Note: Descriptions are shown in the official language in which they were submitted.
CA 02077461 1999-04-07
INHIBITION OF THE FORMATION
OR ACTIVITY OF HUMAN
LEUKOCYTE 12-LIPOXYGENASE PATHWAY
FIELD OF INVENTION
This invention relates to a new form of
12-lipoxygenase (12-LO) RNA and protein in human
adrenal, vascular smooth muscle, endothelial and
mononuclear cells. The invention also relates to the
mediation of angiotensin II (AII) and glucose induced
vascular and renal actions by activation of this
12-LO pathway.
BACKGROUND OF THE INVENTION
Enhanced atherosclerotic cardiovascular and renal
disease continue to be major causes of morbidity and
mortality in patients with diabetes mellitus and
hypertension. All activity and elevated glucose are
known to play a role in increased propensity to these
disorders.
Applicant has found that All can increase the
activity of a 12-lipoxygenase (12-LO) pathway of
- arachidonic acid. The 12-LO pathway can produce
active products including 12-hydroperoxyeicosatetra-
enoic acid (12-HPETE) and more stable 12 hydroxyeico-
satetraenoic acid (12-HETE). In addition, some 12-LO
enzymes can also metabolize linoleic acid to produce
additional active lipids called
hydroxyoctadecadienoic acid (HODES).
The LO products may play a key role in the
development of vascular and renal disease. 12-HETE
and 12-HPETE have been shown to be important
-2-
mediators of All induced effects on inhibition of
renin release from kidney (1) stimulation of F
aldosterone synthesis from rat and human adrenal
cells (2,3) and increase in blood pressure in rats
(4). Furthex'more, 12-HETE and 12-HPETE can lead to
vascular smooth muscle cell migration at
concentrations as low as 10-14M (5), and both
products can inhibit the synthesis of the
vasoprotective eicosanoid prostacyclin (6,7). The
linoleic acid metabolities including 13 and 9 HODS
have been recently found to be capable of producing
mitogenic effects in certain cell types including the
liver and fibroblasts (8,9) and can mediate epidermal
growth factor induced proliferative actions (9).
Recent studies in several species have shown the
presence of two forms of 12-LO (10,11). One type has
been cloned from porcine leukocytes (10), which
shares 85~ sequence homology to a human tracheal
15-LO enzyme (12). Another type of 12-LO found
almost exclusively in human platelets is only 65%
homologous to the porcine leukocyte type of 12-LO
(11). These two forms of 12-LO not only differ in
amino acid sequence, but also show differences in
preferred substrates. The platelet type of 12-LO
exclusively reacts with arachiodonic acid to form
12-HETE. However, the porcine leukocyte type of
12-LO reacts with linoleic acid and to form 9 and
13-HODE as well as arachidonic acid to form 12-HETE.
In a recent study it has also been found that 12-LO
products can mediate AII-induced bovine adrenal cell
proliferation (13).
Prior to this invention it was not known that a
leukocyte type of 12-LO is also expressed in human
tissue.
CA 02077461 1999-04-07
-3-
SUMMARY OF THE INVENTION
This invention includes the discovery of a new form of 12-LO RNA and
protein in human adrenal mononuclear, vascular smooth muscle and
endothelial cells. Activation of this 12-LO pathway plays a key role in
mediating All and glucose induced vascular and renal actions. Products of
this newly discovered 12-LO pathway can directly activate protein kinase C
and led to increased vascular smooth muscle cell growth, a hallmark
atherosclerotic vascular disease.
Another aspect of the invention postulates treatment or prevention of
vascular disease in patients with diabetes mellitus or hypertension by
inhibition of this new form of 12-LO. The invention thus provides a rationale
for development of a new pharmaceutical or molecular method to inhibit this
newly discovered lipoxygenase pathway.
In accordance with an aspect of the invention, isolated and purified 12-
lipoxygenase expressed by human vascular smooth muscle cells, adrenal
cells, mononuclear cells or endothelial cells, wherein the polypeptide
sequence of said isolated and purified 12-lipoxygenase is an expression
product of the nucleotide sequence comprising SEQ ID No. 7.
In accordance with a further aspect of the invention, isolated and
purified human 12-lipoxygenase RNA from human vascular smooth muscle
cells, adrenal cells, mononuclear cells or endothelial cells, wherein the RNA
sequence of said isolated and purified 12-lipoxygenase RNA is transcribed
from the nucleotide sequence comprising SEQ. ID no. 7.
In accordance with another aspect of the invention, a method for
regulating the expression of human 12-lipoxygenase protein and RNA
comprises culturing human vascular smooth muscle cells, adrenal cells,
mononuclear cells or endothelial cells in vitro in a medium in which the
concentration of angiotensin II is controlled.
In accordance with a further aspect of the invention, an in vifro method
for mediating angiotensin II and glucose-induced vascular and renal action on
cells by controlled activation of the expression of the 12-lipoxygenase
according to claims 1 or 5 comprises regulating the levels of ambient glucose
concentration, angiotensin II concentration or both bathing said cells.
CA 02077461 1999-04-07
..
