Note: Descriptions are shown in the official language in which they were submitted.
~U-l~~b~.
WO 91/10696 PCT/US91/00126
Polyanhydrides of Oligomerized
Unsaturated Aliphatic Acids
Background of the ~nveation
This invention is in the area of polymers for
controlled delivery of substances, and is specifically
a polyanhydride prepared from oligomerized unsaturated
aliphatic acids.
There has been extensive research in the area of
biodegradable controlled release systems for bioactive
compounds. Biodegradable, biocompatible matrices for
drug delivery are useful because they obviate the need
to remove the drug-depleted device.
The most desirable polymeric matrix for drug
delivery is one that is hydrophobic, stable, strong,
flexible, soluble in organic solution, has a low
melting goint, and degrades linearly over time. The
polymer must be hydrophobic so that it retains its
integrity for a sufficient time when placed in an
aqueous environment, such as the body, to effect
controlled release. The polymer must be stable to
storage for an extended period before use. The
polymer must be strong, yet flexible enough that it
does not crumble or fragment during use.
Controlled release devices are typically
prepared in one of several ways. In one method, the
polymer is melted, mixed with the substance to be
delivered, and then cooled. Melt fabrication~requires
that the polymer have a melting point that is below
the temperature at which the substance to be delivered
and polymer degrade..or become reactive.
Alternatively,. the device. can be prepared by solvent
casting,~in which the polymer is dissolved in_a
solvent, and thew.:the substance'.to':be delivered is
dissolved or dispersedvin ahe solution:' The solvent
is then evaporated; leaving..the substance in the
polymeric-matrix. .Solvent casting.requires that the
polymer be soluble in organic solvents.
WO 91/10696 PCT/US91/00126
-2-
For controlled drug delivery, the polymer must
degrade by surface erosion and not by bulk erosion.
Surface erosion occurs when the rate of hydrolytic
degradation on the surface of the polymeric structure
is faster than the rate of degradation in the interior
of the polymeric structure. Bulk erosion occurs when
the polymer incorporates water into the center of the
matrix, rendering the polymer sponge--like with hole,
or channels in the matrix. When bulk erosion occurs,
the material to.be delivered is released through the
channels in a rapid, uncontrolled fashion.
Many polymers have been evaluated for use as
controlled drug delivery matrices, including
polyesters, polyamides, polyurethanes,
polyorthoesters, polyacrylonitriles, and
polyphosphazenes. None of these polymers have
exhibited the desired combination of characteristics
for use in the controlled delivery of substances.
Polyanhydrides have also been studied for use in
controlled delivery devices.. See, for example, U.S.
Patent 4,891,225 to Langer, et.al., U.S. Patent
4,886,870 to D'Amore, et al., Leong, et al., J. Med.
Biomed. Mater. Res. 19, 941 (1985); and Leong, et al.,
J. Med. Biomed. Mater. Res. 20, 51 (1986). One of the
first polyanhydrides studied with respect: to
controlled release characteristics was poly(bis(p-
carboxyphenoxy)methane anhydride), as described by
Rosen, et al., ~i~omaterials 4, 131 (1983). The
aromatic polyanhydride exhibited near zero order
(linear)_erosiow and.release kinetics.at 37°C and
60°C, in'vitro.
--Shortly ~thereafter;~-three related::
poly~anhydrides, poly_1,3-(bis(p-carbophenoxy)propane
anhydride:-(p-CPP)~ .(an aromatic polyanhydride) , the
polymer farmed.from.-the.copolymerization of CPP with
sebacic acid (a copolymer of an aromatic diacid and an
~n735fi1
. WO 91 / 10696 PCT/ LJS91 /00126
-3-
aliphatic diacid), and polyterephthalic acid (an
aromatic anhydride) were prepared and studied, as
described by Leong, et al., J. Med. l3iomed. Mater.
Res. 19, 941 (1985).
Tt was found that aromatic polyanhydrides have
unacceptably long degradation rates. For example, it
was estimated that a delivery device prepared from p-
CPP would require more than three years to completely
degrade in vivo. Further, anhydride homopolymers of
aromatic or linear aliphatic dicarboxylic acids were
found to be highly crystalline and have poor film
forming properties. Aromatic polyanhydrides also have
high melting points and low solubility in organic
solvents.
As described in U.s. Patent 4,757,128 to Domb
and Langer, high molecular weight copolymers of
aliphatic dicarboxylic acids with aromatic diacids are
less crystalline than aromatic or linear aliphatic
polyanhydrides, and they form flexible films.
Degradation rates are also increased by
copolymerizing an aromatic dicarboxylic acid with an
aliphatic diacid; however, bulk erosion occurs because
areas of the polymer containing aliphatic anhydride
linkages erode faster than aromatic anhydride
linkages,~leaving channels in the matrix through which
the substance to be delivered is released in an
uncontrolled fashion.w.For example, in the p-CPP
sebacic acid copolymer.,. the aliphatic anhydride bands
are cleaved and all drug released in ten days, while
the aromatic.ragions remained intact for.over.five
months. :y Further,: the:.copolymers have inferior
mechanical properties; they become brittle and crumble
into flakes on exposure to moisture.
