Note: Descriptions are shown in the official language in which they were submitted.
2~75~0~2
F-5819 - 1 -
REACTOR QUENCHING FOR C~TALYTIC OLEFIN
HYDRATION IN ETHER PRODUCTION
This invention relates to olefin hydration,
especially for the production of di-isopropyl ether
(DIPE) from C3+ olefinic feedstocks. Particularly,
this invention relates to a novel technique for
operating a fixed bed multizone reactor with a solid
hydration catalyst.
The need to eliminate lead-based octane enhancers
in gasoline has provided incentive for the development
of processes to produce high octane gasolines blended
with lower aliphatic alkyl ethers as octane boosters.
Supplementary fuels are being vigorously developed in
the petroleum refining industry. Lower molecular
weight alcohols and ethers such as isopropyl alcohol
(IPA~, isopropyl t-butyl ether (IPTBE), and diisopropyl
ether (DIPE) are in the boiling range of gasoline fuels
and are known to have a high blending octane number.
They are useful octane enhancers. In addition,
by-product propene (propylene) from which IPA and DIPE
can be made is usually available in a fuels refinery,
typically as a C3+ aliphatic stream rich in propene and
propane. The petrochemicals industry also produces
mixtures of light olefin streams in the C2-C7 molecular
weight range and the conversion of such streams or
fractions thereof to alcohols and/or ethers can also
provide products useful as solvents and blending stocks
for gasoline.
Adapting available refinery feedstock to produce
these oxygenates simultaneously as octane enhancers can
involve two different olefin hydration and
etherification processes, i.e. propene
hydration-etherification to give DIPE and IPA.
Accordingly, a challenge is provided to explore these
processes to discover how they may be integrated in a
F-5819 - 2 - ~ 2
manner more beneficial to the production of high octane
gasoline.
Catalytic hydration of olefins to provide alcohols
and ethers is established technology for the production
of IPA and DIPE and is of significant commercial
importance. Representative olefin hydration processes
are disclosed in U.S. Patents Nos. 4,334,890 (Xochar),
3,912,463 (Kozlowski et al.); 4,042,633 (Woods);
4,499,313 (Okumura et al.); 4,886,918 (Sorensen et al).
Olefin hydration employing medium pore and large
pore zeolite catalyst is a known synthesis method. As
disclosed in U.S. Patent No. 4,214,107 (Chang et al.),
lower olefins, in particular propylene, are
catalytically hydrated over a crystalline
aluminosilicate zeolite catalyst having a silica to
alumina ratio of at least 12 and a Constraint Index of
from 1 to 12, e.g., acidic ZSM-5 type zeolite, to
provide the corresponding alcohol, essentially free of
ether and hydrocarbon by-product. Acid resin catalysts
such as "Amberlyst ~5" may also be used for the
hydration of light olefins.
Production of ether from secondary alcohols such
as isopropanol and light olefins is known. As
disclosed in U.S. Patent No. 4,182,914 (Imaizumi), DIPE
is produced from IPA and propylene in a series of
operations employing a strongly acidic cation exchange
resin as catalyst. Recently, processes for the direct
hydration of olefins to provide alcohols and ethers
using porous shape selective metallosilicate zeolite
catalyst, such as zeolite Beta have been disclosed in
U.S. Patent No. 4,857,664 (Huang et al.). Prior
processes for hydrating olefins have often been found
to be inefficient with regard to catalyst life.
Poor distribution of water and hydrocarbon reactants
may cause deactivation, especially with solid
metallosilicate catalysts having large pores (i.e. 7+
Angstroms) or medium pores (5-7 A).
2~7~0~2
F-5819 - 3 -
This invention provides a novel process for the
production of ether/alcohol from lower olefins. In the
preferred embodiments, di-isopropyl ether (DIPE) is
produced by the hydration of a feedstock containing
propene, which comprises contacting the propene
feedstock and water in a catalytic reactor having a
series of fixPd bed hydration zones with porous solid
acidic olefin hydration catalyst under olefin hydration
conditions. Improved operation is achieved by
recovering a first liquid effluent stream from the
catalytic reactor; splitting the first liquid effluent
stream into a liquid product recovery stream and a
liquid recycle stream; cooling at least a portion of
the liquid recycle stream; and passing the cooled
liquid recycled stream as interstage quench between
fixed bed hydration zones for controlling reaction
temperature.
The preferred solid catalyst comprises acidic
zeolite, such as zeolite Beta, and hydration zone
conditions comprise temperature of 50 to 220C.