DESCRIPTION OF THE FIGURES
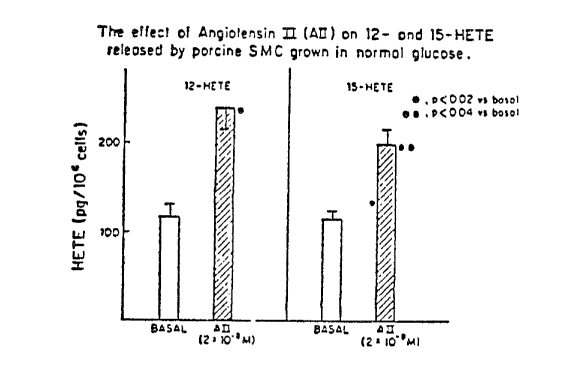
Figure 1 is a bar graph which depicts the effect of All on 12- and 15-
HETE released by porcine smooth muscle cells (SMC) grown in normal
glucose.
Figure 2 is a bar graph which depicts the effect of All on cell associated
12-HETE levels in porcine vascular smooth muscle cells (PVSMC) cultured in
normal (5.5 mM) or high (25 mM) glucose.
Figure 3 is a bar graph which depicts the effect of high glucose (25
mM) on 12- and 15-HETE levels in porcine aortic smooth muscle cells.
Figure 4 is a Western immunoblot which depicts the effect of All (10-'
M) on 12-LO 72K expression in PVSMC in normal (5.5 mM) or high (25 mM)
glucose.
Figure 5 is a Western immunoblot which depicts specific porcine
leukocyte 12-LO protein expression in PVSMC. Lane 1, antigen-porcine 12-
LO; Lanes 2 and 3, PVSMC cytosols. A: with 12-LO antibody
-4-
1:600, B: With 12-LO antibody preincubated with
12-LO antigen.
Figure 6 is a Southern blot analysis of 12-LO
mRNA levels in PVSMC by reverse transcriptase PCR
(RT-PCR). The results show marked increase in 12-LO
expression in cells cultured in high glucose. There
was no effect of glucose on CAPDH (ma:rker gene)
expression.
Figure 7 depicts a Southern blot analysis which
shows regulation of 12-LO mRNA by All in PVSMC.
Figure 8 is a bar graph which depicts the effect
of All on protein synthesis in PVSMC cultures in
normal or high glucose.
Figure 9 is a bar graph which depicts the effect
of baicalein, a 12-LO synthesis inhibitor, on
AII-induced DNA and protein synthesis in PVSMC.
Figure 10 is a bar graph which depicts protein
synthesis after direct addition of 12 and 15-LO
products in PVSMC cultured in normal glucose.
Figure 11 is a bar graph which depicts protein
synthesis after addition of 12 and 15-LO products in
PVSMC cultured in high glucose.
Figure 12 depicts growth curves of PVSMC in
normal and high glucose.
Figure 13 illustrates the regulation of 12-LO
protein expression by All in human adrenal
glomerulosa cells.
Figure 14 is a Southern blot analysis showing
expression of 12-LO RNA in human adrenal glomerulosa
and U937 mononuclear cells.
Figure 15 is a Northern blot analysis that shows
expression of 4.1 kb size 12-LO RNA band in human
adrenal glomerulosa.
~~~~~s~.
-5-
Figure 16 is a Southern blot analysis that
illustrates the regulation of 12-LO RNA levels by All
as determined by RT-PCR.
Figure 17 depicts regulation of 12-LO protein
expression by All in human aortic vascular smooth
muscle (HVSMC) .
Figure 18 illustrates the presence: of leukocyte
type 12-LO in human aortic smooth muscle and
mononuclear cells and also shows induction of 12-LO
expression by All in human vascular smooth muscle
cells (HSMC).
Figure 19 shows the release of 12-LO product
12-HETE by All in human vascular smooth muscle cells.
Figure 20 depicts the effect of baicalein (10-6M)
a 12-LO inhibitor on smooth muscle cell growth in
normal glucose (5.5 mM) and high glucose (25 mM)
conditions. Cell number is significantly reduced by
baicalein in high glucose only.
G High glucose without baicalein
~ High glucose with baicalein
o Normal glucose without baicalein
~ Normal glucose with baicalein
Figure 21 depicts RT-PCR Southern blot analysis
showing the presence of human leukocyte type 12-LO in
human aortic endothelial cells. Lane 1, cDNA
positive control. Lane 2, 12-LO expressed DNAase
treated showing band is not from DNA contamination
and Lane ~ is total RNA from endothelial cells
showing 333 base pair product.
Figure 22 depicts RT-PCR, Southern blot analysis
showing that human aortic endothelial cells do not
express the 15-LO RNA but only the 12-LO RNA of
leukocyte type. Presence of positive control
amplification of 15-LO cDNA but complete absence of
~,
-6-
l5-LO RNA in two separate samples of human aortic
endothelial cells is depicted.
Figure 23 shows specificity of PCR method for
amplification and expression of either leukocyte
12-LO or 15-LO RNA.
DETAILED DESCRIPTION OF THE INVENTION
I. Profile and Mechanism
of LO Product Formation
LO product formation: In PVSMC cells cultured in
normal glucose (5.5 mM, 100 mg/dl), All increased the
release of both 12-- and 15-HETE into the media
(Figure 1). However, All did not increase cell
associated HETE levels in normal glucose (Figure 2).
