Note: Descriptions are shown in the official language in which they were submitted.
2~~~~~~
~~O 91/13949 ~ PCT/US91/01332
Descrio~ion
Motor Fuel Additive Composition
and Method for Preparation Thereof
Background of the Invention
.5 This invention relates to a motor fuel additive
composition and a method of preparing such an
additive. More particularly, this invention relates
to a motor fuel additive composition comprising:
(a) a detergent component selected from the group
consisting of (i) at least one nonionic compound
having a molecular weight in the range of 200-1500;,
(ii) a reaction product component which is the ~ .
reaction product of a substituted hydrocarbon and an
amino compound, and (iii) a polybutylamine or
polyisobutylamine; and (b) a fuel conditioner
component comprising (i) a polar oxygenated
hydrocarbon compound, and (ii) an oxygenated
compatibilizing agent. This invention also relates to
a method of preparing a motor fuel additive which
comprises mixing the above-described reaction produc t
and fuel conditioner components.
Incomplete combustion of hydrocarbonaceous motor
fuels in an internal combustion engine is a common
problem which generally results in the formation and
accumulation of carbon and other deposits in various
places, including the fuel inlet system. Significant
efforts have previously been undertaken to develop
fuel additives to reduce or inhibit deposit formation
in the engine fuel inlet system. Early so called
30. "first generation" additives directed primarily to
cleaning carburetors and injectors include low
molecular weight amine derivatives such as fatty
amines, amides, amido amines and imidazolines. Later
developed so-called "second generation" additives,
directed to cleaning inlet valves as well as
~~'~'~~~~i
WO 91/13949 2 PCT/LJS91/01332 -
carburetors and injectors, have been based primarily
on polyolefinic structures, typically polyisobutenes
and their derivatives. For example, the use of
polybutene succinimides as fuel additives has been
disclosed in U.S. Pat. No. 3,443,918 (Kautsky et al.)
and U.S. Pat. No. 3,172,892 (LeSeur et al.); the use'
of polybutene amines as fuel additives has been
disclosed in U.S. Pat. No. 3,438,757 (Honnen et al.).
For effective deposit control, it has been
customary to use such additives in conjunction with
petroleum based or synthetic carrier oils. Petroleum
based oils useful in this respect include naphthenic
and paraffinic base stock oils of relatively high
viscosity, including so-called Solvent Neutral Oils
such as SNO-500 and SNO-600, as well as so-called top
cylinder oils and the like. Synthetic oils which have
been employed include low molecular weight
polypropylenes and polyisobutylenes, as well as
polyalkyleneoxides.
Although the above-described additives have been
found effective in reducing deposits in the fuel
intake system, the increased use of these additives,
particularly the second generation additives, in motor
fuels has been found to have led to an increase in
combustion chamber deposit formation. The presence of
deposits in the combustion chamber seriously reduces
engine operating efficiency for several reasons.
First, deposit accumulation within the combustion
chamber inhibits heat transfer between the chamber and
the engine cooling system. This leads to higher
temperatures within the combustion chamber, resulting
in increases in the end gas temperature of the
incoming charge. Consequently, end gas auto-ignition
occurs, which causes engine knock. In addition, the
accumulation of deposits within the combustion chamber
reduces the volume of the combustion zone, causing a
higher than design compression ratio in the engine.
This, in turn, also results in serious engine
~~~'~r~ ~~~a
'O 91 /13949 3 PCT/US91 /01332
knocking. A knocking engine does not effectively
utilize the energy of combustion. Moreover, a
prolonged period of engine knocking will cause stress
fatigue and wear in vital parts of the engine. The
above-described phenomenon is characteristic of
gasoline powered engines. It is usually overcome by
employing a higher octane gasoline for powering the
engine, and hence has become known as engine octane
requirement increase (ORI) phenomenon. ~.
In view of the foregoing, it would clearly be
advantageous to employ an additive in motor fuel
compositions which reduces deposits in engine fuel
intake systems and also avoids the formation of
deposits in engine combustion chambers, thereby
reducing or at least modifying the composition of
deposits which tend to cause engine ORI. It is an
object of this invention to provide a motor fuel
additive which is useful in preventing both fuel
intake system deposit formation and combustion chamber
deposit formation. It is a feature of this invention
that the additive comprises a detergent component and
a fuel conditioner component which synergistically
interact to reduce both fuel intake system and
combustion chamber deposit formation. It is an
advantage of this invention that it both reduces
deposit formation in engine fuel intake systems and
ORI associated with combustion chamber deposit
formation.
It is another object of this invention to provide
a method for preparing a motor fuel additive which
reduces deposits in engine fuel intake systems and
also reduces the formation of deposits in engine
combustion chambers, thereby reducing engine ORI. It
is another feature of this invention that such an
additive is prepared by mixing a detergent componen t
and a fuel conditioner component which synergistically
interact to reduce both fuel intake system deposit
~~'~'~b~~
WO 91/13949 4 PCT/US91/01332
formation and ORI associated with combustion chamber
deposit formation.
