Note: Descriptions are shown in the official language in which they were submitted.
HOECHST A~TIENG~SELLSC~T HOE 91/F 275K Dr. SP/pe
Description
Polymer blends of cycloolefin polymer~ and polyacetal~
Cycloolefin polymers are a class of polymexs which have
an outstanding property profile and are distinguished,
inter alia, by hydrolytic stability, low absorption of
water, resistance to weathering, high heat distor~ion
temperature and transparency.
Polyacetals have also been employed for a long time as
multi-purpose materials, above all in the engineering
sector. Because of their good mechanical properties, such
as high rigidity, hardness and strength, as well as the
possibility of producing moldings and shaped articles
within narrow tolerance limits and the good resistance to
many chemicals, they are often suitable as a substitute
for metals. In addition to good electrical and dielectric
properties, they exhibit favorable slip and wear proper-
ties. Because of the good resistance of polyacetals to
many organic solvents, they show only ~light æwelling and
a slight drop in their mechanical properties on contact
with solvents. In practice, the resistance to automobile
gasoline (including that containing methanol), mineral
oil and heating oil is of particular importance, this not
being guaranteed with cycloolefin polymers.
Both polymer classes, cycloolefin pol~mers and poly-
acetals, are proces ed as thermoplastics. Exposure to
high temperatures over an exkended period leads to decom-
position products and vxidation products in the case of
the cycloolefin polymers. Shorter intervals of exposure
to heat during extrusion and injection molding, i.e.
higher output rates by improving the flow propsrties,
would therefore be of advantage. Furthermore, for some
uses of polyacetals, for example as matrix materials for
2 - 2 ~ 7 ~ 41 1
composites, their mechanical properties, for example the
modulus of elas~ici~y or the shear modulus/ are in need
of Lmprovement. In this conneckion, absorption of water
by polyacetals is problematic, since the dimensional
stability is not guaranteed at a high ambient humidity.
A lower absorption of water would therefore be of advant-
age.
It is now known that important properties of polymers,
such as those mentioned above, can be changed by blending
polymers with other polymers. However, the possibility of
reliably predicting the properties of a polymer blend
from the properties of the individual components is as
yet a long way off.
The object of the present invention is therefore to
provide polymer blends of cycloolefin polymexs and
polyacetals having increased flow properties, improved
mechanical properties and a lower absorption of water.
The invention relates to polymer blends comprising at
least two components (A) and (B), wherein
(A) is at least one cycloolefin polymer and
(B) is at lea~t one polyacetal,
ths blends containing (A) in amounts of 1 to 99% by
weight and (B) in amounts of 9g to 1% by weight, and the
relative amounts of (A) and (B) making up 100% by weight
with respect to the total blend.
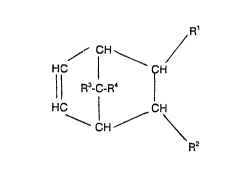
Cycloolefin polymers ~A) which are suitable for the
blends according to the invention comprise structural
units which are derived from at least one monom~r of the
formulae I to VI or VII
"~L ~ Rl
X~
¦¦ R3-C-R- I (I)
3ic 1 ~ ~;
~Y / - R~
2 ~
-- 3 --
C:~ ~ ~2 ~
li R3- C R~ I C'~2
.iC l / C~: /
.~:C--I--C~
¦¦ R3-C-R4 ¦ R5~ 6 ¦ (~II),
HC ¦ ~ eH ¦~ ~H ~
F~ ¦ ~
!1 2~ R~ ¦ -R6 ¦ R7-C-R~ ¦ ( IV),
-~ / ¦ ~ C'.i ~ -- CH ~ Rl
li ?~; ~ p~ l l (V),
r:~ ! c c.~ R2
~6
R5
C.-'-- --'~u~ ¦ ~ ~Rl
C r~ ¦ ¦ 7~ R~ ~ ~2
~6
_.. -- _..
`\ / f~)
( C~2 ) n
in which Rl RZ R3 R4, R5, R6, R7 and Ra are identical or
different and are a hydrogen atom or a Cl--C8-alkyl radical,
it being possible for the 5am~ radicals to have different
2 ~ 7 ~
-- 4 --
meanings, in the various formulae and n is an integer
from 2 to 10.
The cycloolefin pol~ners (A) can comprise, in addition to
the structural units which are derived from at least one
monomer of the formulae I to VII, other structural units
which are derived from at least one acyclic l-ol~fin of
the formula VIII
P~9 RlC
(VIII)
R12
in which R9, Rl, Rl1 and Rl2 are identical or different and
are a hydrogen atom or a Cl-C8-alkyl radical.
Preferred comonomers are ethylene or propylene. Copoly-
mers of polycyclic olefins of the formula I or III and
the acyclic olefins of the formula VIII are employed in
particular. Particularly preferred cycloolefins are
norbornene and tetracyclododecene, which can be substi-
tuted by Cl-C6-alkyl, ethylene/norbornene copolymers being
of particular Lmportance. Of the monocyclic olefins of
the formula VII, cyclopentene, which can be substituted,
is preferred. Polycyclic olefins, monocyclic olefins and
open-chain olefins are also to be understood as meaning
mixtures of two or more olefins of the particular type.
This means that cycloolefin homo- and copolymers, such as
bi-, ter- and multipolymers, can be employed.
It is known that cycloolefins can be polymeri~ed by means
of various catalysts. The polymerization proceeds via
ring opening ~US-A-3 557 072, US-A-4 178 424) or with
opening of the double bond (EP-A-156464, EP-A-283164,
E-A-2gl208, EP-A-291970, DE-A-3922546), depending on the
catalys~.