Polymers prepared.from linear aliphatic diacids
are hydrophilic solids~~that degrade by. bulk erosion,
resulting in a rapid release of the drug from the
WO 91/10696 ~ ~ ~ ~ PCT/US91/0012G
_4_
polymeric matrix. Hydrophobicity is increased by
copolymerizing the linear aliphatic diacids with
aromatic diacids, however this approach results in an
increase in the polymer melting temperature and a
decrease in organic solvent solubility. Furthermore,
it does not improve the drug release profile but
increases the degradation and the elimination time of
the polymer both in vivo and in vitro. Since both
homopolymers and copolymers of linear aliphatic
diacids are very sensitive to moisture, they require
extremely anhydrous. and low temperature storage
conditions.
Several attempts have been made to improve the
cantrolled release characteristics of polyanhydrides
by altering the method of synthesis or the molecular
weight. See, for example, U.S. Patent 9:,857,311 to
Domb and Langer, describing polyanhydrides with-a
uniform distribution of aliphatic and aromatic
residues in the chain, prepared by polymerizing a
dicarboxylic acid with an aromatic end and an
aliphatic end; U.S. Patent 4,888,176 to Langer, et
al., describing the preparation of high molecular
weight polyanhydride controlled delivery devices; and
U.S. Patent 4,789,724 to Domb and Langer describing
the preparation of very pure anhydride copolymers of
aromatic and aliphatic diacids.
There remains is a strong need for a polymer
havingwthe desired.characteristics of hydrophobicity,
stability, strength, flexibility, organic solubility,
low melting. point, and appropriate degradation
profile; ,for. use as: the. matrix: for a controlled
delivery device.
It is therefore :an object of the present
invention to-provide°a biodegradable polymer that is
highly hydrophobic, and-that degrades by: surface
V1'O 91 / 10695
PCT/US91 /00126
erosion to release an incorporated substance in a
controlled manner.
It is another object of the present invention to
provide a polymer that is thermodynamically and
hydrolytically stable and that requires mild storage
conditions.
It is yet another object of the present
invention to provide a strong and flexible polymer
film that degrades in vivo into a soft material that
can be gradually eliminated from the body.
It is a further object of the present invention
to provide a controlled delivery device that is
suitable for intraperitoneal implantation.
8umanary of the Invention
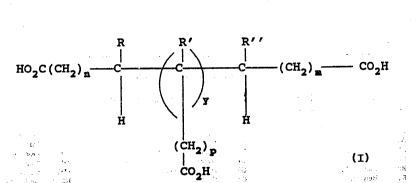
The present invention is a polyanhydride
suitable for use as a matrix material in controlled
delivery devices. The polyanhydride is synthesized
from monomers prepared by linking two or more
unsaturated aliphatic carboxylic acids to form a non-
linear aliphatic di- or polyorganic acid of the
general formula:
R R° R"
I I i
Ho2c(cxa)a--c (cH2)= co2H
y
H ~ H
( IH2)I~
COZH
wherein R, R', and R" are the same or different
aliphatic chain of C~ to C2o or hydrogen; m, n, and p
are integers from 0 and 20; y is 0 or 1; and, if y is
0, one of R or R' is not H.
WO 91/10696 PCT/US91/00126
~~ ~'3~~
°6-
Suitable monomers include the dimers and trimers
of naturally occurring unsaturated fatty acids or non°
naturally occurring unsaturated carboxylic acids.
Naturally occurring and synthetic unsaturated acids
can also be coupled to form a mixed oligomer that is
polymerized. The oligomerized monomer is typically a
hydrophobic liquid.
Films made from these polymers are highly
flexible (can be bent 180° at room temperature without
breaking) and strong (between 1-20 megaPascal), yet
degrade into a soft film that gradually disappears
without forming harmful sharp flakes. The polymers
are soluble in organic solvents and have melting
points in the range of 25° to 65°C. The polymers
degrade without significant bulk erosion over a period
of days. The polymers release incorporated substance
at a rate corresponding to their degradation rate
(zero order release).
Brief Description of thn Figures
Figure d is a graph comparing the percent
release of methotrexate (MTX) from goly(FAD-SA) (6:4,
weight/weight) over time (hours) in phosphate buffer,
pH 7.4, at ~7°C.
Figure 2 is a graph comparing the percent
release of methotrexate from poly(FAT-SA) (6:4,
weightjweight) over time (hours) in phosphate buffer,
pH 7.4, at 3°7°C.
Figure 3 is a graph comparing the percent
release of gentamicin from poly(FAD-SA) (6:4,
~eight/raeight) over time (hours) in phosphate buffer,
pH 7.4, at ~7°C~.
