice, maize, sugar beet, cotton and soya, are
damaged not at all, or only to a negligible extent. For
these reasons, the present compounds are highly suitable
for selectively c~ntrolling undesired plant growth in
plantings for agricultural use.
Moreover, the substances according to the invention have
outstanding growth-regulatory properties in crop plants
They engage in the plant metabolism in a regulating
manner and can therefore be used for influencing plant
constituents and facilitating harvesting in a targeted
manner, for example by triggering desiccation and stunted
growth. Moreover, they are also suitable for generally
- 10- 2~73:~3~
controlling and inhibiting undesirable vegetative growth
without destroying the plants in the process. Inhibition
of vegetative growth is highly important in many mono-
cotyledon and dicotyledon crops since lodging can thereby
be reduced or prevented completely.
The compounds according to the invention can be applied
in the form of wettable powders, emulsifiable concen-
trates, sprayable solutions, dusting agents or granules
in the customary formulations. The invention therefore
also relates to herbicidal and plant-growth-regulating
compositions which comprise the compounds of the formula
(I).
The compounds of the formula (I) can be formulated in
many ways, depending on the prevailing biological and/or
chemicophysical parameters. The following are examples of
formulations which are possible: wettable powders (WP),
water-soluble powderc (SP), water-soluble concentrates,
emulsifiable concentrates (EC), emulsion~ (EW) such as
oil-in-water and water-in-oil emul~ions, sprayable
solutions, suspension concentrates (SC), dispersions on
an oil or water basis, oil-miscible solution~, capsule
suspensions (CS), dusts (DP), seed-dressing agents,
granules for broadcasting and soil application, granules
(GR) in the form of microgranules, spray granules, coated
granules and adsorption granules, water dipersible
granules (WG), water-soluble granules (SG), ULV formula-
tions, microcapsules and waxes.
These abovementioned formulation types are described, for
example in: Winnacker-Ruchler, "Chemische Technologie
tChemical Technology]", Volume 7, C. Hauser Verlag
Munich, 4th Ed. 1986; Wade van ValXenburg, ~Pesticides
Formulations", Marcel Dekker N.Y., 1973; K. Martens,
Spray Drying Handbook", 3rd Ed. 1979, G. Goodwin Ltd.
London.
The formulation auxiliaries required, fiuch as inert
11 2~73:~3~
materials, surfactants, ~ol~ent~ and other additives are
also known and are described, for example, ins Watkins,
"Handbook of Insecticide Dust Diluent and Carrier~",
2nd Ed., Darland Books, Caldwell N.J., H.v. Olphen,
"Introduction to Clay Colloid Chemistry"; 2nd Ed.,
J. Wiley & Sons, N.Y.; C. Marsden, IlSolvents Guide";
2nd Ed., Interscience, N.Y. 1963; McCutcheon~s
~'Detergents and Emulsifiers Annual~, MC Publ. Corp.
Ridgewood N.J.; Si61ey and Wood, ~Encyclopedia of Surface
Active Agents~, Chem. Publ. Co. Inc., N.Y. 1964;
Schonfeldt, 'IGrenzflachenaktive ~thylenoxidaddukte
[Surface-active Ethylene Oxide Adducts]", Wiss.
Verlagsgesell., Stuttgart 1976; Winnacker-Kuchler,
"Chemische Technologie [Chemical Technology] N ~ Volume 7,
C. Hauser Verlag Munich, 4th Ed. 1986.
Based on these formulations, it is also possible to
produce combinations with other pesticidally active
substances, fertilizers and/or growth regulators, for
example in the form of a readymix or a tank mix.
Wettable powders are preparations which are uniformly
dispersible in water and which, besides the active
substance, also contain ionic and non-ionic ~urfactants
(wetting agents, disper~ants), for example polyoxyethy-
lated alkylphenols, polyoxyethylated fatty alcohols and
fatty amines, fatty alcohol polyglycol ether sulfates,
alkanesulfonates or alkylbenzenesulfonates, sodium
ligninsulfonate, sodium 2,2'-dinaphthylmethane-6,6~-di-
sulfonate, sodium dibutylnaphthalenesulfonate, or alter-
natively sodium oleoylmethyltaurate, in addition to a
diluent or inert substance.
