Note: Descriptions are shown in the official language in which they were submitted.
2~7~683
S012-988.550
Process and device for the simultaneous
synthesis of several polypeptides
For rapid evaluation of structure/activity
equations on biologically active peptides by receptor
binding studies and rapid determination of epitopes for
immunology in peptides and proteins, relatively small
amounts (less than 20 mg) of a plurality of peptides are
required. These peptides are conveniently prepared by
solid phase peptide synthesis. This synthesis is based
on the method developed by R.B. Merrifield (G. Barany,
R.B. Merrifield in The Peptides, Analysis, Synthesis,
Biology, Vol. 2, 3-284 (1980), published by Gross,
Meienhofer Academic Press, New York), in which the
peptide chain is synthesised step by step. The
synthesis steps can be summarised as follows:
a) Binding the first amino-acid of the peptide chain
to a polymeric carrier via an anchor group,
b) condensing on the remaining amino-acids of the
peptide chain step by step,
~) intermediate steps between the individual
condensations, consisting of washing, cleaving any
protective groups and neutralising,
d) if desired, acylating terminal amino groups,
e) cleaving the peptide from the carrier.
For this peptide synthesis a period of up to 18
hours, usually up to 4 hours per amino-acid must be
allowed for the synthesis. (The individual
condensations generally take one to two hours' reaction
2~76~g3
time; between the condensations about 10 intermediate
steps are generally required, each of which will require
about 2-15 minutes). The preparation of peptides
consisting of a large number of amino-acids is therefore
very tiresome, labour-intensive and expensive.
A method for the solid phase synthesis of analogous
peptides has been described by R.A. Houghten (Proc.
Natl. Acad. Sci, USA, Vol. 82, pp. 5131-5135, August
1985, Immunology). According to this method the
polymeric carrier for the synthesis is packed into small
porous polypropylene bags in batches of 50-100 mg, the
bags are sealed by fusion, the intermediate steps common
to the syntheses (washing, neutralising, cleaving of
protective groups) are carried out on all the bags
simultaneously in a single reaction vessel and the
individual condensations are carried out separately.
The method may be carried out manually or partly
automated using a peptide synthesizer.
The disadvantage of the method described is that
the handling of the bags is rather laborious, the bags
cannot be reused, the bags have to be separated from one
another for the condensation of the different peptides
and no control samples can be taken throughout the
entire synthesis.
German Patent Application No. P 38 28 576.2 and the
~ublication by G. Schnorrenberg and H. Gerhardt,
Tetrahedron Vol. 45, No. 24, 7759-7764, 1989 describe a
process and a device which allow a number of
polypeptides to be synthesi~ed simultaneously
automatically and avoids the disadvantages mentioned
above. The solid phase synthesis method mentioned above
is modified so that it can be carried out with the aid
of a suitably adapted pipetting robot. Hitherto,
pipetting robots have been used for serial analysis.
For example it is possible to use a pipetting robot made
by TECAN, RSP 5052. Pipetting robots have the following
external components: at least one arm having a metering
2Q7~S~3
pipette, a clamp with storage vessels and a microtitre
plate which may contain up to 96 wells. The robot arm
brings the reagents from the storage vessels and places
them in the respective wells in the microtitre plate and
if necessary sucks liquids out of the wells. The
cannula of the metering pipette may be constructed so
that it is divided into two parts by a partition wall
running from top to bottom. (By means of this
partitioned cannula it is possible to supply two
different metered amounts or to supply one metered
amount and suck out one amount with one arm). The work
pattern of the device is controlled by a computer
programme. According to the above mentioned German
Patent Application No. P 38 28 576.2 the solid phase
peptide synthesis in a pipetting robot of this kind is
carried out as follows: -
carrier material (preferably granulated carrier
material) is placed in the wells of a microtitre plate.
The carrier material may be charged with the initial
quantity of the desired peptide. The liquids required
for the reactions and washing steps are kept in
readiness in the storage vessels of the device. If at
the end of the synthesis the peptide is to be separated
from the carrier and/or if free amino groups are to be
acylated, the reagents required for these reactions
- ~hould also be kept in readiness in the storage vessels.