Advantageously, total liquid recycle is combined with
fresh feed at a weight ratio of 2:1 to 10:1
recycle:feed. Typically, liquid product stream is
separated into at least two streams for recycle to
separate reaction zones, and wherein at least a portion
of cooled liquid recycle is injected between separated
fixed catalyst beds. The fixed bed hydration zones may
be maintained in a vertical downflow reactor; and
wherein the solid catalyst comprises at least one metal
oxide catalyst.
These and other advantages and features of the
invention will be seen in the description and drawing.
Fig. 1 is a schematic process flow diagram of the
improved process.
35The preferred embodiments of the invention are
described with reference to propylene hydration and
2~75002
F-5819 - 4 ~
zeolite catalysts. Metric units and parts by weight
are employed unless otherwise indicated.
The olefins hydration and etherification process
employs the reaction of propylene with water catalyzed
by strong acid to form isopropanol. Reaction may be
allowed to continua in the hydration zone to form
di-isopropyl ether. The operating conditions of the
olefin hydration step include a temperature of 50 to
450C, preferably from 100 to 250~C and most preferably
from 120 to 220C. The pressure is 700 to 24000 kPa
(100 to 3500 psi, preferably 3500-14000 kPa (500-2000
psi)). Water to olefin reactant concentrations are
maintained at mole ratio of 0.1 to 30, preferably
0.3-5.
Olefin hydration to provide ethers and alcohols to
produce DIPE and byproduct isopropyl alcohol (IPA) is
described in U. S. Patents 4,214,107 and 4,499,313. The
preferred catalytic methods for making DIPE employ
porous solid acid catalysts, such as zeolites Y, Beta,
ZSM-35 and/or MCM-22 aluminosilicate. DIPE
etherification conditions may vary widely in choice of
temperature, pressure and reaction time. The preferred
method of Bell et al reacts propene with water in an
adiabatic downflow reactor containing a fixed bed of
25 zeolite Beta at about 90 to 200C and pressure of at
least 4000 kPa. However, it is understood that the
unit operations described herein can be conducted with
any number of specific process steps within the skill
of the art.
The olefin hydration process of this invention are
carried out in liquid phase, sup~rcritical dense phase,
or mixtures of these phases in continuous manner using
a fixed bed flow reactor. Weight hourly space
velocity, based on catalyst weight is maintained in the
range of 0.1 to 10/hour when operating continuously.
Various modifications can be made within the
inventive concept, especially with regard to reactor
2~30~2
F-5819 ~ 5 ~
system configuration. Although a single reactor bed
may be employed, it is advantageous to employ a series
of fixed bed reactor units to permit adequate control
of reaction conditions, especially temperature phase
behavior and flow parameters.
It may be feasible to recover any unreacted olefin
and recycle it to the reactor. Unconverted isopropanol
recovered from the final reactor effluent may also be
recycled to further conversion to ether.
The preferred hydration/etherification catalyst
comprises acidic shape selective porous zeolite having
a pore size of 5-8 x 10 mm (5-8 Angstroms), such as
aluminosilicate zeolite Beta. Also, MCM-22, having
pores similar to zeolite Beta and ZSM-5, is known for
etherification catalysis.
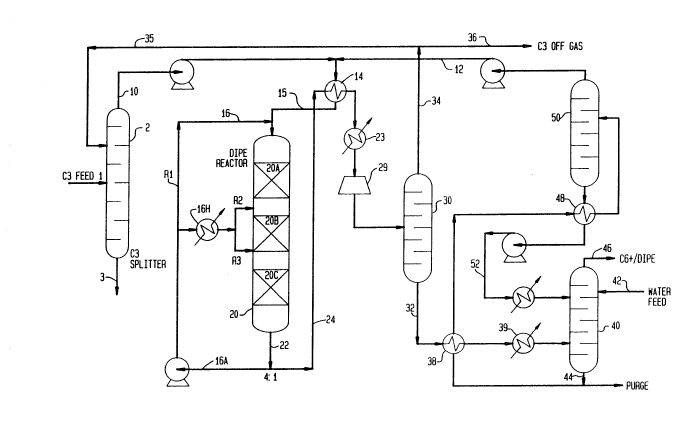
Referring to Fig. 1 of the drawing, a process flow
diagram depicts production of diisopropyl ether by
hydration of fresh olefinic feedstock stream, which is
introduced as a propane-propene mixture via inlet 1 to
feed splitter tower 2 to recover a propane-rich bottoms
stream 3. An overhead stream consisting essentially of
propene (C3=, propylene) is pumped along with water
from stream 12 through heat exchanger 14 to bring the
reactants and recycle stream 16 to the process
conditions for etherification in vertical reactor 20 in
contact with porous solid acidic olefin hydration
catalyst.
