Note: Descriptions are shown in the official language in which they were submitted.
WO91/11197 PCT/US91/00572
- - 207al91
--1--
NEW METHOD FOR TREATING IMMUNODEFICIENCY OR NEUTROPENIA
The present invention is related generally to the
method of treating immunodeficiency. More particularly,
the present invention is related to inducing or
augmenting neutrophil activity by melanoma growth
stimulatory factor (MG5F).
BACKGROUND OF THE INVENTION
Melanoma growth stimulatory ~actor (MGSF) is a growth
related protein from B-thromboglobulin (B-TG)
superfamily. Although ~GSF has a number o~ properties
similar to interleukin-8 (IL-8), another member o the B-
TG family, MGSF has not heretofore been demonstrated to
possess neutrophil chemotactic activity.
SUMMARY OF THE INVENTION
It is, therefore, an object o~ the present invention
to demonstrate neutrophil chemotactic activity of MGSF.
It is another o~ject of the pressnt invention to
provide a new method for stimulating increased neutrophil
number and activity to combat diseases caused by the
condition of immunodeficiency resulting from or
associated with neutrophil dysfunction or neutropenia.
Other objects and advantages will become evident
from the following detailed description of the invention.
~IEF ~EscRIpTl~N QF THE DRA INGS
These and other objects, features and many of the
attendant advantages of t~e invention will be better
understood upon a reading of the following detailed
description when considered in connection with the
accompanying drawings, wherein: "
Figure 1 shows purification of MGSF receptor/binding
prote~n by ligand affinity binding. 125I-membrane proteins
from Hs294T melanoma cells were allowed to bind to rMGSA-
:. ,~ 1 . . .- , ~: ,
~ . . . . : . . . : . ':
.. .., , .
... . .
WO91/11197 PCT/US91/00572
9~
sepharose and eluted as described in Methods. Eluant was
concentrated by lyophilization and subjected to reducing
SDS-PAGE. Gels were dried and exposed to XAR-5 film for
7 days.
Lane a) 125I-Hs294T membrane preparation eluted from 4-
MGSA-sepharose.
Lane b) l25I-Hs294T membrane preparation eluted from
sepharose alone.
Lane c) 125I-Hs294T membrane preparation.
Figure 2a shows MG5F Binding Curve. Human Hs294T
melanoma cells were grown to confluency and incubated in
SF culture medium for 48 hours. Cultures were then
incubated with l25I-MGSA and varying concentrations of
unlabeled MGSA for 2 hours at 25-C. Cells were washed 5X
on ice, lysed and bound radioactivity was determined by
gamma counting.
Figure 2b (Inset of Figure 2a) shows Scatchard
Analysis of Binding of rMGSF to Hs294T Melanoma Cells.
Binding assays of suspension cultures of Hs294T cells
were performed with 12sI-rMGSA and unlabeled rMGSF as
described in Materials and Methods. Cells were wa~hed
once, pelleted through a sucrose cushion and bound
radioactivity was counted on a ga~ma counter. A
Scatchard plot of the binding data is shown.
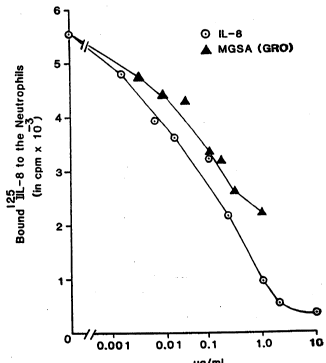
Figure 3 shows MGSF/IL-8 Competitive ~indings Curve.
Human neutrophils (2xlO6) were suspended in binding medium
and incubated with lZ5I-IL-8 wi~ or wi~hout varying
concentrations of unlabeled MGSF or IL-8 for 2 hours at
4-C. Cells were washed once, pelleted through a sucrose
cu~hion, and bound radioactivity was counted nn a gamma
- counter.
