Note: Descriptions are shown in the official language in which they were submitted.
,.0 91/13166 PC~r/US91/00633
-'- 2 0 7 7 .~
ANTIBODY CONSTRUCTS WITH ENHANCED BINDING AFFINITY ~
~ . .
5 Backqround o~ the Invention -
This invention relates to cancér imaging --~
agents and cancer therapeutic agents, and more
specifically, to the preparation of antibody
10 constructs useful as imaging agents and/or cancer
therapeutic agents. In particular, this invention
relates to biosynthetic antibody constructs with
enhanced antigen binding affinities and a short half
life in vivo,
Systemic pharmacotherapy is a commonly used .
mode of therapy. Likewise is the administration of ;'~
labelling agents such as radioisotopes for tumor or -
organ imaging. However, the administration of such -
20 drugs and radiolabels, by themselves, is risky in
that the drugs usually are not target selective.
Limiting the effects of chemotherapeutic drugs and
radioisotopes used in the treatment of cancer are
particularly difficult because these chemicals have
25 the ability to interere with the metabolic processes
of most cells, especially those that are in a !' , ,
proliferative state. Therefore, the art frequently -
suggests coupling such drugs to an agent capable of
targeting a particular tissue or cancerous cell
30 type.
Antibody molecules have been conjugated to
~;~ various drugs, enzymes, toxins, and radioisotopes
(reviewed in Ghose and Bla~r (1~78) J. Natl. Cancer
.
'~ .
, -:: . . ~ . : - . . ~ ,: .. , :; ,,.. .: . , . ; .. . .
.O 91/13166 P ~ /US91/00633
-2- 2077~
Inst. 61:657-676). For example, antibodies have been
successfully employed to image occult tumors not
detected by traditional detection methods such as
magnetic resonance imaging, x-ray analysis, and
5 computed tomography (see Goldenberg (1988) Arch.
Pathol. Lab. Med. 112:580-587).
However, a number of problems have been
associated with the use of antibody molecules as
10 targeting agents. Radiolabeled antibodies having a
specificity for tumor antigen may show accretion
rates of only 0.01% to 0.001% in the targeted tumor
(Goldenberg, ibid.). In addition, there may be a
high level of nonspecific binding of the intact
15 antibody despite its specificity for a particular
tissue-associated antigen. Some of this nonspecific
binding has been attributed to the adherence of the
antibody via its Fc portion, rather than via its
binding domain. Furthermore, intact antibodies have
20 a slow ~learance rate relative to smaller molecules
such as fragments of antibody molecules.
In an effort to all~viate these problems,
the prior art suggests the use of antibody fragments
25 which maintain their specificity and ability to bind ~ --
antigen. Antibody fragments display more rapid
specific targeting than intact antibodies. Wahl et
al. (1983) J. Nuclear Med. 24:317-325). Currently,
enzymatically produced F(ab')2 fragments are most
30 commonly used for such applications. These fragments
lack the potentially troublesome Fc portion of the ~-
intact antibody molecule, and so demonstrate lower -
non-specific accumulation in the liver and spleen and
a faster rate of clearance than intact antibodies.
` ~ O 91/13166 P ~ /~'S91/00633 ;
-3- ::.
2~77348 - -
For example, several days after the administration of
radiolabelled, intact antibody IgG, the level of
circulating and nonspecifically bound radioactivity
is high, and clearance occurs gradually over a period
5 of four days or more (Goldenberg, ibid.). In -
comparison, the use of antibody fragments without Fc
domains permits tumor imaging within 24-48 hours,
thereby permitting the use of radioisotopes with
short half lives, such as 123I and 99mTc (Goldenberg,
10 ibid.).
However, the use of F(ab')2 fragments is not
without pitfalls. Besides being difficult to produce
enzymatically, the binding properties of these
15 fragments may be compromised (~ç~, e.a., Wahl et al.,
ibid. Therefore, what is needed is a targeting
molecule ~aving specificity, enhanced binding
activity, minimal nonspecific binding abilities, and
a shorter half-life in vivo.
Towards this end, monoclonal antibodies with
increased antigen binding activites have been
identified which have mUtationS in their constant
regions ~see, e.g., Pollock et al. (1988) Proc. Natl.
25 Acad. Sci. USA 85:2298-2302). In addition, the
ability to genetically engineer and re-express -
antibodies in transfected cells has made it possible
to combine various portions of the immunoglobulin
molecule as fusion proteins (Neuberger et al. (1984) ,
30 Nature 312:614; Morrison et al. (1987~ Ann. N.Y.
Acad. Sci. 507:187; and Morrison et al. (1988) Clin. ;
Chem. 34:1668). It is also possible to genetically
- encode immunoglobulin deletion mutants such as Fab
(Horwitz et al. (1988) Proc. Natl. Acad. Sci. U.S.A. -
",~
'
.O9l/13166 PCT/US91/00633
4
2077~18
85:8687) or F(ab')2-like fragments. In one study, an
Fd~ fragment, a truncated form of the immunoglobulin
heavy chain, was expressed in E. coli (Cabilly et al.
(1984) Proc. Natl. Acad. Sci. USA 81:3273-3277). In
5 another study, specific constant region domains of
the human Cy3 H chain were either deleted or added in
order to assess the effect on antibody synthesis and
assembly (Morrison et al. (1987) Ann. N.Y. Acad. Sci.
507:1873).
Accordingly, it is an object of the present
invention to provide antibodies which recognize tumor
antigens, and would therefore be useful for the
imaging and/or killing of tumors. It is also an
15 object of the present invention to provide antibody
constructs useful for tumor imaging and/or cancer
therapy which have reduced Fc receptor binding so
that their non-specific accumulation in the spleen
and other parts oS the body is reduced. Additional
20 ob~ects include the provision of antibody constructs
which can bind quickly to tumor antigens and the
provision of antibody constructs with shorter half
lives than intact, whole antibody molecules.
...... ........ . . . . ~ . . -, . . - -.. ,. .- .~. - ~ - . - . . -, . . . . . .. .