2 ~ ?;7 r~
-- 5 --
The cycloolefin polymerizations which proceed with open-
ing of the double bond can be catalyzed using more recent
catalyst systems (DE-A-39 22 54~, EP-A-02 03 799) and also
with a conventional Ziegler catalyst sys~em (DD-A-222317,
DD-A-239409).
The cycloolefin homo- and copolymers which comprise
structural units deri~ed from monomers of the formulae I
to VI or VII are preferably prepared with the aid of a
homogeneously soluble catalyst comprising a metal~ocene,
the central atom of which is a metal from the group
comprising titanium, zirconium, hafnium, vanadium,
niobium and tantalum and which forms a sandwich structure
with two mono- or polynuclear ligands bridged to one
another, and an aluminoxane. The bridged metallocenes are
prepared in accordance with a known reaction scheme (cf.
J. Organomet. Chem. 28B (1985) 63-67 and EP-A 320762).
The aluminoxane ! which functions as a cocatalyst, is
obtainable ~y various methods tcf. S. Pasynkiewicz,
Polyhedron 9 (19~0) 429 and EP-A-302424). The structure
and also th~ polymerization of these cycloolefins are
described in detail in DE-A-3 922 546 and in the earlier-
priority bu~ no~ a~ yet published patent applications
D~ A-40 36 ~64, DE-~-41 06 107 and DE-A-41 07 682. These
are cycloolefin copolymers which differ in their chemical
uniformity and their polydispersity.
Cycloolefin polymers having a viscosity number greater
than 20 cm3/g (measured in decalin at 135C in a concen
tration of 0.1 gtlOO ml) and a glass transition tempera
ture (Tg) of between 100 and 200C are preferably
employed.
The polymer blends can also comprise cycloolefin poly~ers
which have been polymerized with ring opening in the pre-
sence of, for example, cataly~ts containing tungsten,
molybdenuml rhodium or rhenium. The cycloolefin polymers
obtained by this process have double bonds which can be
~7~
-- 6 --
removed by hydrogenation (US-A-3 557 072 and US-A-
4 178 424).
The cycloolefin polymers employed for the polymer blends
according to the invention can also be modified by
grafting with a~ least one monomer chosen from the group
comprising (a) ~,~-unsaturated carboxylic acids and their
derivatives, (b) styrenes, (c) organic silicone compon-
ents containing an unsaturated, preferably olefinic, bond
and a hydrolyzable group and (d) unsaturated e~oxy compo-
nents. The resulting modified cycloolefin polymers haveexcellent properties similar to those of the unmodified
cycloolefin polymers, and can be employed for the alloys
according to the invention either by themselves or
together with the unmodified cycloolefin polymers. The
modified cycloolefin polymers moreover specifically have
a good adhesion to metals and synthetic polymers. The
good compatibility with other pol~mers is to be singled
out. It is even possible to prepare, by reactions carried
out in solution or in melts, graft copolymers of cyclo-
olefin polymers with polyacetals, which can act as
compatibilizers.
Polyacetals (B) which are suit~ble for the polymer blends
according to the invention comprise oxymethylene struc-
tural units [-CHz-O-], preferably in an amount of 80-100%
by weight, preferably 90-100% by weight. Such polyacetals
(B) are described, for example, in EP-A-0 156 285.
Preferred polyacetals (B) are linear, unbranched homo-
and copolymers of formaldehyde or it cyclic oligomers,
such as trioxane or tetroxane, copolymers being under-
6tood as meaning both bi- and multipolymers.
Suitable comonomers are a) cyclic ether6 having 3, 4 or
5, preferably 3, ring members, b) cyclic acetals other
than tri- or tetroxane and having 5 to 11, preferably 5,
6, 7 or 8, ring members snd c) linear polyacetalsl in
each case in amounts of 0.1 to 20% by weight, preferably
~77~
-- 7 --
O.5 to 10% by weight. Particularly preferred copolymers
contain 99.5 to 95% by weight of oxymethylene structural
uni~s and 0.5 to 5% by weight of structural unit~ deri~ed
from one of the abovementioned comonomersO
Homo- and copolymer~ of formaldehyde or its cyclic
oligomers are to be understood as those polymers who e
terminal groups have been converted into ~table groups
after the polymerization.
The polyacetals (B) are prepared by ionic polymerization.
The homopolymer of polyoxymethylene can be prepared by
anionic suspension polymerization of formaldehyde.
Esterification with ace~ic anhydride at about 140C is
then carried out for stabilization of ~he unstable hemi-
acetal end groups. For the preparation of copolymers,
trioxane is first prepared from an approximately 60%
strength aqueous formalin solution in the presence of
sulfuric acid. The monomeric ~rioxane, which has ~een
carefully purified by distillation, can then be polymer-
ized cationically in bulk in the presence of a few
percent by weight of ethylene oxide or 1,3-dioxepane
Ibutanediol formal) at about 70C. ~he unstable hemi-
acetal end groups of the resulting eopol~mer are finally
converted into stable alcoholic end group~. An overview
of the essential process steps and evaluation of the
numerous patents and publications is given in Process
Economic Program (G.~ Haddeland, Report No. 69, Acetal
Resins, Stanford Research Institute, Menlo Park,
California 1971).
The inherent viscosity of the polyacetals (B) is in
general 0.3 to 2.0 dl/g, preferably 0.5 to 1.5 dl/g
(measured in butyrolactone, stabilized with 2~ by weight
of diphenylamine, at 140C in a concentration of 0.5 g/
100 ml) and the melt volume indices (MVI), determined in
accordance with DIN 53~35-B, are in general 0.02 to
70 cm3/10 minute~, measured at 190C under a load of
2~77~
-- 8 --
2.16 kg. The cry6talline melting point (Tm) is in general
140-180C, preferably 150-170C.
The polyacetals employed for the blends according to the
invention can al~o be modified by grafting with sui~able
functional compounds comprising diisocyanates, as coup-
ling agents, and corresponding functional masking agents.