The reaction times needed show that it is advisable to
use a microtitre plate containing not more than 96
wells. Accordingly, a maximum of 96 different
polypeptides can be synthesised in one programmed
operation. In accordance with the programme, which is
adapted to the synthesis of these peptides, the robot
introduces the reagents and washing liquids into the
individual wells and, after the required retention time,
sucks out the supernatant liquid above the carrier. The
process and the necessary adaptation of the device to
the process is described more fully with reference to
207S6~3
the two-armed pipetting robot RSP 5052 made by Messrs
TECAN. However, the application of the process is not
restricted to this device. Pipetting robots of
different constructions, especially one- or multi-armed
robots may be adapted to the process according to the
present invention. A microtitre plate having 96 wells
is used. One well will contain, for example, 10 mg of
resin which may be charged with an amino-acid, and will
hold rather more than 300 ~1 of liquid. This quantity
of resin corresponds to about 5 ~mol of amino-acid or is
suitable for the preparation of about 5 ~mol of peptide.
Conventional carrier materials based on polystyrene or
polyacrylamide can be used. It is convenient to
synthesize peptides containing not more than 20 amino-
acids. The reagent solutions and washing liquids
required for this are prepared in the storage vessels
provided for this purpose. Arm 1 of the device is
equipped with a metering pipette, arm 2 with a suction
channel having a rinsing device. This rinsing device is
preferably connected to a separately standing storage
vessel for the solvent used. Synthesis is carried out
in accordance with the programme in the connected PC.
The arm 1 measures all the reagent solutions which are
taken from open storage vessels. Before the metering
pipette changes from one reagent solution to another,
~he metering pipette is rinsed with solvent in a special
rinsing position. The arm 2 sucks up the reagent and
washing liquids through a cannula fitted with a filter.
In order to prevent resin losses and contamination of
the adjacent wells the outside of this cannula is rinsed
with solvent after each suction process by means of a
line mounted on the side of the cannula. The next
washing process is started at the same time with this
solvent. The cannula is then rinsed in a cannula
rinsing position. In order to separate the peptide from
the resin, trifluoroacetic acid, for example, is
introduced into the wells via the arm 1. After it has
2n~6~3
been split off, the solution is sucked up with the
suction cannula and transferred into a second microtitre
plate from which it is then worked up.
The device described makes it possible to carry out
automatic simultaneous synthesis of a number of
polypeptides. One unsatisfactory feature, however, is
that the liquids cannot be sucked up entirely and that
it takes a relatively long time to suck up the reagents
and washing liquids. The present invention overcomes
these drawbacks. Moreover, it is possible to synthesize
up to 25 ~mol of peptide without changing the reaction
vessels.
The present invention relates to an apparatus for
simultaneously synthesizing a plurality of polypeptides
by the solid phase synthesis method, comprising a
plurality of reaction vessels and a holding device for
the reaction vessels, these reaction vessels being open
at the top and bottom, the bottom opening of the
individual reaction vessels being covered by a filter,
the retaining device being a closable vessel which has a
possible connection for an inner gas feed line and a
suction device and openings in which the reaction
vessels are secured in such a way that their upper
openings are accessible from above for the addition of
the liquids required in the synthesis process whilst the
bottom openings thereof are connected to the interior of
the holding device.
This device may be operated in conjunction with the
pipetting robot described above, for example.
The invention further relates to a process for the
simultaneous synthesis of a plurality of polypeptides by
the solid phase synthesis method using the apparatus
described above, in which polymeric carrier material or
polymeric carrier material charged with the first amino-
acid or a peptide is placed in the reaction vessels,
then the peptides are synthesized in the reaction
vessels in accordance with the solid phase synthesis
20~S3
method which is known per se and, if desired, free amino
groups and/or hydroxy groups of the peptides are
acylated and/or the peptides are subsequently separated
from their carrier material, the reagents or washing
liquids required for the individual steps being
introduced into the reaction vessels by one or more
robot arms with cannulas from the corresponding storage
containers and after the required retention time of the
reagents or washing liquids the liquids contained in the
reaction vessels above the filters are simultaneously
sucked out through the holding device, the individual
steps of the process being controlled by the programme
in the computer attached to the robot. In a preferred
embodiment, inert gas is piped into the holding device
throughout the entire synthesis process, with the
exception of the phases in which the liquids are sucked
out, and this inert gas is kept under such low pressure
that it only prevents the liquids from seeping through
the filters in the reaction vessels.
In a preferred embodiment of the device the holding
device consists of a tub-shaped vessel with a plate-like
cover, this cover having openings each of which holds a
reaction vessel.