Summary of the Invention
The motor fuel additive composition of this
invention comprises a mixture of:
(a) from 5-50 weight percent, based upon
the total weight of the additive, of a detergent
component selected from the group consisting of
(i) at least one nonionic compound
having a molecular weight in the range of 200-1500,
(ii) a reaction product of:
(A) a substituted hydrocarbon of
the formula
R1 - X (I)
wherein R 1 is a hydrocarbyl radical having a
molecular weight in the range of 150-10,000, and X is
selected from the group consisting of halogens,
succinic anhydride and succinic dibasic acid, and
(B) an amino compound of the
formula
H - (NH - (A)m)n - Y - R2 (II)
wherein Y is O or NRS, R5 being H or a hydrocarbyl
radical having 1-30 carbon atoms; A is a straight
chain or branched chain alkylene radical having 1-30
carbon atoms; m has a value in the range of 1-15; n
has a value in the range of 0-6; and R2 is selected
from the group consisting of H, a hydrocarbyl radical
having a molecular weight in the range of 15-10,000,
and a homopolymeric or heteropolymeric polyoxyalkylene
radical of the formula
R3 - ((Q)a(T)b(Z)c)d- (III)
wherein R3 is H or a hydrocarbyl radical having 1-30
carbon atoms, Q, T, and Z are polyoxyalkylene moieties
having 1-6 carbon atoms, a. b and c each have values
ranging from 0-30, and d has a value in the range of
1-50, and
~O 91/13949 5 PC?/US91/01332
(iii) a polybutylamine or
polyisobutylamine of the formula
~R12
R11 - CH2 - N~ R ( IV )
13
where R11 is a polybutyl or polyisobutyi radical
derived from isobutene and up to 20o by weight of n-
butene and R12 and R13 are identical or different and
are each hydrogen, an aliphatic or aromatic
hydrocarbon, a primary or secondary, aromatic or
aliphatic aminoalkylene radical or polyaminoalkylene
radical, a polyoxyalkylene radical or a hetaryl or
heterocyclyl radical, or, together with the nitrogen
atom to which they are bonded, form a ring in which
further hetero atoms may be present; and
(b) a fuel conditioner component
comprising:
(i) from 2-50 weight percent, based
upon the total weight of the additive, of a polar
oxygenated hydrocarbon having an average molecular
weight in the range of 250-500, an acid number in the
range of 25-175, and a saponification number in the
range of about 30-250, and
(ii) from 2-50 weight percent, based
upon the total of the additive, of an oxygenated
compatibilizing agent having a solubility parameter in
the range of about 8.8-11.5 and moderate to strong
hydrogen-bonding capacity.
The fuel conditioner component may additionally
comprise a hydrophilic separant such as a glycol
monoether, and an aromatic hydrocarbon such as xylene
or a xylene. The additive composition may
additionally comprise a carrier oil or fluidizer.
This invention is also directed to a method of
preparing the motor fuel additive of this invention,
which comprises mixing the detergent and fuel
conditioner components to obtain the additive. The
motor fuel additive of this invention is advantageous
in that the detergent and fuel conditioner components
t
WO 91/13949 6 PCT/US91/01332
synergistically interact when employed in a fuel
composition to reduce both fuel intake system deposit
formation, thereby improving engine performance, and
combustion chamber deposit formation, thereby reducing
engine ORI.
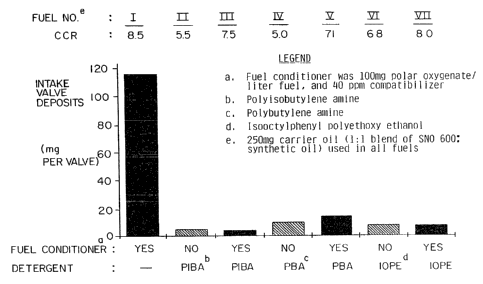
Brief Description of the Drawings
Figure 1 depicts the results of engine test stand
experiments for various motor fuel compositions,
including motor fuel compositions containing the
additive of this invention.
Figure 2 depicts the results of the engine test
stand experiments set forth in Figure 1 for various
motor fuel compositions, including motor fuel
compositions containing the additive of this
invention, as a plot of Combustion Chamber Rating vs.
Intake Valve Deposits (mg).
Description of the Preferred Embodiments
This invention is directed to a motor fuel
additive and to a method for the preparation thereof.
The additive comprises: (a) a detergent component
which is selected from the group consisting of (i) at
least one nonionic compound having a molecular weight
in the range of 200-1500, (ii) the reaction product of
a substituted hydrocarbon and an amino compound,
(iii) a polybutylamine or polyisobutylamine; and (b) a
fuel conditioner component comprising a polar
oxygenated hydrocarbon compound and an oxygenated
compatibilizing agent.
The nonionic compound detergent component, if
employed, is preferably an alkylaryl ether alcohol or
alkylaryl polyether alcohol having a molecular weight
in the range of 200-1500. In a particularly preferred
embodiment, the nonionic detergent compound is an
octylphenyl polyether alcohol or nonylphenyl polyether
alcohol containing 1-10 ethylene oxide moieties.
O 91/13949 ~ PCT/US91/01332
If the reaction product detergent component is
employed, the substituted hydrocarbon reactant used to
prepare the reaction product is of the formula
R1 - X (I)
wherein R1 is a hydrocarbyl radical having a molecular
weight in the range of 150-10,000, preferably a
polyalkylene radical having a molecular weight in the
range of 400-5000, most preferably a polyalkylene
radical having a molecular weight in the range of
600-1500, and X is selected from the group consisting
of halogens, preferably chlorine, succinic anhydride
and succinic dibasic acid. In one preferred
embodiment, R1-X is a polyisobutenyl succinic
anhydride. In another preferred embodiment, R1-X ~s a
chloropolyisobutylene.