The term "functional masking agents" is understood as
meaning monomeric or polymeric compounds having one or
two functional groups per chain, or e~ample hydroxyl,
carboxyl or amino groups, which can undergo addition
reactions with diisocyanates. Suitable functional masking
agents are, for example, mono- and difunctional poly-
al~ylene oxides, polyesters, polyamides, polyolefins,
polydienes and polysiloxanes. Polyacetals modified by
grafting are described in EP-A-0 397 ~93~
These block or graft copol~mers can be employed as
engineering resins per se or as compatibilizers with
other polymers.
The blends according to the invention preerably comprise
5 to 95% by weight, particularly preferably 10 to 90% by
weight, of the cycloolefin polymers (A), and conver6ely
95 to 5~ by weight or 90 to 10% by weight of the poly-
acetals ~B), the relative amounts of components A and B
making up 100% by weight with respect to the total blend.
The blends according to the invention can contain one or
more cycloolefin polymers and one or more polyacetals, as
well as modified cycloolefin polym2rs, modified poly-
acetals and/or block and/or graft copolymers of these.
Blends which comprise modified cycloolefin polymers, in
addition to unmodified cycloolefin polymers and poly-
acetals, exhibit, for example, a finer dispersion, ie.
smaller particle sizes. Extremely fine dispersion occurs
in blends in which the total content of cycloolefin
polymers i9 modified. The~e blend~ al~o have ~urprisingly
2~7~
g
good mechanical pxopertie~.
The blend~ according to the invention are prepared and
processed by standard methods known for thermopla6tics,
for example by kneading, extrusion or injection molding.
The blends according ~o the invention can comprise
additives, for example heat stabilizer~, W ~tabilizers,
antistatics, flameproofing agenc~, plasticizers, slip
agents and lubricants~ pigments, dyestuffs, optical
brighteners, processing auxiliarie~ and inoryanic and
organic fillers, ie. in particular also reinorcing
additives, such as glass fibers, carbon fiber6 or high
modulus fibers.
The blends can advantageously be employed as a matrix
material for composite material6. Moreover, they are
suitable for the production of shaped articles, for
example in the form of sheets, fibers, films and tubes,
by the injection molding or extru~ion process.
The following polymers were prepared by standard methodss
Cycloolefin copolymer Al [COC A1]
A) Preparation of diphenylmethylene-(9-fluorenyl)-cyclo-
pentadienyl-2irconium dichloride (metallocene ~)
All the following working operations were carrled out in
an inert ga~ atmosphere using absolute solvent~ (Schlenk
technique).
12.3 cm3 (30.7 mmol) of a 2.5 molar solution of n-butyl-
lithium in hexane were slowly added to a solution of
5.1 g (30.7 mmol) of fluorene in 60 cm3 of te~rahydrofuran
at room temperature. After 40 minutes, 7.07 g (30.7 mmol)
of diphenylfulvene were added to the orange solution, and
the mixture wa~ ~tirred overnightO 60 cm3 of water were
~0~7~
-- 10 --
added to the d~rk red solution, whereupon the solution
became yellow in color, and the solution was extracted by
shaking with die~hyl ether. The ether phase was dried
over MgS04 and concentrated, and the residue was left to
crystallize at -3~C. 5.1 g (42% by weight) of 1,1-
cyclopentadienyl-(9-fluorenyl)-diphenylmethane were
obtained as a beige powder.
2.0 g (5.0 mmol) of the compound were dissolved in 20 cm3
of tetrahydrofuran, and 6.4 cm3 (10 mmol) of a 1.6 molar
solution of butyllithium in hexane were added at 0C.
After the mixture had been stirred at room temperature
for 15 minutes, the solvent was removed under reduced
pressure, and the xed residue was dried under an oil pump
vacuum and washed several tLme~ with hexane. A~ter the
red powder had been dried under an oil pump vacuum, it
was added to a sus~ension of 1.16 9 (5.00 ~mol) of ZrCl~in 20 ml CH~12
at -78C. After the mixture had been warmed up slowly, it
was stirred at room temperature for a further 2 hours.
The pink-colored 6uspension was filtered over a G3 frit.
The pink residue was washed with 20 cm3 of CH2Cl2, dried
under an oil pump vacuum and extracted with 120 cm3 of
toluene. After the solvent had been removed under reduced
pressure and the residue had been dried under an oil pump
vacuum, 0.55 g of the zirconium complex was obtained in
the form of a pink crystalline powder.
The orange-red filtrate of the reaction mixture was
concentrated and the residue was left to crys~allize at
-35~C. A further 0.45 g of the complex cry~tallizes from
CH2~l2 -
Total yield 1.0 g (36% by weight). Correct elemental
analyses. The mass spectrum showed M~ = 556. lH-NNR
spectrum (100 MHz, CDCl3) 6.90-8.25 (m, 16, Flu-H, Ph-H),
6.40 (m, 2, Ph-H), 6.37 (t, 2, Cp-H), 5.80 (~t 2, Cp-H).
2~7~11
11 ~
B) Preparation of COC A1 (ethylene/norbornene copolymer)
A clean and dry 75 dm3 polymerization reactor with a
stirrer was flushed with nitrogen and then with ethylene,
and 30 1 of an 85% strength by weight toluene ~olution of
norbornene (Nb) were introduced. The reactor was ~hen
brought to a temperature of 70C, while stirring, and
ethylene wa~ passed in until ~ pressure of 3.5 bar was
established.