Emulsifiable concentrates can be prepared, for example,
by dissolving the active ~ubstance in an inert organic
solvent, for example butanol, cyclohexanone, dimethyl-
formamide, xylene or also higher-boiling aromatic
compounds or hydrocarbons, with the addition of one or
more emulsifiers. Examples of emulsifiers which can be
- 12 - 207~
used are: cslcium ~alts of an alkylarylsulfonlc acid,
such as calcium dodecylbenzenesulfonate, or non-ionic
emulsifiers, such as fatty acid polyglycol esters,
alkylaryl polyglycol ethers, fatty alcohol polyglycol
ether~, propylene oxide/ethylene oxide condensation
products, alkyl ethers, sorbitan fatty acid e~ters,
polyoxethylene sorbitan fatty acid esters or poly-
oxethylene sorbitol esters.
Dusts can be obtained by grinding the active substance
with finely divided solid substances, for example talc or
natural clays, such as kaolin, bentonite and
pyrophyllite, or diatomaceous earth.
Granules can be produced either by spraying the active
substance onto adsorptive, granulated inert material or
by applying active substance concentrates onto the
surface of carriers, such as sand, kaoliniteæ or granu-
lated inert materials, by means of binders, for example
polyvinyl alcohol, sodium polyacrylate or, alternatively,
mineral oil~. Suitable active substances can also be
granulated in the manner which is conventional for the
production of fertilizer granules, if desired in a
mixture with fertilizers. Water-dispersible granules are
generally prepared by the customary processes such as
spray drying, fluidized-bed granulation, disk granu-
lation, mixing with high-speed mixers, and extrusion
without solid inert material.
The agrochemical preparations generally comprise 0.1 to
99% by weight, in particular 0.1 to 95% by weight, of
active substances of the formula (I).
The active substance concentration in wettable powders
is, for example, about 10 to 90% by weight; the remainder
to 100~ by weight comprises conventional formulation
components. In the case of emulsifiable concentrates, the
active substance concentration can be about 1 to 85% by
weight, mostly 5 to 80~ by weight. Formulationq in the
- 13 - 2~73~3~
form of dust~ usually contain about 1 to 25% by weight,
mostly 5 to 20% by weight, sprayable solutions about 0.2
to 20% by weight, mostly 2 to 20~ by weight. In the case
of granules, the active substance content depends partly
on whether the active compound is liquid or solid. The
active substance content in the case of the water-
dispersible granules is generally between 10 and 90% by
weight.
In addition, the active substance formulations mentioned
comprise, if appropriate, the adhesives, wetting agents,
dispersants, emulsifiers, penetrants, preservatives,
antifreeze agents and solvents, fillers, colorants and
carriers, defoamers, evaporation inhibitors, pH regula-
tors and viscosity regulators which are conventional in
each case.
For use, the formulations, present in commercially
available form, are diluted, if appropriate, in a cus-
tomary manner, for example by means of water in the case
of wettable powders, emulsifiable concentrates,
dispersions and water-dispersible granules. Preparations
in the form of dusts, granules for soil application or
for broadcasting as well as sprayable solutions are
usually not further diluted with other inert substances
before use.
The formulations are applied, for example, to the plants,
parts of the plants, ~eeds of the plants (seed dressing)
or the area under cultivation.
The application rate required of the compounds of the
formula (I) varies as a function of the external con-
ditions such as, inter alia, temperature, humidity, andnature of the herbicide used. It can vary within wide
limits, for example between 0.001 and 10.0 kg/ha or more
of active ingredient, but it is preferably between 0.005
and 5 kg/ha.
