Note: Descriptions are shown in the official language in which they were submitted.
Wo91/15209 PCT/US91/01951
TITL~ OF TH~ INVENTIONSUBSTITUTED PYRIMIDINES, PYRIMIDINONES AND PYRID9-
PYRIMIDINES ~
SUMMAR~ OF ~H~ INVENTI~N
The is a continuation-in-part of copending
Application S. N. 50l,580, filed March 30, l990.
This invention rslates to novel substituted
pyrimidine, pyrimidinone and pyridopyrimidine
compounds and derivatives thereof which are useful as
angiotensin II antagonists in the treatment of
elevated blood pressure and congestive heart
failure. The compounds of the invention are also
useful-as ocular antihypertensives.
- , . ...
-
... . . - ..
. . . ,
~ .. . ..... .. . . ... .. . .. .
~. .. ~ - . . . . . :
. - - ' ~ ,
:
. .
,
.
wos1/15209 PcT/us9l/ols
2~ ?~ 2 -
The compounds of this invention also have
central nervous system (CNS) activity. They are
useful in the treatment of cognitive dysfunctions
including Alzheimer's disease, amnesia and senile
dementia. These compounds also have anxiolytic and
antidepressant properties and are therefore, useful
in the relief of symptoms of anxiety and tension and
in the treatment of patients with depressèd or
dysphoric mental states.
BACKG~0UND OF~EE_l~y~NTION
Renin-angiotensin system (RAS) plays a
central role in the regulation of normal blood
pressure and seems to be critically in~olved in
hypertension development and maintenance as well as
congestive heart failure. Angiotensin II (A II), an
octapeptide hormone is produced mainly in the blood
during the cleavage of angiotensin I by angiotensin
converting enzyme (ACE) localized on the endothelium
of blood vessels of lung, kidney, and many other
organs, and is the end product of the RAS-A II is a
powerful arterial vasoconstrictOr that-exerts its
action by interacting with ~pecific receptors present
on cell membranes. One-of the possible modes of
controlling the RAS is angiotensin II receptor
antagonism. Several peptide analogs of A II are
known to inhibit the effect of this hormone by
competitively blocking the receptors, but their
experimental and clinical applications have been ~ -
limited by partial agonist activity and lack of oral
absorption [M. Antonaccio. Clin. Exp. ~ypertens. A4,
27-46 (198Z); D. ~. P. Streeten and G. H. ~nderson,
wosl/l5209 PCT/US91/01951
~; t, ~ $ ~,, !~
- 3 - ~ -
Jr. - Handbook of Hypertension, Clinical
Pharmacology of Antihvpertensive Drug~, ed. A. E. -
Doyle, Vol. 5, pp. 246-271, Elsevier Science --
Publisher, Amsterdam, The Netherlands, 1984].
Recently, several non-peptide compounds have
been described as A II antagonists. Illustrative of
such compounds are those disclosed in U.S. Patents
4,207,324; 4,340,598; 4,576,958; and 4,582,847; in
European Patent Applications 028,834; 245,637;
253,310; 291,969; 323,841; and 324,377; and in
articles by A.T. Chiu, et al. tEur. J._Pharm Exp.
Therap, 157, 13-21 (1988)] and by P.C. Wong, et al.
~J. Pharm. ~a~ Therap, 247, 1-7(1988)]. All of the
U.S. Patents, European Patent Applications 028,834
and 253,310 and the two articles disclose substituted
imidazole compounds which are generally bonded
through a lower alkyl bridge to a substituted
phenyl. European Patent Application 245,637
discloses derivatives of 4,5,6,7-tetrahydro-2E-
imidazo[4,5-c]-pyridine-6-carboxylic acid and analogs
thereof as antihypertensive agents. '
DETAILED D~SCRIPTION OF T~ INVENTION .
This invention relates to novel substituted
pyrimidine, pyrimidinone and pyridopyrimidine
compounds and derivatives thereof which are useful as
angiotensin II antagonists, as antihypertensives, in ~
the treatment of congestive heart failure and in the . .
treatment of elevated intraocular pressure. The
compounds of t~is in~ention have the general formula
,j~- j
. ` ., : '.' . ,'. ~ . ., .'. ~ : !, ' . ,
.'~ . ' ' :''' ' , , ' ~ " `.' ' : ,
WO91/15209 PCT/US91/~1951
.~
2 ~J.~.'~
-- 4 --
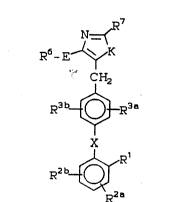
N ~
R6- E~K
CH2
R3 b - ~R3
R2 b~R1
R2 a ~. .
(I)
wherein: ~
K is -N(R8a)-C(=M) or -N=C(R8b)-wherein M is 0 or : .
NR22 . ::
Thus, the compounds of formula I can also be
e~pressed as compounds having the following formulae
(Ia), (Ib), and ((Ic) if R7 and R8a are joined) -
~
.
- . . .
.
-
~, .
.;, .
!
WO 91/15209 PCr/US91/01951
~y~
R7 R7
N~N,Ra a N~N
R6 _ E~M R6 _ E~ 8 b ~ ~ `
CH2 CH2
R3 b~ -R3a ~3b~3 _R3a
X . ` .
R2b~R1 ~,R
~R26
( Ia) NlN~~ Ib)
R6 E~M : '-
CH2 .,
R3b$l--R3-
R2 b~R1
R :
( Ic)
~` , .
.,........................................................... ~ ~
~ .
.
WO 91/15209 PCr/US91/01951
J~ 6 -
wherein:
Rl i s ( a ) -Co2R4,
( b ) - So3R5,
( c ) -NHS02 ( C l-C4-polyf luoroalkyl )
( d ) -PO ( oR5 ) 2 ~
( e ) - So2-NH-R9,
(f ) -CoNHoR5,
OH O
(g) -C--P-oR5,
R9 oR5
(h) -CN,
(i) -S02N~-heteroaryl,
(j~ -C~I2S02NH-heteroaryl,
(k) -So2NH-Co-R23,
(1) -C~I2S02NH-CO-R23,
(m) -CONEI-S02R23,
(n) -CHzCON~I-S02R23,
( o ) -N~IS02N~CO-R23,
(p) -NEICON~S02-R23,
.~, . .
, ,' ' ' ' , . ''' , ` :' ", , ` ,' , ' ::
WO 91/15~0~ PCr/US91/0195
-- 7 --
N-N
) ~N,N ~
Rl 1 .
N--N
( r ) ~ CH2 ~N~N
Rl1 ~
N--N
( s ) - CON~ N
H R~
t ) - CONHN~ O2 CF3 ,
,
N--N
( u) N CF3
H
N~N
( v) ~
R12
.
: ~ .
W091/15209
PCT/US91/019~1
2~'?'~ 8
wherein heteroaryl is an unsubstituted, monosub- ~ ~ :
stituted or disubstituted five or six membered
aromatic ring comprising ~rom l to 3 heteroatoms
selected from the group consisting of 0, N or S and .
wherein the substituents are members selec~ed from
the group consisting of -OH, -SH, -Cl-C4-alkyl,
-Cl-C4-alkoxy, -CF3, Cl, Br, F, I, -N02, -C02H,
-C02-Cl-C4-alkyl, -NH2, NH(Cl-C4-alkyl) and
-N(Cl-c4-alkYl)2;
R2a and R2b are each independently
(a) ~,
(b) Cl ,Br, I or F,
(c) N02,
.
(d~ NE2, :
(e) Cl-C4-alkylamino,
(f) di-(Cl-C4-alkyl)amino
(g) So2NHR9,
(h) CF3,
(i) Cl-C4-alkyl, or
(j) Cl-C4-alkoxy;
R3a is
(a) E,
(b) Cl, 2r, I, F
(c) Cl-C6-alkyl,
(d) Cl-C6-alkoxy,
(e) Cl-C6-alkoxy-Cl-C4-alkyl; : .
'
, .
.
. .
WO91/15209 PCTiUS91/01g51
_ 9 _ .
R3b i S
(a) H
(b) Cl, Br, I, F
(C) N02,
(d) Cl-C6-alkyl,
~e) C2-C6-alkanoyloxy,
(f) C3-C6-cycloalkyl, :
(g) Cl-C6-alkoxy,
(h) -MHSo2R4,
(i) hydroxy-Cl-C4-alkyl, ~','
(j) aryl-Cl-C4-alkyl,
(k) Cl-C4-alkylthio,
(1) Cl-C4-alkylsulfinyl, ''-~
(m) Cl-C4-alkylsulfonyl,
(n~ NH2,
(o.) Cl-C4-alkylamino,
(p) Cl-C4-dialkylamino,
(q~ CF3,
(r) -So2-NHR9,
(s) aryl or
(t) furyl;
wherein aryl is phenyl or naphthyl unsubstituted or
~: substituted with one or two substituents selected
from the group con~isting of Cl, Br, I, F,
Cl-C4-alkyl, Cl-C4-alkoxy, NO2, CF~, Cl-C4-alkylthio,
0~ NH2~ -NH(Cl-C4-alkYl). -N(Cl-C4-alkyl)2~ -CO2H,
Cl-C4 polyfluoroalkyl, C3-C6-polyfluorocvcloalkyl~
-CO2-Cl-C4-alkyl or
-; Nl It `
H :
~' ~
~:
WO91/15209 PCT/US91/019~1
2~
- 10 -
R4 is H, Cl-C6-alkyl, benzyl or phenyl;
R4 0
R5 is H, -CH-o-C-R4;
E is a single bond, -NRl3(CH2)S-, -S~O)x~CH2)s-
where x is 0 to 2 and s is 0 to 5,
-C~(OH)-, -O-, -CO-; ~
R6 iS
(a) Cl-C6-alkyl, C2-C5-alkenyl or C2-C5-alkynyl -
each of which can be substituted with a
substituent selected from the group -.
consisting of aryl, C3-C7-cycloalkyl, Cl, - :
Br, I, F, -0~, CF3, CC13. -NX2,
-NH(Cl-c4-alkYl). -N(cl-c4-alkyl)2,
-N~I-S02R4, -CooR4, -S02NHR9, Cl-C4-alkoxy, ' .