580 cm3 of a toluene solution of methylaluminoxane (10.1%
by weight of methylaluminoxane having a molecular weight
of 1300 g~mol according to cryoscopic determination) were
then metered into th~ reactor, and the mixture was
stirred at 70C for 15 minutes, the ethylene pressure
being kept at 3.5 bar by topping up. In parallel with
lS this, 500 mg of metallocene L were dissolved in 500 cm3
of a toluene solution of methylaluminoxane (for the
concentration and quality, see above) and were preacti-
vated by being left to stand for 15 minutes. The solution
of the complex (catalyst solution) was then metered into
the reactor. Hydrogen may be used to regulate the mole-
cular weight. Polymerization was then carried out at 70C
for 140 minutes, while stirring t750 revolutions/minute~,
the pressure in the reactor being kept at 3.5 bar by
topping up with ethylene. The contents of the reactor
were then drained rapidly into a stirred ve~sel, into
which 200 cm3 of isopropanol (as a stopper) had been
introduced. The mixture was precipitated in acetone and
stirred for 10 minutes, and the suspended polymeric solid
was filtered off.
A mixture of two parts of 3 N hydrochloric acid and one
part of ethanol was then added to the polymer which had
been filtered off, and the mixture was stirred for 2
hours. The polymer was filtered off again, washed neutral
with water and dried at 80C under 0O2 bar for 15 hours.
An amount of product of 4500 g was obtained.
7, o ri 7 ~
- 12 -
Cycloolefin copolymer~ A2 [COC A2] and A3 [COC A3]
A) Preparation of rac-dimethylsilyl-bis-(1-indenyl)-
zirconium dichloride (metallocene A)
All the following working operations were carried out in
an inert gas atmosphere usin~ ab~olute ~olvents (Schlenk
techni~ue).
80 cm3 (0.20 mol) of a 2.5 molar solution of n-butyl-
lithium in hexane were added to a solution of 30 g
(0.23 mol) of indene (industrial grade, 91%), which had
been filtered over aluminum o~ide, in 200 cm3 of diethyl
ether, while cooling with ice. The mixture was stirred at
room temperature or a further 15 minuteæ, and the
orange-colored solution was passed via a cannula into a
solution of 13.0 g (0.10 mol) of dimethyldichlorosilane
(99~ pure) in 30 cm3 of dieth~l ether in the course of 2
hours. The orange-colored suspension was stirred over-
night, and extracted three times by shaking with 100-
150 cm3 of water. The yellow organic phase was dried twice
over sodium sulfate and evaporated in a rotary evapora-
tor. The orange oil which remained was kept at 40C under
an oil pump vacuum for 4 to 5 hours and freed from excess
indene, a white precipitate being obtained. A total of
20.4 g ~71% by weight) of the compound (CH3)2Si(Ind)2
could be isolated as a white to beige powder by addition
of 40 cm3 of methanol and crystallization at -35C.
Melting point 79-81C (2 diastereomers).
15.5 cm3 ( 3~.7 mmol~ of a 2.5 molar solution of butyl-
lithium in hexane were slowly added to a solution of
5.6 g (19.4 mmol) of (CH3)2Si(Ind)2 in 40 cm3 of tetra-
hydrofuran at room temperature. One hour after the
addition had ended, the deep red solution was added
dropwise to a suspension of 7.3 g (19.4 mmol) of ZrCl4-2-
tetrahydrofuran in 60 cm3 of tetrahydrofuran in the course
of 4 6 hours. After the mixture had been ~tirred for
2 ~
- 13 -
2 hours, the orange precipitate was filtered off with
suction over a glass frit and recrystallized from OEI2Cl2.
1.0 g (11% by weight) of rac-(CH3) 2Si ( Ind~ 2ZrC12 was
obtained in the form of orange cry~tals, which gradually
decompose above 200C. Correct elemen~al analyses. The
mass spec~rum showed ~ = 448. lH-NMR spectrum (CDCl3):
7.04-7.60 (m, 8, aromatic H~, 6.90 (dd, 2, ~-Ind H), 6.08
(d, 2, ~-Ind H), 1.12 (s, 6, SiCH3).
B) Preparation of COC A2 and COC A3 (e~hylene/norbornene
copolymers)
COC A2 and COC A3 were prepared analogou~ly to COC Al,
some of the conditions summarized in Table 1 being
changed.
~07~
- 14 --
. ~ _" _ .
o o o
o o
~D
o _. ~ ~
.0
o o
N E3 ~ OD t`~
_l
.
,, ~ ,, o o a)
~ ~ ~ U~ O
u~ _ h
g ~ ~ ~ o
~r
.
. _ _ _ S~
U~ ~1 N
Ul t~l ~`I Il~ _~
~ ~ _ _l C~l
~: .. _ 1~:
~ ~ U~ O
O _i Q
~1 _ . e _I
h 0 ~ la
o o ~
~ ,~ t- ~
- -- -
_~
O _~~1 t~) N~
U'~
h _ 1::
I ~ ~
_l O ~1 ~ ~
~ ~3 ~ _1
~0~ _
E~
2~77~ ~
- 15 -
The physical parameters of the three cycloolefin copoly-
mers which comprise structural units of the formula Fl
and F2
CH2 - CH~ ~ ~ CH CH ~
HC CH
CH2 7
H2C CH2
Fl ~2
can be seen from Table 2.
~able 2
. . _
Cyclo- Incorporation ~N <Mw> <Mn> <~w> Tg 0
ole:Ein of
copoly- Ethyl- Nor- 10 5 1o~4 . ~ . . [ oC]
mer ene bornene
[mol %] [mol %] [cm3/g] ~g/mol] [g/mol] ~Mn>
_
A1 49 51 166 2.25 10.2 2.2 178
_ _
A2 55 45 91 0.92 4.5 2.0 143
A3 59 41 83 0.82 3.5 2.3 114
determined ~y 13C nuclear magnetic resonance spectro-
scopy
VN: ~iscosity number determined in accordance with DIN
53728
~Mw>, ~Mn>: gel permeation chromatography: 150-C ~LC
Millipore Waters Chromatograph
Column set: 4 Shodex columns AT-80 M/S
Solvent: o-dichlorobenzene at 135C
2~7~
16 -
Flow rate: 0.5 ml/minute, concentration
0.1 g/dl
RI detector, calibra~ion: polyethylene (901
PE)
0 Heating up and cooling down rate: 20/minute
Other charac~eristics of the cycloolefin copolymers A1,
A2 and A3 can be seen from the examples.