- 14 - 2~73~3~
If appropriate, mixtures or mixed formulation~ with other
active substances such as, for example, insecticides,
acaricides, herbicides, safeners, fertilizers, growth
regulators or fungicides are also possible.
A. Chemical examples
a) Sodium salt of 3-(4,6-dimethoxypyrimidin-2-yl)-
l-t3-(N-methyl-N-methylsulfonylamino)pyrid-2-yl-
sulfonyl]urea (Tabulated Example 1)
3.0 g (0.13 gram-equivalent) of sodium are dissolved
in 300 ml of methanol. 58.0 g (0.13 mol) of 3-(4,6-
dimethoxypyrimidin-2-yl)-1-[3-(N-methyl-N-methylsul-
fonylamino)pyrid-2-ylsulfonyl]urea are added to this
solution at room temperature, and the mixture is
subsequently refluxed for 30 minutes. The mixture is
cooled, and the solvent is removed in vacuo, giving
60.7 g (100% of theory) of the abovementioned sodium
salt of melting point 203-205C (decomp.).
b~ Triethylammonium salt of 3-(4,6-dimethoxypyrimidin-
2-yl)-1-~3-(N-methyl-N-methyl 8U lfonylamino)pyrid-
2-ylsulfonyl]urea (Tabulated Example 12)
To a solution of 2.23 g (5 mmol) of 3-(4,6-di-
methoxypyrimidin-2-yl)-1-[3-(N-methyl-N-methylsul-
fonylamino)pyrid-2-ylsulfonyl]urea in 50 ml of
dichloromethane there is added 0.51 g (5 mmol) of
triethylamine, and the mixture is refluxed for
30 minutes. The mixture is cooled, the solvent is
removed in vacuo, and the residue is triturated with
diethyl ether. This gives 2.5 g (91% of theory) of
the abovementioned triethylammonium salt of melting
point 99-101C.
c) Lithium salt of 3-(4,6-dimethoxypyrimidin-2-yl)-
l-t3-(N-methyl-N-methylsulfonylamino)pyrid-2-yl-
sulfonyl]urea (Tabulated Example 2)
2073~3~
- 15 -
~o a solution of 0.21 g (5 mmol) of lithium
hydroxide (monohydrate) in 50 ml of methanol there
are added 2.23 g (5 mmol) of 3-(4,6-dimethoxypyri-
midin-2-yl)-1-[3-(N-methyl-N-methyl 8ul fonylamino)-
pyrid-2-ylsulfonyl]urea, and the mixture i8 refluxed
for 30 minutes. The mixture is cooled, the solvent
i8 removed in vacuo, and the residue is triturated
with diethyl ether. This qives 2.2 g (98% of theory)
of the abovementioned lithium salt of melting point
247-249C (decomp.)
The compounds in Table 1 below are obtained analogously
to the processes of Examples 1-3.
2 ~
-- 16 --
~ r-
~¦ N
c~æ . _--_ ___
2~73~3~
-- 17 -
=_ _ _ ____ _ _ _ _
~ _ ~ __- ___ _- _ _ . _ _
:i~ I . I I z ~:
N =
~ O = = = = = = = . = = = =
X O = = = = = = =
I . . = = = =
I = = = = = = =
~ _ ~ = ~ e ~ ~
r~
-- 18 -- 2 ~ 7 3 1 ~ ri
1~ .
~--~ ~ ~
1-- I' . __
:, .~ ~ ~1 ~ ("~ L~
N (,) __=. = =
= = = =
I
x O = = r =
~: I
_ I
~ O = = = = =
U N N N N N
2 ~ 3 ~
-- 19 --
r _ ~ __ l r ~ _
~ u ~
1~ æ L ~ L ~ l
. ~ ~
~- I _ _ _ = . _ _ T _
X I _ _ O' _____T . _
C~: I I : : _ ~
I _ _ - _ . T _
~ L
- 20 - 2~3~3~
_____ ............... __ __ ~ _ __
N _ , __ N N _ ~ _ ~ N _
I _ ___ _ ~ I
S V S~ Z ~ 5 g I IN Z ~ S O
__ __ _ _
= = Z = ~ . = . V .. . =
T _ _ _ __
~ O ~ _ = s O = = =
~ r
1~
_ _ .