WO 91/10696 Q ~ PCT/US91/00126
Detai3ed Dnsoription of th~ Invention
The disclosed invention is a polyanhydride
suitable for use as a matrix material in controlled
delivery devices. The polyanhydride is prepared from
oligomerized, unsaturated, naturally occurring or
synthetic aliphatic organic acids: The monomers are
prepared by linking two or more unsaturated aliphatic
carboxylic acids to form a non-linear aliphatic di- or
polyorganic acid of the general formula:
R R° R°°
HOZC ( CHZ ) n C C C ( CHZ )'---~ COZH
y
H H
(iH2)P
C02H
wherein R, R°, and R°' are the same or.a different
aliphatic chain of Cl to Czo or hydrogen; m, n; and p
are integers from 0 and 20; y is 0 or 1; and, if y is
0, one of R or R° is not H.
If the polymer is used for medical applications,
monomers should be chosen that are biocompatible and
biodegradable. Suitable monomers include the dimers
and trimers of naturally occurring unsaturated fatty
acids, such as: oleic, erucic, lauroleic-, myristoleic,
gadoleic, ricinoleic, palmitoleic, linoleic,
linolenic,.. and:.-arachidonic:.acids.- These fatty-acid
derivatives should be=_readily eliminated:=from the body
through :the. normal:-metabolic. pa~ways. 'for' fatty acids .
:Diacids.-or.triacids can. also: be-:synthesized from
non-naturally occurring carboxylic acids, such'as ~ '-=
acrylic, methacrylic,'fumaric;:~crotanic; vinyl acetic
(3-butenoic),'-isocrotonic,-allylacetic (4-pentenoic),
CA 02073561 2001-05-16
WO 91/10696 PCf/US91/00126
-8-
hexenoic and undecylenic acids. Naturally occurring
and synthetic unsaturated acids can be coupled to form
a mixed oligomer. The oligomerized monomers are
typically hydrophobic liquids.
Dimers and trimers of oleic acid are available
from commercial sources. Unichema Chemicals, Inc.
(Chicago, Illinois) sells the dimer as PripoIT"" 1009
(99% diacids; molecular weight 556). It sells the
trimer as PripoIT"' 1025 (containing 25% by weight of
trimer and 75% dimer) and PripoIT"" 1040 (78% trimer and
22% dimer). Henkel Corporation (LaGrange, Illinois)
sells the following oligomerized oleic acid monomers:
VersadymeT"" 213 (50-70% trimer, 25-40% dimer) ; VersadymeT"~
288, 58, and 52 (97% dimer) ; VersadymeT"" 204 (83% dimer,
17 % monomer ) ; and Versadyme T"" 216 and 2 2 8 ( 8 8 % d imer ) .
The oligomerized unsaturated carboxylic acid
monomers can also be synthesized from the
corresponding unsaturated acids by methods known to
those skilled in the art. See for example Advanced
organic Chemistrv J. March, editor, 3rd ed., John
Wiley & Sons, New York, New York 1985.
Oligomerized unsaturated aliphatic acids can be
polymerized by methods known in the art, including
melt polycondensation and solution polymerization. In
the method of melt polycondensation, described by
Domb, et al., in J. Poly. Sci 25, 3373 (1987), a
prepolymer is prepared by heating the diacid with
acetic anhydride to form a diacetyldianhydride. The
prepolymer is then heated neat under vacuum to form
the polymer. Mixtures of diacetyldianhydrides can
also be polymerized with this method.
Solution polymerization is described in U.S. Patent No. 4,933,431,
entitled "One Step Preparation of Poly(amide-anhydride)" to Domb et al.,
issued on June 12, 1990. Solution
WO 91/10696
PC.'T/iJS91 /00126
_g_
polymerization involves the coupling of diacids with
phosgene in an organic solvent. Poly(4-vinylpyridine)
is added to remove the HC1 from solution. For
example, diphosgene (0.5 equivalents) is added
dropwise to a stirred mixture of diacid (1.0
equivalent) and poly(4-vinylpyridine) (2.5
equivalents) in 20 ml of chloroform. The solution is
stirred for 3 hours at 25°C. The insoluble PVP~HC1 is
removed by filtration. The solvent is then removed
and the precipitate is isolated, washed with ethyl
ether, and then dried at 25°C for 24 hours in a vacuum
oven.
The physical properties of the polyanhydride are
often improved by copolymerizing the oligomerized
unsaturated aliphatic acid with another dicarboxylic
acid, for example, sebacic acid (SA), isophthalic acid
(ISO), adipic acid (AA), 1,10-dodecanoic acid (DID), or
1,3 bis(p-carboxyphenoxypropane) (CPP).
Biodegradable biocompatible polyanhydride films
prepared as described herein can be used as a physical
barrier for adhesion prevention using the method of
Linsky, et al., J. Reprod. Med. 32, 17 (1987)); or for
targeted release of drugs to specific organ surfaces.
Examples are films containing heparin fox the
prevention of blood clotting, and films releasing
dexamethasone or cyclosporin to prevent organ
transplant rejection. Biodegradable films can also be
used in. guided tissue regeneration in periodontal
disease using the method reported by Nyman et al., J.
Clin: Perio. 9, 290 (1982); Nyman~ et al., J. Clin.
Perio...T3, 604 (1986); Nyman et al., J. Clin. Perio.