Cycloolefin graft copolymers ~3-Pl CCOC ~3 P1] and A3-P2
[COC A3-P2+3 and A3-P3 [COC A3-P3]
1. Preparation of COC A3 (A3-P1 and A3-P2+), grsfted with
maleic anhydride
20 g (108.1 g/l) of COC A3 were dissolved in 150 ml of
toluene (thiophene-free, absolute) in a clean and dry
500 ml two-necked flask connected to an inert gas ~upply
(consisting of an oil pump for generating a high vacuum,
and an argon feed for aeration). When the copolymer had
dissolved completely, 8 g (438.6 mmol/l) of maleic
anhydride (MA, 99% pure) were added and dissolved, before
2 g (2701 mmol/l) of dilauroyl peroxide (dissolved in
35 ml of toluene (thiophene-free, absolute~) were added.
The contents of the flask were dega~sed completely at
-196C (four freezi~g/degassing cycles). Argon was then
applied and the reac~ion solution wa~ introduced into an
oil bath preheated at a controlled temperature of 80C.
Free radical qr~fting of MA onto COC A3 wa~ ended after
~ hours by precipitation in 2 l of acetone. For working
up, ie. purification of the polymer, thi~ was reprecipi-
tated in acetone four times r 16.3 g of MA~grafted cyclo-
olefin polymer A3 (A3-Pl~ being obtained after drying at
130C (72 hours/oil pump vacuum).
A3-P2~ was prepared analogously, 2.8 g (38.0 mmol/l) of
dilauroyl peroxide being employed. Yield: 15.9 g
~7~
-- 17 --
FT-IR [cm 1] 5 1865 ss / 1790 ss ~C=O, anhydride)
VN [ cm3 / g ]: in ac c ordance with D IN 5 3 7 2 8
A3-Pl : 85 . 7; A3~P2+ : 84 . 2
2. Prepara~ion of COC A3 (A3-P3), grafted with maleic
anhydride
A clean and dry 2 1 two-necked ~lask was ~illed with
argon. 70 g (90.0 g/l) of COC A3, dissolved in 700 ml of
toluene (absolute, were introduced in an argon counter-
current. 19.0 g (250.3 mmol/l) of maleic anhydride (M~,
99% pure) were then added in countercurrent to the inert
gas and dissolved, before 6.68 g (21.8 mmol/l) of
dilauroyl p~roxide (dissolved in 70 ml of ~oluene (abso-
lute)) were likewise added in a coun~ercurrent of argon.
~he reaction solution was introduced into an oil bath,
preheated at a controlled temperature of 80~C, and
stirred vigorously with a precision glass stirrer. Free
radical grafting of N~ onto COC A3 was ended after 5
hours by precipitation in 5 1 of acetoneO For working up,
ie. purification of the polymer, the latter was repre-
cipitated four times in acetone, 66 mg of MA-grafted
cycloolefin copolymer A3 (A3-P3) being obtained after
drying at 130C (72 hours/oil pump vacuum).
FT-IR tcm~1]: 1865 ss / 1790 ss tC-O~ anhydride)
VN [cm3/g]: in accordance with DIN 5372
A3-P3 : 86.7
The absolute contentE of MA in the graft copolymer were
determined by means of potentiometric titration.
2~77~
- 18 -
Table 3
Sample~A contentAvarage n~mber of MA/
t4 by weight]polymer chain A3
A3-Pl 1.07 4
A3-P2+ 1.95 7
A3-P3 0.64
Polyacetal B having structural unit~ of the formula F3
and F4
H H H
---C ~ --C ---C --O--
F 3 F4
in which the content of oxymethylene struc~ural units is
98% by w~ight and the content of oxyethylene structural
units is 2% by w~ight.
Such a polyacetal B is commercially avail~ble. It is
marketed, for example, as Hostaform C 2521 (~ = registered
trademark) by Hoechst AG, Frankfurt am Main.
The polymers described above wsre first dried (130~C,
24 hours, vacuum) and then kneaded or extruded under a
protective gas (Ar~ in a measuring kneader (HAAKE
(Karlsruhe), Rheocord Sy tem 40/Rheomix 600) or measuring
extruder (HAAKE (Karlsruhe), Rheocord System 90/Rheomex
TW 100). The resulting ground or granulated blends were
dried (130JC, 24 hours, vacuum~ and then pres~ed (vacuum
pre~s: Polystat 200 S, Schwabenthan (Berlin)) to give
~7~ ~
-- 19 --
sheets (120 x 1 mm). The ground or granulated blends were
introduced into the press, which had been preheated to
230C (blends with A1 and A2) or 190~C (blends with A3
and graft copolymers A3-Pl* and A3-P2~ or blends with
graft copolymer A3--P3), a vacuum was applied and the
blends were melted in the course of 10 minutes. The
material was pressed together under a pressure of 150 bar
at the above temperature, this pressure was maintained
for 5 minutes and the shee~s were then cooled to room
temperature in ~he course of 10 minu~es. The resulting
melt-pressed sheets were investigated in respect of their
physical properties.
The following apparatus was used for this:
A differential scanning calorLmeter (DSC-7) from Perkin-
Elmer (~berlingen) for measurement of, for example, glass
stages, melting points and heat~ of fusion.