, . - ... ,.. - ,. . -. , ~: . : ., .:~ . ............. .. . . .
: , .:,. . . - - . -: ~ , ... . , , .. . . :
- ~O9ltl3166 PCT/US91/00633
20773~8
... ... . . . .
summary of the Invention
It has been discovered that antibody
constructs retaining their CHl and CH3 domains, but
5 lacking their CH2 domain, surprisingly have an
enhanced binding affinity as well as a shorter
half-life and an unexpectedly low level of
non-specific binding relative to an intact antibody
molécule with the same specificity. This finding has
10 been exploited to develop the present invention which
features antibody constructs having specificity for
an epitope, present on various tumor antigens.
Native antibody molecules of the IgG and IgD
15 classes are composed of two heavy (H) and two light
(L) chains which are held together by covalent
(disulfide) bonds and non-covalent interactions. The -
variable (~) domains 2t the amino termini of the two
chains together form the antigen binding region. The
20 first constant ~C) domain of the H chain (CH1)
interacts with the C region of the L chain through
hydrophobic interactions and also through a disulfide
bond. The ne~t H-chàin domain, adjacent to the
hinge, is called CH2. This domain reportedly
25 contains many o the effector functions of the
antibody including the sequences responsible or
complement fixation and Fc receptor-mediated -
antibody-dependent cellular cytotoxicity (ADCC), and
is the sole N-linked glycosylation site in human Cyl
30 chains. The CH3 domain at the carboxy terminus of
the H-chain is thought to play a major role in the
assembly of H chains. :
-
,
~`. ' ' `'.,, ', ' "`" ' . '~` ;~' .,,'.' '.' .
:_0 91tl3166 PCI/US91/00633
-6-
2077~48
The antibody constructs of the present
invention include a first portion having an
immunoglobulin binding region of predetermined
specificity. This binding region has plural
5 hypervariable regions homologous with hypervariable
regions of a native antibody specific for the same
epitope. The antibody constructs further include a
second portion consisting essentially of a CHl domain
and a hinge whose C terminus is linked to the N
10 terminus of a CH3 domain, i.e., a hybrid constant
domain which omits the CH2 domain normally present in
a native antibody. ~.ntibody constructs with the CH2
domain deleted are referred to hereinafter as ~CH2
constructs. ~CH2 constructs are characterized by
15 enhanced binding activity, at least twofold greater
than the binding activity of an antibody construct
with the same specificity but having an intact
constant domain.
. .
In one preferred embodiment, the binding
region of the ~CH2 antibody construct includes the
binding region of a nonhuman immunoglobulin such as a
murine immunoglobulin. In one aspect o~ the
invention, the immunoglobulin binding region is-one
25 which has a predetermined specificity for mucin such - -
as the binding region of monoclonal antibody B72.3,
or has a predetermined specificity for the GD2 - -
ganglioside such as the binding region of monoclonal
antibody 14.18. In another embodiment of the
30 invention, the immunoglobulin binding region has a
predetermined specificity for a melanoma-specific
proteoglycan. The preferred altered constant region - -
- includes a human immunoglobulin constant domain.
. . . . . . . . . . . . ~ . . . . . . . , . . . . , . . . . . . . . = . . ~ - . . . . . .
~: ' ' ,.. ': ' " ' ' ''., ': .. , . ''' ' ';, . .' .. ~ ~ ' ' ' ' ,' '. '. " ". ' , ". '."'., '; ' , .. ""
,~'091/13166 PCT/US91/0063~ ~
_7_ 2Q77~`~18
The antibody construct typically includes an
immunoglobulin heavy chain disulfide-linked to an -
immunoglobulin light chain. -
The antibody construct further may include a
third portion bonded to the carbo~y terminus of the
the second portion comprising a tumoricidal protein.
This third protein portion preferably is lymphotoxin,
interleukin-2, epidermal growth factor ~EGF), active
lO analogs thereof, or active fragments thereof. In a
preferred embodiment of the invention, the third
portion is peptide bonded via an endopeptidase-
cleavable amino acid residue, such as lysine, to the
carbo~y terminus of the CH3- domain of the second
15 region. Alternatively, the third portion may be
linked to the second portion by chemical
crosslinking, for esample.
... . ..
In yet another embodiment, for use as an
20 imaging agent, the ~CH2 antibody construct includes a
radioactive label attached thereto.
~-
'-
.
:
~.091tl3166 PCI/I,IS91/00633
20773~8
Brief Description of the Drawing
The foregoing and other objects of the -
present invention, the various features thereof, as
5 well as the inventions thereof may be more ~ully ~ ~
understood from the following description when read ~ --
together with the accompanying drawings in which:
FIG. 1 is a schematic representation showing
10 the construction of a ~CH2 deletion mutant form of ,
the hu~an Cyl gene: (A) is a map of the HindIII to
PvuII fragment containing the genomic Cyl gene
segment and restriction sites; (B) is the ~CH2
deleted gene missing the AflIII to AvaII fragment
15 (the CH2 exon) after restriction and ligation; (C)
shows the SmaI site engineered in the CH3 gene; (D)
is a map of a SmaI to PvuII linker which is used to ~
at~ach the (E) PvuII to XhoI fragment containing an :
I~2 gene; and ~F) is is the remainder of the vector
20 including an XhoI site and polyA region;
,
FIG. 2 is a graphic representation of the
antigen binding activity of variaus ~C~2 chimeric
constructs: (A) ~he ~CH2 chl4.18 antibody construct;
25 (B) the ~CH2 chimeric B72.3 (chB72.3) antibody -
construct;
FIG. 3 is a graphic representation of the
~ competitive antigen binding analyses of the intact
30 chl4.12 antibody and the ~CH2 chl4.18 antibody
constructs after (A) 2 hours incubation at 37C; and
(B) 18 hours incubation at 4C;
.
:~ - .'. .