In contrast, cells grown in high glucose (25 mM, 450
mg/dl) showed markedly elevated levels of cell
associated 12- as well as 15-HETE (Figure 3)
(12-HETE; 1413~174 normal glucose vs 2600~181 high)
and 15-HETE: (1530*134 normal vs 3322-1225 high
pg/106 cells, both p<0.01 high vs normal glucose).
Figure 2 also shows that All further increased the
cell associated 12-HETE concentrations in high
glucose.
These results indicate that All stimulates 12-
and 15-LO product (HETE) formation in PVSMC. In
addition, PVSMC cultured in elevated glucose media
produce more LO products and have an enhanced LO
response to AII.
LO ~arotein expression: Figure 4 is a Western
immunoblot using an antibody against porcine
leukocyte 12-LO showing effects of high glucose
(25mM) and All (10-7M) on 12-LO enzyme (72 KD)
expression at 45 hours. It is clearly seen that
basal 12-LO enzyme expression is markedly increased
in PVSMC cultured in high glucose. In addition, All
caused a significant stimulation in 12-LO expression
_7_
in normal ad high glucose. The specificity of these
results using antibody blocking studies was also
confirmed. Figure 5 shows that the bands obtained
with authentic porcine leukocyte 12-LO enzyme
(lane 1) as well as with PVSMC cytoso7.s (lanes 2
and 3) (Aj all disappeared when treated with 12-LO
antibody which had been preincubated 9:or two hours
with the 12-LO enzyme (S).
These results indicate that PVSMC express the
leukocyte form of 12-LO and that porcine 12-LO enzyme
expression is increased by high glucose as well as
AII.
LO mRNA expression: The invention also includes
the discovery that AII, as well as high glucose, can
upregulate 12-LO mRNA expression. To demonstrate
this discovery, a specific reverse transcriptase
polymerase chain PCR procedure was designed for
evaluating basal and stimulated 12- and 15-LO mRNA
levels in PVSMC, human adrenal glomerulosa, human
vascular smooth muscle and monocytes.
The sequences of the primers and the probes were
designed based on known gene sequences (10,12,13,14),
and selected from regions displaying most divergence
between porcine 12-LO and 15-LO sequences (11).
Human 15-LO (Ref. 12). All sequences are 5°-3'.
SEQ ID. 1: Primer 1:5'AACTCAAGGTGGAAGTACCGGAG3'
nucleotides 146 to 168
SEQ ID. 2: Primer
2:5'ATATAGTTTGGCGCCAGCCATATTC3' complementary to
nucleotides 453 to 477
SEQ ID. 3: Probe: 5'AGGCTCAGGACGCCGTTGCCC3°
complementary to nucleotides 305 to 326.
Porcine Leukocyte 12-LO (Ref. No. 10).
SEQ ID. 4: Primer 1:5' TTCAGTGTAGACGTGTCGGAG3'
nucleotides 145 to 165.
CA 02077461 1999-04-07
-g-
SEQ ID. 5: Primer 2:5' ATGTATGCCGGTGCTGGCTATA
TTTAG 3' complementary to nucleotides 451 to 477.
SEQ ID. 6: Probe: 5' TCAGGATGCGGTCGCCCTCCAC 3'
complementary to nucleotides 301 to 322.
Total RNA from both human adrenal glomerulosa
tissue and cultured cells was extracted with
guanidium thiocyanate-phenol-chloroform using RNAzol*
(Cinna/Biotecx Laboratories International, Inc.,
Texas). Poly (A)+RNA was purified by oligo (dT)
cellulose chromatography column (5 prime ~ 3
Prime, Inc., West Chester Pennsylvania). 1 ~g of
total RNA or mRNA was mixed with the PCR buffer (10
mM Tris-HC1, pH 8.3, 50 mM KC1, 1.5 mM MgCl2, 0.001%
gelatin), 200 ~M of each of the four deoxynucleotide
trisphosphates, 25 pmole each of 5' and 3' primers
5'TTCAGTGTAGACGTGTCGGAG3' (SEQ ID. 4) and
5'ATGTATGCCGGTGCTGGCTATATTTAG3' (SEQ ID. 5), 2 units
of Avian Myeloblastosis Virus reverse transcriptase
(20 U/ul, Lie Sciences, St. Petersburg, FL) and 2.5
units Taq polymerise (Perkin Elmer Cetus), in a final
volume of 50 ~1. In some reactions, 5 pmole of each
5' and 3' primers of ~2 microglobuiline or GAPDH were
added as an internal standard. The samples were
placed in a thermal cycler at 37°C for 8 minutes for
the reverse transcriptase reaction to proceed. Then
conditions used for PCR were a denaturation step at
94°C for 1 minute, annealing at 50°C for 2 minutes
and extension at 72°C for 2 minutes for 25-30 cycles.
Blank reactions with no RNA template, or with no
reverse transcriptase were carried out through the RT
nd PCR steps. RNA samples from HEL cells or IM-9
cells were run as controls in both PCR nd in Northern
analysis. The human 15-LO cDNA, porcine leukocyte
12-LO cDNA and human platelet 12-LO cDNA
amplifications were carried out by mixing 2-5 ng of
* trademark
-g-
cDNA in 50 ,ul volume containing 10 mM Tris-HC1, pH
8.3, 50 mM KC1, 1.5 mM MgCl2, 0.001 gelatin, 200 ~aM
of each of the four deoxynucleotide triphosphates, 25
pmole of 5' and 3° primers, and 2.5 U of Taq
polymerase.