A torsion pendulum machine from Brabender (Duisbur~) for
measurement of the shear modulus, damping and linear
expansion.
A tensile stress-elongation test machine (type: Instron
4302) from Instron (Offenbach).
A melt index tester MPS-D from Goettfert (Buchen) for
measurement of flow properties in accordance with DIN
53735-MVI-B (plunger loadttemperature variable; barrel:
internal diamter 9.55 (+ O.01) mm, length at least
115 mm, discharge nozzle ~.095 (+ 0.005) mm, melting tLme
5 or 10 minutes).
The water content was determined in accordance with AS~M
D 4019-81.
~7~
- 20 -
Example 1.
The cycloolefin copolymer (Al~ and the polyacetal ~B)
were kneaded together, after intensive drying, in various
weight ratios under an argon a~mosphere ~y means of -the
measuring kneader. The follow.ing tables show~ the thermal
properties determined on the blends.
COC Al POM B Cooling 2nd Heating
t% by weight] [~ by weight] ~m AHm Tm ~Hm Tg
POM B POM B COC
[C] [J/g] [C] [J/g] [~C
_ .
100 __ __ __ __ 17g
1~143.~ 16837.7 179
_
14088.7 168 75-9 *
_
141125.5 167114.7 *
100 ~42168.~ 168171.2 - ;
Heating up and cooling down rate: 20/minute
not measurable (sensitivity of the apparatus too low)
Example 2:
The cycloolefin copolymer (Al) and the polyacetal (B)
were mixed together, after intensive drying, in various
weight ratios under an argon atmosphere by means of the
measuring kneader, and the mixture was then ground. Aftex
intensive drying, the ground products w~re used to
measure the flow properties.
207r~
- 21 -
_
COC Al POM B MVI
[% by weight~ [% by weight~21.6 kg/230C
~cm3/10 minutes]
Melting tLme 5 minutes
_ _ ._
100 0 23.5
110.6
305.7
391.1
100 74.4
Example 3:
The cycloolefin copolymer (A2) and the polyacetal (B)
were extruded together, after intensive drying, in
various weight ratios under an argon a~mosphere by means
of the twin-screw extruder, and the extrudate was granu-
lated. After intensive drying, the granules were used to
measure the flow properties.
2 ~
- 22 -
COC A2 POM B MVI
[% by weight] [% by weight]21.6 kg/220C
[ cm3 / 10 minutes]
Nelting tLme 5 minutes
100 0 1 14.2
198.9
445.4
_
not measurable (~ 450)
0 100 62.0
Example 4:
The cycloolefin copolymer (A2) and the polyacetal ~B)
were extruded together, after intensiv~ drying, in
various weight ra~ios under an argon atmosphere by means
of the twin-screw extruder, and the extrudate was granu-
lated. After intensive drying, the granule~ were th~n
pressed to give pressed sheets. The following table shows
the mechanical data determined on the blends in the
tensile stress/elongation e~perLment.
~7~ ~ ~
- 23 -
COC A2 POM B Modulus of Yield stress
elasticity
[~ by weight] ~% by weight~ [GPa] [MPa~
_
100 ~ 3.2 6~
3.0 59
_
10 50 50 2.8 58
_
2.9 58
~_
0 100 2.8 58
Example 5:
The cycloolefin copolymer (A3) and the polyacetal (B)
were kneaded together, after intensive drying, in ~arious
weight ratios under an argon atmosphere by means of the
measuring kneader. The following table shows the thermal
properties determined on the blend~.
~7~ ~ ~
- 24 -
CoC A3 POM s Cooling 2nd Heating
[% by weight] [~ by weight] Tm ~Hm Tm ~Hm Tg
POM s POM B COC
5lC~ [~/g~ [oc] ~J/g] [C]
_
10~ 0 __ __ __ - 114
_
142 ~ 168 x114
.
141 x 167 x *
r__ _
142 x 168 x *
0 100 142 168.4 168 171.2 ~
Heating up and cooling down rate: 20C/minute
x Separation not possible (melting point, gla~s stage)
* not measurable tsensitivity of the apparatus too low~
Example 6:
The cycloolefin copolymer (A3) and the polyacetal ~B)
were kneaded together, after intensive drying, in various
weight ratios under an argon atmosphere by means of the
measuring kneader. The ground products were dried inten-
sively and, after storage at 23C and 85~ relativehumidity for at least 240 hours, the absorption of ~ater
was determined.
2 ~ ',J~
~ 25 -
COC A3 POM B Water content
~ by weight] [% by weight] [% by weight]
100 0 0.01
_
~.10
0.16
0.29
~ . _
0 100 0.41
_
Example 7:
The cycloolefin copolymer (A3) and the polyacetal (B)
were kneaded together, after in~ensive ~rying, in various
weight ratios under an argon atmosphere by means of the
measuring kneader. The ground products were dried inten-
20 sively and pressed to give pressed heets. The followingtable shows the mechanical data of the blends during the
torsion pendulum test.
2 0 7 7 ~ ~ 1
-- 26 --
__ _ _____
o al I~ co
~r i I ~ ~D ~r
o l l ~ ,~ o~
_l l l _
_~ U~ U~ o~ o C~
~1 c:~ ~ r~ ~ ~
_l U~ ~ ~ ~
F _I u~ ~ ~D D CD
_l a~
~1 _J ~` U~ U~ d' r~
~o
~ _J ~ O~ ~ I`
a~ c~ cO O ~r I~
0~ U:~ D Ln
~~)
o o o ~7 _~ CO U~
.~ _. o ,~ ~ s~ ~ t~
o~ r~ ~o ~9 In
o ~ h
x ~ ~ _l r~
D ~t Q~ ~ In O~
~n cn r` I~ r
~ ~, o ~ ~ cn ~
tn ~ o n ~ ~ co ~D
~r ~n a~ ~o c~ c~
o
O co sn _~
o a~ ~ ~ o o
s~
o ~ U~
,~ u~ o~ c~ ~r a:~
U~ I ~ o~ o ~ ~
~1 C~ ;- ~D I
~D O O ~ ~ O~
l _l ~ _
_l ~1 ~D U~ ~D r~
er ~ ~ O ~ 0
_~ U~ D ~ r~ S_
_ I ~_ _l ~_ ~_
.~
~d~ O O ~ O O
o~n ~ rr~ U~ r~ o
dP
_. _ _ ,_ _ _
.
f¢ ~ ~ o o o
0~ o r u~ ~
d~
_ _ _ _ _ . __ .