The amino compound reactant used to prepared the
reaction product is of the formula
H - (NH - (A)m)n - Y - R2 (II)
wherein Y is 0 or NRS, where R5 is H or a hydrocarbyl
radical having 1-30, preferably 1-22 carbon atoms, A
is a straight chain or branched chain alkylene radical
having 1-30, preferably 1-15 carbon atoms, m has a
value in the range of 1-15, preferably 1-12, n has a
value in the range of 0-6, preferably 0-5, and R2 is
selected from the group consisting of H, a hydrocarbyl
radical having a molecular weight in the range of 15-
10,000, preferably 15-2000, and a homopolymeric or
heteropolymeric polyoxyalkylene radical of the formula
R3 - ((Q)a(T)b(Z)c)d- (III)
wherein R3 is H or a hydrocarbyl radical having 1-30,
preferably 1-22 carbon atoms. Q. T, and Z are
polyoxyalkylene moieties having 1-6 carbon atoms, a,
b, and c each have values ranging from 0-30, and d has
a value in the range of 1-50, preferably 1-25.
WO 91/13949 -8- PCT/US91/01332
Various preferred embodiments of the amino
compound reactant of formula (II) are given in Table 1
below:
Table 1
1. A=CH , m=2, n=3, y=NR , R =H, R =H yields an amino
compound reactant of ~he ~ormuld~: .
NH2 - (CH2)2 - NH - (CH2)2 - NH - (CH2)2 - NH2
2. A=CH , m=3, n=1, y=NR , R =H, R =oleyl radical
yields an amino compound reactant of the formula:
NH2 - (CH2)3 - NH - oleyl
3. A=CH , m=6, n=1, y=NR , R =H, R =H yields an amino
compound reactant of ~he i~ormuld~:
NH2 - (CH2)6 - NH2
4. A=CH , m=12, n=1, y=NR , R5=H, R =H yields an
amin~S compound reactant of the f~Srmula:
NH2 - (CH2)12 - NH2
CH3
5. A=(CH ) - CH - (CH ) , m=1, n=1, y=NRS, R =H,
R =H fields an amine L~ompound reactant of the
formula:
CH3
NH2 - (CH2)2 - CH - (CH2)3 - NH2
' iH3 iH3
6. A = (CH ) - CH - CH - CH - CH , m=1, n=1, y=NR ,
R =H, R2=~i yields an2amino compound reactant of 5
tt~e for~lula
iH3 iH3
NH2 - (CH2)2 - CH - CH2 - CH - CH2 - NH2
O 91/13949 9 PCT/US91/01332
In another preferred embodiment, R2 is the
above-described homopolymeric or heteropolymeric
polyoxyalkylene radical of formula (III). As used in
this description and in the appended claims, the terms
homopolymeric and heteropolymeric refer to
polyoxyalkylene compounds, which in the case of
homopolymeric compounds contain one recurring
polyoxyalkylene moiety. and in the case of
heteropolymeric compounds contain more than one
recurring polyoxyalkylene moiety, typically having 1-6
carbon atoms, such as ethylene oxide (E0), propylene
oxide (PO) or butylene oxide (BO). Thus, for example,
in one embodiment R2 may be a homopolymeric
polyoxyalkylene radical of the formula
R3 - ((EO))d-
wherein in formula (III), a=1, b=0, c=0, Q=ethylene
oxide, and R3 and d are as previously defined. In
another embodiment, R2 may be a heteropolymeric
polyoxyalkylene radical of the formula
R3 - ((EO)a (PO)b (BO)c)d-
wherein, in formula III, Q=ethylene oxide, T=propylene
oxide, Z=butylene oxide, and a, b, c, d and R3 are as
previously described.
In yet another preferred embodiment, the above-
described amino compound reactant is selected from the
group consisting of polyethylene polyamines,
polypropylene polyamines and mixtures thereof. In yet
another preferred embodiment, such polyamines are
monoalkylated.
The reaction product component is preferably
prepared by reacting the substituted hydrocarbon R1-X
to the amino compound in a mole ratio in the range of
0.2:1 - 20:1, more preferably in the range of 0.5:1 -
10:1. The reaction product component may be prepared
under reaction conditions (including e.g. reaction
times, temperatures, and reagent proportions) as are
well known by those skilled in the art for preparing
such amino compound-substituted hydrocarbon reaction
CA 02077666 2002-05-06
68086-563
products. the method for psepasing such reaction
products is described. For example. in U.S. Pat. No.
3,17x.892 (LeBeur et al.). ~l.S. Pat. No. 3.438.'157
(Honnen et a3..),~and U.S. pat. No. 3,443,918 (Kautsky
5 et al. ).