14,.. 618 (1987) , ~ Nyman:~et a.l. , J. Clan. Perio. ~ 15; 288
(1988), or as tubes~for guided'nerve'regeneration
using the method ~f 'U. S. Patent- No. ',-4, 870, 966 to
Dellon L: and Mackinnon~ S~E..
WO 91/10696 2 0'~ 3 5 G..1
P~'T/1JS91/00126
-10-
Other medical applications for the polyanhydride
films described here including coatings for
implantable devices, i.e. stents, catheters,
artificial vascular grafts, and pacemakers. The
coating can incorporate antibiotics, anti-
inflammatories, or anti-clotting agents for release at
a predetermined rate, to decrease complications caused
by the implanted devices. Controlled delivery devises
prepared from these polyanhydrides can also be used as
ocular inserts for extended release of drugs to the
eye.
The polyanhydrides of the present invention are
described in greater detail in the following non-
limiting working examples using dimers and trimers of
oleic acid, a naturally occurring fatty acid, for ease
of illustration.
Oleic acid dimer (referred to below as F~1D)
and trimer (referred to below as FAT) were obtained
from Unichema Chemicals, Inc. (Pripol 1009 and Pripol
1025, respectively). Both are liquids at room
temperature.
Infrared spectroscopy was performed on a Perkin-
Elmer 1310 spectrophotometer (Perkin-Elmer, CT.).
Polymeric samples were film cast onto NaCl plates from
a solution of the polymer in chloroform. Acids and
prepolymer samples were either pressed into KBr
pellets or dispersed in nujol onto NaCl plates. A
Perkin Elmer DSC 7 Differential. Scanning Calorimeter
calibrated with indium was, used to determine the glass
transition temperature;.(Tg), the-melting temperature ,
(Tm),.and the heat of fusion.of the-polymers. The_:..
standard heating rate,.for all; polymerswas-10°C/min
under,nitrogen atmosphere. The decomposition.
temperatures: were determined on a Dupont 951-
Thermogravimetric Analyzer (TGA).ata.heating rate-of.
20°C/min. in a nitrogen atmosphere. The molecular
WO 91/10696 ~ ~ ~ '~ ~ PC'Jf/US91/00126
_11_
weights of the polymers were estimated on a Waters GPC
system (Waters, MA) consisting of a Waters 510 pump
and Waters programmable multiwavelength detector at
254 nm wavelength. Samples were eluted in
dichloromethane through two Styrogel(TM) columns
(Waters, Linear and a 10a A pore sizes) in series at a
flow rate of 1.0 mL/min. Molecular weights of
polymers were determined relative to polystyrene
standards (Polysciences, P.A., molecular weight range,
400 to 1,500,000) using Maxima 840 computer programs
(Waters, M.A.). 1H NMR spectra were obtained on a
Varian 250 MHz spectrophotometer using deuterated
chloroform containing tetramethylsilane (TMS) as the
solvent for the pplymers and prepolymers. UV
absorbencies were determined on a Lambda 3B
spectrophotometer (Perkin Elmer, CT). Degradation
studies were performed at 37°C, using melt molded or
film cast samples containing drug placed in a 20om1
solution of phosphate buffer at pH 7.40. The release
of drug to the medium was determined by the W
absorption or HPLC (high pressure liquid
chromatography) of the drug.
Eacample Z - Bseparation of lPsepolymess of Oligomessized
oleic Aoid.
The fatty, acid FAD and FAT oligomers were
separately purified by extraction of a dichloromethane
solution of the oligomer (50% w/v) with deionized
water. The purified monomers were then separately
refluxed in acetic anhydride (100..8 in 500 ml) for one
hour and. evaporated.to~dryness.
The infrared spectra for both grepolymers
included the_..following absorbances: (cm-1, film cast)
1800,, 1740 (sharp) . The proton NMI2 for the
prepolymers contained the following chemical shifts:
WO 91/10696 PCT/US91/00126
20?35~~~
-12-
0.9(m,6H), 1.3(s,52H), 1.7(m,2H), 2.2(s,4H),
2.4(m,4H).
Prepolymers of sebacic acid, isophthalic acid,
adipic acid, 1,10-dodecanoic acid, and 1,3 bis(p-
carboxyphenoxypropane) were prepared as described by
A. Domb and R. Langer in f. Polym. Sci. 25, 3373
(1987).
Exampl~ a - Polyaaerization of the Prepolymers
Polymers of varying combinations of oligomers
and aliphatic diacids were synthesized by melt
polycondensation of diacetyldianhydride prepolymers or
by solution polymerization of the diacids.
The melting point, molecular weight, and
appearance of the polyanhydrides prepared by melt
polycondensation are provided in Table 1. The melting
point, molecular weight, and appearance of
polyanhydrides prepared by solution polymerization are
provided in Table 2. The ratio of monomers was
calculated on a weight/weight basis. The following
abbreviations are used in the.tables: SA, sebacic
acid; CPP, 1,3-bis(p-carboxyphenoxy)propane; DD, 1,10-
dodecanoic acid; and rSO, isophthalic acid.