2~
- 27
Example 8:
The cycloolefin copolymer (A3) and the polyacetal (B)
were kneaded together, after intensive drying, in various
weight ratios under an argon atmosphere by means of the
measuring kneader. ~fter intensive drying, the ground
products were u~ed to measure the flow properti2s.
_ _ _
CoC A3 POM s MVI
[% by w~ight] [~ by weight]21.6 kg/190C
0 [ cm3/ 10 minutes]
Melting time 5 minutes
100 0 15.3
. . ~
15 70 30 89.4
_ _ _ _ _
50159.0
_ _
70189.3
0 10038.2
Example 9O
The cycloolefin copolymer (A3), the graft copolymer A3-Pa
and A3-P2+ and the polyacetal ~B) were kneaded together,
after intensive drying, in various weight ratios under an
argon atmosphere by means of the mea~uring kneader. The
following table shows the thermal properti~s determined
on the blends.
~7~
- 28 -
COC A3 COC POM B Cooling 2nd ~eating
[% by A3-P1 /2+ [% by Tm AHm Tm ~Hm l'g
weight] [~ by weight] weight] POM B POM B COC
[C~ [J/g] [C] [J/y] [C]
_. _ _ _ I
100 0 0 ____ ~ 114
_ _
10 ~ 1 0 30 142x 167 x 114
56 14~ 30 142~ 167 x 113
. ._
18 12+ 70 143x 167 x V
_
15 30 0 70 142x 167 ~ V
: _
100 142 168.4 16B 171.2 -- .
Heating up and cooling down rate: 20C/minute
x Separation not possible (melting point, glass stage)
* not measurable (sensitivity of the apparatus too low~
Example 10:
The cycloolefin copolymer ~A3), the graft copol~mer (A3-
Pl and A3-Ps+) (compatibilizer) and the polyacetal (B)
were kneadad together, after intensive d~ying, in various
weight ratios under an ar~on atmosphere by means of the
measuring Xneader. After intensive drying, the ground
products were used to measure the flow properties.
~7~
-- 29 --
COC A3 CO(: POM B MVI
[% by A3-Pl~/-P2~ [% by 21.6 kg/190C
weight [% by weight3 weight] [cm3/10 minutes]
Melting time:
. . 5 / 10 minutes
100 O O 15.3 / 15.5
O 30 89.4 / 96.7
56 1~ 30 64.3 / 71~2
18 12+ 70 171.~ / 181.6
0 70 189.3 / 188.9
_ ~
O ~ 100 38.2 / 37.2
Example 11 t
The cycloolefin copolymer (A3), the graft copolymer (A3-
Pl and A3-P2+) and the polyacetal tB~ were kneaded
together, after intensive drying, in various weight
ratios under an argon atmosphere by means of the measur-
ing kneader. After intensive drying, the ground productswere pressed to give pressed sheets. ~he following table
shows the mechanical data determined on the blends
with/without the compatibilizer (A3-P1 and A3-P2+) in the
torsion pendulum test.
~7~
-- 30 --
. _ ___ ~ ,~--~
o CO ,1 o~
C~ l l l U~ ~ ~
,~ U~ U~ ~ er O O
~ O _~ cr) r~ ~ ,~
_ ~ U~ ~ ~ ~ ~ ~
_~ u~ ~ n u~ ~D OD
, o _l a~ co r~ ~r ,~
~ ~ I~ U~ In d' ~P ~
~o u~ cn r~
~:: ~ ~a 0 a~ o~ ~ r
1:~ CO ~ ~D U~ U~ ~r
O ~ ~ O~ ~ ~D
O co r~ r~ ~D ~ U~
D ~ _~ I~ D r~ U~
_l C~ O t` U~ G~
O o ~ r~ CD r I~ u7
_, ~ o o ~ ~n ~ o. ~D
~ ~ ~ U~ ~ 1` O 0~ U:~
c~ X ~ :n o~ c~ ~ o~ c~
~ ~ O rr~ O ~ ~ ~_ ~
co o~ ~ _l o ~
o~ ao o~ c~ o ~o
_~ ~
o ~o U~ U~ ~ oo
o a~ ~ u~ ~ 1` ,~ ~ c~
~ E~ I o ~n o
5~ _1 ~/ _1 ~ _1
~ _I ~ r~ ~ o~ o t~
a~ ~ o~ ~ ~ ~ u~ ~r
I o o ~ ~ U~
U~ ~I ~ _~ _l _l
~1 ~1 ~D OD ~D ~D I`
~r d' ~ ~ ~1 ~ OD
~D ~ r~ r~ r~
l _I_ _I _l .
t~
. m ~
O
O ~ ~ ~ I~ r~ o
dP _ __ _
C ~ rl
U ~ ~ +
0 ~ O O ~ N O O
'¢ I¢
. _ _ __ _ __
~ rl
~ ~ O O ~D CO O O
.~il O 1- In _~
~
_. _ _ _ __
2 ~
~ 31 -
Example 12:
The cycloolefin copolymer ~A3), the graft copolymer
tA3-Pl~ and A3-P2+) and the polyacetal (B) were kneaded
together, after intensive drying, in various weight
ratios under an argon atmosphere by means of the measur-
ing kneader. After intensive drying, the ground products
were pressed to give pressed ~heets. The following table
show~ the mechanical data determined on the blends with/
without a compatibilizer (A3-Pl~ and A3-P2+) in the
tensile stress~elongation experiment.