The detergent compound may sled be a
po7.ybutylamine or polyisobutylaatine of the Formula
R '
Rll - C~~ - N ~ iZ (IV)
1Q R13
where Rll is a polybutyl- or polyisobutyl radical
derived from isobutene and up to 20% by weight of n-
butene, acid RlZ and R13 are identical or different and
are each hydrogen, an aliphatic or aromatic ~ '
hydrocarbon, a primary or.secondary, aromatic-or
aliphatic aminoalkylene radical or polyaminoaikylene
radicalr a polyoxyalkylene 'radical or a hetaryl or
heterocyclyi radical, or, together with the nitrogen
atom to which they are bonded, form a ring in which
further hetero stoma may be present.
Compounds of the general formula (IVY and the
method of preparation thereof are disclosed, for
example, in U.S. pat. No. 4,832,702 (Kumrner et al.),
incorporated herein by reference. Compounds of the
Z5 general formula-(IV) are preferably prepared in
accordance with the method disclosed in U.S. Pat. No.
- 4,832.702, wherein an appropriate pOlybutene or
polyisobutene ie hydroforroylated with a rhodium or
cobalt catalyst in the presence of CO and I12 at from
about 80-200°C and CO/H2 pressures of up to 600 bar,
and the oxo product thereby formed is then subjected
to a Mannich.reaation or amination under hydrogetlating
conditions, wherein the 4tmination reaction is
advantageously~carriQd out at 80-200°C and under
pressures up to 600 bar, preferably 80-300 bar.
The fuel conditioner component employed in
admixture with the detergent component to proc9uce the
additive of this invention may preferably be the fuel
CA 02077666 2002-05-06
68086-563
11
conditioner previously disclosed in U.S. pat. No.
4,753,661 (Nelsa» et a1.).
This fuel conditioner comprises a polar
oxygenated hydrocarbon compound and an oxygenated
aompatibilixing agent.
The polar oxygenated hydrocarbon portion of the
fuel conditioner signifies various organic mixtures
arising from the controlled oxidation of petroleum
liquids with air. Often these air oxidatiorts of
liquid distillates.are carried out at a temperature of
from about 100°C to about 150°C with an organo-
metallic catalyst, such as esters of manganese,
copper, iron, cobalt, nickel or tin, or organic
catalysts, such as tertiary butyl peroxide. The
result is a melange of polar oxygenated. Compounds
which may be divided into at least three categories:
volatile, saponifiable and non-saponifiable.
The polar oxygenated compounds preferable for use
in the present inventiop may be characterized in a
least three ways, by molecular weight, acid number,
and saponification number. Chemically these axidatio»
products are mixtures of acids, hydroxy acids,
lactones, esters, keto»es, alcahois, anhydrides, and
other oxygenated organic coritpounds. Those suitable
for the present invention are compounds and mixtures
with an average molecular weight between about 250 and
500. With an acid number between about x5 and about
1Z5 (ASTM-D-974), and a sapanification number from
about 30 to about 250 (ASTM-a-974-5Z). Preferably the
polar oxygenated cotxpounds of the present invention
have an acid number from about 50 to about 100 and a
saponification number from about 75 to about 200. An
Gxa~tple of a polar oxygenated hydrocarbon within this
preferred range is ALpX~"400L (Alox Corporation,
Niagara Falls. New Yoxk).
Suitable cotnpatibilizing agents for use i» the
fuel conditioner component of the instant invention
are organic compounds of moderate solubility parameter
2Q'~'~666
WO 91/13949 12 PCT/US91/01332
and moderate to strong hydrogen-bonding capacity.
Solubility parameters, d, based on cohesive energy
density are a fundamental descriptor of an organic
solvent giving a measure of its polarity. Simple
aliphatic molecules of low polarity have a low d of
about 7.3; highly polar water has a high d of 23.4.
Solubility parameters, however, are just a first
approximation to the polarity of an organic solvent.
Also important to generalized polarity, and hence
solvent°power, are dipole moment and hydrogen-bonding
capacity: Symmetrical carbon tetrachloride and some
aromatics~with low gross dipole moment and poor
hydrogen-bonding capacity have a solubility parameter
of about 8.5.' In contrast, methyl propyl ketone has
almost the same, solubility parameter, 8.7, but quite
strong hydrogen-bonding capacity and a definite dipole
moment. Thus, no one figure of merit describes the
"polarity" of an organic solvent.
For the practice of the present invention a
compatibilizing agent should have a solubility
parameter from about 8.8 to about 11.5 and moderate to
strong hydrogen-bonding capacity. Suitable classes of
organic solvents are alcohols, ketones, esters, and
ethers. Preferred compatibilizing agents are
straight-chain, branched-chain, and alicyclic alcohols
with from six to 14 carbon atoms. Especially
preferred compounds for compatibilizing agents are the
hexanols, the heptanols, the octanols, the nonyl
alcohols, the decanols, and the dodecanols.
The fuel conditioner component of this invention
may additionally include a hydrophilic separant which
decreases the amount of water in the hydrocarbon fuel,
thus improving combustion. Suitable separants for
practicing the current invention are ethers of glycols
or polyglycols, especially monoethers. Monoethers are
preferred over diethers in the practice of the present
invention.