, ,, ,,, / ,, ,~ , . ., .,, " . .. , . ~. . . , . . - -. - :
. .~'091/13166 PCT/~'S91/00633
2 ~ 7 7 ~
FIG. 4 is a graphic representation of the
antibody-dependent cellular cytoxicity (ADCC) of - -
intact antibodies and ~CH2 antibody constructs;
FIG. 5 is a graphic representation of the
complement-dependent cytotoxicity (CDC) of intact -,
antibodies and ~CH2 antibody constructs; .
FIG. 6 is a graphic representation of the
lO biodistribution of (A) intact chl4.lB antibody and
(B) the chl4.18 ~CH2 antibody contruct over time :
within athymic nude mice bearing M21 xenographic .
tumors;
lS FIG. 7 is a graphic representation of the :~
biodistribution of F(ab')2 fragments and the ~CH2 :
antibody construct within nude mice bearing M21 .
~enographic tumors; and
FIG. 8 is a graphic representation of the . .
clearance of the AC~2 antibody construct, intact
antibody, and F(ab')2 fragments from the circulation
of athymic nude mice bearing M21 senographic tumors.
. .
: : ,
- ~ , .
- ' .
.; .. , .. , ,,, ..... .. , .. .. ,, .. ;, .,,,.,, . . .. ., . ,~. .-. . -~, . ... ..
,. . , .- - . .. . , ~ , ., : -. , .. . , ~
- , . ~ . . ~ : ,
.,. ... , . , . - .. - . -: .
, , . . . ;.,. . ~. . : . ,: : . . :. . ~ . : .
. .
,O91/13166 PCT/US91/0063~
-lO- 2077 3~18 -:
Descri~tion of the Invention
The present invention features truncated
antibody constructs having specificity for a
5 tumor-associated or other antigen, and demonstrating
the in vivo properties of an enhanced binding
activity, a relatively short half-life, and a
relatively low level of nonspecific binding. These
~CH2 constructs are well suited as tarqeting agents
10 for tumor imaging and systemic pharmacotherapeutic
treatment.
The invention provides antibody constructs
with binding specificity for an epitope on a human
15 tumor antigen. The specificity of the antibody
preferably is for a tumor antigen which enables the
selective targeting of that ~umor, i.e., the `
construct binds to a unigue marker for the tumor.
Unfortunately, only a few antigen specific markers
20 or human cancers have been identified. However,
human tumor cells do have tumor-associated antigens
which are present in smaller quantities in certain
normal cells such as embryonic cells. Such antigens
include alpha fetoprotein (AFP) and carcinoembryonic
25 antigen (CEA). Antibody constructs which recognize
these or other tumor-associated antigens such as
mucin (B72.3 antibody) and the disialoganglioside GD2
(14.18 antibody) are included in this inven'ion.
The ~CH2 constructs of the present invention
can be prepared by genetic manipulation of the DNA ~
which encodes the various domains of an antibody ~- -
(see, e.a., Neuberger et al. (1984) Nature 312:604;
Morrison et al. (1987) Ann. N.Y. Acd. Sci. 507:187; -
;, , , - - - - . , , ~ ; . . , . :
~'091/13t66 PCT/~'S91/00633 ~ ~
-11- 20773'~8 ::
.:' '". ',
Morrison et al. (1988) Clin. Chem. 34:1668; Horwitz -
et al. (1988) Proc. Natl. Acad. Sci. 85:8678). -
. . . .
In order to reduce immunogenicity of the
5 ~CH2 constructs in humans, the ~CH2 constructs were
designed to ha~e a minimal amount of nonhuman
protein. This may be accomplished by creating -
chimeric antibody molecules having at least human
constant regions and mammalian, e.g., mouse, variable
10 regions.
:.
The production of recombinant chimeric
antibodies with predetermined specificity has
typically involved the use o cloned genomic DNA
15 fragments. For example, the genomic DNA sequences -
encoding H and L chains can be cloned in their
rearranged forms (i.e., in the DNA sequence that
results from recombination events during B Cell
maturation). As such, these genomic sequences
20 contain the information necessary for their
expression, ~i.e. the 5' untranslated sequences,
promoter, enhancer, protein coding region, and donor
splice site). The donor splice signals at the 3' end
of the V gene segments are compatible with the splice
25 acceptor signals at the 5' end of the Ig regions of
other species. That is, the splice product between
the two maintains the correct reading frame. For
example, when a murine V and a human Ck segment are
joined and transfected into the appropriate host cell -
30 type, the primary transcript is correctly spliced and
results in a mature messenger RNA (mRNA) molecule
with an open reading frame through both the V and C -~
regions. ~i -
:
~ ' - . .
..091/13166 PCT/US91/00633
2~773;18
A preferred method of producing the chimeric -
~CH2 constructs involves the preparation of an
immunoglobulin constant region-encoding cassette
including a DNA sequence which enables the splicing
5 of that immunoglobulin constant region-encoding
segment to an immunoglobulin variable region-encoding
DNA segment having a compatible splice sequence,
thereby allowing subsequent transcription and
translation of an immunoglobulin heavy or light
lO chain. This method is described in co-pending patent
application serial number 409,889 entitiled "Method
of Producing Fusion Proteins", filed September 20,
l9~9, herein incorporated as reference.
Briefly, a DNA cassette is prepared by
- reconstructing the 3' end of a splice donor site, and
its attachment to the 3' end of a DNA seguence or -
eson encoding a variable ~V) region. The cassette is
transfected with expressable DNA (structural ge~e)
20 for a constant ~C) region gene having a compatible
splice acceptor site at its 5' end.
During the sequence of events leading up to
expression in the transected cell of the fusion
25 protein, mRNA derived from the two DNA sequences are
spliced to produce a mature mRNA having a 5' end
encoding a complete VH or VL and a 3' end encoding a
human CH or CL domain. The resulting single chain ~
polypeptide is a fusion of the v region and the -
30 constant domain encoded by the exon of the structural
gene. In addition, a tumori~idal agent-encoding
cassette may be spliced to an Ig C region which, in
turn is spliced to an immunoglobulin V region,
.~ ,
~O91/13166 PCT/US91/00633
-13-
2077 3 48
resulting in a ~'magic bullet"-type pharmacotherapeutic ~;~
agent.