The reactor vessel 20 contains a series of fixed
bed adiabatic hydration reaction zones 20A, 20B, 20C
maintained under olefin hydration conditions. Static
mixers and liquid distributors may be employed before
each bed to promote operation in a single homogeneous
phase, as localized high concentrations of water or
propylene are known to deactivate acidic catalysts
(both zeolites and resins). Preferably, at least one
hydration reaction zone contains porous zeolite
catalyst, such as zeolite Beta.
2~7 ;Y~2
F-5819 - 6 -
A fluid handling system is operatively connected
for recovering a liquid reactor effluent stream 22 from
the last zone 20C. This can be achieved by splitting
effluent stream 22 into a liquid product recovery
stream 24 and a liquid recycle stream 16A, which is
recycled to the multizone reactor 20 as a plurality of
flow-controlled recycle streams R1, R2, R3. Heat
exchanger 16H cools the interstage quench streams R2,
R3 below the process reaction, thus balancing the
adiabatic heat of reaction from the preceding zone.
Reactor 20 is operated continuously by passing the
liquid recycle stream 16A substantially unfractionated.
Reaction temperature can be controlled in zones 20B,
20C by varying the degree of cooling and/or flow rate
of the recycle stream in unit 16H.
The amount of unfractionated liquid recycle stream
16 may be sufficient to maintain a substantially
homogeneous single liquid reaction phase in the primary
hydration zone 20A under reaction conditions. Use of
DIPE, IPA containing product for quench will also
promote single phase operation. The first liquid
product stream 24 is passed via exchangers 14, 28 and
fluid handling unit 29 to the product fractionation
system, as described. Effluent stream 24 is
ractionated in the product recovery system first in
column 30 to recover ether-rich stream 32 and a
propene-rich vapor stream 34, which may be recycled to
tower 2 via line 35. Some of the steam may be purged
from the system via line 36. It is advantageous to
recover isopropanol for recycle to the reactor to
provide isopropanol byproduct stream for further
conversion to di-isopropyl ether. In the DIPE system
depicted, unfractionated liquid recycle stream 16A is
passed to reactor 20 at a rate which is about four
times the total weight of propene and water reactants
in the product recovery stream 24: the exact quantity
depends an conversion targets, feed properties, etc..
2~7~0(~2
F-5819 - 7 ~
Ether-rich stream 3~ containing byproduct
isopropanol, unreacted propene, water and C6+
hydrocarbon oligomer is further separated after passing
through heat exchangers 38, 39 to extractor unit 40,
where it is contacted with fresh feed water 42 and
water recycle stream 52 to extract isopropanol in an
aqueous phase 44. A product stream 46 consisting
essentially of DIPE and byproduct C6+ propene oligomer
is recovered from the extraction unit 40. The extract
phase 44 is passed via exchangers 38, 48 to
distillation column 50 to obtain an overhead
isopropanol recycle stream 12 and liquid aqueous stream
52 for use in extraction unit 40.
The process flow scheme for olefin hydration to
ethers over a zeolite Beta catalyst as disclosed
utilizes product recycle and reactor quench for
improved catalyst life and temperature control. The
example system utilizes three catalyst beds in a single
vertical reactor shell with mixing with inter-bed
quench to reduce cost while controlling system phase
behavior in comparison to other designs (tubular
reactor).
The example shows improved catalyst life and yield
benefits when recycling reactor product (pumparound)
for propene hydration to di-isopropylether (DIPE) over
typical porous acid solid catalyst. Product pumparound
is advantageous as a means of controlling reactor
temperature rise and therefore reduces equipment cost,
as compared to a conventional isothermal tubular
reactor or the like. The use of the product recycle
for inter-bed quench eliminates inter-reactor coolers
while giving benefits in control of phase behavior.
The three 'oeds are contained in a single reactor
vessel. This reactor design is lower cost than other
reactor designs.
This preferred process design, which is suitable
for commercial design, utilizes feed purification,
2~7~0~32
F-581g - 8 -
product recycle, and reactor quench for hydration of
propene to DIPE to achieve high propylene conversion
(~95% overall). The design also takes advantage of
reactor recycle and quench with interstage
mixing-distribution to provide reduced catalyst aging
and reactor temperature control (heat removal).