WO91/11197 PCT/US91/00572
207~
DETAILED DESCRIPTION OF THE INVENTION
The above and various other objects and advantages of
the present invention are achieved by the demonstration
of neutrophil chemotactic activity of the MGSF and making
use of this new property of MGSF for inducing or
augmenting neutrophil number and activity resulting in
increased immune function.
Unless defined otherwise, all tachnical and
scientific terms used herein have the same meaning as
commonly understood by one of ordinary skill in the art
to which this invention belongs. Although any methods
and materials similar or equivalent to those described
herein can be used in the practice or testing of the
prQsent invention, the pre~erred methods and materials
are now described. All publications mentioned hereunder
are incorporated herein by reference. Unless mentioned
o~herwise, the techniques employed or contemplated herein
are standard methodologie~ well known to one o~ ordinary
skill in the art. The material~, methods and examples
20 are illustrative only and not limiting. .
It is pointed out that a recombinantly made MGSF
(rMGSF) and natural MGSF exhibited equivalent growth
stimulatory activity. Hence, in the ~tudies described
herein, either r~GSF or natural ~GSF was employed as
Pound convenient or as noted herein. ~he specifics of
the methods are now described.
~A~ a~ ODS
P~epa~at~on o~_~lasma Membrane-enriçhed Fract~on _~om
~s294T Melanoma Cel~s
3~ Confluent P-150 dishes (5 per preparation~ of Hs294T
cells were maintained on serum-~ree medium for a minimum
o~ 48 hours. After washing with serum-free medium, the
, . . , - . ,~
''~ , '.. .. I ' ' ' , ,' , .
, ' ' ;' ~ , ~ , ,,, ' ; ' ' '
WO91/11197 PCT/US91/00572
,_
~ 4
cells were removed by scraping and sedimented by
centrifugation. The weights of the cell pellets were
determined and the cells were resuspended (l:lO w/v) in
a hypotonic buf~er solution (50 mM mannitol, 5mM Hepes,
lOug/ml leupeptin, ~% aprotinin pH 7.4). The cell
suspension was passed through an 18 gauye needle, l M
CaCl2 was added to ~hi5 suspension to a final
concentration of lO mM and the suspension was maintained
on ice for lO minutes. The suspension was then passed
through a 25 gauge needle five times. Insoluble material
was removed by microcentrifugation at 12,500 xg in
Eppendorf microfuge tubes for l minute at 4~C. The
supernatant was ultracentrifuged at 105,000 xg for 90
minutes at 4'C producing a fine translucent pellet that
was resuspended in hypotonic bu~fer and stored at -80C.
Iodination of Hs2~4T Nembr~nes
A suspension of Hs294T membranes (10.8: ug) was
microfuged for 15 min. at 4C. The pellet was
resuspended in 2.5 ~l 0.5 M sodium phosphate. pH 7.2. To
the suspended membranes, 20 ~l of lactoperoxidase, lO ~l
o~ glucose oxidase, and lO~l 125I-Na (l mCi) were added.
The reaction was initiated by the addition of 20 ~l B-D-
gluco~e (l8 mg/ml H20 freshly prepared) followed by
incubation of the reagents for l5 min. at room
temperature (about 22--24-C). The membranes were then
solubilized by the addition of 1 ml 0.05 M sodium
phosphate, 1% Triton X-lO0 pH 7.2. This solutio~ was
trans~erred to a PD-lO column that was previously washed
with 50 ml H2O, 2 ml 0.05 M sodium phosphate, 0.1% Triton
X-lO0, 20 mg/ml bovine serum albumin pH 7.2 followed by
30 ml 0.05 M sodium phosphate, 0.1% Triton X-lO0 pH 7.2.
Material w~s eluted using 0.05 M sodium phosphate, 0.1%
: .: ,: :,:, - . .