The holding device and reaction vessels are
conveniently made from glass, metal (preferably
~tainless steel), polypropylene, teflon or polyamide 66
or a combination of these materials.
The retaining device and reaction vessels must be
matched to one another so that the reaction vessels fit
securely in the holding device. This may be achieved,
for example, by screwing or fitting the reaction vessels
into the holding device.
In a preferred embodiment of the device the cover
of the holding device is provided with suitable conical
openings and is preferably made of teflon or polyamide
66. The reaction vessels are cylindrical glass
containers which have at the bottom a ground glass
2~766,~3
-- 7
section which is inserted in the holding device. The
filters which cover the bottom openings in the reaction
vessels are fritted glass or teflon filters. (It is
advantageous to use reaction vessels which have a bore
in the bottom into which the fritted teflon filter can
be placed or pressed. After the end of a cycle of
synthesis these fritted filters can be replaced by new
ones).
When the apparatus according to the invention is
operated with the pipetting robot mentioned above it is
advisable to construct the apparatus with not more than
48 reaction vessels.
However, the application of the process is not
restricted to this apparatus. Pipetting robots of
different constructions, particularly one-armed or
multi-armed robots, may also be adapted to the process
according to the invention.
In order to carry out the process according to the
invention, 10-50 mg of resin, for example, are placed in
each reaction vessel. This quantity of resin
corresponds to about 5-25 ~mol of amino-acid or is
suitable for preparing about 5-25 ~mol of peptide.
Conventional carrier materials based on polystyrene or
polyacrylamide may be used. It is advisable to
synthesize peptides containing not more than 30 amino-
~cids. The reagent solutions and washing liquids
required for this are prepared in the storage vessels
provided for this purpose. The arm of the pipetting
robot is fitted with a metering pipette. Synthesis is
carried out in accordance with the programme in the
attached PC. In this way all the reagent solutions are
metered in, having been taken out of the storage vessels
sealed with teflon partitions. Before the metering
pipette changes from one reagent solution to another, it
is rinsed with solvent in a special rinsing position.
The washing liquids required are introduced analogously.
The removal of the desired peptide from the carrier
2~6~3
-- 8 --
using trifluoroacetic acid may similarly be carried out
automatically or manually.
The sucking out of all the liquids from the
reaction vessels for the particula- reaction or the
washing steps is carried out simultaneously in all the
reaction vessels by means of the suction device attached
to the holding means (eg. water jet pump or diaphragm
pump).
Generally, DMF or N-methylpyrrolidone is used as
solvent. Accordingly, the reaction vessels and any
rinsing device provided must be made from solvent-
resistant material, eg. glass, polypropylene or teflon.
In the standard commercial equipment the metering
pipettes are made from stainless steel. This material
is suitable for the process according to the invention.
The process is carried out, for example, with the
following means (quantity per well): starting material
is 10 mg of resin charged with Fmoc amino-acids
(particle size 200-400 mesh); Fmoc-protected amino-acids
are used in an excess of up to 10 times for each
individual coupling step, ie. 200 ~1 of a DMF solution
of 50 ~mol Fmoc amino-acid and 50 ~mol of 1-
hydroxybenzotriazole and 100 ~1 of a DMF so]ution of 75
~mol of N,N-dicyclohexyl-carbodiimide are added; the
coupling time is about 1 hour. The Fmoc protecting
~roup is split off by means of 300 ~1 of a 40% solution
of piperidine in DMF. The cleaving time is about 20
minutes. The washing steps are each carried out with
300 ~1 of DMF.
The acylation of free groups (NH2, OH) may be
carried out analogously by adding suitable acid
anhydrides, eg. acetanhydride and pyridine. After these
reactions have ended the solution is suction filtered
and taken for processing.
The finished peptide can be removed from the
carrier in the reaction vessels by the manual addition
of trifluoroacetic acid (20 minutes' reaction time).
2~7~3
The peptides are isolated separately. As will become
clear from the above explanation, all the steps of the
peptide synthesis are carried out in open vessels. The
method of synthesis according to the invention
nevertheless produces the peptides in a very high degree
of purity.
Figures 1 to 3 diagrammatically show an example of
the apparatus according to the invention.