. .
The cDNA encodes a H or L chain V region
5 (most likely non-human, e.g., murine) specific for a
tumor antigen while the C region exon(s) encode the H
or L chain C region (CHl, CH2, and CH3 domains) of
another (most likely human) Ig species. Useful V
regions include the VH and VL domains of murine
10 monoclonal antibody 14.18 ~Mujoo et al. (1987) Cancer
Res. 47: 1098-1104) which recognizes the
disialoganglioside GD2 on the surface of many
neuroblastoma, melanoma, glioma, and small lung
carcinoma lines and tissues. Other useful V regions
15 include the VH and VL domains of murine monoclonal
antibody B72.3 (Johnson et al. (1986) Cancer Res.
46:850) which recognize a mucin-like structure on
many breast and colon carcinomas. These V region can
be combined with various C regions such as that of
20 the Ig human gamma (H~ and kappa (L) chains.
Alternatively, the V reqion may be of human origin.
Espression of H and L constructs in a single
competent host cell results in production of intact
chimeric immunoglobulins having a desired specificity
25 and, for example, an intact human constant region.
To produce the ~CH2 construct, a V region
cassette is constructed and placed in an appropriate
vector, together with a C region-encoding DNA
30 sequence. This C region specifically lacks the CH2
domain and is designed as shown in FIG. 1. FIG. lA
shows a map of the HindIII to PvuII fragment
containing the human genomic Cyl gene segment. FIG.
lB shows the DNA encoding the C region of the ~CH2
.
, , - ` : : .
..... . -: -: : ..
-..091/13166 PCT/US91/00633
' . :
20773~8 ~ : :
construct after restriction and ligation of the two
fragments with a synthetic linker (to join the Af lIII ~ .
and AvaII ends).
Vectors encoding this mutant C region are
transfected into an appropriate host capable of
espressing the vectors. Upon co-expression of the
two DNAs, the host cell nuclear enzymes produce mRNA
by implementing the normal splicing events, resulting
10 in an mRNA encoding a fused protein. In the case of
a chimeric binding protein, the mRNA may encode (5'
to 3') a VH or VL domain, which may be identical to
the native sequence up to the VC junction, attached
directly to another polypeptide such as at least a
15 portion of at least one C region domain, which for
example, may comprise human sequences. The 3~ half
of the donor splice site (and any nucleotides
downstream) and the 5' half of the acceptor splice
site ~and any nucleotides upstream) are removed as an
20 intron, resulting in an mRNA encoding the properly
fused protein.
:
Normally both exons are placed on the same
vector under control of a single regulating -~
25 sequence. Also, it is preferred to coexpress both L ;
and H chain constructs so that the host cell secretes
an intact fusion protein. The method requires use of
a host cell-having the enzymes which recognize the ~-
DNA splice signals and effect proper splicing.
Particular vector construction, host cell ~ ~ -
selection, transformation, and methods of expression
do not, per se, constitute an aspect of the --
invention, but can be selected and implemented by -
, ..
..
: - :, ~ ' . . . . -:
' ;~ ' . , : .~ ' . . : ' , , ' ' ,, ' ', ~ ' ' ' : . - ':
~'091/13166 PCT/US91/00633
-15- 207~3`~ -
skilled workers based on personal preference and
convenience. Techniques adaptable for use in the
invention are disclosed, for example, in Current
Protocols in Molecular Bioloqy (Greene Publishing
5 Associates, 430 Fourth Street, ~rooklyn, N.Y.,
1989). Useful vectors include any number of known
plasmids which contain the correct signals for
transcription and translation of the genes of
interest. Enhancer elements may be present, and
lO additional signals or polyadenylation and splicing
must be present in cases where they are not provided
by the gene itself. For e~ample, all of the signals
for the expression of functionally rearranged Ig
genes are present on a continuous stretch of DNA and
15 include the transcription promoter, the splicing
signals for excision of the intron sequences and the
polyadenylation and termination sites. Additional
information that must be provided by the vector is a
selectable marker gene. This gene must also contain ~-
20 the signals for e~pression of the selectable
phenotype (usually resistance to the lethal effect of
a tosic drug such as methotresate, for example).
Therefore, if the vector encodes both the first and
second polypeptides, it is necessary that it provide
25 the sequence information for three separate
transcription units in a limited amount of space.
The recombinant cassette-containing vector
is transfected into an appropriate host cell. The
30 choice of host cell line, in addition to the
criterion noted above, is based on its ability to
grow in a growth media, preferably one that is
commercially available and serum-free as well as its
ease of selectivity after transformation. For the
.
- - - ~ -
.
: , . .
.-: : - . : ~ : . .
:- , . .. . . .. .. .
-,iO 91/13166 P ~ /US91/00633
-16-
2~773~
production of chimeric antibodies, useful host cells
include myelomas or hybridomas such as, for example,
the murine non-Ig-producing Sp2~0 Ag 14 hybrido~a
cell line, yeast, bacteria, and plant cells. Useful
5 host cells are widely available in repositories and
from commercial sources and may be isolated readily
from natural sources by those skilled in the art.
One method for introducing recombinant DNA
10 into cells is electroporation (see, e.g., Potter et
al. (1984) Proc. Natl. Acad. Sci. USA 81:7161-7165),
which requires specialized equipment and the
availability of highly purified DNA. However, many
different lines can be transformed using this method
15 if conditions are optimized for the specific cell
type.
Another transfection method is protoplast
~spheroplast) fusion ~see, e.g., Sandri-Goldin et al.
20 (1981) ~olec. Cell. Biol. ~:743-752). Bacteria
harboring the recombinant plasmid of interest are
fused to the lymphoid cells with a chemical agent,
generally 45-50% polyethylene glycol i~ a buffered,
isotonic solution. This method is simple and does
25 not require extensive purification of plasmid DNA. ~-
In addition, very high transformation frequencies can -
be obtained, and the time for obtaining highly
productive transfected cell clones is reduced because
this transfection method is likely to give
30 transfectants containing multiple copies.