Cl-C4-alkyl-S. -CF2CF3;
(b) C3-C5-cycloalkyl;
(c) polyfluoro-Cl-C4-alkyl;
.
R7 is (a) hydrogen,
(b) aryl,
(c) heteroaryl, ~'
(d) Cl, Br, I, F, ::
(e) -C02H, ~ : .
(f) -C02R4,
(g) NX2,
(h) -NH(Cl-C4-alkyl),
(i) -N~Cl-C4-alkyl)2,
( j ) -So2NR9R10, ,~
(k) -N~IS02-51-C4-alkyl,
( 1 ) -S ( O )x-Cl-C4-alkyl,
~ .
.
W091/15209 pcT/ussl/ol9s
(m) -OH,
(n) -SH,
(o) -S(O)x-aryl, , .:
(p) ~Cl-C4-alkyl or -O(Cl-C4-alkyl) or
-S(Cl-C4-alkyl) each of which can be
substituted with aryl, heteroaryl, :
-OH, -NH2, -CF3, G3-C5-cycloalkyl,
-NH(Cl-c4-alkYl)~ -N(cl-c4-alkyl)
-C02H, -Co2R4, Cl, ~r, I, or F,
(q) C3-C5-cycloalkyl or
(r) -CF3;
R8a is (a) aryl,
(b) heteroaryl,
(c) Cl-C4-alkyl either unsubstituted or
substituted with aryl, heteroaryl,
-OH, -NH2, -NH(Cl-C4-alkyl),
N(Cl-C4-alkyl)2, -C02H, -Co2R4 Cl, Br,
I, or F, or
(d) Cl-C4-alkylaryl either unsubstituted
or substituted with Co2R4;
or R7 and R8a when alkyl groups on adjacent atoms may
be joined together with the atoms to which they are
bound to form a pyridine ring which may~be
unsubstituted or.substituted with R26 or R27 or R26 ~
and R27 wherein R26 is ~ ~.
-.-(a) R7
- (b) -NHCO(C~-C5-alkyl),
~c) :-NHCO(C3-C6-cycloalkyl),
(d) .~-NHCO(aryl), .-
(e) -NHCO(heteroaryl),--.
(f) -N(Cl-C~-alkyl)CO~Cl-C5-alkyl~
.
.. ~ . . .. .
wosl/ls2o9
PC~r/ussl/o1s
~_,
Z ,~
- 12 -
(g) -N(Cl-C5-alkyl)CO(C3-C6-cyclo-
alkyl),
(h) -N~Cl-C5-alkyl)CO(aryl),
(i) -N(Cl-C5-alkyl)CO(heteroaryl).
and R27 is Cl-C4-alkyl, Cl, Br, F, I, -CF3, aryl or
heteroaryl;
R8b is (a) -OH,
(b) -NH2,
(c) -N~(Cl-C4-alkyl),
(d) -N(Cl-C4-alkYl)2~
(e~ -MHC02-Cl-C4-alkyl,
(f) -NHCO-Cl-C4-alkyl,
( g ) -N~S02-Cl-C4-alkyl,
(h) -NHS02-aryl, ;
(i) -MHS02(Cl-C4-polyfluoroalkyl),
(j) -C0zH,
(k) -Co2R4,
(1) Cl, Br, I, F,
(m) -CONHS02-aryl, ~ ~ .
(n) -CONHS02-heteroaryl,
(o) -CONHS02-Cl-C4-alkyl,
(p) -CONHS02(Cl-C4-polyfluoroalkyl),
(q) -C~20~
(r) -CH20CoR4,
(s) -0-Cl-C4-alkyl-, unsubstituted or
substituted with Co2R4,
(t) -S(0)x-aryl unsubstituted or -
--substituted with C02R4,
~u) -S(0)~-Cl-C4-alkyl unsubstituted or l~:
substituted:with C02R4, I
(v) ---S02NHR25,
-. ~) -CN,. . . i-
'~'
WO91/15209 pcT/vss
(x) tetrazol-5-yl;
R9 is H, Cl-C5-alkyl, phenyl or benzyl;
R10 iS H, Cl-C4-alkyl;
Rll is H, Cl-C6-alkyl, C2-C~-alkenyl, Cl-C4-alkoxy
alkyl, or -CH2-C6H4R20;
R12 is -CN, -N02, -Co2R4, or -CF3;
R13 is H, C2-C4-alkanoyl, Cl-C6-alkyl, allyl,
C3-C6-cycloalkyl, phenyl or benzyl;
R14 is H, Cl-C8-alkyl, Cl-C8-perfluoroalkyl,
C3-C6-cycloalkyl, phenyl or benzyl;
R15 is ~, Cl-C6 alkyl;
R16 is H, Cl-C6-alkyl, C3-C6-cycloalkyl, phenyl or -~
benzyl;
Rl7 iS -NR9R10, -OR10, -NHCONH2, -NHCSNH2,
- N~SO2~CH3 or - N~02~ ;
R18 and R19 are independently Cl-C4-alkyl or taken
together are ~~CH2)q~ where q-is 2 or 3;
20 is H, -NO2, -NH2, -O~ or -OcH3;
R21 is (a) -CO-aryl, ~-
(b) -CO-Cl-C4-alkyl,
(c) -COCF3,
(d) -CO-heteroary',
(e) heteroaryl; .
R22 is the same as R8a or -H;
,, - , - . .
',
.: .
. .
W091/1520~ PCT/VSs~/01951
14 -
R23 is (a) aryl,
(b) heteroaryl,
(c) C3-C7-cycloalkyl,
(d) Cl-C6-alkyl unsubstituted or
substituted with a substituent
selected from the group consisting of
aryl, heteroaryl, -0~, S~,
-C1-C4-alkyl, -O(Cl-C4-alkyl),
-S ( Cl-C4-alkyl ), -CF3, Cl, Br, F, or I,
-N2 . -C02H, -C02-Cl-C4-alkyl, -NH2
-NH(Cl-C4-a~kYl). -N(Cl-C4-alkyl)2,
-P3~2, or -po(oH>(o-cl-c4-alkyl);
R25 is (a) H,
(b) Cl-C4-alkYl;
X is
(a) a carbon-carbon single bond,
(b) -CO-,
(c) --O--,
(d) -S ,
~e) -N-, :~
R13
(f) -CON-,
RlS
(g) -NCO-,
R15 ' - ~ ~,
(h) -OCH2-,
( i ) -C~I20-
(j) SC~2-, ~ .,, .. " ~ '
(k) -C~2S-, --
( 1 ) -NHC ( R9 ) ( Rl ) -,
.
WO91/l5209
PCT/US91/01951
-- 15 --
(m) -NR9So2-,
(n) -So2NR9-,
(o~ -C(R9)(RlO)N~-,
(p) -C~=CH-,
(q) -CF=CF-,
(r) -CH=CF-,
(s) -CF=CH-,
(t) -C~2CH2-'
(U ) -CF2CF2-,
(v) ~ / C~2 `C~
,C\ ¦ or ¦ / CH2
C~2 / CH
oR14
(w) -CEI-,
ocoR16 . ,::
(x) -CH-,
NR17
i, .
~ (y) -C-, or
R180 oR19
(z) --C--;
~ ~ Z is 0, NR13 or S; or,
: : :
a pharmaceutically acceptable salt thereoP.
The terms "alkyl", "alkenyl", ~alkynyl" and
the like include both the straight chain and branched
chain species of these generic terms wherein the
number of car~on ~toms in the species permit. Unless
otherwise noted, the specific names for these generic
terms shall mean the straight chain species. For
example, the term "butyl" shall mean the normal butyl
;;substituent, a-butYl.
~ ~ .
U'O 91/1~209
PCT/I~S91 /01 9' 1
.~:~, '? .~ ~
324/WHN33 - 16 -
One embodiment of the novel compounds is
that of formula Ib wherein K is -N=C(R8b)
In a class of this embodiment,
N-N
Rl is -COOH; ~ " -NH-SO2CF3; -Co2R4; ~ ~-
H
-S02N~- heteroaryl or CH2S02N~- heteroaryl
wherein the heteroaryl is an unsubstituted, :
monosubstituted or disubstituted 5- or
6-membered aromatic ring 1 to 3 heteroatoms
selected from O, N and S and wherein the
substituents are members selected from the group
consisting of OH, S~, Cl-C4-alkyl, Cl-C4-alkoxy,
CF3. Cl, Br, F, I, NO2, C2~, C2-Cl-C4-alkYl'
NH2, NH(Cl-C4-alkyl) and N(Cl-C4-alkyl)2;
-SozNHCoR23;-CH2SO2N~COR23; -CoNHS02R23;
-CH2CoNEsS02R23; -N~So2NECoR23; or -MEsCOMHSo2R23; : -
R2a and R2b are H, F, Cl, CF3, Cl-C4-alkyl
Cl-C4-alkoxy;
~3a is Es~ F or Cl; :
R3b is H, F, Cl, CF3, Cl-C4-alkyl, Cl-C4-alkoxy, ~:
-COOC~3, -COOC2Hs, -S02-CE3, MH2, :.
-N(Cl-c4-alkYl)2 or -NH-so2cEs3;
.
E is a single bond, -O- or -S-;
.... .. .~ .. .. ~ . . . . . . ... .. .
: .
-::
wo 91/15209 PCl`/US91/01~1
Z'~, ~i ~ .J ''_ ~
-- 17 --
R6 is
(a) Cl~C5-alkyl, C2-C5-alkenyl or C2-C5-alkynyl
each of which can be substituted with a
substituent selected from the group
consisting of, CF3, CF2CF3, -0-CH3, -OC2H5,
-S-CH3, -S-C2H5, phenyl and C3-C5-cyclo-
alkyl;
~b) C3-Cs-cycloalkyl; and
(c) polyfluoro-Cl-C4-alkyl;
X is a C-C single bond.