Polyanhydrides prepared according to this method
have low melting temperatures, in the range of 25°C to
65°C, and high solubility in chloroform,
dichloromethane, tetrahydrofuran, ethyl acetate and
methyl ethyl ketone. These characteristics allow the
polymer to be easily fabricated into controlled
delivery devices. High molecular weight polymers, in
the range of 18,300 to 243,100, were obtained by melt
polymerisation:
All polymers displayed the typical.IR-
absorbances for aliphatic polyanhydrides (2910, 2860,
1810, and 1750 c~ i). Copolymers containing an
aromatic unit or fumaric acid had an additional sharp
WO 91/10696
PCT/ US91 /00126
-13-
peak at 1600 c~ 1. The H-NMR spectra of the polymers
were consistent with their polymeric structures.
None of the polyanhydrides prepared had a glass
transition temperature (Tg) in the temperature range
of -50 to +50°C. In addition, all had a law heat of
fusion (<5 Jouls/gram), indicating low crystallinity
(<5%). P(FAD-SA) (50:50) and P(FAD-DD) (50:50)
decamposed at 292°C and 296°C, respectively.
WO 91/10696 ~. , PCT/CJ891/00126
Table 1: Analxsis of Polygnhgdridea Prepared by
Melt Condensation.
Polymer Melting Molecular Weight Physical
Point (°C) Mn Mw Appearance
P(FAD) 25-35 18,600 88,700 soft clear
sticky
P(FAD-SA)50:50 60-65 18,200 243,100 clear
flexible
P(FAD-SA)33:66 42-47 16,900 175,200 soft clear
rubbery
P(FAD-CPP)50:50 35-40 8,900 18,300 Sticky Soft
white
P(FAD-CPP)80:20 30-35 9,100 23,600 sticky soft
white
P(FAD-ISO)50:50 38 -42 15,700 48,800 sticky
clear soft
P(FAD-SA-CPP) 55-60 10,300 86,200 strong
45:45:10 flexible
P(FAD-DD)50:50 38-42 20,100 123,700 clear
flexible
P(FAD-AA)50:50 25-30 9,300 24,600 sticky
clear
semisolid
P(FAD-FA)50:50 25-30 10,400 26,300 sticky dark
semisolid
P(FAT) a insoluble insoluble clear
rubbery
P(FAT-SA)50:50 58-63 15,300 85,900 flexible
hard solid .
P(FAT-SA)33:66 56-60 1.1,200 74,700 flexible
hard solid
P(FAT-CPP)50:50 a insoluble insoluble rubbery
P(FAT-CPP)80:20 a insoluble insoluble rubbery
P(FAT-SA-CPP) 54-58 10,700 55,700 flexible
40:50:10 hard solid
P(FAT-SA-CPP) 61-64 10,400 53,700 flexible
40:40:20 hard rubber
P(FA~-FAD-SA) 4i-44 15,700 88,800 sticky hard
3:1:1 rubber
P(FAT-DD)50:50 41-45 8,500 35,200 soft
breakable
rubbery
a: melting temperature in excess of 200oC
WO 9t/10696 PCT/1US91/00126
~o~~:~s~~
-15-
Table 2. Analysis of Polyanhydrides Prepared by
Solution Condensation.
Polymer Melting Molecular WeightPhysical
Point (C) Mn Mw Appearance
P(FAD) 25-355,600 13,400soft clear
stic3~y
P(FAD-SA)50:50 60-658,600 24,300soft
f lexible
P(FAD-SA-CPP) 52-606,700 17,400strong
45:45:10 flexible
P(FAD-DD)50:50 38-427,900 26,500clear
flexible
a. The polymers were synthesized in
dichloromethane solution using phosgene as coupling
agent and pyridine as an acid acceptor.
Exampl~ 3 - Preparation of Polyanhydrids Films
Polyanhydride films were.prepared by melt or
solvent casting using the following methods.
sole~at castings The polyanhydrides were
dissolved in dichloromethane (1 g dissolved in 5 ml)
and cast on a Teflon(TM) coated Petri dish. When drug
was incorporated into the film, the drug powder was
dissolved or dispersed in the polymer solution prior
to casting. On solvent evaporation,, transparent
strong films were obtained from most of the polymer.
compositions described in Table 1.
Malt Casting: 200 Milligrams. of polymer were
compressed between two hot plates heated to the
melting point of trie compressed polymer. Partially
crosslinked polymers based on trimer fatty acids
prepared from Pripol 1025 (example 2, polymers 12-19)
formed flexible and strong films ~xsing_the melt
technique.
2.~17;3~5 61
WO 91/10696 PCfJUS91/00126
-16-
Polyanhydride films prepared as described above
are useful as laminates for degradable or
nondegradable fabrics. FAD°SA copolymers are
particularly useful for this purpose. As an example,
poly(FAD-SA) (50:50) was laminated onto a
biodegradable oxidized cellulose (Surgicell TM) by
solvent casting or melt compression (0.5 gram on 2
gram of fabric) to form a solid nonporous sheet. The
sheet can be used as a degradable physical barrier for
adhesion prevention.
Erample 4 ~ Release of Metlhotrexate (MTg) groin
Polyanhydrides.