The size of the amplified fragmen~k: is 333 by for
both of 12-LO and 15-LO. The 333 by PCR amplified
fragment obtained with porcine leukocyte 12-LO cDNA
or with human 15-LO cDNA as a template could be seen
in an ethidium bromide stained gel after 25 cycles of
amplification (data not shown). However, using RNA
samples from the human cells, the 333 by amplified
product could not be seen in an ethidium bromide
stained gel even after 35 cycles of amplification.
The product could only be detected by autoradiography
of a blot hybridized with a porcine leukocyte 12-LO
oligonucleotide probe.
Since the amino acid sequences of porcine
leukocyte 12-LO and human 15-LO are highly
homologous, the porcine leukocyte 12-LO cDNA probe
could not readily distinguish the 333 by amplified
products corresponding to porcine leukocyte 12-LO or
to human 15-LO (data not shownj. Moreover, human
15-LO oligonucleotide and porcine leukocyte 12-LO
oligonucleotide probes can cross hybridize to the
12-LO or 15-LO amplified product, respectively using
12-LO or 15-LO eDNA as templates of amplification.
At high stringency, e.g., hybridized membrane wash
temperature of 60°C, the 333 by PCR amplified
products of porcine 12-LO and human 15-LO were
distinguished by the cDNA probe.
Figure 23 depicts comparison autoradiograms of
PCR of cDNA for human 15-LO and cDNA for porcine
leukocyte 12-LO. cDNAs samples were amplified for 25
cycles with specific primers for the gene (Table 1j
-1~_
and were hybridized with a labeled porcine leukocyte
12-LO o:ligonucleotide probe (panel A) or with a
labeled human 15-LO oligonucleotide probe (panel. B).
Lane 1 is porcine leukocyte 12-LO primers on cDNA for
porcine leukocyte 12-LO. Lane 2 is human 15-LO
primers on cDNA for human 15-LD.
Direct DNA sequencing of PCR product
PCR amplification: RNA was reverse transcribed
as follows: the reactian mixture contained porcine
leukocyte 12-LO complementary primer
5°ATGTATGCCGGTGCTGGCTATATTTAG3° (SEQ ID. 5), dNTP and
2 ug of RNA in a final volume of 9 ~1. The mixture
was heated to 80°C for 5 minutes and cooled to 37°C.
Two units of AMV reverse transcriptase was added and
maintained for three minutes at 37°C. Then an
additional two units of AMV reverse transcriptase was
added, the sample was heated to 95°C to denature, arid
then amplified for 40 cycles by PCR as described
before. The PCR product was analyzed by
hybridization. In order to obtain sufficient amount
of PCR product for sequencing, 1 ~l of total product
of the PCR reaction was used as a template for
secondary PCR amplification with 5° and 3° primers,
(145-165 and 451-477) primers for U937 cells;
(177-198 and 428-450) primers far human adrenal. The
reaction conditions were as described before except
that 30 cycles was used.
Preparation of DNA for sequencing: The products
of secondary PCR were purified by electrophoresis on
nondenaturing 8% polyacrylamide gel. The isolated
DNA fragment was directly used for sequencing.
Sequencing: Two approaches were used for
sequencing.
CA 02077461 1999-04-07
-11-
(A) Sequencing reaction of purified PCR product
of U937 cells was set in the presence of 0.5% NP-40
detergent and [y32-P] ATP labeled porcine leukocyte
12-LO oligonucleotides 145-165, 451-477 and 301-322.
DNA sequencing reactions were performed by the
dideoxynucleotide chain termination method using
Sequenase (United States Biochemicals, Cleveland,
Ohio), sequencing in both directions with 5' primer
and 3' primers.
(B) Sequencing reaction of PCR products of human
adrenal glomerulosa tissue and U937 cells was
performed by a cycle sequencing method of AmpliTaq(trade-mark)
DNA polymerase with a cycle sequence kit (Perkin
Elmer Cetus, Norwalk, CT). 15 ng of human adrenal
PCR product and 2 pmole of [~32-P] ATP labeled
porcine leukocyte 12-LO oligonucleotide (177-198 or
428-450) were used. The cycling program was 1 minute
at 95°C, and 1 minute at 60°C for 20 cycles.
As Figure 23 shows, amplification of their cDNAs
has confirmed that specific expression of leukocyte
type 12-LO and human 15-LO is accomplished.
Regulation of Porcine 12-LO mRNA:
Another aspect of the invention is the regulation
of porcine leukocyte-type 12-LO mRNA in PVSMC
cultured in normal (5.5 mM, NG) or high 25 mM, HG)
glucose using quantitative RT-PCR. Figure 6 is a
Southern blot analysis of the RT-PCR (25 cycles)
amplified products from PVSMC total RNA.
Hybridization was performed with the porcine
leukocyte type 32P-labeled 12-LO oligonucleotide
probe. It is seen that cells cultured in high
glucose have a much greater expression of the 333 by
12-LO PCR amplified product than those cultured in
normal glucose. GAPDH mRNA amplification was used as
an internal standard (280 bp). Densitometric
-12-
analysis revealed nearly a 20-fold greater 12-LO
expression in high glucose. In addition, experiments
were performed to study the regulation of 12-LO mRNA
expression by All (10-~M) at 24 hours using RT-PCR.