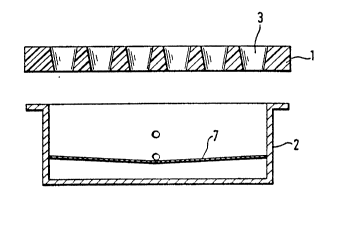
Figure 1 shows the holding device consisting of a
tub (2) and cover (1). The tub (2) can be firmly closed
by means of the cover (1), preferably by screw closures
which screw the flange of the tub to the cover. The
cover (1) has openings (3) which are arranged over the
entire surface at regular intervals. (The drawing shows
only some of the openings). The reaction vessels (4)
are inserted in these openings. If the process is
carried out in a number of reaction vessels which is
less than the number of openings in the cover, the
unused openings are sealed off by means of ground glass
stoppers.
Two connections (5) and (6) are provided in the
wall of the tub (2). Connection (5) is attached to the
suction device and connection (6) serves for the supply
of inert gas.
Figure 2 shows a cross section of a preferred
-embodiment of the holding device. This contains a guide
vane (7) which is mounted like a second base in the tub
(2), just below the connection (5). It is arranged to
slope slightly towards the connection (5), so as to
allow all the liquids sucked out to drain away entirely.
Figure 3 shows a diagrammatic view of a reaction
vessel (4). The upper part of the vessel is
cylindrical. The lower part of the vessel tapers and is
fixedly connected to a ground glass section (9). The
bottom opening in the reaction vessel is covered by a
fritted glass or teflon filter (8).
In this embodiment, the tub (2) is made of metal
20~83
-- 10 --
(preferably V2a steel), the cover (1) is made of teflon
or polyamide 66 and the reaction vessels (4) are made of
glass. The openings (3) are conical and correspond to
the precise size of the ground glass sections (9) of the
reaction vessels. The filters (8) are fritted glass
filters G2 or G3 or fritted teflon filters made by G.T.
Baker Chemikalien, DE-6080 Gro~-Gerau, order number
7329/03. If the apparatus is used together with the
pipetting robot made by TECAN, RSP 5052, the tub (2) is
conveniently produced with dimensions of about 165 x 127
x 45 (mm). The reaction vessels then have a total
height of about 70 mm, of which about 25 mm consists of
the cylindrical part above the fritted glass filter.
The diameter of this upper part is 13 mm. It is
convenient to construct the apparatus for not more than
48 reaction vessels.
The holding device is connected via connection(5)to
a suction device, eg. a diaphragm pump having a 5 litre
suction bottle provided in front of it. The holding
device is also connected to an inert gas supply (eg.
nitrogen bottle) via the connection (6~ A pressure
relief valve (usually set to about 0.1 bar) is provided
in this line. Adjusting means are also provided in the
lines, adjustable by means of a PC, for example.
An alternative embodiment of this device is
~onstructed so that the tub (2) has only one connection
which is connected to the two lines (inert gas/suction
means) by means of a PC-controllable 3-way valve.
The example which follows illustrates the course of
the process according to the invention: the apparatus
described above is used in conjunction with a pipetting
robot. The process is computer-controlled. Carrier
material already charged with a protected amino-acid or
a short peptide is placed in the reaction vessels.
2 ~ 3
-- 11 --
Part of the synthesis cycle:
Step Operation
1 Valve N2 open, vacuum shut
2 Metering of DMF 3 min. Washing
3 Valve N2 shut, vacuum open Suction
4 Valve N2, open, vacuum shut
Metering 40% piperidine in DMF Cleaving of
3 min. protective groups
6 Valve N2 shut, vacuum open Suction
7 Valve N2 open, vacuum shut
8 Metering 40% piperidine in DMF Cleaving of
20 min. protecti-~e groups
9 Valve N2 shut, vacuum open Suction
Valve N2 open, vacuum shut
11 Metering of DMF 30 sec. Washing
12 Valve N2 shut, vacuum open Suction
13 Valve N2 open, vacuum shut
14-40 Repetition of steps 11-13 Washing 9x
41 Metering of desired Fmoc-
amino-acid/HOBt in DMF 1st coupling
42 Metering of DIC/DMF 40 min 1st coupling
43 Valve N2 shut, vacuum open Suction
44 Valve N2 open, vacuum shut
A5-48 Repetition of steps 41-44 2nd coupling
49-78 Repetition of steps 11-13 Washing 10x
Compared with the process and apparatus according
to the German Patent Application No. P 38 28 576.2
referred to above, the apparatus according to the
invention substantially reduces (approximately by half)
the time taken for the synthesis cycle. The synthesis
is reliable and clean since the liquids are sucked up
completely. The course of the synthesis can easily be
monitored as the reaction vessels are open.