Methotrexate (MTX; x.00 mg) was uniformly
dispersed in a molten sample of poly(FAD-SA) (6:4
weight/weight) (0.9 g) and separately in a molten
sample of poly(FAT-SA) (6:4 weight/weight) (0.9:g).
The samples were cast to tough yellow slabs (6 x 10 x
1 mm, about 200 mg). The ~n vitro release of
methotrexate from the polyanhydride was determined in
phosphate buffer (pH 7.4) at 37°C by HPLC analysis
(C18 column, mobile phase: ammonium persulphate pH
3.5, ~. ml/min). The rate of release of MTX from
poly(FAD-SA) (6:4) and poly(FAT°SA) (6:4) as well as
the degradation rates of the polymers, are illustrated
in Figures 1 and 2, respectively.
As shown, MTX is released from the polymers at a
linear rate approximately corresponding to the
degradation rates of the polymers. - Over 80% ~of the
drug was released from poly(FAD-SA) (6:4) in 250
hours, ~ and over 95%~-of the drug was released from
poly (FAT-SA) ( 6 4 ) 'in tlie~ same time period o
Bxampl~ 5~ - Rehaaae of Gentamici~ from P~lga~hydrides.
Gentamiciri sixlphate (200 mg) was uniformly
distributed in a-'molten sample of poly(FAD-SA) (1:1)
WO 91/10696
Pcrms9lioolz
-I7-
(1.8 g). The sample was cast to tough but flexible
off-white rods (3 X 25 mm). The rods were cut into
200 mg samples. The in vitro release of gentamicin
was determined in phosphate buffer at pH 7.4 and 37aC,
by radioimmunoassay (RTA). The degradatian rate of
the polymer was determined from the weight loss of the
sample. The rate of release of gentamicin from
poly(FAD-sA) (1:1) as well as the degradation rate of
the polymers is illustrated in Figure 3.
As shown, gentamicin was consistently released
from the polyanhydride over a period of approximately
days. After 15 days, the polymer was completely
degraded, leaving the water insoluble FAD as a semi-
liquid mass.
To investigate whether the polymer was eroding
predominately from the surface, at various time
intervals, polymer samples were removed from the
gentamicin containing slab and cut in the middle for
observation. The samples were found to have a solid
core, containing anhydride bonds,,that decreased in
size with time, leaving a soft shale of the FAD
degradation products (as determined by IR and H-Nit
spectroscopy) indicative of surface erosion.
Exmmple 6 -.Release ~~ cisplatin and carbgplatin from
Polym~ra.
Poly(FAD-SA). (50:50) samples containing the
anti-cancer agent Cisplatin or Carboplatin (3 mg in a
30 mg circular tablet, ~3 x 3 mm) were prepared by melt
casting. The in vitro, release rates were determined
as described in Example 5. The release of drug was I
monitored by at~mic absorption:spectroscopy. The
drugs were released at a linear rate. approximately
corresponding to the degradation..rates of the
polymers, over a period of 14 days:
2~ 4'~ 3 5 G
CVO 91/10696 - P~CT/US91/00126
_18-
E$ampie 7 - Films containing Heparin.
Heparin containing films were prepared by mixing
heparin powder (121 units/mg, particle size less than
65 microns) into molten polymer (300 mg, Poly(FAD:SA)
(1:1) weight/weight) and then melt pressing the
mixture to form films (0.1 mm thick and 12 cm2 wide)
containing 2, 5, 10, and 15 units per mg of film. The
mechanical properties of the film, i.e., flexibility
and strength, were not affected by drug incorporation.
The release of heparin from these films was
determined in vitro by immersing a film sample in 0.1
M phosphate buffer at pH 7.4. The amount of heparin
released into the buffer was determined by increasing
inhibition of blood clotting. Thirty percent of the
heparin was released from films containing 5 units/mg
in one hour; 55% was released in six hours; 78% in 18
hours; 85% in 24 hours and 100% in 48 hours. No~drug
remained in the polymer after 48 hours.
The rate of release of heparin from the films
can be manipulated by compressing heparin loaded films
between two drug free films or by varying the drug
concentration or thickness of the film. Films that
release heparin over a period of 4 days were obtained
using these methods.
To obtain drug release from one side of the film
only, the drug loaded film was compressed with a drug
free filmwon one side. These films were effective in
vivo to prevent adhesions and clotting prevention.
EHaynple ~ - Hydrolytic Stability of' ~olyanby~iride
8ilms
vThe hydrolytic: stability of: the polyanhydride
films . ( o . 1. mw. thickness) - were tested. by. storing the
polymers at 25°C under 70%: relative humidity,-and
monitoring the changes in the film properties over
time. The results are summarized in Table 3.
-'~x'091/10696 ~ ;~., _ PC1'/US91/00126
_19_
Polyanhydrides of fatty acid oligomers (films 5-
11) are flexible and strong when prepared, and remain
flexible and relatively strong after 5 days at room
temperature exposed to air. For comparison, under
similar conditions, films of linear aliphatic
homopolymers and copolymers with aromatic diacids
became brittle and fragmented after 2~ hours.