In a more preferred class of this embodiment are
those compounds wherein:
E i~ a sin~le bond;
R2a R2b R3a and R3b are each H; and
X is a single bond.
~ .
Exemplifying this embodiment are the
following compounds of the Formula II shown in Table ::
, .:
.- R7
I
~: N~N
~, ~
~R8 b
WO 91/15209 pcl/ussl/olss
n,~
- 18 -
Table 1
Compd.
No. Rl R6 R7 R8b
__~__ _
II-l tetrazol-5-yl Bu Me -COOMe
II-2 tetrazol-5-yl Bu Me -COOEt
II-3 tetrazol-5-yl Bu Me -COOH
II-4 tetrazol-5-yl Bu Me -CHO
II-5 tetrazol-5-yl Bu Me -CH20H
II-6 tetrazol-5-yl Bu Me -NHS02CF
II-7 tetrazol-5-yl Bu Me -NHso2cF2cF3
II-8 tetrazol-5-yl Bu Me -NHS02Ph
II-9 tetrazol-5-yl Bu Me -CONHS02Ph ~ :
II 10 tetrazol-5-yl Bu Me -S02NHCOCYPr
II-ll tetrazol-5-yl Bu Me -SOzNHSO2CF3
II-lZ tetrazol-5-yl Pr Me -COOMe
II-13 tetrazol-5-yl Pr Me -COOEt
II-14 tetrazol-5-yl - Pr Me -COOH :
II-15 tetrazol-5-yl Pr Me -CHO
II-16 tetrazol-5-yl Pr Me -C~20H
II-17 tetrazol-5-yl Pr Me -NHS02CF3
II-18 tetrazol-5-yl Pr Me NHS02CF2CF
II-l9 tetrazol-5-yl Pr Me -NHS02Ph :
II-20 tetrazol-5-yl Pr Me -CONHS02Ph .
II-21 tetrazol-5-yl Pr Me -S02NHCOCYPr
II-22 tetrazol-5-yl Pr Me -S02NHSO CF3
II-23 -NHS02CF3 Bu Me -COOMe
II-24 -NHS02CF3 . Bu Me -COOEt .. -. .II-25 -NHS02CF3 Bu Me -COOH
II-26 -NHSO~CF3 Bu Me -CHO
:'
'
. . .
'':,"',-"'''',.''''',"``,,'''' . . '"`
W O 91/15209 2r~,$ ~ PCT/US91/01951
-- 19 -- : .
Compd.
No. Rl R6 R7 R8b
_ I
!~ ~
II-27 -NHS02CF3 Bu Me -CH20H
II-28 -NHS02CF3 Bu Me -NHS02CF3
II-29 -NHS02CF3 Bu Me -NHS02CF2CF3
II-30 -NHS02CF3 Bu Me -NHS02Ph
II-31 -NHS02CF3 Bu Me -CONHS02Ph
II-32 -NHS02CF3 Bu Me -S02NHCOCYPr
II-33 -NHS02CF3 Bu Me -S02NHS02CF3
II-34 -NHS02CF3 Pr Me -COOMe
II-35 -NHS02CF3 Pr Me -COOEt
II-36 -NHS02CF3 Pr Me -COOH
II-37 -NHS02CF3 Pr Me -CHO
II-38 -NHS02CF3 Pr Me -CH20H
II-39 -NHS02CF3 Pr Me NHS02CF3
II-40 -NHS02CF3 Pr Me -NHS02CF2CF3
II-41 -NNS02CF3 Pr Me -NHS02Ph
II-42 -NHS02CF3 Pr Me ~CONHS02Ph
II-43 -NHS02CF3 Pr Me S02NHCOCYPr
II-44 -NHS02CF3 Pr Me -S02NHS02CF3
II-45 -S02NHCOCYPr Bu Me -COOMe
II-46 -S02NHCOCYPr Bu Me -COOEt `~
47 -S02NHCOCYPr Bu Me -COOH
II-48 :-S02NHCOCYPr: Bu Me --CHO
:II-49 -S02NHCOCYPr Bu. Me -CH20H
II-50 -S02NHCOCYPr -Bu Me -NHS02CF3 -
II-51 -S02NHCOCgPr Bu. Me: -NHS02CF2CF3 .
II-52` -S02NHC~CYPr Bu - Me -NHS02Ph ~ .
II-53 -S02NHCOCYPr Bu Me -CONHS02Ph
II-54 -S02NHCQCYPr Bu - Me -S02NHCOCYPr -
.
.
~:
W O 91/15209 P~T/US9~/01951
.~ ,
2 ~ 20 -
Compd~ - .
No. Rl R6 R7 R8b ::. -:
II-55 -S02NHCOCYPr Bu Me S02NHS02CF3
II-56 -S02NHCOCYPr Pr Me -COOMe : .
II-57 -S02NHCOCYPr Pr Me -COOEt
II-58 -S02NHCOCYPr Pr Me -COOH
II-59 -S02NHCOCYPr Pr Me -CHO
II-60 -S02NHCOCYPr Pr Me -CH20H
II-6l -S02NHCOCYPr Pr Me -NHS02CF3
II-6Z -S02NHCOCYPr Pr Me -NHS02CF2CF3 -~
II-63 -S02NHCOCYPr Pr Me -NHS02Ph
II-64 -S02NHCOCYPr Pr Me -CONHS02Ph
II-65 -S02NHCOCYPr Pr Me -S02NHCOCYPr
II-66 -S02NHCOCYPr Pr Me -S02NHS02CF3
II-67 tetrazol-5-yl' Pr -CF2CF3 -COOMe
II-68 tetrazol-5-yl Pr -CF2CF3 -COOEt
II-6g tetrazol-5-yl Pr -CF2CF3 -COOH
II-70 tetrazol-5-yl Pr -CF2CF3 -C~O
II-71 tetrazol-5-yl Pr -CF2CF3 -CH20H
II-72 tetrazol-5-yl Pr -CF2CF3 -NHS02CF3 ~:~
II-73 tetrazol-5-yl Pr -CF2CF3 HS02CF2CF3 ~
II-74 tetrazol-5-yl Pr -CF2CF3 -NHS02Ph : :
II-75 tetrazol-5-yl Pr -CF2CF3 -CONHS02Ph
II-76 tetrazol-5-yl Pr -CF2CF3 -S02NHCOCYPr
II-77 tetrazol-5-yl Pr -CF2CF3 -S02NHS02CF3 :
II-78 -NHS02CF3 Pr -CF2CF3 -COOMe
II-79 : -NHS02CF3 Pr -CF2CF3 - -COOEt -~
II-80 -NHS02Cr3 Pr -CF2CF3 -COOH~
II-81 - -:NHS02CF3 Pr -CF2CF3 -CHO .'
II-82: -NNS02CF3 --Pr . -CF2CF3 -CH20H -
:
; ~
'~ ; '
: . ~ ' . :
WO 91/15209 PCr/US91/01951
~.,, ~.,
- 21 -
Compd.
No~ Rl R6 R7 RBb
II-33 -NHS02CF3 Pr-CF2CF3 NHS02CF3
II-84 -NHS02CF3 Pr-CF2CF3 -NHS02CF2CF3
II-85 -NHS02CF3 Pr-CF2CF3 -NHS02Ph :~
II-86 -NHS02CF3 Pr-CF2CF3 -CONHS02Ph
II-87 -NHS02CF3 Pr-CF2CF3 -S02NHCOCYPr
II-88 -NHS02CF3 Pr-CF2CF3 -S02NHS02CF3
II-89 -S02NHCOCYPr Pr -CF2CF3 -COOMe
II-90 -S02NHCOCYPr Pr -CF2CF3 -COOEt
II-91 -S02NHCOCYPr Pr -CF2CF3 -COOH
II-92 -S02NHCOCYPr Pr -CF2CF3 -CHO
II-93 -S02NHCOCYPr Pr -CF2CF3 -CH20l~
II-94 -S02NHCOCYPr Pr -CF2CF3 -NHS02CF3
II-95 -S02NHCOCYPr Pr -CF2CF3 -NHS02CF2CF3
II-96 -S02NHCOCYPr Pr -CF2CF3 -NHso2ph
II-97 -S02NHCOCYPr Pr -CF2CF3 -CONHS02Ph
II-g8 -S02NHCOCYPr Pr -CF2CF3 -S02NHCOCYPr
II-99 -S02NHCOCYPr Pr -CF2CF3 -S02NHS02CF3
II-100 -S02NHCOPh Pr Me -COOMe
II-101 -S02NHCOPh Pr-CF2CF3 -COOMe
II-102 -S02NHCO- Pr Me -COOMe.
.` (CH2)5NH2 -'
II-103 -S02NHCO- Pr-CF2CF3 -COOMe
(~H2)5NH2 . j ' - .
II-104 -S02NHCOPh Pr..... Me -NHSO CF3 -
II-105 -S02NHCOPh Pr . -CF2CF3 -SO NHCOCYPr
II-106 -S02NHC~ Pr- Me - - -NHS02CF3
(CHZ)5NHz
II-107 -S02NHCO- Pr -C ~ F3 -S02NHCOCYPr
(CH2)5N~2
,:
j- .
WO91/15209 PCT/US91/01951
2 ~ J ~?~
- 2~ -
In another embodiment of the novel compounds
is that of formula Ia (M=O) wherein K is -N(R8a)-CO-.
In a class of this embodiment
N--N
Il ~ ` , . ~
Rl is -COOE; ~ ~N ; -NH-SO2CF3; Co2R4;
H ~ -
.