W O 91/11197 P ~ /US9~/00572
207~191
Triton X-100 pH 7.2. Fractions (2 ml) were collected
into tubes containing 1 ml o.05 M sodium phosphate, 0.1%
Triton X-100, 20 mg/ml bovine serum albumin pH 7.2. A 5
~1 aliquot of each fraction was counted in a gamma
counter, then diluted with 2X laemmli electrophoresis
sample buffer for visualization by standard SDS-P~GE and
autoradiographic analyses,
Puri$ication Q~ NGSF
Natural MGSF was purified ~rom Hs294T conditioned
medium as described by Thomas et al (A. Mol Cell_Endo.
57:69-76, 1988.) For some studies recombinant MGSF was
used (rMGSF). The rMGSF was puri~ied from conditioned
media from chinese hamster ovarian (C~0) cells stably
transfected with the MGSF gene placed under the control`
15 of the cytomegalovirus promoter as described by Balentien ;~
et al, supra.
PreParation of Sepharose-~ound Recombinant MGSF ~'
Cyanogen bromide activated sepharose (O.1 g) was
incubated overnight (about 12-16 hrs) with 15 ml lmM HCl.
The swollen resin was washed three time~ with 2.5ml
aliquots of 1 ~M HCl ~hen suspended in 375 ~1 of coupling
buf~er (98.4 g sodium bicarbonate, 29~2 g sodium chloride
per iiter water, pH 8.3~. Recombinant MGSF (5 ~g per 375
~1 coupling bu~fer) was added to the suspended resin and
agitated overnight at 4-C. The resin was pelleted by
centrifugation at 500 rpm in a Beckman ~J-6 centrifuge.
The supernatant was removed and the resin was washed two
times with coupling bu~fer. The resin was incubated
overnight at 4-C with 1 ml of monoethanolamine. A~ter
removing monoethanolamine, the resin was washed with
ac~tate bu~fer (8.2 g sodium acetate, 58.4 g sod~um
chloride per liter water, pH 4.0) ~ollowed by Tris bu~fer
WO91/11197 PCT/US91/00572
(6.075 g Tris, 11.6 g sodium chloride per liter water, pH
8.0). The acetate ~u~fer - Tris buffer cycle was
repeated three times. The resin was stored in o.05 M
sodium pho~phate buffer, pH 7.2
~ LJ~L_ o~ l2sI-La~ d ~s22~ ~nes to r~GSF-
Seph~rose
rMGSF-sepharose was suspended in 30 ~1 of binding
buffer (0.8g ~odium chloride, 0.115 g dibasic sodium
phosphate, 0.02 g potassium chloride, 0.02 g monobasic
potassium phosphate, 0.01 g magnesium chloride, o.o1 g
calcium chloride, 1.O g bo~ine serum albumin, 1.0 mg
leupeptin, and 10,000 kallikrein inhibitory units of
aprotinin per 100 ml water, pH 7.2). 125I-labelled Hs294T
membranes (35 ~1, 5,000,000 cpm) were added to rMGSA-
sepharose and a control sepharose resin. These wereincubated at 4-C with agitation for 4 hrs. The resin was
pelleted by centrifugation and the supern~tant was
aspirated. The resins were washed with three X 1.5 ml
aliquots of ice cold binding buffer. Material bound to
the resins was eluted by incubating with 0.4 ml aliquots
100 mM HCl for 15 min. A~ter pelleting the resin the
supernatant was removed, the elutio~ was repeated and the
aliquots were combined and frozen. The solution was
dried by speed vac and the material was redissolved in 20
~1 4 mM HC1. Laemmli 2X electrophoresis sample buffer
(40 ~1) was added, the solutions were boiled for 5 min.,
microfuged for 2 min., and the supernatants were
electrophoresed in a 7.5% polyacrylamide gel. After
staining with Coomassie blue R-250, the gel was dried and
exposed to Kodak XAR5 film for ~isualizatio~ of the
radiolabelled proteins.