Cells which are successfully transformed -~ -
with the vector must then be isolated from those
which are not. Many methods are available for the
,',~
... .. . .
. ~
. . , - .. ~ . . . .: . . , ~ .: . ~ .. .. , ,.,; . . . . ... :
~091/13166 PCT/~S91/00633
-17- 20773 1~
selection of transfected cells. For esample, the
guanine phosphoribosyl transferase (gpt) and neomycin
(neo) resistance markers may be used for selection
purposes in lymphoid cells. The gene encoding the
5 marker would be included on the V-region encoding
vector. The resistant form of dihydrofolate
reductase (DHFR) can also be used for the selection
of hybridoma cell transformants as well as for
subsequent amplification of the marker and flanking
10 product qenes.
The transfected cell is then cultured to
express the polypeptide encoded by the cassette.
Culturing may be in vitro, or in the case of
15 recombinant antibodies, may be accomplished by
employing other strategies such as n vivo culturing
in ascites fluid.
The CH2 domain of the human Cyl heavy chain
20 which is de~eted from the intact antibody molecule
inclùdes the following seguences: the sequence
conferring long half-life; the sequence responsible
for binding the Fc receptor on e~ector cells; the
sequence to which the single N-linked carbohydrate
25 chain is attached; and the sequence to which the
first complement component, Clq, binds.
To determine to what extent these sequences
are necessary for various antibody functions, the
30 ~C~2 constructs were examined closely. When the
supernatants of cells expressing the intact and ~CH2 ~ :
antibodies were first quantitated by measuring
associated human ~ chain to normalize the data to the
number of antigen binding domains (antibody molecules ~ -
. .-. - : . - ., . . .; ;; - .
- .. - . .. ; . . ......... . .
. . .:. . : :; ~ . : ,: . :
~'0 ~1/13166 PCT/US91/00633
-18- 2~7 73ll~
.. . .
contain one ~ light chain per antigen binding site),
and then compared in a direct antigen binding assay,
the ~CH2 constructs demonstrated much higher
activity, as shown in FIG. 2. ~he chl4.18 ~CH2
5 construct was assayed on GD2-coated plates, while the
chB72.3 ~CH2 construct was assayed on mucin-coated
plates.
The antigen binding activity of the ~CH2
10 construct was tested further in a competitive binding ;!~ ;
format in order to rule out the possibility o~
non-specific interactions with the ELISA plates. As
seen in FIG. 3, the chl4.18 ~CH2 construct competes
-much more efficiently with the labeled intact chl4.18
15 antibody for antigen than the normal antibody
competes with itself. When the length of the binding
assay is estended from 2 hours (FIG. 3A) to 18 hours
(~IG. 3B), the difference in binding is not as
dramatic, suggesting that the rate of antigen binding
2~ and not the overall affinity is increased by the
removal of the CH2 domain.
It is hypothesized that the increased
antigen binding of aCH2 antibody constructs reflects
25 conformational changes in the antibody molecule. By
removing the CH2 domain, the Fab re~ion (including
both V region, CL and CHl sequences) is no longer
restricted by interactions with portions of the CH2
domain. Removal of these inter-domain interactions
30 appears to increase the rate of association with
antigen. In addition, the removal of the
carbohydrate moiety of the CH2 domain may reduce -
steric interactions between the antibody and the
- antigen.
' :., '. -
:, . '
' i , ~ . ' . . .! ; ` . - ; . .. . .
', . , ': . ! ' :. . . !:: ' ..':: ':.: ~ ' . ' , ' ', -
': ' . ' ''':: . .. .. ' :: . .' . : -.' . . .. .' .:- '' ' - ' : ':. '' . . '' ` .: i . .. '. . ' : ' " . . ' .. :
~091/13166 PCT/~S91/00633
.
20773 !1 g
That the effector functions attributed to
the CH2 domain are no longer retained in the ~CH2
antibody can be confirmed by ADCC assay using the -
intact chl4.18 antibody as a basis for comparison.
5 The results of a typical ADCC assay, which measures
the ability of unfractionated peripheral blood
leukocytes (PBLS) to lyse melanoma target cells as a
function of the amount of added antibody, are shown
in FIG. 4. Here, the specific lysis o M21 human
~0 melanoma cells in the presence of the indicated
concentrations of chl4.18 antibody or ~C~2 construct
is shown for an effector to target ratio of lOO~
The average of triplicate samples is shown for each
data point. The intact chl4.18 antibody is a very
lS potent mediator of ADCC, even at levels as low as l ~
ng/ml. Surprisingly, the ~CH2 construct exhibits a -
very small amount of ADCC activity.
The loss of ADCC activity also reflects a
20 loss of the ability to bind to Fc receptors. Since
the site for binding of the high-affinity receptor
~FCR) has been mapped to the CH2 domain, adjacent tc
the hinge, it is not surprising that the ~CH2 mutant
had greatly reduced activity. For the radioactive
25 imaging of tumors, it is important that Fc receptor
binding be reduced so that there is minimal
nonspecific accumulation in the spleen, where many
Fc-receptor-bearing cells localize. The ~CH2
construct here described is therefore useful in this
30 regard.
As expected, the ~CH2 construct also was
found to have a reduced ability to mediate the
complement lysis of melanoma target cells. FIG. 5
~. , ... ,, - . ~ .. . .. .. ... . . .
-.~'091/13166 PCT/US91/00633 ~ ~
-20- 2~773~8 ;
shows that the ~CH2 mutant had no measurable ability -~-
to lyse M21 cells in the presence of human complement
relative to the very potent chl4.18 antibody.
Anti-tumor aCH2 antibodies, having the
ability to specifically and rapidly localize to the
tumor, also serve as ideal vehicles for the delivery
of therapeutic agents. For example, as shown in FIG.
l(C)-l(E), the DNA sequence encoding human
lO interleu~in-2 (IL2) may be attached to the carboxy
terminus of an anti-tumor ~CH2 construct, thereby
resulting in the expression of a ~CH2/IL2 fusion
protein that serves to deliver IL2 to the tumor
site. The local accumulation of IL2 activates
15 T-cells at the tumor site, thereby hastening its
destruction. Although the half-life of aCH2 ~ -
antibodiès is shorter than whole antibodies, the -
~CH2/IL2 fusion protein should be much longer than
the half-life of IL~.