~ _
~ ~ ~ ~ O ~ ~ ~ ~ ~ ~ ~ ~ ~ O
2~731~
-- 21 --
T = C C Z C C C C C C Z C
L~ ~., T T Z (.) Z
T _ o _ I oN o ~
_ T
X O _ O = = _ O O O ~ O O O
i:1: I I . I . . . . I
a: O = O ~ ~ I = D = = = =
~: _ __ _ _____ _
-- 22 --
~ ~ 2 D 7 1~ ~
E ~ ~
I _ _ ~ I
N I _ _ ~ __ T
X r _ _ __ __ = I
~ T _ _ ~ _ _ _ _ _ _
_ I T ON
t~ ( \ = O N (.) r~ O~N t.) ~,)N T
~'~ _ _ ___
1~ _ ~ _ ~D tD ~D O ~_ N _ I~
` - 23 - 2 ~ 7 ~ 1 3 ~
B. Formulation Examples
a) A dusting agent i8 obtained by mixing 10 parts by
weight of a compound of the formula (I) and 90 part6
by weight of talc as inert substance, and
comminuting the mixture in a hammer mill.
b) A wettable powder which i8 readily dispersible in
water is obtained by mixing 25 parts by weight of a
compound of the formula (I), 64 parts by weight of
kaolin-containing quartz as the inert substance, 10
parts by weight of potassium lignin6ulfonate and
1 part by weight of sodium oleoylmethyltaurinate as
the wetting and dispersing agent, and grinding the
mixture in a pinned disk mill.
c) A dispersion concentrate whi¢h is readily disper-
sible in water is obtained by mixing 20 parts by
weight of a coI~pound of the formula (I) with 6 parts
by weight of alkylphenol polyglycol ether (Triton
X 207), 3 parts by weight of isotridecanol poly-
glycol ether (B E0) and 71 parts by weight of
paraffinic mineral oil (boiling range, for example,
about 255 to above 277C), and grinding the mixture
in a ball mill to a fineness of below 5 microns.
d) An emulsificable concentrate is obtained from
15 parts b~ weight of a compound of the formula (I),
75 parts by weight of cyclohexanone as the solvent
and 10 parts by weight of oxethylated nonylphenol as
the emulsifier.
e) Water-dispersible granules are obtained by mixing
75 part~ by weight of a compound of the formula
(I),
" " " of calcium ligninsulfonate,
5 ~ I of sodium lauryl sulfate,
3 - - 1~ of polyvinyl alcohol and
7 " " " of kaolin,
2 ~ rj~ 3 ~ 2~
- 24 -
grinding the mixture on a pinned disk mill, and
granulating the powder in a fluidized bed by spray-
ing on water a~ granulation liquid.
f) Water-di~persible granules are also obtained by
homogenizing and precomminuting
25 part(s) by weight of a compound of the formula
(I),
" " ~ of sodium 2,2'-dinaphthyl-
methane-6,5'-disulfonate,
2 ~ of sodium oleoylmethyltaurate,
1 ~ of polyvinyl alcohol,
17 ~ ~ " calcium carbonate and
" " " of water
in a colloid mill, subsequently grinding the mixture
in a bead mill, and atomizing the resulting suspen-
sion in a spray tower by means of a single-substance
nozzle, and drying it.
g) Extruder granules are obtained by mixing 20 parts by
weight of active sub~tance, 3 parts by weight of
sodium ligninsulfonate, 1 part by weight of car~
boxymethylcellulo~e and 76 parts by weight of
kaolin, grinding the mixture, and moistening it with
water. This mixture i8 extruded and subsequently
dried in a ~tream of air.