Polymers prepared from fatty acid oligomers
remained stable when stored in an aluminum foil pouch
under dry argon at 5°C for at least 2 months.
WO91/10696 ? JC.~~., PCT/US91/0012,6
-20-
O O
b
v v N N p N
N N
W .4~ .1-~~.1 J~ v v v v v v
~ .4~ .1~
!~ j~ ~ ~ ~ C'~wr~l r-~ r4 ri '-i r~
~.i C~.a
v v v v p N .p t7 .Q .p .p .4
v v
~-1 ~-1 ~-1 ' k' X k k' SC
' ?~ '~ ?G ~ '
?C ''
ro ro r r r n v . v , k
ro ro ro ro ro v ro v ro .. v
ro ro ro ro ro ro ro v ro
ro ro
~ N ~ w w w
~
1NW WNW w w wl 3 3 w3 w3 w3 w3
V.. n
~a ~ ro ~ ~a
01 v v N v N N
v .d.a W ri .1a ~1 .8.~ ~ v Q1 v v v ~ 4l
J~., dJ ~"., ~ ri .f", ~'., ~ ~', ~ r~9 r~ r1 r~ r~ rl
v ~.-1 N O) v ~ N v v v ~.-I N .f~ .Ll ~ tT ,Ll .Ct ,Cl
.C 3 .~ ~ N ~ ~-~4 ~ ~ 3 .~ x ~ ~k b ~~C O ~k c~0 ~>C ~ ~k ro
ro ro ro ro ro ro ro ro ro ro v v v v v s~ v v v v v v
eo t~ o ~ s~ sa o s~ s.a I~ ~ >,J o ,~ ~ ~ ,~ r, ~ +~ ~ ~
d' W ~ W 4-a W W W W N W W d.~ W W U W U W N . W U W U '.sa U
t<l .
fi
r1
~r1 't~ T5 ~r1 T3 'E3 'C9
W v v '>:f v N v
..N ..~, ..u ~i v v ai v ai
a~ v>~ v~v vt~ v>~ v~
b ~ v ~ v .A ~ cu ~ v ~ v ,o s~ is .a ~r ,~ .a tr~ .o
u.~'c ~o~ ~~r~ ~~ ~~ ~~ ~k~ ~~to ~xo ~ero ~xo ~xro
b v ~~~ ro ~~~ ro v ~~a ro ~.o ro ~~I ro v v v 1~ v 1~ v v v s~ v v
w~ s~~ s,as'a~ ~~ fry via ~~ ~w ~w ~
.C W N l3 W ~ k.t 3 .0 W .fa V-1 .G W W U W N W N W U W N W U
a ro
N
r-1 v
p ~rt
t~ .I-I il!
f-I rf
W v .1.~ ...
O W +s ,~
'~11 ~.1 r'~d ri v ~.d ~~' r~i r-~~ r°) r-~i r~-i r~-I r-~~i
ae ,~ d~ r~ .S~ W .G .G ~ . .9 L'S~ .» 4~ .t~ $~ .4 b~ to ~
~ed ~i-1 ~.i ~ ~r~1 ~.~1 !~" ~n-1 C,' ~ri '1.,' ~.~1 C'., ~r~l C; ~r-i T.,
~s~ x x .a, Na x xo xo xo xo xo xo
~ v v ~~~ Nro v vs~ v~ v~ vs~ vs~ vs~
,o -.a r, r~~ !.a v r~ rl ~I .aJ .-a .~ .a +~ ra +~ .-~ .u
nA w 4-1 W j1 r1 y W W N W N w N 4-I N W N W N
st' , e-d d' v-I ri Ri', ri e-1 tV
00 00 (~ ~~
1'°~ T~~'I ~ 1~'I f"I I p'~' r'I P')
,~. W ~ . ~w ~ r ~I is s1
~~~o~t~J~ ~ ~ v~J~U~erAap
v 1 1 I-t I f 1 ~ I 1 1
p, W ... ... 1 D H H ~f1 v7 E-~ H
W W ~ GI iaC Et,' ~ ~ ~ r~ ~ a;
C~ U !'P1 C5 CI5 i~ 6sr C~ O Gc, trr t:e
v ..r wr v wr d. ~ v. w,s
~7 ~i al- a1 ~1 ~1 ~1 ~1 Q! ~ Ad pd ~1
~a'~35~1
VVO 91/10696 PCT/US91/00126
21
Example 9 - Hydrolytio Degradation ofi Polyanhydrides
' in Buffer solutions.
The hydrolytic degradation characteristics of
polyanhydrides described in Table 3 were evaluated by
immersing the films in buffer at pH 7.4 and 37°C, and
monitoring the physical properties of the polymers
over time.
Polyanhydrides prepared from olig0merized fatty
acids (films 6-11) did not fragment after 5 days in
buffer. At day one, the films were as flexible as at
time zero, and they were relatively strong. After 2
days the films were still flexible and strong enough
to be handled. After 5 days the films became weak and
soft, and when mixed with a spatula formed a soft
semi-solid mass.