Figure 7 shows that 12-LO mRNA expression 333 by is
much greater in high glucose (with little basal
expression in normal glucose). In addition, All
caused a significant 3-4 fold increase in expression
in both normal and high glucose. These results
represent the first demonstration of regulation of
12-LO mRNA and indicate that glucose and All regulate
12-LO protein expression at the transcriptional
level. The size of the transcript (4.0 kb) was
confirmed using Northern analysis (data not provided).
II. Effects on Hypertrophy and Hyperplasia
Effects on hypertrophy: Figure 8 shows that All
(10-6M) increased total cell protein (126 of
control) in PVSMC cultured in normal glucose.
Similar results were obtained with All 10-~ and
10-$M. However, the effects of All on total cell
protein were significantly greater in PVSMC grown in
elevated glucose (147 of control). These results
indicate that elevated glucose enhances the
hypertrophic response of AII.
The hypertrophic response of All is mediated at
least in part by activation of the 12-LO pathway.
The role of the LO pathway in AII-induced
hypertrophic effects is illustrated by Figure ~ which
shows that AII-induced protein synthesis in normal
glucose was blunted by a specific 12-LO inhibitor
baicalein. Similar results were obtained in high
glucose. In addition, Figure 10 shows that the 12-LO
product 12°HETE could directly increase protein
synthesis with the same potency as All in normal
glucose. Moreover, the effect of not only AII, but
-13-
also 12-HETE was enhanced in elevated glucose
(Figure 11). 15-HETE was less potent than 12-HETE
showing significant effects only in e7.evated glucose
(Figures 10 and 11).
Effects on hyperplasia: Growth curves in PVSMC
in 5.5 mM and 25 mM glucose are shown in Figure 12.
The proliferation rates were approximately 30% faster
in cells grown in elevated glucose. rioreover, in
Figure 20 it is seen that the specific 12-LO
inhibitor baicalein (10°6M) attenuated the growth
responses suggesting that LO product formation may
play a role in the proliferative response.
The effect of All alone and with LO inhibition on
DNA synthesis as determined by [3H] thymidine
incorporation has been measured. All caused a small
but significant increase in DNA synthesis in normal
glucose. The 12-LO inhibitor, baicalein, blocked
AII-induced DNA synthesis (Figure 9), suggesting that
products of the 12-LO pathway may mediate in part,
AII-induced proliferative effects. AII-induced DNA
synthesis was also enhanced in elevated (25 mM)
glucose (normal glucose, 146~7% vs high glucose
174~8% control, p<0.05) (data not shown).
Therefore elevated glucose enhances basal
proliferation and also enhances AII-induced
proliferative responses. In addition, blockade of
the 12-LO pathway can reduce the proliferative
actions of glucose and AII.
III. Mechanism of Action of LO Products
Table 1 shows the results of Protein Kinase C
(PKC) activity measurements in PVSMC grown in normal
and high glucose.
-14- ~'~d ''d ~ ~.
TA~~E 1
The Effect of Various Agents on PKC
Activity in PVSMC (Normal oar High Glucose)
Normal ~ Hfgh
rune i c:vzosol t Membrane
Control 185 63.2 126 94.5
TPA (lOnM) 109 181 72.4 242
All (lOnM) 159 65.1 97.3 78
All+9HODE(100nM) 142 89.8 109 121
AIT+13HODE(100nM1 127 102 120 117
It is seen that in comparison to normal glucose,
cytosolic activity in high glucose is lower, while
membrane activity is higher indicating increased PKC
activity in high glucose. Cells were treated with
agents for 15 minutes. All alone showed only slight
activation of PKC relative to TPA. However, in
combination with 13- or 9_HODE, All showed increased
activity in membrane fraction with reciprocal
decrease in cytosolic activity, Immunoblotting
experiments show that PVSMC express a and a isoforms
of PKC but not p or y forms.
These results show that cells cultured in high
glucose show increased PKC activity and All can
induce higher PKC activity in combination with LO
products. Therefore, increased activity of certain
PKC isoforms may be a key mechanism for vascular cell
proliferation in response to glucose All and the LO
products.
IV. Studies in Human Adrenal, Mononuclear
Vascular Smooth Muscle and Endothelial. Cells
Previously published studies show that All
induced aldosterane synthesis in rat and human
adrenal glomerulosa cells is mediated by activation
-15-
of a 12-LO pathway (2,3). This application presents
new evidence that the particular isoform of 12°LO in
human glomerulosa cells is a '°porcine leukocyte
type". Figure 13 shows the effect of All (10-7M) on
the expression of the 12-LO protein in normal human
adrenal glomerulosa cells as assessed by Western
immunoblotting. All increased the expression of
12-LO (Fig. 13A) approximately two-fold over basal as
determined by densitometric analysis (Fig. 13B).
Thus, the 12-LO protein is present in cultured human
glomerulosa cells as seen using an antibody against a
porcine leukocyte 12-LO. Furthermore, the 12-LO
protein ea:pression is increased in cells cultured in
the presence of All for 30 hours. A: shows
immunoblot of data while B: represents a
densitometric analysis of the data in A.