In the following example, 65% zeolite Beta is used
in extrudate form with an alumina binder; however,
other binders such as silica, zirconia, etc may be
used. Continuous runs are made, with weight hourly
space velocity of 0.33 charged to catalyst basis for
propene. Unless otherwise indicated, reaction
conditions include reactor inlet temperature of about
165C and pressure of about 10,000 kPa. comparative
runs include a single zone adiabatic downflow reactor
with fixed bed extrudate catalyst. Tables 1 and 2 give
data for a typical DIPE production system, based on 100
parts by weight of fresh propene feed.
2~7~g2
F-5819 - 9 -
TABLE 1
Stream Component Flowrates
Stream No. 1 3 10 12
C3= 100 4.24 237.910.00
C3 91.27 90.77 59.60o.oo
Water 50.12
IPA 51.37
DIPE Trace 5.49
c6=+ Trace 0.50
TOTAL191.27 95.01 297.51107.48
Stream No.15 24 35 32
C3=237.91 142.64 142.14000.498
C3 59.60 59.60 59.10200.498
Water50.12 32.67 0.000032.670
IPA 51.37 Sl.S2 0.000051.621
DIPE 5.49 106.23 0.0022106.234
C6=+ 0.5 12.22 0.000212.220
TOTAL404.99404.99 201.2500203.740
Stream No.46 44 52 42
C3= 0.498 0.000 0.00 0.00
C3 0.498 0.000 0.00 0.00
Water0.748 176.560 126.4318.20
IPA 0.249 51.370 0.00 0.00
DIPE100.748 5.490 0.00 0.00
c6=+11.720 0.498 0.00 0.00
TOTAL114.463233.92 126.4318.20
F-5819 - 10 - 2~7~002
TABI~E 2
Reactor Recycle/Ouench Component Flowrates
Rl R2 R3
Recycle to Recycle to Recycle to
First Bed Second Bed Third Bed
C3= 49~.8 71.6 79.3
C3 208.2 29.9 33.2
Water114.0 16.5 18.2
IPA 180.3 25.9 28.7
10 DIPE371.1 53.1 59.1
C6=+ 42.6 6.2 6.7
TOTAL1415 203.2 225.2
Average
Temperature,C (F) 165(330) 54(129) 54(129)
T, C (F)/bed11(20) 11(20) 11(20)
Bed Inlet
Temp. C (F)(SOC) 155(310~ 155(310) 155(310)
Bed Inlet
Temp. C (F)(EOC) 177(350) 177(350) 177(350)
Bed Inlet
Temp. C (F) (SOC) 165(330)165(330)165(330)
Bed Inlet
Temp. C (-F) (EOC) 188(370)188(370)188(370)
2~75~
F-58l9
In fixed bed hydration of propene to
di-isopropylether (DIPE), catalyst aging has been found
to limit cycle length and catalyst life during single
pass operation. When effluent recycle with mixing and
quenching are employed, propene conversion and DIPE
yield increase and aging is significantly reduced.
This effect is unexpected, since pump-around type of
reactor operation (i.e., back mixed) generally reduces
conversion compared to plug flow (single pass).
Catalyst life is increased compared to conventional
process flow schemes. Product pumparound recycle is
ordinarily expec~ed to lower yields (back-mixed versus
plug flow) and reduce catalyst life (coke/polymer
formation). The opposite occurs in both instances.
This advantage in yield and catalyst aging is
unexpected. Product recycle is believed to improve
overall distribution in the reactor due to higher flux
and improving phase behavior (a single liquid phase
versus water-rirh and water-lean phases). The
~ increased DIPE at the reactor inlet might act as a
solvent to remove coke precursors. Temperature control
by product recycle guench is also a primary benefit for
process with a small window of operation.
This flow scheme provides significant benefits
beyond simple heat control. It is possible to adjust
pumparound composition by adjusting reactor effluent
temperature, separating out a water rich phase, and
recycling a separated ether-rich phase which may
further improve performance. This same effect in water
reduction might also be accomplished by reducing the
water content or amount of the IPA/water recycle from
distillation.
A theoretical explanation for the observed
increase in yields results from the strong solvents
isopropanol/ether sa~urating the catalyst pores. This
prevents the formation of any separate water or olefin
2~7~2
F-5819 - 12 -
phases in the pores during operation. The aqueous or
hydrocarbon phases can cause catalyst deactivation.
The water phase may attack the crystalline structure of
the catalyst, while a highly olefinic phase would
deactivate the catalyst via rapid coke formation. The
isopropanol/ether mixture also allows controlled
quantities of water and propylene to be present
homogeneously in the catalyst pores, which allows the
reactions to proceed properly at reaction temperature.
For the recycle techni~ue to be effective, the recycled
liquid must dissolve the water and olefin present in
fresh feedstock and the quench must be used with good
mixing and distribution.