In contrast, the films of linear aliphatic
homopolymers and copolymers with aromatic diacids
(films 1-5) became white and rigid after 1 day, and
crumbled into sharp small flakes after two days.
Example to - Biocompatibility studies.
Twelve female Sprague-Dawley rats (250=300 gram
weight, 8-14 weeks of age) were implanted with 200 mg
melt molded polymer discs (1 mm thick; poly(FAD-SA)
(l: l), poly(FAD-SA-CPP) (5:4.:1), and clinical grade
poly(CPP-SA) (20:80)) using the following procedure.
After prepping and draping the rat under sterile
conditions, an.8 cm midline incision was~made on the
dorsum. Using fine tissue scissors, a pocket area was
created 6 cm away from the midline incision. One 200
mg disc or no:disc°was implanted, and'the wound was
then closed: using surgical' clips. w= Seven -days after
implantation;:. the rats were: sacrificed -and- thewsite of
implantation was°examined.
In Groups 1 (control animals) ,- 3/3 sites- were
found to be~normal;-with no.swelling or'redness. In
Group 2:(poly(FAD-SA):(1:3).disc), 3/3 sites were also
sormal. Most of the film material remained in the
WO 91/10696 2 ~ ~ 3 ~ 6 PCT/US91/00126
22
site of implantation as a waxy semi-solid. In Group 3
(poly(FAD-SA-CPP) (5:4:1) discs), 2/3 sites were
normal, and one site showed some red foci. In Group 4
(poly(CPP-SA) (20:80} disc), 2J3 sites were normal,
and one site had red foci.
Both poly(FAD-SA) (1:1) and poly(FAD-SA-CPP)
(5:4:1) copolymers displayed similar or better
biocompatibility than the reference biocompatible
polymer, poly(CPP-SA) (20:80).
Example ii - =ntraperitoneal =mplaatation of the
Polyanhydrides.
Films (1 x 1 cmZ, 0.12 mm) of poly(FAD-SA)
(1:1), poly(FAD-SA-CPP) (5:4:1), and clinical grade
poly(CPP-SA) (20:80) as reference, were implanted into
the peritoneal cavity of rats as described in example
10. Animals were sacrificed and examined after one or
five weeks. After one week, the reference films
(poly(CPP-SA) (20:80)) had completely crumbled to
white flakes and had spread out in the peritoneal
cavity. In contrast, the fatty~acid based ffilms
remained as saft materials somewhat stuck to the
surface of the organ they had been placed. The site
of implantation was clean with no tissue response,
indicating that the polymers were highly
biocompatible.
The animals sacrif iced after f five: :weeks showed
no',inflammation at the site of implantation. Further,
most of_ the;polymer had disappeared.by-this:time.
Ezampi~i ~x2. -, Tensile; Strength of polymer.: films.
The _tensile~ strength .ofsolvent casted= ors melt
compressed: films ~af-_polymers prepared asp in vExample =~2-
(numbers 2, 7, 8, 12, and 16)wwere:. ested:;..The.
results.are provided in.:Table 4:
Solvent:casted:films:were preparedrfrom l0% w/v
solutions in dichloromethane:at room: temperature.
WO 91/10696 ~,. ,
~.1 (; , ~~f~ :.. 1'~/US91/00,126
23
After drying, the films were stored under vacuum for
12 hours.
Alternatively, polymer samples (200 mg) were
compressed between two Teflon coated hot plates to
form films using a Carver laboratory press. Tensile
measurements were made using an Instron Tensile Tester
Model 1122 at room temperature according to ASTM D882-
83. Tensile strength, tensile modules, elongation at
yield and break were determined. The tensile values
were calculated from the arithmetic average of at
least four measurements obtained from four separate
specimens per polymer sample.
As seen in Table 4, films made by melt
compression are stronger than films prepared by
solvent casting. All films were transparent and very
flexible. Adding a trifunctional monomer (FAT) almost
doubled the tensile strength of the films. Additional
increase in the tensile strength can be achieved by
incorporating small amounts of an aromatic monomer
(CPP) in the polymer.
Table ~1. Meohanioal Prop~rties of Fatty Acid
Polyanhydride Films
Polymer Method' Tensile Tensile Elongation
Strength Modules yield break
(MPa) (MPa) (%) (%)
1-2.P(FAD-SA) A 4 45 14 85
1-2.P(FAD-SA) B 3 35 18 115
50:50 ...
1-7.P(FAD-DD) A 5 50 13 77
50:50
1-8 . P (FAT-SA)A 7 . .. . 75 . 12 _8p
.
50: 50 .. - .- _~. r .,
.
. .. . .... _m _. ~ _~ _ .:. '.~
1--16.~P(FAT-SA-. il.a ... ... ~..:h:~
' 11 x.20 , .
~ ..
15 88_
. . .
CPP)A':~._.._..;..._: ., ... -: _ -~:=., :,
. . :;-.._i .: .
. : .:: _:
.: . _::
., 40; 50:.10 ..
.
,
a. Films prepared by melt compression (A) or by
solvent casting (B).