CA 02077666 2002-05-06
68086-563
13
Examples of such compounds which may be used are
the ma»oethers of ethyle»e glycol, propylene glycol,
trimethylene glycol, alphabutylane glycol, I,3-
butanediol, beta-butylene glycol, isobutylene giycal,
tetramethylenc glycol. hexyiane glycol, diethylene
glycol, dipropylene glycol, tripropylene glycol,
triethylene glycol, tetraethylene glycol, 1,5-
pentanediol, 2-methyl-2-ethyl-1,3-propanedial, 2-
ethyl-1,3-hexanediol. ,Some monoethers include
ethylene glycol monophenyl ether, ethylene glycol
monomethylether, ethylene glycol monoethyl ether,
ethylene glycol mono-(n-butyl) ether, diethylene
glycol monomethyl ether. diethylene glycol monoethyi
ether, diethylene glycol mo»o-(n-butyl) ether.
propylene glycol monomethyi ether, dipropylene glycol.
monomethyl ether, diethylene glyCOl
monocyclohexylether, ethylene glycol monobenzyl ether,
triethylene glycol monophenathyl ether, butylene
glycol mono-(p~(n-butoxy) phenyl) ether, trimethylene
glycol rnono(a~lkylphenyl) ether, tripropylene glycol
monomethyl ether, ethylene glycol mono-isopropyl
ether, ethylene glycol rnonoisobutyl ether, ethylene
glycol monohexyl ether, triethylene glycol monobutyl
ether, triethylene glycol m»vnomethyl ether,
triethylena glyCO1 monoethyl ether, 1-butaxyethoxy-2-
propanol, aaonophenyl ether of polypropylene glycol
having an average molecular weight of about 975 to
1075..and monophenyl ether of polypropylene glycol
wherein the polyglycol has s average raolecular weight
of about 4p0 to 450, monophenyl ether of polypropylene
glycol wherein the polypropylene glycol has an average
molecular weight of 975 to 3075. Such compounds are
sold commercially undor trade names such as Butyl
CELLOSOLVE; ethyl CELL060LVE; xexyi CELLOSOLVE:" Methyl
CARBITOL'; Butyl CAFIEITOL~; DOWANOL~"Glyaol ethers, and
tho like.
In the practice of the current invention. it has
been found useful to include an aromatic hydrocarbon,
WO 91/13949 14 PCT/US91/01332
or a mixture of such, in the fuel conditioner
component of the present invention. Any aromatic
hydrocarbon blend that is liquid at room temperature
is suitable. Among these are benzene, toluene, the
three xylenes, trimethylbenzene, durene, ethylbenze'ne,
cumene, biphenyl, dibenzyl and the like or their
mixtures. The preferred aromatic constituent is a
commercial mixture of the three xylenes, because it is
cheaper than any pure xylene. As used in this
description and in the appended claim, the word
"xylene" means not only the three specific xylene
compounds o-xylene, m-xylene and p-xylene, but also
includes aromatic "cuts" or distillates of aromatic
hydrocarbons containing not only xylene but benzene,
toluene, durene and naphthalene which may be mixed in
the xylene. Aromatic naphthas are also useful.
Without being limited to any theory or hypotheses for
the use of an aromatic hydrocarbon, it has been found
that the presence of an aromatic hydrocarbon in the
conditioner promotes clean and efficient combustion cf
the fuel.
The composition of this invention may
additionally comprise a suitable amount of a carrier
oil or fluidizer selected from the group consisting of
petroleum-based oils, mineral oils, polypropylene
compounds having a molecular weight in the range of
500-3000, polyisobutylene compounds having a molecular
weight in the range of 500-3000, polyoxyalkylene
compounds having a molecular weight in the range of
500-3000, and polybutyl and polyisobutyl alcohols
containing polybutyl or polyisobutyl radicals derived
from polyisobutene and up to 20~ by weight of n-
butene, corresponding carboxylates of the polybutyl or
polyisobutyl alcohol, and mixtures thereof. Petroleum
based oils which may be employed include top cylinder
oils as well as both natural and synthetic napthenic
and paraffinic base stock oils of relatively high
viscosity, including so-called Solvent Neutral Oils
CA 02077666 2002-05-06
68086-563
(SNO) such as ENO-500 and SNO-60o. Mineral. ails which
may be emp3.oyed include so-called "light" mineral
oils, i.e. those petrolQUm, aliphatic or alicyclic
fractions having a viscosity less than 10,000 SUS at
S 25°C. A mixture of hydrocarbon fractions may also be
employed in place of a base stock. Tho above-
described polybutpl and pelyisobutyl alcohols include
those disclosed in Q.S. Pat. No. 4,859,210 (Franc et
a1.). As used in
10 this description and in the appended ciaiars, the terms
"carrier oil" aad "tluidixer" are interchangeable, as
will be readily understood by those skilled in the
art.