: : ,:. ~j,,, :,
.. : ,, j ~ .: .,,, : ,
WO91/1~197 PCT/US91/00572
2~7~191
Competitive Bindinas Assays
~ MGSF was iodinated by either Bolton-Hunter or the
chloramine-T procedure ~Bolton et al, ~iochem J. 133:529-
539, 1973: Hunter et al, Nature (London) 194, ~95 1962).
Studies of l2sI-MGSF (specific activity 6.7Xl05 cpm/ng)
binding were performed on confluent cultures of Hs294T
malignant melanoma cells in 24 well Falcon plates at
25-C. Incubations were performed in a binding buffer
containing phosphate buffered saline + 30 mM H~PES + 1% .
BSA, p~ 7.2. After the appropriate length of time, the
binding buffer was aspirated from the monolayers and the
cells were washed ~X with ice cold binding buffer. The
monolayers were then treated with 1 ml of lysis buffer
(o.5 g ~DS, 0.121 g Tris, 0.037 g E~TA/lOO ml H2O) at room
temperature for 15 min. The lysis bu~fer was transferred
to 12 x 7.5 mm tubes. The wells were rinsed with a
second aliquot (0.5 ml) of lysis buf~er. The aliquots
were combined and the amount of l2sI-MGsF found was
determined in a gamma counter. Non-specific binding was
determined in the presence of lOO fold excess crude MGSF.
Studies of the binding of l25I-MGSF to several normal
and malignant cell lines were per~ormed on suspension
cultures ~1XlO6 cells!ml) to enable a constant cell number
for comparison among cell types. The methods were
essentially the same except that cells were recovered by
centri~ugation between incubations and washes. The lysis
step was eliminated. Data are expressed as cpm bound to
each cell line divided by cpm bound to Hs294T cells x
100~ .
M~SF ~ioresponse ~ssay
The bioresponse elicited by exogenous MGSF in various
cells lines was determined by 3H-thymidine incorporation
: . , ., : .
- .
~: .
wos~/11197 PCT/US~1/00572
assay or in a si~-day cell number assay. For tritiated
thymidine incorporation assays 104 cells were plated into
96 well paltes (Falcon) în DME~ medium (Gibco) containing
1~ fetal bovine serum and incubated overnight at 37C in
a water jacketed C02 incubator. The following morning
aliquots of ~GSF or fetal bovine serum were added, the
incubation was continued for an additional 18 hours, and
then 5 ~Ci of 3H-thymidine was added to each well.
Cultures were incubated for an additional 24 hours at
which time each of the monolayers was washed twice with
lOo ~l phosphate buffered saline and fixed with 5%
trichloroacetic acid (TCA) for 30 min on ice. The TCA
- was aspirated and 200 ~l of 100% methanol was added to
each well. After 30 min the methanol was aspirated and
cells were solubilized in lOO ~l of 0.3N NaOH for l hr at
37-C. Aliquots were added to Beckman Ready Fluor and
counted by liquid scintillation counting.
For cell number assays, the cells were seeded (5000
cells/well) into 24 well Falcon plates in media
containing 10% serum. After a 72-hour incubation~ the
cells were washed once and placed on serum-~ree medium.
Twenty-four hours later, the medium was aspirated and
replaced with serum-free medium with and without aliquots
of ~GSF. A background count was done on well containing
only serum-~rQQ medium. On thQ third and sixth days
a~ter growth factor additions, cell number was
determined. Tho~e cultures that were not counted on day
three were ~ed at that ~$me.
Chemot~xls_AssaY
~he nautrophil chemotaxis assay was performed as
describQd by Larsen et al., Science 243:1464-1466, 1989
and Falk et al, J. Immunol. Method. 33:239-247, 1980,
WO91/11197 PCT/US91/00572
.
2075~91
except that rMGSF wa~ used.
5I-IL-8 Competitive Bindin~ Assav
Recombinant IL-8 has been expressed in E. coli and
purified to homogeneity with specific activity of
2XlO~/mg (Furuta et al, J. Biochem. 106:436-441, 1989).