In Figure 14, the identification of the RNA for
this form of 12-LO in human glomerulosa cells and
mononuclear leukocyte type cells (U937 cells) can be
seen using the previously described PCR assay, which
is specific for this form of 12-LO. RNA samples were
amplified for 30 cycles with SEQ ID. 4 and 5 porcine
leukocyte 12-LO primers. Membranes were hybridized
with internal porcine leu3cocyte 12-LO oligonucleotide
probe (SEQ ID. 6). Panel A, lane 1 represents total
RNA from normal human adrenal glomerulosa using
RT-PCR. Lane 2 is a negative control without
template and lane 3 is a negative control using human
15-LO cDNA. Samples in panel B are mRNA or total RNA
from human U937 cells. Lanes 1 and 5 represent
negative controls without reverse transcriptase (RT)
for mRNA and total RNA respectively. Lane 2, mRNA
and lane 6, total RNA are true RT-PCR. Lane 3 is a
positive control using the porcine leukocyte 12-LO
-16-
cDNA. Lane ~ is another negative control without RNA
template.
Figure 15 is a Northern analysis using the 12-LO
probe (SEQ ID. 6). 20 ug of RNA from human adrenal
glomerulosa in lanes 1 and 2 showing that the size of
the RNA expressed (approximately 4.1 kb) is similar
to the porcine leukocyte 12-LO RNA size.
Figure 16 shows regulation of 12-;LO mRNA levels
by All determined by RT-PCR. Total RNA was extracted
from cultured adrenal glomerulosa cells that were
incubated alone or with 10°~M All for 24 hours. RNA
samples were amplified for 25 cycles with primers
amplifying porcine 12-LO. All reactions in the '
experiment also contained primers amplifying human
GAPDH. Controls without RNA or with RNA pretreated
with RNAase were simultaneously run. The position of
the specific products are indicated by arrows. 284
by and 333 by represent amplified products of human
GAPDH.and porcine leukocyte 12-LO respectively.
Panel A is the autoradiogram of the blot hybridized
with oligonucleotide probe specific for the porcine
12-LO gene. Panel B is the autoradiogram of the same
blot subsequently hybridized with oligonucleotide
probe for the GAPDH. Lanes 1 and 4 are glomerulosa
cells in the control incubation. Lanes 2 and 5 are
glomerulosa cells incubated with 20-~M AII. Samples
in lanes 4 and 5 were treated with RNase A prior to
the RT-PCR. Lane 3 is without RNA. These results
Shaw that 12-LO gene is present in human glomerulosa
and monocytes and that the RNA is upregulated by AII.
To confirm that the amplified PCR product in
human monocytes and adrenal cells is not due to
contamination of the porcine 12-LO cDNA, the
amplified product was sequenced. As shown in
.Cable 2, the sequence in human cells is 2 base pairs
different than the porcine sequence.
_ 17 --
U
U
7 t~ U ~
C -U -
7 -C o
C7 - C7
C9 --
N C7
~1'
U H H-H t7-L9
M O
U-U
~
~
~
t
7 -i
7
~ U
N
~ C H-H~
O 7-~
H
N U U U
~ U U ~
~ M
r - - 7
-1 0
-r 7
i -
U C
t
.N H U U-U (~-C7
U d
v
QW C7--C7 H--H H-H
~
1a
N
d~ C7-C7 U-U U-U
O
23
t1
~
.N ~-~ C7-C9
N O
U
S~
O U -U ~cC C9
la In -~ -i9
~
O
U N U
~~ H-H -Hcw
O ~
~
O i7-C7 -U C7-C7
.~ M O
N
U
~
~
U-U H-H ~-~
~1 M
N H-H ~- ~7C9
1 ~
.tl
N
N _ U
O U U
U
~ H - 7 7
~ H - -
R C C
a C 7
7 4
N ~ V-
~
~
R4 ~-H -
cr
rt
f'~ ~
e-1 -
4-1 ~
M M
~
n
M
N ~ ~ ~ H ~7 ~-~
H ~ ~7
v
WU r-1 t0 - -C U-U
'~ U-U C
O U'
-U'
rl~ rc3 N H--H H-H H-H
tn M
~1v O a~ H - C7 H
t~' ~ H N - -
C7 H
o
~CO N ~ O U' -C7 (7 C7
N -U' -U'
o
H4 ~-I tn H U
H
M
~ ~ H --H - -
O
N V U U
a1 V ~ U
Q ~
~
O
= 7 7
C --C
7
C
N C7 -C7 U
C ---U
rl
G7
~
U
~ N H-H U-U
~ ~
~
N L U-U H-H -
~ 7-C o
O 7
~ H-H
Sp N
O
, Q'.-.Q',C7-U' U-U C7-C9
a 01
N
Cla
1
.a C7-C7 H-H U--U H -H
z1 N o
N
.~
U Ch-U' U-U U-U U -U
O ~
~
~
Rf
rtJ ~-~ U-U ~--~ ~ = o
O M
O U-U U-U U-C9 M
O
N
O
d H-H U-U H-H H -H ~r
3
+~
fa
'w H-H H-H ~&-~C c9 -C7
~
.~
~
c6
O U-U H-H C7-C7 c7 -C9
La o
cU
U
U U-U Ch-C7 U-U C7 -C7
.L1 ~
O
N
N 0 H H H
~ 7
n U-U o U -
.. o '
O ~- -C
~
U
N U-U U-U t7-C7 U -C7
O N 0
O
~
rI ~C -~C H H C9 -C9
.1 -H -H
rl m
N
S~ U-U H-H C7-C7 U -U o
1 M
~
~
rtf t7-C7 t9-C7 ~-~ H -H N
N
N
N
f1 U' -C7 FC U A,'-~,' d~
rl -~C -U
~1
W
U U-U ~-~ CU7.--~ H -H
~ o
'
O - N C7-C~ U-U U -U
O
~ ~
a ~ a ~ a
0-~1 ri e-I wl N
N N N
Qa ~; G~ L4
f~la
_ag_
Furthermore, amplified genomic DNA from human
leukocyte nuclei shows that the gene size in the
segment amplified (1 Kb) was substantially larger
than the expected size in the same region in the
porcine gene.