Given the presence of the many constituents
15 described above, a wide var~ety of proportions are
suitable for the additive composition of this
invention. Below a "Useful Range" and a "Preferred
Range" are given in weight percent, based upon the
total weight of the additive compositions
xo
Peeferred
continent UsefuiRanae ~anae
Detergent Component 5-50 10-30
polar Oxygenated Compound z-50 5-25
Coatpatibili~ting Agent 2-50 5-25
Hydrophilic Separant 0-40 0-30
Arotaatic ftydrocarbon o-80 0-30
Carrier 031 0-80 0-6o
The additive composition of this invention may be
employed in a wide variety of hydrocarbon or modified
hydrocarbon (e.g. alcohol-containing) fuels for a
variety of engines. Preferred motor fuel compositions
for use with the additive composition of this
invention are those intended for use iri spark ignition
internal combustion engines. Such motor fuel
compositions, generally referred to as gasoline base
stocks. preferably comprise a mixture of hydrocarbons
boiling in the gasoline boiling range, preferably from
WO 91/13949 -16- PCT/US91/01332
about 90-450°F. This base fuel may consist of
straight chains, branch chains, paraffins,
cycloparaffins, olefins, aromatic hydrocarbons, and
mixtures thereof. The base fuel may be derived from,
among others, straight run naptha, polymer gasoline,
natural gasoline, or from catalytically cracked or
thermally cracked hydrocarbons and catalytically
reformed stock. The fuel may also contain synthetic
hydrocarbons, ethers such as methyl tertiary butyl
ether (MTBE), ethyl tertiary butyl ether (ETBE) and
the like, alcohols such as methanol, ethanol, TBA and
the like, and other functional organic compounds such
as ketones, esters and the like. The composition and
octane level of the base fuel are not critical and any
conventional motor base fuel may be employed in the
practice of this invention. In addition, the motor
fuel composition may additionally comprise other
additives typically employed in motor fuels, such as
anti-knock compounds (e. g. tetraethyl lead), anti-
icing additives, upper cylinder lubricating oils,
carburetor detergents, anti-corrosion additives, de-
emulsifying agents, odor suppressors, and the like.
Having described this invention above, it is now
illustrated in the following examples. These
examples, however, do not limit the application of
this invention, which may be carried out by other
means in other systems.
Example 1
(Comparative Example)
Two automobiles (a Ford Escort and a Chevrolet
Cavalier) were driven for 7000 miles (30% town
driving, 70~ highway driving) using the same unleaded
base gasoline containing no detergent additives, the
gasoline having a (RON + MON)/2 octane number - 87.
Before beginning the test, the engines were
disassembled and the combustion chambers thereof were
NO 91/13949 17 PCf/US91/01332
completely cleaned. They were then reassembled with
new spark plugs and new valves.
After the 7000 mile driving test the engines were
disassembled again and the deposits accumulated on the
tulip of the inlet valves and in the combustion
chamber were collected and weighed. The results are
summarized below:
Car 1 Car 2
Automobile Make/Model Ford Escort Chevrolet Cavalier
Cylinders I-4 V-6
Injector FI PFI
Inlet Valve 1.1 1.35
deposits (g)*
Combustion Chamber 0.95 1.1
deposits (g)*
* per unit
Example 2
(Comparative Example)
The identical test performed in Example 1 was
repeated except that each vehicle used an unleaded
base fuel containing a different commercially
available fuel detergent additive package at its
recommended level. After 7000 miles, the following
results were obtained:
20~~~~
WO 91/i3949 18 PCT/US91/01332
Car 1 Car 2
Additive Type A* B**
Inlet Valve 0.5 <0.1
deposits (g)
Combustion Chamber 1.45 1.7
deposits (g)
* Additive A = 250 ppm of a polybutene succinimide type
additive*** + 500 ppm SNO 600.
** Additive B = 350 ppm of a polybutene amino type
additive*** + 600 ppm top cylinder oil.
*** Reaction products of a substituted hydrocarbon and an
amino compound.
The results above indicate that although
commercial additives A and B improved inlet valve
cleanliness in comparison with Example 1, combustion
chamber deposit formation actually increased, thereby
detrimentally enhancing engine ORI.
Example 3
(Comparative Example)
The identical test performed in Example 1 was
run, except that each vehicle used an unleaded base
fuel containing 500 ppm of the fuel conditioner
component of this invention, as disclosed in U.S. Pat.
No. 4,753,661 and available from Polar Molecular Corp.
(Saginaw, Mi.) under the DurAlt Fuel Conditioner trade
name.* The following results were obtained:
CA 02077666 2002-05-06
68086-563
19
Car ~1 C,ar ~2
Inlet Valve 1.27 i.45
deposits t91
Co~abustion Chamber 0.48 0.5
deposits (g)
* Contained 30t (by weight of fuel Conditioner) ,
active polar oxygeri~ted compound.
example 4
1p jInventianl
The identical test performed in Example 1 was
run, except that each vehicle used an unleaded base
fuel containing its respective atdditive (A or 8? as~~in
Example 2 plus 50G pit of the fuel conditioner
Component of this invention sv~tilable from Polar
Molecular Corp. (Saginaw, Mi.) under the DurAlt Fuel
Conditioner trade name. The following results were
obtained:
~'ar ~1 fir- ~2
ZO Additive 'type ' A+500ppm DurAlt B+500ppm DurAlt
Inlet Valve 0.35 c0.1
deposits (g)
Combustion Chambex 0.45 0.4
a~pog~ts t9)
The results above show that the additive
composition of this invention, when employed in a
motor fuel comgosition, reduces both inlet valve
deposits and combustion chamber deposits, arid hence
tends to reduce~engine ORI. These results are
30~ unexpected, in that the use of additives A and H
without the DurAlt~"fuel conditioner (as in Example 2)
showed touch greater (i.e. 3-4 times greater)
combustion chamber deposit formation, and hence
~~7~~
WO 91 / 13949 2 0 PCT/US91 /01332
greater ORI tendencies. Thus, the combination of the
detergent and fuel conditioner components in the
additive composition of this invention synergistically
acts to reduce both intake valve deposit formation and
combustion chamber deposit formation.