~0 . . ':
In another example, the anti-tumor cytokine
lymphotoxin or tumor necrosis factor B ~TNF B), could
be used to the carboxy terminus of a ~CH2 antibody.
This fusion protein also serves to specifically --
25 localize this cytotoxic.protein to a tumor, thus
reducing its potential adverse systemic effects.
Likewise, other toxic proteins could be fused to ~CH2
antibodies. These include protein toxins such as ;
ricin, diphtheria toxin, and Pseudomona exotoxin or
30 those portions of the whole toxin molecule that is
responsible for toxicity, i.e. the ADP-ribosylating -
enzyme activity that leads to the inactivation of
mammalian cell elongation factor 2.
~091/13166 PCT/US91/00633
-21- 207~3~8 ~
The specificity and half-life of various
antibody constructs in vivo can be characterized by
performing biodistribution studies. Intact, ~CH2, or
F(ab')2 are injected into M21 xenographic tumor- -
5 bearing animals, and the amount of construct in
various body organs and tissues are monitored over
time or at a particular time. FIG. 6 and TABLE 1 ~. .-
show the biodistribution of (A) intact chl4.18
antibody and ~B) a ~CH2 construct with time, and
lO demonstrates that the latter targets quickly (within
4 hours) and specifically to the tumor.
TABLE 1
Time
Antibody SamDle 4 hr. 24 hr. 96 hr.
~A) chl4.18
tumor/blood 0.18 0.56 l.08
liver/blood 0.79 2.02 4.55
20 t~) ~CH2
tumor/blood 0.86 2.20 l.l9
liver/blood 2.98 7.37 2.28
FIG. 7 shows the biodistribution of human Ig
F(ab')2 fragments and chl4.18 ~CH2 constructs 24
hours after injection, and demonstrates the
specificity of targeting of the aCH2 constructs.
The half-life of the ~CH2 antibody
constructs in circulation may be determined by
injecting them into the circulation of tumor-bearing - -
animals and monitoring their presence in the blood ~
',
: .
. ' ~ .,:, .' , ' ' .' :'. ,, ' ' ' . ' ~ ' ' ' . ' .' . ',, ' ,. ' ' '
~O9ltl3166 PCT/~S9l/0063
-22- 2 0773ilg ~ ;
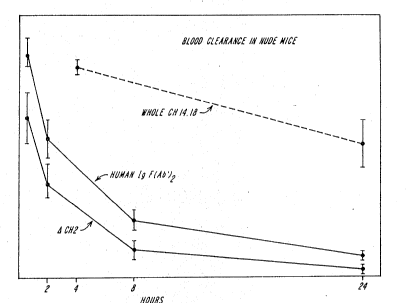
with time. FIG. 8 demonstrates the short half-life
of ~CH2 constructs relative to F(ab')2 fragments and
intact antibodies in the circulation of nude mice
bearing M21 xenographic tumors.
Because the ~CH2 antibody constructs are
able to bind quickly to a tumor epitope with
relatively little non-specific binding to other
tissues, they can be used to target various
10 therapeutic agents to tumors. Particularly useful
therapeutic agents include those having direct or
indirect tumoricidal activity such as interleukin-2, : -
lymphotoxin, or epidermal growth factor. For ;
example, EGF is fused to an antibody that binds to -
15 and activates a cytoto~ic T-cell, thus cross-linkir.g
the T cell and EGF receptor-bearing cell. Fragments
and biosynthetic analogs of these proteins having
tumoricidal activity also are useful. These proteins
may be chemically linked to the CH3 domain via, for
20 example, various cross-linking agents known in the
art, or, as disclosed herein, may be peptide-bonded
to the carboxy terminus of the CH3 domain. For
example, the vectors constructed to encode the ~CH2
constructs may further encode the tumoricidal
25 protein.
.:
The increased binding activity of the ~CH2
construct, together with its shorter half-life ln
v vo, and its low level of nonspecific binding make
30 this molecule extremely useful in the radioactive
imaging and/or treatment of tumors. The construc's :
may be labeled with, for example, radionuclides of
low energy such as iodine-123, indium-lll, or
technetium-9gm, or with any radioactive isotopes that~- ;
. .
'
~ . .
: . -
,,, . , . ,-: . ... . .
- ~'0 91/13166 PCl /US91/00633
-23- 20773~ -
will not change the binding characteristics of the
construct when attached thereto, such as iodine-125.
When labelled with a suitable radioisotope the ~CH2
antibody constructs enable detection of the locus of
5 a tumor-related antigen by external detection of
emitted radiation. Radioactive gamma emitters are
preerred, although in some circumstances positron
emitters, such as 64CU, 11C, and 150 may be used. It
is also contemplated that the QC~2 antibody
10 constructs may be labelled with a paramagnetic
substance such as gadolinium so that concentrations
of the reagent localized about a tumor may be imaged
by nuclear magnetic resonance imaging techniques.
lS Targeting of a construct with a tumoricidal
agent and/or radionuclide may require a higher
concentration of construct having a longer half-life
than does imaging. The level of radioactivity
required for imaging depends in part on the ability
20 of the antibody const~uct to selectively label tumor
relative to surrounding tissue, the size of the
tumor, and the distance of the tumor from the
injection site.
The ~CH2 antibody constructs of the
invention can be labelled with such agents using
conventional techniques used to label other proteins
known to those skilled in the art. One currently - -
preferred technique is to express a ~CH2 antibody
30 construct, covalently linked on either its 3' or 5'
end to a lysine rich polypeptide sequence comprising,
for e~ample, 2-20 residues, to serve as a site of
attachment for the remotely detectable moieties
., ..... ,. . . , - . .. - . , . . ~ ., . ~ .- . .: . .