Recombinant IL-8 was labelled with l25I by Bolton-Hunter
reagent as previously reported (Samanta et al, J. Exp.
Med. 169:1185-1189, 1989; Bolton et al., suPra: Falk et
al, supra). Ability of rMGSF to compete with 125I-IL-8
10 (1. 0 X 107 cpm/~g) to human neutrophils was examined. In
standard binding assays, 2xl06 cells were incubated in
duplicate with 4 ng 125I-IL-8 (40,000 cpm) in RPMI 1640
medium containing lO mg/ml BSA and 20 mM HEPES bu~fer pH
7.2 in a total volume of 200 ~l. Samples were mixed and
incubated at 4-C for 2 hours. After incubation, the
tubes were microcentrifuged for 15 sec. at lO,000 rpm.
The supernatant was aspirated and the pellet was
suspended in 180 ~l binding medium and layered on to 800
~l cold D-PBS containing 10% sucrose. ~he tubes were
centrifuged for l min by microcentrifugation. The
supernatant was aspirated carefully and the pellet was
collected by cutting the tip of the tube and the
radioactivity present in the pellets was measured by a
gamma counter.
RESULTS
Identification of MGSF Bindinq Proteins by the MGSF
Affinity Column
MGSF binding proteins have been iden~ified with an
MGSF-sepharose affinity column. When Hs294T-~embrane
preparations were iodinated by the lactoperoxidase
~ethod, bound to MGSF-sepharose, and eluted with lO0 mM
HCl, ~ollowed by lyophilization and reducing SDS-
-; . ~ . :
- ; WO91/11197 PCT/US91/00~72
polyacrylamide gel electro-phoresis prior to
autoradiography. The results indicate 12sI-membrane
proteins of >200,000 and -65,000 molecular weight bind
MGsF-sepharose suggesting that these two proteins are the
major MGSF binding proteins. Proteins in these molecular
weight ranges had a slight affinity for sepharose alone,
as is shown in Fig. l.
Bindina o~ 125I-MGSF to Cells
Binding ~tudies with natural l2sI-MGSF and Hs294T cells
consistently produced low levels of binding. Tests with
several different binding buffer preparations by altering
~Ca++], [~g++], [BSA], ~ovalbumin], pH, ionic strength,
and other parameters indicated that optimal binding could
be achieved with a binding buffer of Pss, ph 7.2,
containing l0 mg~ml BSA and HEPES (30 mM). Binding
assays performed on sub-confluent cultures of Hs294T
cells in 24 well plates for 2 hours at 25~ C typically
specifically bound about 0.74 fmoles of MGSF per lo6 cells
~-445 receptors per cell). Prewashing cultures 5 times
prior to binding helped remove endogenous MGSF and
enhanced l25I-MGSF binding. Suramin pretreatment followed
by 5 washes did not improve over the prewash treatment
alone. Half maximal binding was achieved in the presence
of l0 ng unlab~lled natural MGSF.
When ~25I-rMGSF binding studies were performed on
suspensions o~ Hs294T cells using the protocol for IL-8
bindings studies, the results indicated approximately 480
high affinity receptors per cell. the calculated Kd was
0.5 x l01M, as compared to the Kd of IL-8 for the
30 neutrophil IL-8 receptor of 8.0 x l01M. One difficulty
enaountered in the binding studies using rMGSF is that
concentrations of cold rMGSF of 200 ng/ml inhibited
:`' . ' '' ' .~ ~ .: . : ~ " ' ; . . ,
WO91/11197 pcT/uss1ioo572
207~191
11 .... ,~
binding to the same extent as 1 ~g/ml or 2 ~g/ml. this
is at least partially the result of the tendency of MGSF
to form dimers and tetrameres at neutral pH so that the
concentration of free monomer available for binding does
not actually increase as the unlabelled MGSF increases to
2 ~g/ml. Additionally, there could be a very large
number of low affinity and perhaps less specific binding
sites on the cells studies (Fig. 2b).