Example 5
(Invention)
The identical test performed in Example 1 was
run, except that each vehicle used an unleaded base
fuel containing its respective additive (A or B) as in
Example 2 plus 300 pm and 100 ppm, respectively, of
the DurAlt fuel conditioner. The following results
were obtained:
Car 1 Car 2
Additive Type A+300ppm DurAlt B+100ppm DurAlt
Inlet Valve 0.37 <0.1
deposits (g)
Combustion Chamber 0.40 0.85
deposits (g)
The results above again show that the additive
composition of this invention, when employed in a
motor fuel composition, reduces both inlet valve
deposits~and combustion chamber deposits, and hence
tends to reduce engine ORI. These results are again
unexpected, in that the use of additives A and B
without the DurAlt fuel conditioner (as in Example 2)
showed much greater (i.e. 2-3 times greater)
combustion chamber deposit formation, and hence
greater ORI tendencies. Thus, the combination of the
detergent and fuel conditioner components in the
additive composition of this invention synergistically
acts to reduce both intake valve deposit formation and
combustion chamber deposit formation.
CA 02077666 2002-05-06
68086-563
21
Example ,ø
( Comoa,~~~~ve Example 1
The identical test performed in Example 1 was run
for Car ~2, except that Car ~z used an unleaded base
fuel having additive package R plus 500 ppm of a
commercial fuel additive as disclosed in U.S. Patent
Nv. 4,548,614 (Sung et al.), the additive being a poly
(oxyethylene)(oxypropylene) polyol. Such additives
are available from sA6F Wyandotte Corp, under the
PLUitONIC series trade name. The following results
were obtained:
Additive Type B+500 ppm PLURONIC L-31*
Inlet Valve X0.1
I5 deposits (g)
Combustion Chamber 1.z
deposits (g)
x PLURONIC L-31, a product of HASP Wyandotte Corp.,
is a poly (oxyethylene)-poly (oxypropylene)-poly
(oxyethyle~le) polyol having a molecular weight of
about 950 containing about 10 wt. % derived from
poly (oxyethylene) and about 90% derived from poly
(oxypropylene).
The above results show that a combination of
additive package B plus poly (oxyethylene) poly
(oxypropylene) polyol (PLURONICTML-31.) is less
effective in controlling combustion chamber deposit
foriaation, and hence engine ORI, than the additive of
this invention. as exemplified iri Examples 4 ~cnd 5.
~x~,mnle 7
i invention
A Honda Accord, I-4 engine with 2-barrel
carburetor a»d mileage of 44,550 miles, run primarily
on leaded gasoline containing commercially available
CA 02077666 2002-05-06
68086-563
22
additives, was found to have knocking problems. This
vehicle was then run for 2000 miles on a fuel
containing an additive composition of this invention,
namely.an additive composition comprising additive H '
as set forth in Example 2 (i.e. 35o~ppm polybutene
amino type additive and 600 ppm top cylinder oil) + .
500 ppm~of DurAlt FC. The knocking problems totally
disappeared, thus showing-a clear reduction in engine '
ORI for this vehicle. .This eXample illustrates the
utility of this invention in so-called "clean-up"
applications, ~herein,use of the invention improves
performance of engines which have already demonstrated
engine ORI.
Additional experimental results were obtai~sd
using a ES6500 Honda generator engine testing sysxem.~
Two identical engine systems having identical loading
(1500 and X500 watt electrical resistance hot water
heaters) were employed, in accordance with the testing
procedure set forth in M. Megnin et al., "development'
ZO of a Gasoline Additive Screening Test for Intake Valve
Stickiness and Deposit Levels,".S~ Paper No: 892121
(presented at the Int'1 Fuels and Lubricants Meeting,
Baltimore, Md. Sept. 1989),,whiah was modified as follows.
The engine was prepared for testing by first
being disassembled. The intake valves were then
cleaned with gum solo~nt consisting of a mixture of
1/3 acetone. 1/3 toluene, and 1/3 methanol to remove
any lube oil on the valves. The valves were then
stored in a desiccator for at least one hour, and
thereafter weighed. to the nearest 0.1 mg, just prior
to engine assembly. The co~abustion chambers and parts
were cleaned with a suitable wire brush, as ware the
tops of the pistons. The clean cylinder heads were
reassembled and installed onto the Qngine, and the
remainder of the engine was reassembled. The ail and
oil filter were replaced prier to testing.
J 91/13949 2 3 PCT/US91 /01332
The fuel composition to be tested was prepared
and poured into the fuel tank. The engine was started
and allowed to idle for 30 second to warm up. The
engine was then allowed to run under a 1500 watt load
for two hours. At the end of the two hour period, the
generator load, coolant in/out temperature, oil
temperature, exhaust temperature for cylinders 1 and
2, and manifold vacuum were recorded. The engine was
then run for an additional two hour period under a
2500 watt load. At the end of this two hour period,
the above-described data were again recorded. The
above-described four hour test run procedure was
repeated (with intervening refueling) for 16 hours of
engine running per day. for five consecutive, days for
a total of 80 hours of engine running. At the end of
the 80 hour period, the generator fuel tank was
drained and added to the remaining fuel mix for the
run. The total volume of fuel remaining was measured
to calculate the amount of fuel used during the 80
hour run.