COC A3 COC POM B Modulus of Yield Elongation
[% by A3-P1~ [% by ela~ticity stress at break
weight3 A3[~P2y weightJ [GPa] [GPa~ [%]
_ _ weight]
100 0 0 3.~ 60 4.9
_
30 2~9 ~ 5.0
56 14~ 30 3.0 57 3.6
18 12+ 70 ~.g 60 10.~
~ _ _
0 70 2.8 56 5.4
0 0 100 2.8 58 35.0
Example 13:
The graft copolymer A3-P3 and the polyacetal (B) were
kneaded together, after intensive drying, in variou~
weight ratios under an argon atmosphere by means of the
measuring kneader. The following table show~ the ~hermal
~7~
- 32 -
properties deter~ined on the blends.
_. _
COC Cooling 2nd Heating
A3~P3 POM B Tm QHm Tm aHm Tg
5 [~ by weight~ [% by weight] POM B POM B COC
[~C] [~/g] [C] ~J/g] [C]
. , __ ~ .__~
100 0 __ __ -- -- 113
_
83/114~ x 162 33 112
143 x 168 x V
0 100 14~ 168.4 168 171.2 -
15 ______________ _ _ _ _
Heating up and cooling do~ rate: 20C/minute
x Separation not possible (melting point, glass stage)
V not measurable (sensitivity of the apparatus ~oo low)
+ several minLma (2nd main minimum)
Example 14:
The graft copolymer A3-P3 and the polyacetal (B) were
kneaded together, after intensive drying, in various
weight ratios under an argon atmosphere by means of the
measuring kneader. After intensive drying, the ground
2S products were used to measure the flow properties.
2~7~ ~
COC POM B MVI
A3-P3 [~ by weight]21.6 kg/190C
[% by weight] rcm /10 minutes3
S Melting time
5 / 10 minutes
100 0 14.6 14.8
7.8 7.9
_
9.3 7.0
_ _
0 100 38.~ 37.2
_ _ _ _ _
~ foam-like mat~rial
Example 15:
The cycloolefin copol~mer A3 or the graft copolymer A3-
P3 and the polyacetal (B) were kneaded together, after
intensive drying, in various weight ratios under an argon
atmosphere by means of the measuring kneader. After
intensive drying, the ground product~ were pressed to
give pres6ed sheet6. The following table shows the
mechanical data determined on the blends in the torsion
pendulum test.
2~77~
-- 34 --
_____ ~ ~D
_l l l l l ,~
o t~ ,1
N ~ l l l ~D ~ ~
r~l l l l l ~1 ~1 ~`I
_~ U~ ~I 111 ~O tr~ C~ O
~1 O I~ _l ~1 t~l ~1 ~1
_ ~ u~ r~ ~ ~ ~ ~ r~
_~ Ul U~ ~ ~ ~ ~D ~0
~ O _l U~ U~ ~ ~ d' r-
_l ~ I` ~ U~ ~D ~:P
::1
U~ ~ CO ~ O 0 ~ 1_
~ CO N O~ Cl:~ ~ OD ~ r~
~1 CO 1` D ~` U~ U~
O O U~ ~ ~ ~ 00 ~D
o~ ~ ~ r~
O c~ c~7 1~ r- ~D U~ U~
ta ~ ~D ~r r~ _l _~ ~ ~ u~
5~ ~1 D C~ Lrl U~ U~ a~
O O o~ co ,~ ao r~ r~
-- E3 o o ~D ~ O- ~ cn u~
r u~ O ~ ~1 O~ ~0 ~D
x S~ cn ~ a~ ~ co OD ~
O~) ~D O O CO i~ t`~
CO d'O~ O~ ~ ~ C~l
Z h a~cn c~ o~ o o o
,1 O o U~ o~ ,1 C~ o~
o a) ~ ~ ~ O~ _, a~ ~ o~
~ I O O ~ _~ N ~ ~1
~ _l ~ rv~ ~` ~ ~ O t-
(U ~D tD 1~ ~` ~1 ~0 U') ~r
I O ~ O ~ U~ U~ O~
U~ _1 _~ ~1 ~1 ~1 _~ ~1
_l _l t~ ~D ~ Ct~ U~ r~
d~ ~ ~ ~ ~ ~ C~
_l In ~ ~ CO r-
l _l ~ ~1
.. . _ _ . _ _ __ .
.C
a:l Q)
~ 3 o o ~ ~ ~ o o
P1 ,~ ~1
d~
.. _ . _ , _ _
.~
V ~ ~
O ~ ~ o o l o ~ l o
~¢~ ,1
-y~- _ ,__ _ _ _ _ _
8 3 o o ~ L o o
~077~1 ~
- 35 -
Example 16:
The graft copolymer A3-P3 and the polyacekal (B) were
Xneaded together, after intensive drying, iIl various
weight ratios under an argon atmosphere by means of the
measuring kneader. After intens iV8 drying, the ground
products were pressed to give pressed sheets. The fol-
lowing table shows the mechanical data determined on the
blends in the tensile str~ss/elongation experiment.
10COC POM B Elasticity Yield Elongation
A3 P3 [% by weight] modulus stress a~ break
[~ by weight] [GPa] [MPa] [%]
_ _ _ _ . _ _
100 0 3,1 61 5.0
_ .
2.9 5~ 7.9
2.9 58 17.8
_
0 100 2.8 58 35.0