. . ,.... .- . .... . . -. . ~ . : :: . ~ - . - . - -
.- . . .. . - . - : , . . . . .. . . ..
.~091/13]66 pcT/us91/no633
2 0 7 7 3 ~
discussed above. The gamma or positron emitting ions
are preferably attached to these linkers by ionic
interaction or chelation with the free amine groups -
on the lysine residues. -
For example, diethylenetriamine penta acetic :;
acid (DTPA) may be used to label with Tc-99 or
In-lll. In or I isotopes may be attached using the
oxine salt (8-hydro~y quinoline). Iodogen (l, 3, 4, "'"!,
10 6 tetrachloro - 3, 6a diphenylglucoluril - Pierce -
Chem. Co., Roskville, Ill) may be used for labeling
with 125I. (~, e.g., Thakur et al. Thromb. Res.
9:345 (1976); McFarlane, J. Clin. Invest. 42:346
(1963); Knight et al., Radiopharmaceuticals, N.Y.
15 SoC. of Nuclear Medicine, 1975, pp. 149; and Harwig
et al., Int. J. Appl. Radiat. Isot. 27:5, 1976.)
Other methods are known to those skilled in the art.
ln view of the foregoing, it will be
20 apparent that other types of known reagents can be
linked using conventional techniques to ~CH2 antibody
contructs. Examples inclùde therapeutic drugs which
promote healings such as calcitonin, epidermal growth
factor, tumor growth factor alpha and beta, platelet
25 derived growth factor, insulin-like growth factor l
(Somatomedin-C), connective tissue activating
peptide, and human collagenase inhibitor.
The dosage and means of administration of
30 the family of ~C~2 antibody contructs produced in
accordance with the invention will necessarily depend
on the nature of the drug involved, the degree if any
that its bioactivity is reduced when it is present as
a conjugate, the immunological tolerance of specific
. .
. ' ' . ' ' . ' ~ .'.'. ' ~ . , '. . ' ! '
~ U'O9l/13166 PCT/~'S91/00633
... .
-25- 207 ~ 3 `~8
patients, and the nature of the diseased condition in
question. When the polypeptide is linked to an
imaging agent, it should be administered in an amount
sufficient to be detected by scintillation scanning ' ,~
5 or other external radiation detection means capable
of detectin~ the localized radiation present in the
tumor, such as autoradiography. In general, about
5-20 microcuries should be administered; most
preferably about 10 microcuries. The actual amount
lO depends on the location of the tumor-associated
antigen as is well known in the art. Additional
radioactive peptide may be injected if necessary up
to the amounts limited by prevalent standards of
safety.
Tumors may be imaged or trea,ted by systemic
administration of the ~CH2 construct via intravenous
injection. Their preferred dosage is in the range of '
from about 0.~1 to lO mg per kg body weight. ~ '
The invention will be further understood ,'
from the following, non-limiting esamples.
EXAMPLES
'
l. Plasmid Construction ',
.
The human Cyl gene, subcloned in pBR322 as a
HindIII to PvuII fragment (Gillies et al. (1989) J.
30 Immuno. Meth. 125:191-202) was used for the
construction of the H chain mutants. The deletion
mutant lacking the CH2 domain (~CH2) was made by
joining the HindIII to AflIII fragment (containing
the CHl and hinge exons) to an AvaII to PvuII
. ~
'O91/13~66 PCT/US91/00633 --
.. -. :
-26-20773~g '' '".-"
fragment (containing the CH3 exon) using a synthetic
double-stranded oligonucleotide fragment. The ACH2
construct was checked by DNA sequence analysis and
then introduced into the chimeric antibody expression
5 vector pdHL2-VCylC~ (Gillies et al. i~id.) containing
the V regions of the mouse monoclonal antibody 14.18
(Mujoo et al. (1987) Cancer Res. 47:1908).
A ~CH2-IL2 fusion construct was constructed
10 by first introducing a SmaI site by mutation near the
carboxy terminus of the ~CH2 human IgGl gene (FIG.
l(C). The mutation changes nucleotide 2373
(according to the numbering in the Huck et al.,
(1986) Nucl. Acids. Res. 14:1779-178g) from a T to a
15 C in the wobble position of a serine codon and thus
does not change the amino acid sequence. A synthetic
DNA fragment was used to link the mature IL2 protein ~,
sequence to this SmaI site (FIG. lD-lE). The
sequence includes, from 5' to 3', the SmaI site, the
2b remaining seguence of the human gamma 1 gene (three ,
additional amino acids) Sused directly to the first
amino acid ~alanine) of the mature I~2 protein
sequence, and the amino terminal sequence o IL2 up
to the unique PvuII site. The remaining IL2 sequence
2S was then joined at the PvuII site and extends through
the coding sequence to a unique XhoI site located 3' -'
of the translational stop signal. The XhoI end of
the joined DNA was ligatéd to a vector containing the
3' untranslated region and polyA addition site of the
30 mouse ~ constant region (FIG. lF). The resulting
construct encodes a ~CH2 human gamma 1 chain to which
IL2 is attached at the carboxy terminus.
,
;
.,, ~, ' ~ ' ' ' ' 1 . '; . ' ' ': '
.', , . . : ` '
~ U'09t/13166 PCT/US91/00633
-27- 20773~8
Transfection and drug selection were carried
out essentially as described in Gillies et al.
(Bio/Technol. (1989) 7:799-804), herein incorporated
as reference. Briefly, the non-Ig-producing murine
5 hybridoma line, Sp2/0 Agl4, was maintained in
Dulbecco~s modified Eagle~s medium (DMEM) containing
10% fetal bovine serum. Plasmids were introduced
into cells by protoplast 'fusion essentially as
deiscribed in Gillies et al., (1983) Cell 33:
lO 717-728). Transfectants were selected in growth
medium containing methotrexate at an initial
concentration of O.l ~M. MTX-resistant clones were
tested for the secretion of human antibody by ELISA '~'
assay. Microtiter plates were coated with goat
15 anti-human IgG (H and L specific-Jackson
Laboratories) and incubated with conditioned medium ~'
from tran~fected cells. Goat anti-human IgG
~Fc-specific-Jackson Laboratories), conjugated to '
horseradish perosidase, was used for detection of
20 human antibody. The clones that produced the '
greatest amount of antibody were cultured in medium
containing increasing concentrations of MTX (from O.l
~M to 1 ~M and after adaptation, to 5 ~ and finally
10 ~M MTX). After a few passages in lO ~M MTX, cells
25 were subcloned by limiting dilution.