-S02NH- heteroaryl or C~2S02NH- heteroaryl
wherein the heteroaryl is an unsubstituted, :
monosubstituted or disubstituted 5- or 6-membered
aromatic ring comprising contain l to 3
heteroatoms selected from O, N and S and wherein .
the substituents are members selected from the
group consisting of O~, SH, Cl-C4-alkyl,
Cl-C4-alkoxy, CF3, Cl, Br, F, I, N02, CO2~ 9
C02-Cl-C4-alkyl, NH2, NH(Cl-C4-alkyl) and
N(Cl-C4-alkyl~2; -So2NHCoR23;-CH2So2N~coR23;
-CoN~So2R23; -CH2CoN~So2R23; -N~So2N~CoR23; and
-N~CoNHSo2R23;
R2a and R2b are H, F, Cl, CF3, Cl-C4-alky
Cl-C4-alkoxy; .
R3a is H, F or Cl; --
R3b is Hi F, Cl. CF3. cl-C4-alkyl. Cl-C4-alk~i`
.-COOCH3, -COOC2H5.--S02-CH3, NH2,
N(Cl-C4-alkyl)2 or -NH-SO2CH3;
. . .
,~ ' .
W091/15209 PCT/US91~01951
- 23 -
E is a single bond, -O- or -S-;
R6 is
(a) Cl-C5-alkyl, C2-C5-alkenyl or C2-C5-alkynyl
each of which can be substituted with a
substituent selected from the group
consisting of Cl, CF3, CF2CF3, CCl3,
-O-C~3~ -C2x5~ -S-CH3, -S-C2Hs, phenyl and
C3-C5-cycloalkyl;
(b> C3-C5-cycloalkyl;
(c) polyfluoro-Cl-C4-alkyl;
R7 and R8a are as defined above or together with the
atoms to which they are bonded may be
joined to form a pyridine ring which can be
subætituted with R26 and R27; and
X is a C-C single bond.
In a more preferred clas~ of this
embodiment are those compounds wherein:
E is a single bond;
R2a R2b, R3a and R3b are each H; and
X is a single bond.
.,
Exemplifying this embodiment are the
following compounds- of the Formula III shown in Table
2: :~
.
.. . .
- ' ` -~' ~
.
WO 91/15209 PCT/US91/01951
,~, .
2~
-- 24 -- -
~1~ R~ a
R ~ O
~ Rl : :
III
Tabl e 2
Compd.
No. ~ R6 R7 R8a : :
III-l tetrazol-5-yl Bu Me H
III-Z tetrazol-5-yl Pr Me 2-CF3-phenyl
III-3 tetrazol-5-yl Pr . Me 2-Cl-phenyl
III-4 tetrazol-5-yl Pr Me 2,6-diCl-phenyl
III-5 tetrazol-5-yl Pr -CF2CF3 2-CF3-phenyl :
III-6 tetrazol-5-yl Pr -CF2CF3 2-Cl-phenyl
III-7 tetrazol-5-yl Pr Me 2-COOH-phenyl
III-8 tetrazol-5-yl Pr Me F2CF3
III-9 -NHS02CF3 . Bu Me H :
III-IO -NHSO2CF3 Pr Me 2-CF3-phenyl :
III-ll -NHSO2CF3 Pr Me 2-Cl-phenyl
III-12 -NHSO2CF3 Pr Me 2~6-diCl-phenyl
III-13 -NHSO2CF3 Pr CF2CF3 2-CF3-phenyl
III-14 -NHSO2CF3 Pr -CF2CF3 2-Cl-phenyl : :
III-15 -NHSO2CF3 Pr Me ~ ~ 2-COOH-phenyl
--: '. , '
W O 91/15t09 PCT/US91/01951
2 ` ~
- 25 -
Compd.
No~ Rl R6 R7 R~b
III-16 -NHSO2CF3 Pr Me CF2CF3
III-17 -S02NHCOCYPr Pr Me 2-CF3-phenyl
III-18 -S02NHCOCYPr Pr Me 2-Cl-phenyl
III-l9 -S02NHCOCYPr Pr Me 2,6-diCl-phenyl
III-20 -S02NHCOCYPr Pr -CF2CF3 2-CF3-phenyl
III-21 -S02NHCOCYPr Pr -CF2CF3 2-Cl-phenyl
III-22 -S02NHCO~YPr Pr Me 2-COOH-phenyl
III-23 -SQ2NHCOCYpr Pr Me -CF2CF3
III-24 -S02N~COPh Pr Me 2-CF3-phenyl
III-25 -S02NHCOPh Pr Me 2-Cl-phenyl
III-26 -S02NHCOPh Pr Me 2,6-diCi-phenyl
III-27 -SO2NHCOPh Pr -CF2CF3 2-CF3-phenyl
III-28 -S02NHCOPh Pr -CF2CF3 2-Cl-phenyl
III-29 -SO2NHCOPh Pr Me -C~2CF3
III-30 -SO2NHCOPh Pr Me 2-COOH-phenyl
III-31 -S02NHCO- Pr Me 2-CF3~phenyl
(CH2)5NH2 : :"
III-32 -S02NHCO- Pr CF2CF3 2-Cl-phenyl
(CH2)5NH2 : :
Also exemplifying this embodiment are the
following compounds of the Formula IV shown in Table ~ -
3- :
-
..
..... .. ~ . . . . , -.. . .
:.. , . .~ - :
. . -. `, . . " . . -~ . . . - . .. . . , .. , . , . . . . - . . . . .. .. . . . . . . . . . . .
W 0 91/15209 PCT/US91/01951
2 .~ J',~ ~~
: '
- 26 -
.
9 ~ 27 :~
N 6
R6 ~ O
IV
Table
Compd.
No. Rl R6 RZ6 R27 ~:
. _
IV-l tetrazol-5-yl Bu H 7-Me ;
IV-2 tetrazol-5-yl Bu H 7-iPr ::
IV-3 tetrazol-5-yl Pr H 7-N(Pen)COPh .
IV-4 tetrazol-5-yl Pr H 7-N(Pen)CO(4-Cl-Ph)
IV-5 tetrazol-5-yl Pr H 7-N(Pr)C02-iBu
IV-6 tetrazol-5-yl Pr H 7-N(Bn)COBu
IV-7 tetrazol-5-yl Bu 8-Cl 7-S02Me :
IV-8 tetrazol-5-yl Bu . H . . 8-Cl
IV-9 -NHS02CF3 Bu H 7-Me
IV-iO -NHS02CF3 Bu H 7-iPr :
IV-ll -NHS02CF3 Pr H 7-N(Pen)COPh
IV-12 -N~S02CF3 Pr H 7-N(Pen)COt4-Cl-FhJ
IV-13 -NHS02CF3 Pr H 7-N(Pr)C02-iBu :
IV-14 -NHS02CF3 Pr H 7-N(Bn)COBu :
~? IV-15 -NHS02CF3 Bu 8-Cl 7-S02Me .
IV-16 -NHS02CF3 Bu H 8-Cl :~
IV-l7 -S02NHCOCYPr Bu H 7-Me
'.
- '
W O 91/15209 PCT/US91/019~1
Compd.
No. Rl R6 R26 R27
-
IV-18 ~S02NHCOCYPr Bu H 7-iPr
IV-l9 -S02NHCOCYPr Pr H 7-N(Pen)COPh
IV-20 -S02NHCOCYPr Pr H 7-N[Pe~1)CO~4-Cl-Ph)
IV-21 -S02NHCOCYPr Yr H 7-N~Pr)C02-iBu
IV-22 -S02NHCOCYPr Pr H 7-N(Bn~COBu
IV-23 -S02NHCOCYPr Bu 8-Cl 7-S02Me
IV-24 -S02NHCOCYPr Bu H 8-Cl
IV-25 -S02NHCOPh Bu H 7-Me ~
IV-26 -S02NHCOPh Bu H 7_ipr :
IV-27 -S02NHCOPh Pr H 7-N(Pen)COPh - .
IV-28 -S02NHCOPh Pr H 7-N(Pen)CO(4-Cl-Ph)
IV-29 -S02NHCOPh Pr H 7-N(Pr~C02-iBu :.
IV-30 -S02NHCOPh Pr H 7-N(Bn)COBu
IV-31 -S02NHCOPh Bu 8-Cl 7-S02Me
IV-32 -S02NHCOPh Bu H 8-Cl :
IV-33 -S02NHCO- Bu H 7-iPr
(CH2 )5NH2
IV-34 -S02NHCO- Bu 8-Cl 7-S02Me . :
(CH2)5NH2
IV-35 -S02NHCO- Bu H 8-Cl
(CH2 )5NH2
IV-36 -S02NHCO- Pr H 7-N(Pen)CO(4-Cl-Ph)
(CH2)sNH2
IV-37 -S02NHCO- Pr . . ~ 7-N(Pr)C02-iBu
(CH2)5NH2
IV-38 -S02NHCO- Pr Me ; . 2-CF3-phenyl
(CH2)5NH2
IV-39 -S02NHCO- Pr -CF2CF3 2-Cl-phenyl
( CH2 )5NH2
:
'' ' '
~. .
WO 91/15209 PCr/US91/01951
.,
~ ?~ 28 -
In another embodiment of the novel compounds
of this invention is that of formula Ia
(M is NR22) wherein K is -N(R8a)-
C(=NR22)-
In a class of this embodiment:
N-N
~N~N; : :
Rl iS --COOH; H -NH-SO2CF3; Co2R4;
.
-S02NH-heteroaryl or -CH2SO2NH-heteroaryl wherein the ~
heteroaryl is an unsubstituted, monosubstituted or
disubstituted 5-or 6-membered aromatic ring
comprising 1 to 3 heteroatoms selected from O, N and
S and wherein the substituents are members selected
from the group consisting of OH, SH, Cl-C4-alkyl,
Cl-C4-alkoxy, CE3, Cl,Br, F, I, NO2,CO2H, CO2-Cl-C4-
alkyl, NH2, MH(Cl-C4-alkyl) and N(Cl-C4-alkyl)2; .