C. Biological Example~
1. Pre-emergence effect on weeds
Seeds or rhizome pieces of monocotyledon and dicotyledon
weed plants were placed in sandy loam soil in plastic
pots and covered with soil. The compounds according to
the invention which were formulated in the form of
wettable powders or emulsion concentrates were then
applied to the surface of the soil cover in the form of
aqueous suspensions or emulsions at an application rate
of 600 to 800 1 of water/ha ~converted), in various
2 ~ 7 3 -~ 2~
- 25 -
dosages.
After the treatment, the pots were placed in a greenhouse
and kept under good growth conditions for the weeds.
After the test plants had emerged, the damage to the
plants or the negative effect on the emergence wa~ scored
visually after a test period of 3 to 4 weeks by
comparison with untreated controls. As shown by the score
figures, the compounds according to the invention have a
very good herbicidal pre-emergence action against a broad
range of grass weeds and dicotyledon weeds. Compared with
those 6ulfonylureas which correspond structurally to the
salts, the salts according to the invention, for example
those of Examples 1, 2, 3, 12, 13, 15, 29 and 52 of Table
1 show in some cases a considerably better herbicidal
action in the case of monocotyledon weed species such as
Avena, Alopecurus, Echinochloa, Digitaria, Setaria,
Cyperus, Bromus or Sorghum or in the case of dicotyledon
weed species such as Galium, Viola, Veronica, ~amium,
Stellaria, Amaranthus, Sinapis, Pharbitis or Convolvulus.
2. Post-emergence effect on weeds
Seeds or rhizome pieces of monocotyledon and dicotyledon
weeds were placed in sandy loam 80il in plastic pot~,
covered with soil and grown in a greenhouse under good
growth conditions. Three weeks after sowing, the test
plants were treated in the three-leaf stage.
The compounds according to the invention which were
formulated as wettable powders or as emulsion concen-
trates were sprayed in various dosages on the green parts
of the plants at an application rate of 600 to 800 1 of
water/ha (converted) and, after the test plants had
remained in the greenhouse for about 3 to 4 weeks under
ideal growth conditions, the effect of the preparations
was scored visually by comparison with untreated con-
trols. The agents according to the invention also have a
good herbicidal post-emergence action against a broad
_ 26 - 2 0 7 3 ~. 3 (j
range of economically important grass weeds and
dicotyledon weeds. Compared with those sulfonylureas
which correspond structurally to the salts, the salts
according to the invention, for example those of Examples
1, 2, 3, 12, 13, 15, 29 and 52 of Table 1 show in some
cases a considerably better herbicidal action in the case
of monocotyledon weed species such a~ Avena, Alopecurus,
Echinochloa, Digitaria, Setaria, Cyperus, Bromus or
Sorghum or in the case of dicotyledon weed species such
as Galium, Biola, Veronica, Lamium, Stellaria, Amaran-
thus, Sinapis, Pharbitis or Convolvulus.
3. Tolerance by crop plants
In further greenhouse experiments, ~eeds of a substantial
number of crop plants and weeds were placed in sandy loam
soil and covered with soil.
Some of the pots were treated immediately as described
under 1., and the remaining pot~ were placed in a green-
house until the plants had developed two to three true
leaves and then sprayed with various dosages of the
substances according to the invention, as described under
2.
Visual scoring four to five weeks after the application
and after the plants had been in the greenhouse revealed
that the compounds according to the invention did not
inflict any damage to dicotyledon crops ~uch as, for
example, soya, cotton, oilseed rape, suqar beet and
potatoes when used pre- and post-emergence, even when
high dosages of active substance were used. Moreover,
some substances also left Gramineae crops such as, for
example, barley, wheat, rye, Sorghum species, maize or
rice unharmed. The compounds of the formula (I) therefore
have a high selectivity when used for controlling
undesired plant growth in agricultural crops. The com-
pounds of Examples 1, 2, 3, 12, 13, 15, 29 and 52 of
Table 1 showed, for example, good selectivity properties
- 27 - 2 ~ 7 ~
in cereal crops such as wheat and barley as well as in
rice, soya and maize.