When lZ5I-MGSF was tested ~or ability to bind to a
number of cell types, a low level of binding was obtained
with melanoma, polymorphonuclear leukocytes (PMN's), and
Balb/c 3T3 fibroblasts, lymphocytes, nevus cells, luny
carcinoma, prostatic carcinoma, NRK49-F and glioblastoma
cells, while other cell types tested exhibited a very low
binding capacity for 125I-MGSF (neuroblastoma and FS-4
human fibroblasts) (Table 1).
~iores~onse to MGSF
Numerous cell lines were examined ~or ability to bind
125I-MGSF and exhibit a bioresponse to MGSF. Bioresponse
to MGSF was assessed by either cell number or 3H-thymidine
assay. 3H-thymidine incorporation assays were performed
on serum depleted confluent cultures. Cell lines which
exhibited a strong MGSF response were MeWo LCl, A~27 lung
carcinoma, nevus 106, Calu-6 adenocarcinoma of the lung,
PC3 prostatic carcinoma, NRK-49F normal rat kidney, FS4
human ~ibroblasts. One human fibroblast culture did not
respond and the Al72 glioblastoma cell line did not
respond. Nevus 106, Hs294T melanoma and ~alb/c 3T3 cells
gave a modest response to exogenous MGSF (Table 1).
... . . . .. .. ....
WO9~/11197 ~ ~9~ PCT/US91/00572
Competition of rMGSF _with 125I-I~-8 Neutrophil Bindin~
~ssay
The observation that ~GSF binds to human neutrophils
and lymphocytes ~uggested that there may be some
relationship between MGSF and other members of the B-TG
family which are chemotactic for neutrophils.
Consequently, the ability of MGSF to compete with 125I-IL-
8 f or its binding ~ites on the neutrophil was examined.
125I-IL-8 specifically binds to human neutrophils and lOO
ng unlabelled IL-8 displaces approximately 50% of the
labelled 125I-IL-8 from these cells. rMGSF competed with
1Z5I-IL-8 for binding to its receptor with 50% competition
at 500 ng/ml. ~his represents a concentration -5 times
that of IL-8 for 50% inhibition of binding of i25I-IL-8 to
this receptor (Fig. 3).
Neutro~hil Chemotactic Activitv in rMGSF
Since IL-8 shows in vitro neutrophil activity, the
possible chemotactic activity of rMGSF for human
neutrophils was tested. Concentrations of as little as
0.5 ng/ml of MGSF resulted in a chemotactic index of 3.0
while the peak response was at 50 ng/ml with a
ch,emotactic index of 17.6. There was a decline in the
chemotactic index with 500 ng/ml of MGSF. For comparison
10'7M fMLP resulted in an index of 21.2. This was
comparable to the 50 ng/ml concentration of MGSF (Table
2). Thus, the dose dependent potency of neutrophil
chemotactic activity o~ ~GSF is essentially equal to
recombinant IL-8 (Larsen et al, supra~.
~SF_Bioactivity 'n IL-8 PreParations
Based upon the demonstration that rMGSF binds to the
IL-8 receptor and exerts chemotactic activity, it was
desired to be determined whether IL-8 might also be
-WO 91tlll97 PCT/US91/00572
2~7~jl91
13
capable of eliciting an MGSF biological response in the
Hs294T melanoma cultures using the MGSF bioassay as
described in Bordoni, et al, J. Cell Biochem. 39:241-24~, -
1989. Therefore, the ability of rIL-8 to stimulate 3H-
thymidine incorporation into DNA in Hs294T calls was
examined. IL-8 preparations exhibit MGSF bioactivi~y at
concentrations of 60 ng/~l and 6 ng/ml, but 0.6 ng/ml and
0.06 ng/ml were inactive. The percentage of stimulation
was equivalent to that of 0.6 ng/ml preparations of
rMGSF, yielding a modest stimulation of 3H-thymidine
incorporation into DNA in low density serum free Hs294T
melanoma cultures (Table 3).