Figure 17 shows identical procedures as outlined
previously for protein expression that All can
increase 12-LO protein expression in human aortic
smooth muscle cells. The increase of expression was
seven fold as measured using a computerized video
densitometric system. Figure 18 shows expression of
12-LO RNA in human vascular smooth muscle cells using
a similar RT-PCR procedure. Lane 5 shows expression
of the expected 333 base pair 12-LO band in human
vascular smooth muscle cells, while lane 3 shows an
identical RNA band in samples taken from mononuclear
cells. Basal expression of 12-LO in unstimulated
smooth muscle cells is below the detection limit of
this experiment (lane 7). However, smooth muscle
cells stimulated by All show a marked increase in
12-LO expression (lane 5). Panel B represents an
ethidium bromide stain of the RT-PCR experiment
showing internal marker RNA (B2 micoglobulin) for
these experiments (lanes 2, 4, 6). Figure 19
illustrates the stimulatory effect of All at 10°9 and
10-8 M on 12-HETE synthesis and release from human
aortic smooth muscle cells. 12-HETE was assayed by
HPLC and specific radioimmunoassay.
These studies confirm that a new gene has been
found in several human tissues which encodes a 12-LO
which is distinct from the one already found in human
platelets. These studies indicate that this 12-LO
gene and protein is present in human adrenal,
mononuclear and aortic smooth muscle cells and that
All markedly upregulates both protein and RNA
_19_
expression of 12-LO in several of these tissues
(Figures 13, 16, 17 and 18).
In further studies using RNA from human aortic
endothelial cells and the RT-PCR procedure described
a leukocyte 12-LO was found to be expressed in these
cells. Figure 21 depicts RT-PCR Southern blot
analysis showing the presence of human leukocyte type
12-LO in human aortic endothelial cells. Lane 1,
cDNA positive control. Lane 2, total RNA from
endothelial cells that have been treated by DNAase
showing that band is not from DNA contamination and
Lane 3 is total RNA from endothelial calls showing
333 base pair product. These results suggest that a
12-LO is expressed in this key vascular wall. Figure
22 depicts the evidence against a 15-LO being
expressed in human aortic endothelial cells. 'Osing
15-LO specific primers and probes (SEQ ID. 1-3
respectively) revealed specific amplification of the
15-LO cDNA used as a template. However, in two
separate experiments no 15-LO RNA band is seen when
RNA from endothelial cells is used. Therefore, only
a leukocyte type of 12-LO is expressed in human
aortic endothelial cells.
The presence of a new form of 12-LO gene in human
tissues has been described. This 12-LO gene appears
to encode a protein which forms active products that
mediate angiotensin II and glucose-induced vascular
and probably renal actions.
No agent has yet been developed with the
indication of blocking the activity or formation of
this 12-LO pathway for prevention of hypertensive and
diabetic vascular and renal disease. Several
compounds are available for in vitro use that do
block 12-LO activity. However, none have been
developed for clinical use.
~20-
Increasing evidence suggests that All and
elevated glucose are each factors involved in
accelerated vascular and renal disease. In
addition, the mechanisms for increased
atherosclerotic cardiovascular and renal disease in
patients with diabetes remains unknown. The
invention described here is apparently the first
therapy designed to reduce or prevent a critical
pathway involved in these disorders.
-21°
REFERENCES
1. Antonipillai, I, et al., J. Endocrinology
125:2028-2034 (1989).
2. Nadler, J.L., et al., J. Clin. Imrest,
80:1763-1769 (1987).
3. Natarajan, R., et al., J. Clin. Endocrinol.
Metabl. 67:584-591 (1988).
4. Stern, N., et al., J. Am. J. Physiol.
257:H434-443 (1989).
5. Nakao, J., et al., Atherosclerosis 44:339-342
(1982).
6. Setty, B,N,Y., et al. J. Clin. Invest. 77:202-211
(1986).
7. Hadjiagapiou, C., et al. ProstaGlandins 31:1136
(1986).
8. Ku, G., et al., Clin. Res. 39:335A (Abstraa~t)
(1991).
9. Glasgow, w.C., et al., Mol. Pharmacol. 38:503-510
(1990).
10. Yoshimoto, T., et al., Proc. Natl. Acad. Sci. USA
87:2142-2146 (1990).
11. Funk, C.D., et al. Proc. Natl. Acad. Sci.
X7:5638-5642 (1990).
12. Sigal, E., et al., Biochem. Bio hys. Res. Commun.
157:457-464 (1988).
13. Natarajan R., et al., Endocrinology September
1992.