-So2N~CoR23; -CH2So2NHCoR23; -CoNHS02R23; -
-CH2Co~S02R23; -N~So2NXCoR23; a~d - N~CoNHso2R23;
R2a and R2b are H, F, Cl, CF3, Cl-C4-alkyl
Cl-C4-alkoxy;
R3a is - H, F or Cl;
R3b is H, F, Cl, CF3, Cl C4-alkyl- C5-C6-
cycloalkyl, -COOCH3, -COOC2H5, -S02CH3;
NH2, -N(Cl-C4-alkyl)2 or -NH-SO2CH3;
E is -a single bond, -O- or -S-;
,. - :~ , .
. . . ~ . . .
:: .
: -- -
!
. , .. . .. . .. . . .. .. ~ . ~
WO91/15209 PCT/US91/019~1
2 ~` ' J/~'~'~'~
- 29 -
R6 is
(a) Cl-C5-alkYl- C2-Cs-alkenyl or
C2-C5-alkynyl each of which can be
substituted with a substituent selected
from the group consisting of Cl, CF3,
CCl3, -O-CH3, -OC2Hs, -S~CH3, -S-C2Hs,
phenyl, and C3-C5-cycloalkyl;
(b~ C3-C5-cycloalkyl;
(c) perfluoro-Cl-C4-alkyl;
R7 and R8a are as defined above or together with the
atoms to which they are bonded may be
joined to form a pyridine ring which may
be substitùted with R26 and R27; and
X is a C-C single bond.
In a more preferred class of this
embodiment are those compounds wherein.
E is a single bond;
R2a R2b, R3a and R3b are each ~; and
X is a single bond.
Exemplifying this embodiment are the :
following compounds of the Formula V shown in Table 4:
- ~-R7 .:
N ~ ,R~
R6 J~NR2 2 .
'? .~
V ~ d"
~ . : '
~,
WO 91/15209 pcr/us91/ol95
, ,'J?..~
- 30 -
Tabl e 4
Compd.
No. Rl R6 R7 R8a R22
... .
V-l tetrazol-5-yl Bu Me - H -CF3-phenyl
V-2 tetrazol-5-yl Pr Me 2-CF3-phenyl Me .
V-3 tetrazol-5-yl Pr Me 2-Cl-phenyl Me
V-4 tetrazol-5-yl Pr Me 2,6-diCl-phenyl Me ;
V-5 tetrazol-5-yl Pr -CF2CF3 2-CF3-phenyl Me
V-6 tetrazol-5-yl Pr -CF2CF3 2-Cl-phenyl Me
V-7 tetrazol-5-yl Pr Me 2-COOH-phenyl Me
V-8 tetrazol-5-yl Pr Me -CF2CF3 2-pyridyl
V-9 -NHSO2CF3 Bu Me H 2-CF3-phenyl ~
V-10 -NHS02CP3 Pr Me 2-CF3-phenyl Me ..
V-ll -NIIS02CF3 Pr Me 2-Cl-phenyl Me
V-12 -NHS02CF3 Pr Me 2,6-diCl-phenyl Me
V 13 -NHS02CF3 Pr -CF2CF3 2-CF3-phenyl Me
V-14 -NHS02CF3 Pr -CP2CF3 2-Cl-phenyl Me
V-15 -NHSO2CF3 Pr Me 2-COOH-phenyl Me .~:. :
V-16 -NHS02CF3 Pr Me -CF2CF3 Z-pyridyl
V-17 -S02NHCOCYPr . Pr Me 2-CF3-phenyl Me
V-18 -SOzNHCOcYPr Pr Me 2-Cl-phenyl Me
V-l9 -SOzN~COcYpr Pr Me 2,6-diCl-phenyl MeV-20 -S02NHCOCYPr Pr CF2CF3 2-CF3-phenyl Me
V-21 -S02NHCOCYPr Pr -CF2CF3 2-Cl-phenyl Me
:V-22 -SO2NHCOCYpr Pr Me 2-COOH-phenylMe
V-23 -S02NHCOCYPr Pr Me -CF~CF3 2-pvr
V-24~ -SO2NHCOPh Pr Me 2-CF3-phenyl M~ l
V-25 -SO2NHCOPI. Pr I Me 2-Cl-phenyl Me .
V-26 -S02NHCOPh Pr Me 2,6-diCl-phenyl Me
V-27 -S02NHCOPh Pr -CF2CF32-CF3-phenyl Me .
.,.
~ '
W 0 91/1S209
PCT/US91/01951
- 31 -
Compd .
No. Rl R6 R7 R8a R22
V-28 -S02NHCOPh Pr -CF2CF3 2-Cl-phenyl Me
V-29 ~S02NHCOPh Pr Me -CF2CF3 Me
V-30 -S02NHCOPh Pr Me 2-COOH-phenyl Me
V-31 -S02NHCO- Pr Me 2-CF3-phenyl Me ~:
t CH2 ) 5NH2
V-32 -S02NHCO- Pr -CF2CF3 2-Cl-phenyl Me
(CH2)5NH2
Also exemplifying this embodiment are the
following compounds of the Formula VI shown in Table
8 ~2~
g ~ ~27
N~N 6
R6 ~ NRa2
~)
VI
Table 5
Compd.
No. Rl R6 R22 R26 ~ R27 . .
: . - , .
: ,., ,_ ............................. ...
VI-l tetrazol-5-yl Bu CYpr H . . 7-Me -;
VI-2 tetrazol-5-yl Bu cypr H 7-iPr
.
Vl-3 tetrazol-5-yl Pr Me H 7-N(Pen)COPh ~
': :
W O 9l/15209 PCT/US91/Ot951
- 32 - ..
;~ b ~ ~
Co~pd.
No. Rl R6 R22 R26 R27
VI-4 tetrazol-5-yl Pr Me H 7-N(Pen3C3-
(4-Cl-Ph)
VI-5 tetrazol-5-yl Pr Me H 7-N(Pr)C02-iBu
VI-6 tetr2zol-5-yl Pr Me H 7-N(Bn)COBu .:
VI-7 tetrazol-5-yl Bu cypr 8-C1 7-S02Me
VI-8 tetrazol-5-yl Bu CYpr H 8-Cl
VI-9 -NHS02CF3 Bucypr H 7-Me
VI-10 -NHS02CF3 Bucypr H 7-ipr :~
VI-ll -NHS02CF3 Pr Me H 7-N(Pen)COPh ~.
VI-12 -NHS02CF3 Pr Me H 7-N(Pen)CO-
(4-Cl-Ph) .
VI-13 -NHS02CF3 Pr Me H 7-N(Pr)C02-iBu
VI-14 -NHS02CF3 Pr Me H 7-N(Bn~COBu
VI-15 -NHS02CF3 Bucypr 8-C1 7-S02Me
VI-16 -NHS02CF3 Bucypr H 8-Cl
VI-17 -S02NHCOCYPr Bucypr H 7-Me
VI-18 -S02NHCOCYPr Bucypr H 7-iPr
VI-l9 -S02NHCOCYPr . Pr Me H 7-N(Pen)COPh
VI-20 -S02NHCOCYPr Pr Me H 7-N(Pen)CO-
~ .~,1
(4-Cl-Ph)
VI-21 -S02N~COC~Pr Pr Me H 7-N(Pr)C02-iBu
VI-22 -S02NHCOCYPr Pr Me H 7-N(Bn)COBu
VI-23 -S02NHCOCYPr Bucypr 8-C1 7-S02Me
VI-24 -S02NHCOCYPr Bucypr H 8-Cl
VI-25 -S02NHCOPh Bucypr H 7-Me
VI-26--S02NHCOPL -- -- Bu - cypr -H- - 7-iPr
VI-27 -S02NHCOPh Pr Me H 7-N(Pen)COPh
Vl-28 -S02NHCOPh ` Pr Me H ~ -N(Pen)CO-
(4-Cl-Ph) :.
W O 91~15209 PCT/US91/01951
-- 33 ~
Comp~.
No. Rl R6 R22 R26 R27
VI-29 -S02NHCOPh Pr Me ~ 7~N(Pr)C02-iBu
VI-30 -S02NHCOPh Pr Me H 7-N(Bn)COBu
V1-31 -S02NHCOPh Bucypr 8-C1 7-S02Me
VI-32 -S02NHCOPh Bucypr H 8-Cl
VI-33 -S02NHCO- Bucypr H 7-i2r ~-
(CH2)5NH2
VI-34 -S02NHCO- Bucypr 8-C1 7-S02Me
(CH2)sNH~ : -
VI-35 -S02NHCO- Bucypr H 8-Cl
(CH2)sN~2
VI-36 -S02N~CO- Pr Me H 7-N(Pen)CO-
(CH2)5NH2 (4-Cl-Ph)
VI-37 -S02NHCO- Pr Me H 7-N(Pr)C02-1Bu
(CH2 )5NH2
VI 38 -S02NHCO- Prcypr H 7-iPr
( CH2 )5NH2
VI-~9 -S02NHCO- Prcypr H 7-S02Me
(CH2)5NH2
Several methods for preparing the
compounds of this invention are illustrated in the
ensuing Schèmes. .
'' ' ' ' `
- Abbrçviations Used in Schemes ~
.. . ...
Reagen~ ? -- - ,
NBS-- . - N-bromosuccinimide
AIBN Azo(bis)isobutyronitrile
DDQ ~ - ~ Dichlorodicyanoqi~inone
Ac~O acetic a~hydride
TE~ triethylamine -
W091/15209 PCT/US91/019S
2~ ?~ ~
- 34 -
DMAP 4-dimethylaminopyridine
PPh3 triphenylphosphine
TFA trifluroacetic acid : :
TMS-Cl trimethylsilyl chloride - :.
Im imidazole
AcSK potassium thioacetate
p-TsOH p-toluenesulfonic acid
Solvents~
DMF dimethylformamide ~.