In short, the results presented herein cIearly
demonstrate the neutrophil chemotactic activity o~ MGSF.
This newly established properly of MGSF now allows for
the first time to use MGSF as an inducer of neutrophil
activity which may be desired in such conditions as
Neutropenia or in those conditions which result from
neutrophil dys~unction.
It is understood that the examples and embodiments
described herein are for illustrative purposes only and
that various modifications or changes in light thereof
will be suggested to persons skilled in the art and are
to be included within the spirit and purview of this
application and scope of the appended claims.
'. `~ " ' '' '; ' ',;'''.'.'',..'~"'',,.'', :' '' ,7 ""~' .;" '.' " i' `'
,. ' ~ ~ ~
~ ., ' ' ,' ' : ,
; WO91/11197 PCT/US91/00572
:
Table l
Relative 125 I-MGSA MGSA Bioresponse
Cell Type Bindina as ~Hs294T Assayl
Hs294T melanoma 100% *159% (l.8 ng/ml)
MeWo LCl melanoma 45% **221% (.15 ng/ml)
SK-N-Mc neuroblastom~38% ND
Al72 glioblastoma 2~3% **125% (6ng/ml)
PC3 prostatic carcinoma 56% *260% (1.8 ng/ml)
A427 lung carcinoma139% **188% (.6 ng/ml)
Calu 6 Adenocarcinoma 170% *263~ (l.8 ng/ml)
(probable lung)
Nevus 106 62% *157~ (.6 ng/ml)
NRK-49F rat fibroblasts 277% *257% (0.18 ng/nl)
Balb c/3T3 mouse
fibroblasts 204% **150% (6ng/ml)
FS-4 human fibroblasts 22% **333% (6ng/ml)
LeSans human fibroblast ND no response
~0
lymphocytes-human PB 68% ND
granulocytes-human PB 90% ND
** bioresponse data from cell number experiment
* bioresponse data from 3H-thymidine incorporation
assay.
Bioassay data shown are as percent of binding
bu~fer control for the concentration yielding
the maximal response.
WO91/11197 PCT/US91/00572
2075~1
.Table 2
Chemotaxis Assay
Migrated
Cell Nu~ber/High Power
Field (40XlO)
Chemotatic*
~GSA Contraction ~n=6. mean yalue ls shown) Index
0 5.8
0.05 ng/ml 6,0 l.l
0.5 ng/ml 17.5 3.0
5.0 ng/ml 48.3 8.3
15 50.0 ng/ml lOl.8 17.6
500 ng/ml 86.5 14.9
fMLPlO7M 123.0 21.2
*Chemotactic Index=
Miqrated cell number in the presence of chemoattractant.
Migrated cell number for media alone.
: : ;
W~91/11197 ~ PCT/US91/00572
:`
Table 3
Effect of IL-8 and r~GSA
on Hs294T 3H-Thymidine Incorporation
IL-8 c~m~S.D. ~ BSA_Control
1) 60 ng/ml 2136+174 179%
2)` 6 ng/ml 2167~407 173%
10 3) 0.6 ng/ml 2204+481 133% ~:
4) 0.06 ng/ml 1761+231 112%
rMGSA
1) 6 ng.ml 1607+415 134%
2) o6 ng/ml 2164+565 173%
15 3) .06 ng.ml 2192+173 133%
4) .006 ng/ml 2078+420 132%
BSA dilutions
1) 12.5 ug/ml 1209~328
2) 1.25 ug/ml 1253+203
20 3) .125 ug/ml 1655+454
4) 0.125 ug/ml 1578+586
.
~ Samples are initially aliquoted with 50 ug BSA so
that as each sample is diluted for bioassay, the BSA
concentration diminishes. Therefore, the data are
normalized to a dilutions o~ BSA aliquots equivalent
to the ~SA in the growth factor samples.