3. Purification of Proteins
The chl4.18 intact antibodies were purified ~-
30 by binding to and elution from protein A Sepharose -
(Repligen~. The ~CH2 mutant antibody was purified by
immunoaffinity chromatography on an anti-human ~ -
monoclonal antibody-Sepharose column. Both proteins
were >90~ pure when analyzed by SDS-PAGE or HPLC.
`-~Uosl/l3l66 PCT/US9l/00633
-28-
2a7~3ls
4. Antigen Binding Assays
Direct antigen binding assays as well as
competitive binding assays were performed with the
5 disialoganglioside GD2 antigen (14.18 antibody) or
submaxillary gland mucin (Sigma) (B72.3 antibody)-
coated microtiter plates as described in Gillies et
al. (1990, ibid.). In both cases, the secondary
(detecting) antibody was a goat anti-human IgG,
10 Fc-specific polyclonal antiserum (Jackson
ImmunoResearch), labeled with horse radish peroxidase
(HRP). ~riefly, the GD2-coated plates were first
blocked with 5~ bovine serum albumin (BSA) and 5%
goat serum in P8S for 2 hr at 37C. Unlabelled goat ~
15 antibodies were diluted in assay buffer (1% BSA, 1% 7 , .'
goat serum in PBS) and 25 ~1 was added to each well.
ASter inculbation at 37C for 4 to 5 hours, 25 ~1 of -
HRP-conjugated 14.18 chimeric antibody (6.3 ng) was
added to each well. Plates were covereed and -
20 incubated overnight at 4C, washed sis times with
PBS, and developed with 0-phenylenediamine (OPD)
substrate.
5. Cytoto~icity Assays
~ `
ADCC assays were carried out using 5lCr-
labeled M21 human melanoma target cells or any cell
line which e~presses GD2. A fixed number of labeled
targets (2 x 105 cells/ml in 50 ~L) and varying
30 concentrations of human effector cells (peripheral
blood leukocytes from normal donors) in 50 ~L were
mi~ed with 100 ~L of diluted chimeric antibodies in
round-bottom microtiter plates. Following a 4 hr
incubation at 37C, the plates were centrifuged, and
"
, . : . .
~091~13166 PCT/US91/~0633
,. . .
-29-
20773~8
lO0 ~L samples were removed for counting in a LKB
model 1272 gamma counter.
Complement-mediated lysis of labeled M21
5 melanoma cells was carried out by mixing 50 ~L of
cells (2 ~ lOs cells/mL) with an e~ual volume of each
antibody dilution. After a 15 min incubation at
37C, lO0 ~L of human complement (1:4 dilution of
fresh huma~ serum) was added to each well. Plates
lO were incubated for an additional hour at 37C, and
the amount of released s~Cr was determined by
centrifuging the plates and counting lO0 ~L aliquots
of the supernatants. The percentage of cytoto~icity
was determined as:
: ',
Experimental cpm - Spontaneous cpm ~
- X 100
Total cpm - Spontaneous cpm
,, .
20 6. Biodistribution and ~lood Clearance Studies
, :
8 to lO weeks old female athymic mice
~nu/nu) ~the National Cancer Institute, ~ethesda, MD)
were injected subcutaneously with 2 ~ lO6 M21 tumor
25 cells. Tumors of 50-150 mg weight grew within lO
days. At this time, the animals received i.v.
injections into the lateral tail vein of 2S ~g and -
3-4 ~Ci l25I-labeled antibody. At designated time
points after injection, groups of 3 animals were
30 anesthetized with halothane, and blood samples were
obtained by retro-orbital bleeding. For
biodistribution of radiolabeled antibodies, groups of
3 animals were sacrificed at various time points
after injection. Tumors and major organs (heart,
.:
. . , -; .
,
' .. , ' ' '~ , . . ' .,, ~ , . . ,. . ' . ' . ~ . ' ' , . . .
~-~'091/13166 PCT/~IS91/00633
.
-30-
2077348
skin, muscle, bone, lung, liver, spleen, thyroid,
kidney, and intestine) were removed and weighed. All
tissue samples were assayed in a gamma counter for
l25I activity. The results were calculated as -
5 percent of injected dose per g tissue and as -
localization ratios (cpm/g tumor : cpm/g tissue).
.. . .
7. Radioactive Labeling
Chl4.18, chl4.lB-~CH2, and F(ab')2 fragments
of human IgG were labeled with l25I. Briefly, 500 ~9
antibody was incubated for 25 min on ice with 0.5 mCi
l25I (lO0 mCi or 3.75 Gaq/ml, Amersham Corp.,
Arlington Heights, IL) in polystyrene tubes coated
15 with lO0 ~g Iodo-Gen reagent (Pierce Chemical Co.,
Rockford, IL). Unincorporated l25I was removed by
gel filtration on PDlO columns (Pharmacia Fine ~
Chemicals, Piscataway, NJ). Specific activity was -
typically 1.5 - 0.5 nCI/ng antibody.
The invention may be embodied in other
specific forms without departing ~rom the spirit or
essential characteristics thereof. The present
embodiments are therefore considered to be in all
25 respects as illustrative and not restrictive, the
scope of the invention beinq indicated by the
appended claims rather than by the foregoing
description, and all changes which come within the
meaning and range of equivalency of the claims are
30 therefore intended to be embraced therein.
; ~ What is claimed is:
'
: '
... . , ,~ . - ... i . -, ~ .. , . ;. ., , . -
.. - . - . . . : ... . . ~ . , ~
. ~ . .. .. : . - ., . ... , . ~ : :
- . - . , -
~ . . .. .. . . . . . . . . .