HOAc (AcOH) acetic acid '
EtOAc (EtAc) ethyl acetate
Heæ hexane
THF tetrahydrofuran
DMSO dimethylsulfoxide
MeOH methanol
iPrOH isopropanol
Others:
rt room temperature
T8DMS t-butyldimethylsilyl
OTf OS02CF~
OTs OS02-(~-methyl)phenyl
OMs OSo2cH3
Ph phenyl
FAB-MS (FABMS) Fast atom bombardment mass
spectroscopy
NOE . Nuclear Overhauser Effect
SiO~ silica gel
tri~yl triphenylmethyl
' . Pyrimidinones substituted in the 2,4,5,
: and 6 positions may be prepared as shown in Scheme l. ''
The dianion of ethyl hydrogen malonate is made usin~
two equivalents of butyllithium in THF at - 780C. It
~' is then quenched with an acyl chloride then acidified ~ ' '
giving the necessary'~-ketoester as shown. l The~
-ketoéstër'is`'then`alkylated with the appropriate
~ : . .
:
~ .
. ~ .
WO91/15209 PcT/uss1/019s1
2; ;~ ~
- 35 -
sidechain using sodium hydride in DMSO (or other
suitable base in a suitable solvent) to give
intermediate l- Intermediate 1 may then be treated
with an appropriate R7-amidine, guanidine, O-alkyl or
aryl isoureat or S-alkyl or aryl isothiourea, to ~ive
the 2,5,6-trisubstituted pyrimidin-4(3H)-one 2 2
Pyrimidin-4(3H)-one 2 itself may be an
A-II antagonist but may also be used as an
intermediate for the preparation of
2,3,5,6-tetrasubstituted pyrimidin-4(3H)-ones as
indicated in Scheme 2. Intermediate 2 may be
deprotonated in DME with sodium hydride (or other
suitable solvent and base) and alkylated with an R8a
ele~trophile to afford the pyrimidin-4(3H)one 3.3
Scheme 3 illustrates an alternative
preparation of pyrimidinone 3. An R7 nitrile can be
converted to an imidate then to an amidine with an R8a
amine. This can then be condensed with ~-ketoester l
to give 3. Similar procedures also exist for the
preparation of isoureas, isothiuronium salts, and
guanidines.4 Other methods are also available for the
introduction of substituents at the 2-position of the
pyrimidine.5
- Scheme 4 shows how pyrimidlnone 2 can be
converted to 4-chloropyrimidine 4, which is a useful
intermediate for the preparation of other
4-substituted pyrimidines. One could also envision
using triflic anhydride and a suitably hindered amine
base to give the corresponding pyrimidine triflate
that could be used~in a similar fashion to the
4-chloropyrimidine.
. -. ,
.
. "
WO9ltlS209 PCT/US91/01951
2~ 36 -
Scheme 5 shows how nucleophilic
displacement would be achieved using an R8b
nucleophile which could be an amine, alcohol, thiol,
or carbon nucleophile with or without a Ni2~ or PdO
catalyst, to give the 2,4,5,6-tetrasubstituted
pyrimidine 5.7
Scheme 6 provides a route to the useful
intermediate ~-ketonitrile 6. Cyanoacetic acid can be
condensed with an R6 acyl chloride to give the
a-unsubstituted R6 ~-ketonitrile.8 This can then be
a alkylated using Na~ in DMS0 (or other suitable base
and solvent) and the appropriate sidechain: :
electrophile to afford 6.
Scheme 7 shows how the ~-ketonitrile 6 can
be condensed with an amidine or isourea to give
4-aminopyrimidine 7. The 4-aminopyrimidines such as 7
can be converted to pyrimidin-4(3H)-ones simply by .~
diazotizing them with nitrous acid.9 .
Scheme 8 shows an alternative pyrimidinone
synthesis via an intermediate isoxazole.10 The
~-ketonitrile 6 can be converted to the
5-aminoisoxazole 8 upon treatment with hydroxyl- .
amine. Acylation with an R7 acyl halide gives
intermediate isoxazole 9~which upon~xeduction and
: .heating~-gives:pyrimidinone 2. . ::
: .Pyridopyrimidinones such as 10 can be ~ -
o~tained by condensing variouæly substituted
2-aminopyridines with ~-ketoesters 1 as shown in;
Scheme 9.11 ~ '
Scheme lO.illustrates-a preparation of a
4-carboxy or 4-carboalkoxy pyrimidine. Ethyl hydrogen
malonate can be doubly deprotonated using ~wo
: ~;
, . : - . . i
WO91/15209 PCT/US91/01951
- 37 - ,
equivalents of butyllithium. The dianion can then be
used as a nucleophile on which to add the electrophile
sidechain to give ethyl ester 11. The ester can then
be deprotonated and be added to diethyl oxalate to
give the diethyl oxalacetate derivative-12.12
Condensation of this material with an R7 amidine or
isourea would give the 6-carbo-ethoxypyrimidin-4(3H)-
one 13.13 Conversion of this material to the 4-chloro
(or 4-trifluoromethane- sulfonato) derivative
followed by nucleophilic displacement by an R6-E: '
nucleophile such as an amine, alkoxide, or thiol,
would give the 2,5,6-trisubstituted-4-carboethoxy- ~
pyrimidine 14. Hydrolysis of the ester would give the '-
corresponding 4-carboxypyrimidine.
Conversion of the 4-carboxypyrimidine to
the 4-acetyl derivative followed by peracid oxidation
and hydrolysis would give pyrimidinone 15 as
illustrated in Scheme 11. Scheme 12 shows how the
2,3,5,6-tetrasubstituted pyrimidin-4(3H)-one 16 could
be prepared from pyrimidinone 15. Scheme 13 shows how
the 2,4,5,6-tetrasubstituted pyrimidine 17 can be
prepared from intermediate 15 with the R8b ~ucleophile
as described above.l4 .Alternatively, one could use
triflic anhydride and hindered-amine base in place of
POC13.
- Scheme 14 illustrates how the pyrimidine ,~
ring system can be built onto what would become the
5-sidechain. Conversion of the bromide 18 to a-
Grignard reagent,`organo-zinc reagent, organo-lithium
, ,,reagent, or o.her-related or~ano-metal reagent
, ~ollowed by.addition of diethyl oxalate would give the
pyruvate derivative l9. -Addition of metho~y- or ~^
ethoxymethylenetriphenyl-phosphorane or related
.
.: . . . . . : . . ..
WO91~152~9 PC~/US91/01951
2~ 38 -
reagent would give the ethyl ~-ethoxyacrylate
derivative 20. Condensation of this material with an
R7-amidine or isourea would provide pyrimidinone 21 .
Conversion of the 4-hydroxy function to the methoxy
followed by addition of a Grignard reagent or
alkyllithium and oxidation with dichlorodicyanoquinone
would afford the 2,5,6-trisubstituted~4-methoxy-
pyrimidin-4(3H)-one 23. Conversion of the methoxy
back to a hydroxy then provides pyrimidinone 2.
Scheme 15 describes the preparation of ..
4-methoxy-5-bromopyrimidines 25 that may either be
converted into nucleophiles and added to the
electrophilic sidechain as shown in Scheme 16 or used
as electrophiles as illustrated in Scheme 17.
Condensation of ~-etho~yacrylate with R7-amidine or
isourea would provide pyrimidinone 24. Conversion of
this material to the 4-methoxy-5-bromopyrimidine 25 is
straight forward as.shown. .
: In Scheme 16 the 5-bromopyrimidine i~
converted to a Grignard, organolithium, or related
reagent then added to the electrophilic sidechain (a . -
catalyst or stoichiometric reagent such as CuCN may be
added to enhance nucleophilicity or selecti~ity if
.~ necessary) to give-pyrimidine 22 which may be used as
illustrated previously.
~ Scheme 17 provides a route to the same -
intermediate 22 by conversion of the bromide l8 to the
organo-metal reagent followed by addition .to the :
: ,.eIectrophilic 5-bromopyrimidine 25.
S.cheme-2l:illust-rates one specific method
used to prepare...two of-the more-preferred compounds in
T~ le l...Scheme 22:..illustrates another-specific :
method used to prepare a:~preferred compound in Table 3.
: ~ .
-: .
:
~ ,
'
WO91/15209 PCT/US91/01951
. z~ ;"/l
- 39 -
IDENTIFI~ATION OF REFERENCES CITED IN SCHEMES
W. Wierenga, ~.I Skulnick, ~g~_~yn_ (1982) 61 5.
D.J. Brown, The Pyrimidines, (1962), J. Wiley &
Sons, pp. 48-51.
3 S. Hirokami, T. Takahashi, K. Kurosawa, M.
Nagata, J. Org. Chem. (1985) 50 166.
T. Takahashi, S. Hirokami, M. Nagata, T.
Yamazaki, J. Chem. ~oc.. P~rkin Tr~ns. I (1988)
2653.
4 S.S. Ahmad, S.I. Haidea, I. Fatima, Svn Çomm.
(1987) 17 1861.
G.D. Daves Jr., F. Baiocchi, R.K. Robins, C.C.
Cheng, J. Org. Chem. (1961) 26 2756.
6 J.R. Marshall, J. Walker, J. Chem. Soc. (1951)
1004. `.. :`
7 T. Sakamoto, H. Yoshizawa, H. Yamanaka, Chem.
Phar~ ull. (1984) 32 2005.
8 J.C. Krauss, T.L. Cupps, D.S. Wise, L.B.
Townsend, Syn~hesis (1983) 308.
9 D.J. Brown, The Pyrimidines, (1962), J. Wiley
Sons, p. 333. -~
G. Shaw, G. Sugowdz, J. Chem. ~oc. (1954) 665.
Y. Honma, Y. Sekine, T. ~ashiyama, M. Takeda, Y. `~
Ono, K. Tsuzurahara, Chem. Pharm. Bull. ~1982) 30 ~
4314. -
11 M. Shur, S.S. Israelstam, J. Org. Chem. (1968)
3015.
F. Fulop, I. Hermec ~ Z. Mes~aros, G. Dombi. G.
Bernath, J. ~et._Chem. ~1979) 16 457.