After completion of the 80 hour run, the engine
was disassembled, including removal of the cylinder
head, cam shaft, and rocker arm assembly. The amount
of deposits on the intake system, consisting of the
carburetor throttle plate, intake manifold, head
runners, head ports, and intake valves were rated
using the method described by Coordinating Research
Council (CRC) Rating Manual No. 16, Atlanta, Ga. 1987
("CRC Rating"), which is well known to those skilled
in the art. The combustion chamber and piston tops
were similarly rated using the CRC Rating system. The
sequence for rating, valve weighing, and photographing
was as follows: the piston tops, cylinder head,
combustion chamber, head runners, intake manifold, and
throttle plate were all initially rated, and
photographed. The combustion side of the intake
valves were thereafter cleaned, and the intake valves
were removed carefully so as to not disturb any
WO 91/13949 -2 4- PCT/US91/01332
deposits residing thereon. The valve stems were wiped
with gum solvent to remove tube oil, and thereafter
photographed. The valves were then placed in a
desiccator for one hour and then removed and
immediately weighed, with the weight being recorded to
the nearest 0.1 mg. The valves were then put back
into the desiccator for an additional 0.5 hour and
reweighted, this process was repeated until valve
weighings were within 0.5 mg. The valve was then
cleaned with gum solvent and wire brush, and stored in
a desiccator until ready for final weighing. The
engine was thereafter reassembled with a new set of
intake valves, and the engine runs were repeated.
A base fuel composition obtained from the Sun
Refining and Marketing Company was used in all of the
following examples. The analysis of the base fuel
composition was as follows:
Item Result
API Gravity, ASTM D287 56.1
Research Octane No., ASTM D2699 95.2
Motor Octane, ASTM D2700 84.2
Sensitivity, (R-M) 11.0
Octane, (R+M)/2 89.7
Reid Vapor Pressure, psi
ASTM D323 Automated 9.1
Distillation, ASTM D86
Automated
IBP 93
loo Evap. 131
50% Evap. 220
90~ Evap. 336
FBP 411
Hydrocarbon Composition,
Vol. °s, ASTM D1319
Aromatics 30.4
Olefins 17.1
Saturates 52.5
The base fuel composition, without any additives,
was tested in each of the two Honda engine systems to
obtain comparative results. Both intake valve deposit
weights (in milligrams) and combustion chamber ratings
'O 91 / 13949 - 2 5 - PCT/US91 /01332
. (according to the CRC method, as previously described)
were obtained for each of the two engines as follows:
Example 8
(Comparative Example)
Engine No. 1 Engine No. 2
Intake Valve Deposits (Mg)* 108.8 97.7
Combustion Chamber Rating 8.1 8.0
(CRC)**
* Each is average of two runs
** In accordance with the CRC Rating Method, the
combustion chamber is rated from 1-10, with "1"
being very "dirty" (i.e. very heavy deposits] and
"10" being completely free of deposits. It is well
known to those skilled in the art that reduction of
combustion chamber deposits (i.e. high CRC Rating
Number) can have a significant positive effect on
ORI of vehicles as well as reduced amounts of
exhaust emissions.
A variety of fuel compositions, including fuel
compositions containing the additive composition of
this invention were tested using the above-described
procedure in the Honda generator engines in order to
obtain intake valve deposit measurements and
combustion chamber rating measurements. These test
results are set forth below:
Example 9
Motor fuel compositions were prepared using the
base fuel composition from Sun Refining and Marketing
Company, and additionally having the following
additives:
WO 91/13949 2 6 PCT/US91 /01332
Detergent
Component Fuel
(250 mg/1 Conditioner** Carrier Oil*
Fuel No. fuel Component (250 mq)
I - yes yes
(compara-
tive)
II polyiso-
(compara- butylamine - yes
tive)
III polyiso-
(inven- butylamine yes yes
tion)
IV poly-
(compara- butylamine - yes
tive)
V poly-
(inven- butylamine yes yes
tion)
VI isooctyl-
(compara- phenyl poly- - yes
tive) ethoxy ethanol
VII isooctyl-
(inven- phenyl poly- yes yes
tion) ethoxy ethanol
* In Fuel Nos. I-VII, carrier oil used was 250 mg of
1:1 blend of SNO-600 oil and synthetic oil.
** Fuel conditioner component was 100mg/1 fuel polar
oxygenated hydrocarbon plus 40ppm compatibilizer
(hexanol).
Fuels Nos. I-VII were rated in terms of the
amount of intake valve deposits (in mg) and for
combustion chamber (CRC) rating, as summarized in
Figure 1. As is clear from Figure 2, which plots the
data set forth in Figure 1, Fuel Nos. III, V, and VII
(i.e. the fuel compositions comprising the additive of
this invention) exhibited superior properties both in
terms of combustion chamber rating (i.e. high CRC
rating) and reduction of intake valve deposits (i.e.
low value of mg of deposits on intake valves).
O 91/13949 2 ~ PCT/US91/01332
It will be evident that the terms and expressions
employed herein are used as terms of description and
not of limitation. There is no intention, in the use
of these descriptive terms and expressions, o
excluding equivalents of the features described and it
is recognized that various modifications are possible
within the scope of the invention claimed.