14. Tzumi, T., et al., Proc. Natl. Acad, Sci. USA
87:747?-7481 (1990).
15. Tso, J.Y., et al., Nucleic Acid Research
13:2485-2502 (1985).
_22_
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: Jerry L. Nadler
Rama Devi Natarajan
Jiali Gu
(ii) TITLE OF INVENTION: Inhibition of the
Formation or Activity of Human Leukocyte
12-Lipoxygenase Pathway
(iii) NUMBER OF SEQUENCES: &
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: City of Hope
(B) STREET: 1500 East Duarte Road
(C) CITY: Duarte
(D) STATE: California
(E) COUNTRY: United States of America
(F) ZTP: 91010°0269
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: 3M Double Density 5
1j4" diskette
(B) COMPUTER: Wang PC
(C) OPERATING SYSTEM: MS-DOS (R) Version
' 3.30
(D) saFTWARE: Microsoft (R)
(vi) CURRENT APPLICATION DATA:
(A} APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION: Unknown
(vii) PRIOR APPLICATION DATA: None
~~~r~.
°23°
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Irons, Edward S.
(B) REGISTRATTON NUMBER: 16,541
(C) REFERENCE/DOCKET NUMBER: None
(ix) TELECOMMCTNICATTON INFORMAT~:ON:
(A) TELEPHONE: (202) 785°6938
(B) TELEFA?C: (202) 785-5351
(C) TELEX: 440087 LM WSH
(2) INFORMATION FOR SEQ ID NO: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 23
(B) TYPE: Nucleotide
(C) STRANDEDNESS: Single
(vii) IMMEDIATE SOURCE: Synthetically produced
(xi) SEQUENCE DESCRIPTION: SEQ TD NO. 1:
AACTCAAGGT GGAAGTACCG GAG
INFORMATION FOR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 25
(B) TYPE: Nucleotide
(C) STRANDEDNESS: Single
(vii) IMMEDIATE SOURCE: Synthetically produced
(xi) SEQUENCE DESCRIPTION: SEQ ID N0. 2:
ATATAGTTTG GCCCCAGCCA TATTC
_2~_
INFORMATION FOR SEQ ID NO: 3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21
(B) TYPE: Nucleotide
(C) STRANDEDNESS: Single
(vii) IMMEDIATE SOURCE: Synthetically produced
(xi) SEQUENCE DESCRIPTION: SEQ ID NO. 3:
AGGCTCAGGA CGCCGTTGCC C
INFORMATION FOR SEQ ID NO: 4:
( i ) SEQUENCE 'CHARACTERISTICS
(A) LENGTH: 21
(B) TYPE: Nucleotide
(C) STRANDEDNESS: Single
{vii) IMMEDIATE SOURCE: Synthetically produced
{xi) SEQUENCE DESCRIPTION: SEQ ID NO. 4:
TTCAGTGTAG ACGTGTCGGA G
INFORMATION FOR SEQ ID NO: 5:
(i) SEQUENCE CHARACTERISTICS:
(A) LED1GTH: 27
(B) TYPE: Nucleotide
(C) STRANDEDNESS: Single
(vii) IMMEDIATE SOURGE: Synthetically produced
(xi) SEQUENCE DESCRIPTION: SEQ ID NO. 5:
ATGTATGCCG GTGCTGGCTA TATTTAG
_25_
INFORMATION FOR SEQ ID NO: 6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22
(B) TYPE: Nucleotide
(C) STRANDEDNESS: Single
(vii) IMMEDIATE SOURCE: Synthetically produced
(xi) SEQUENCE DESCRIPTION: SEQ ID NO. 5:
TCAGGATGCG GTCGCCCTCC AC
INFORMATION FOR SEQ ID NO: 7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 239
(B) TYPE: Nucleotide
(C) STRANDEDNESS: Single
(vii) IMMEDIATE SOURCE: Synthetically produced
(xi) SEQUENCE DESCRIPTION: SEQ ID NO. 7:
AAACGGCACC TCCTTCAGGA TGACGCGTGG TTCTGCAATT GGATCTCCGT
GCAGGGTCCG GGAGCAAACG GGG,ACGAGTT CAGGTTCCCC TGCTACCGCT
GGGTGGAGGG CGACCGCATC CTGAGCCTCC CTGAGGGCAC TGCCCGCACA
GTGGTCGATG ACCCTCAAGG CCTGTTCAAG AAACACAGGG AGGAGGAGCT
GGCAGAGAGA AGGAAGCTGT ATCGGTGGGG TAACTGGAA
INFORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 239
(B) TYPE: Nucleotide
(c) STRANDEDNESS: single
-26°
(vii) TMMEDIATE SOURCEc Synthetically produced
(xi) SEQUENCE DESCRTPTIONs SEQ ID NO. Sa
AAACGGCACC TCCTTCAGGA TGACGCGTGG TTCTGCAATT GGATCTCCGT
GCAGGGCCCG GGAGCAAATG GGGACGAGTT CAGGTTCCCC TGCTACCGCT
GGGTGGAGGG CGACCGCATC CTGAGCCTCC CTGAGGGCAC TGCCCGGACA
GTGGTCGATG ACCCTCAAGG CCTGTTCAAG AAACACAGGG AGGAGGAGCT
GGCAGAGAGA AGGAAGCTGT ATCGGTGGGG TAACTGGAA