P.L. Ferrarini~ C. Mori, O. Livi, G. Biagi, A.M.
~arini, J. ~et. Chem. (1983) 20 1053.
H. Antaki, V. Petrow, J. Chem. Soc. (1951) 551. --
: :,.', .
: ~ .. ~; .. .
.
W091/15209 PCT/US91/01951
- 40 -
12 M.W. Goldberg, F. Hunziker, J.R. Billeter, H.R.
Rosenberg, Helv. Chim. Acta. (1947) 30 200.
13 W.K. Hagmann, F.Z. Basha, M. Hashimoto, R.B. -
Frye, S. Kojo, S.M. Hecht, J. Org. Chem. (1981)
46 1413.
T.A. Riley, W.J. Xennen, N.K. Dalley, B.E.
Wilson, R.K. Robins, S.B. Larson, J. Het. Chem.
(1987) 24 955.
Y. Muraoka, T. Takita, K. Maeda, H. Umezawa, J. ~:
Antibio~ics (1970) 23 253.
M. Otsuka, S. Kobayashi, M. Ohno, Y. Umezawa, H.
Morishima, H. Umezawa, Chem. Pharm. Bull. (1985) :
~3 515.
G.D. ~avies, Jr., R. Baiocchi, R.K. Robins, C.C.
Cheng, J. ~Fg~ Chem.. (1961) 26 2755.
14 E. Ochiai, H. Yamanaka, Chem. Pharm. Bull. (1955)
3 173.
'; ' ' .
- ', .
: ; ,
- .
- .
.
: , ~ ,,
. :
: ~ ` ~ ; , - , I
..
~ ~ ~"" , , ~
Wo 91/1520~ PCr/USsl/01951
~ r~
- 41 -
S C~EME 1
~COOEt BuLi O O
COOH R6COCl R6~OEt
NaH, DMSO O
LGR ~ OEt R7--(/
CH2 NH2
CH2 ~ ::- .
R3b~R3a R ~}R3"
RZ b~
R7
N~N-H
R6 /~
CH2 --- LG=Cl, I, Br, - :
R3b_~}R3a OTf, OTS, OM~;
X : .. :
R2 b~
R2a ` ,
.'
'
. .
WO 91/15209
P~/US91/019~1
4 2 -
S CEIEMl~; 2
:.
R7 R7 R8 a
N~N- H N~N~
R6 /~ R6 ~ ,
CH2 NaH, D~ CH2
R3b~R3a R~ L" R3b~} _3_
~,Rl 1 P'~
R2 b~J R2 b~
~ ~ -
.
~ .
WO 91/15209 PCr/US91/01951
:
_ 4 3 ~ s ~L
S CEEME 3
R7-CN 1 )HC1, M~OH 7
R
2 ) R8 a NH /R~ a
H ;
; .,. ~
.,
_~ R7
NJ~N_ ~8 a
R~OEt R6 /~
CH2 CH2
R3b~R3a
X 1 X
1 3 . .
R2a R2a ...
,~
-~: .' '~'
:~ :
I '' -
WO 91/15209 PCr/US91/019~1
:
.
-- 44 --
S C~EME 4
R7 R7
N-- NH N ~
R ~O R6 ~1
CH2 POC13 C~I2
R3 b~R3 a r e f 1 ux R3 b~R3 a
X 2 X 4
J~R1 ~ ,R1
R2b~ R2b~
R2a R2a
- . '.
- , . ,:
WO 91/1~209
PCl/US91/019~1
-- 45 --
S CHEME 5
R7 R7
N-- ~ N ~ ~
R6 J~c1 R6 ~ ';
CHz CH2
R3b~}R3~ R3~ R3b~
X 4 X 5 -
~2 ~R
E~ a R2 a
. . .
, ~
!
- : .,
1 .''
WO 91/15209
PCr/USgl/01951
f~ ?.~7 ?~ - 46
S CHEME 6
~CN 2 BULi o
COOH R6COCl R6~N
NaH, DMSO
.. ... .
LG R,jJ~C
CH2 CH2
R3b~3--R3a R3b ~R3a ~ '
X X 6
R2b~Rl R2b~R1 '-'
R2a R2a
,.. . .. .. .
WO 91~15209 PCr/lJS91/019~1 ~
.
- 47 ~ ?;~
S CH~ME 7
O
R6 ~,C 1 )CH2N2 R7 :
CH2 R7 N~ N -~
R3b~R3a -- 2) HN NH2, R6
X CH2
R2a R3b~R3~i
~ ,R
R2b~ , . ' " .
R .
,: -,
" ,
.
: . ''' ;" . '
~ .;~ '.
.,
, .
-,~,,,,F
:: ~ : '~
: ' .,
~ ~ '' ""
WO 91/15209
PCrJUS9 1/0195 1
-- 4~3 --
2~ ?~ ~
S CHEMEQ
O N--O
f N R~
(CH,)r . (C~2),
~N-OH
Rlb~X R~ ~b_~-R~'
R7 b_~
R7 - COCl N--O O ~ Pd - C
---- - o~ R thon h~t:
(C~)r
_~R
R~' :
R7
N~{
~ .
Cl ~)r
X ':
R~r~ R'
. .
I -. .
. ". .. ., ,: ., , .. ' , , : ' : ' `':
WO 91~15209 PCT/US91/0195 1
- 4g - :
S CHEME 9
~26
,~R2 7
O O :, ' .
R6 /~OEt R2 6 ~O
R3b~R3~ HzN~ R3b~--R3a ~ ~
X X :
,, .,
R2 a R2 a ; .
1 0 . ,`:
'.,`.`'
s~
.
..
,~
WO ~1/15209 PCI/US91/019~1
. W~
Z' ~ 5 0
,~, ... .
SCHEME 10
Et OOC ~
COOH 2 BULi
Et OOC
then
LG CH2
CH2 R3 b~R3a
R3b~l?3a X 1 ,,
X RZ
R2 ~
then H30+
t hen heat
Base O
t henEt OOC~l COOEt
EtO~/ R3b~_R3a ~r~
1 2
2 b~
1?2a
.~ .
WO91/15209 ~,~ "~, ~ P~/US91/01951
; `~ ,.. ~:,
-- 51 --
'
SCHEME 10 (Cont ' d)
R7 R
~ ~ ''',.:
CH2 1 )POC13, h2at ~COOEt
1 3 R3b~R
R2~R R' R
~ ''' ' ~
.,
.:
' ' ' ~
~ ~ .:. ,
~ . ,' .
WO 91/15209
PCI/US91~019~1
- 52
SCHEME 11
R7 R7
~$CooEt 2) ~3Li ~EJ~O
lH2 3 ) RCO3H :lH2
R3b--~$}R3~ 4~ NaOH R3b-~_R3~
X 14 15
R2b~ _~Rl :
R2~ . '
~E=-S-)
~: .
: : .
' : .. .
WO 91/15209 P~/US91/01951
f~r.~!~,'q ~
- 53 -
S t;HEME 12
R7 R7 ~8a
N~ NH N ~ N
R E~ E~O
CH2 NaH, DMF CH2
R3b--~?3a R~-LG R3b ~
X 15 X 16
R2 b~ R2 b~ ~ :
RZa R2a
?~,
.', ', ': '' , . " . ,. .. , - ; . .' ' ~ :' '. , , .'' ' . - . '
WO 91/1~209
P~US91/01951
-- 54 --
SCHEME 13
R7 R7 :
R ~E ~o `E--~R8 b
CH2 1 ) Pocl3 CH2 ~ -~
2): R8b ,~
R3 b~ R3a R3b~3--R3a
X 15 X 17
R2b~R' R2b~Rl ..
R2a R2a '~:.::
~, , ', '
~: :
~.
: ',.,
..
: . :` '
WO 91~15209 PCJ/US91/01951
:
'~ .
-- 55 --
' , '
Sc EME 14
~r M
~;_ Et O ~OEt ~OEt
X R3 b~ R3n
R2~ R2 b_~
18 EtO O R2~ J~ .
N NH
OEt ~
- - CH2 1 0
_~_R3~ R7R3b--~; R
- RZ' X
_
,, .
~,
:
. .
:.
. . . . ., .. ... . . , . . ., .. ... , . _ .. .. ... . . . .. ...... . ..... . . , . . . . ~ . . ... . . ..
. . . .. .. . . .
WO 91/15209
Pcr/US9l/0195
SC~IEME 14 ~Cont ' d 2
1 )POC13 or ~$ 1 )R6-M
Tf OTf OMe
CH2 2 ) DDQ
2)M~ONa
R3b~R3a
R2 b~R1 .
R2a :
R 22 R7
N~ N~NH
R6/~OM~ NaI/AcOH R )~O
CH2 .. ~ CH2
R3b~ R30 R3b~R3a
~ R1 ~R -
R2 b~ R2 b ~ , , ' . .
R a R2a
23 2 !: ~
.
: ~ .
, . .... ... , . .. - .. , .. ~, . . .. - ...... . ... . ... .. , . ~ . . .. . .
:
Wo 91/15209 Pcr/U~9l/01951
2~
SCHE.ME 15
R7 7
EtO HN/J NH N/ NH
~o
,
COOEt 2
- 7
R
1 )POC13 or TfOTf N N
I I
2)M~ONa ~OM~
3 ) NBS Br
: :: Ac OH
: '
: ~
WO 91/15209 P(~/U.S91/D1951
2~
-- 58 --
S CHEME 16
N~ 1 ) M ~
N ' N
~OMe 2 ~ C UC N~ ,~ -:
~r t hen ~r
CH2
LG
2 5 CH2 R3b_~R3a
R3b~R3a X
~Rl
X R2 b~J
R2 b~ R2a
2 :
:~?2 a 2
" '
wo 91/15209 pcr/us91/ol95
- 59 -
S CEEME 17
N~N
Br ~O~b
CH2 1 ) M CH2
R3b~}R3a 2)c talyst+ R3b_~R3a
~R2~ 13r ~R~
18 25 22
: ~ ,
~ ~;:' ',''
~ '' . ~ .
Wo91~15209 PCT/US91/0l951
- 60 -
. .
Where condensations of N-C-N group (amidines,
isoureas, isothiuronium salts, etc.) with the C-C-C
group (generally the ~-keto esters) fail because of
initial addition of N-C-N group to the ester rather
than the keto group, the ~-keto esters may be
converted to ~-acetoxy, ~-ethoxy~ ~-enol phosphate,
~-enol triflate, or similar ~-leaving group
~,~-unsaturated esters. [E. Piers, et al,
Tet.L~., 25, 3155 (1984); M. Alderdice, ~1, Org.
Syn. 62, 14 (1984)] Such a reaction is illustrated
in Scheme 18. The N-C-N group may then be condensed
with the C-C-C group to give the expected
pyrimidinone or pyridopyrimidine.
.
'~. '
WO 91/15209 P(-r/US91tOt951
- 61 -
S CHEME 1 B
( Et ) 2P~
R~OEt
CH2 CH2
NaH, THF
R3b~R3a ( I;tO32POCl R3b_~R3a
X X
{~Rl R2 b ~ 26
R2a R2a
R7
N ~ NH
- R6 ~o
CH2
173~3b~}R3~
HN~NH2 X 1 2
R ~R
R2a :
, . . . :
- . .....
.... .. . . . . . . . . .. . .. .
. ~ .. . . ..
- : ....
.
WO 91/15209
PCT/US91~01951
2.. ~
- 62 -
Scheme 19 provides a route for the
preparation of acyl sulfonamides 27. The carboxylic
acid can be activated by conversion to the acid
chloride by various methods including treatment with
refluxing thionyl chloride or preferably with oxalyl
chloride and a catalytic amount of DMF at low
temperature.15 Activation by conversion to the acyl
imidazole can be achieved upon treatment of acid 26
with carbonyldiimidazole. N,N-Diphenylcarbamoyl
anhydride intermediates may be prepared as activated
carbonyls.16 Treatment of the activated carbonyls
with alkali metal salts of alkyl or aryl sulfonamides
or with the sulfonamide and DBU will give the
expected acyl sulfonamide 27.17
Scheme 20 provides a route to the isomeric
acyl sulfonamides 33. The commercially available
bromobenzenesulfonyl chloride 28 may be converted to
the corresponding sulfonamide upon treatment with
ammonia or ammonium carbonate. Protection with the
triphenylmethyl group gives sulfonamide 29.
Palladium catalyzed cross-coupling gives the biaryl
3Q 18 Treatment of this material with
N-bromosuccinimide and catalytic AIBN in refluxing
CC14 will give the alkylatin~ agent 31. The bromide
31 may now be used as the alkylating agent shown in
previous scheme~ to give intermediate 32.
Deprotection and acylation will give the acyl
sulfonamide 33.
.
A.W. Burgstahler, LØ Weigel, C.G. Shaefer, Synth~is
(1976~ 767.
16 F.J. Brown, et al, European Patent Application # EP 199~43 -
K.L. Shepard, W. ~alczenko, J. Het. Chem. (1979) 16 321~;~
17 J.T. Drummond, G. Johnson, Tet. Lett. (1988) 29 16~3.
18 T.R. Bailey, Tet. ~ett. (1986) 27 4407.
I.P. Beletskaya, I. Qr~a~ometallic Chem. (1983) 250 551.
-......
~ . . .
'.. .. .
WO 91/15:209 PCr/US91/01951
.
~r ~ n,~,~ A
-- 63 -
S C~EME 19
N~(R N~?
R_ E--~K R- E--~K
CHz CH2
R3b~_R30 R3 b~--R3
~ 1) ?Cnrboxyl oot~votlon ~
X X
~COOH 2) ~9OZN~ ~CON~ o2R23
R2b~ ' ~ b~- R2b_~ :
R2~ R20
26 27
.:
,
:: :
: :
*:~ a. SOC12, reflux
.: : ~ b. ~(COC1)2, DMF, -~0C
c. N (N,N- Diphenylcarban~I) p~ridinium chl~rido/
agueous. NaOH :~
: d. : carbonyl diimidazole
** :base can be NaEj KH, DBU
WO 91/15209 P~T/US91/0195
2~ ?;'~ 64-
S CEEME 2 0
.
~3r ~3r
~SO2Cl 1 ) NH3 or ,~S02NHCPh3
( NH4)2CO3
2 ) Ph3CCl
28 29
'~ .:
M3 ~*
Snl~3 ~/SOzNHCPh3 N~S, AI13N cat.
Pd ~ ~J CCl4 r~f lux
~3r ~ 7
R6
~sozNHcPh~ 2
~SO2NHCPh3
31 I~J
, . j, . . .
' "
-:
wo 91/15209 PCr/USsl/01951
SCHEME 20 (Cont. )
R6_E 1~,K
1 ) AcOH/HzO
2) R23 cocl CH2
~), ,": , ,,
I So2N~CORZ3
~/
.
33
: -
~ - ;
. .,, ~
- ~--s ~ ~,~ ~ ., . i: r,, ~, ! ~ , ~
WO 91/15209
PC~/US91/01951
2~,~ 66 -
;~, ~ ... . --
SC~EME 21 ~ -
~COOEt 2 eq. ~3uLi. THF O
COOH t hen ~
\~ COCl \/` OOEt
then H30~ - :
NaH, DMF
~3r ~COOEt ~
DMF, beat
M~ '
N~N 1 ) POCl3, heat
2) CuCN : .
3) H2O2, NaO~ M;OH : .
4) An~erlystR -15, M30~ heat
.
, .
WO 91/15209 PC~/US91/01951
-- 67 --
SC~IEMI: 21 (CONT':~)
Tr Tr
N~ `NN--N N--N
N~N NpN
`13
TEIF, ~Ph3P)2PdCl~(cat. ),
reFlux, 4 h
N~N
--~CO0~3 AcO~ H20 35 NaOE~ ~30H, 36
N I
N - N
Tr ME~
~ ~R
35 R=t~ .
U
..... ,.. :.:'. ' '
... .
~ ' .
: :
! :
WO 91/15209 PCI/US91/01951
:
. .
- 68 -
SC~IEME 22
~ ".
Et H2 N~NJ N~N ~ ~
~ PPA, hea t ~\~ ~ ~ -
34 ~ ~ :~
~ , .
Tr Me
N--N N--N 1 -
N~,~N N~N N~ `N
~Zn~, ~COO~b
~ ~ ~q ".
W~
THF, (Ph~,P)2PdCl2(cat- ),,N ~:J
ref lux. 4 h N
N--N ,
'
~b ,
NlN , . .
AcOH, H20 \[~
3 7,1~
N `H ::
~'' ,,." ': .
':. '
;
W091/15209 ~ ~ ~ PCT/US91/019~1
- 69 -
The compounds of this invention form salts
with various inorganic and organic acids and bases
which are also within the scope of the invention.
Such salts include ammonium salts, alkai metal salts
like sodium and potassium salts, alkaline earth metal
salts like the calcium and magnesium salts, salts
with organic bases; e.g., dicyclohexylamine salts,
N-methyl-~-glucamine, salts with amino acids like
arginine, lysine, and the like. Also, salts with
organic and inorganic acids may be prepared; e.g.,
HCl, ~Br, H2S04, H3P04, methanesulfonic,
toluensulfonic, maleic, fumaric, camphorsulfonic.
The non-toxic, physiologically acceptable salts are
preferred, although other salts are also useful,
e.g., in isolating or purifying the product.
The salts can be formed by conventional
means~such as by reacting the free acid or free base
forms of the product with one or more equivalents of
the appropriate base or acid in a solvent or medium
in which the salt is insoluble, or in a solvent such
as water which is then removed i~ ~acuo or by freeze-
drying or by exchanging the cations of an existing
salt for another cation on a suitable ion exchange
resin.
- Antiotensin II-(AII) is a powerful arterial
vasoconstrictor,-and it exerts its action by -
interacting with specific receptors present on cell
membranes. The compounds describediin-the present
invention act as competitive antagonists of AIISat~
the receptors. In order to identify AII antagonists
and determine their efficacy in vitro, the following
two ligand-receptor ~ ding assays were established.
': '
, '
.
`
. ~ ' ' , , .
wosl/l~2o9 PCTtUS91/01951
2 r; ~ 70
Receptor binding assay using rabbit aortae membrane
preparation:
Three frozen rabbit aortae (obtained from
Pel-Freeze Biologicals) were suspended in SmM -
Tris-0.25M Sucrose, pE 7.4 buffer (50 ml), ~ , '
homogenized, and then centifuged. The mixture was
filtered through a cheesecloth and the supernatant
was centrifuged for 30 minutes at 20,000 rpm at 4C.
The pellet thus obtained was resuspended in 30 ml of
50mM Tris-5 mM MgC12 buffer containing 0.2% Bovine
Serum Albumin and 0.2 mg/ml Bacitracin, and the
suspension was used for lO0 assay tubes. Samples
tested for screening were done in duplicate. To the
membrane preparation (0.25 mI) there was added
l25I-SarlIle8-angiotensin II [ovtained from New
England Nuclear] (lO ~l; 20,000 cpm) with or without
the test sample, and the mixture was incubated at
37~C for 90 minutes. The mixture was then diluted -
with ice-cold 50mM Tris-0.9% NaCl, p~ 7.4 (4 ml) and
filtered through a glass fiber filter (GF/B Whatman
2.4",diameter). The filter was soaked in
seintillation cocktail (lO ml) and counted for
radioactivity using Packard 2660 Tricarb liquid
scintillation counter. The inhibitory concentration
(IC50) of potential AII antagonist, which gives 50%
displacement of the total specifically bound : '
l25I-SarlIle8-angiotensin II, was presented as a
measure of the efficacy of such compounds as'`AII ,,
antagonists. - - ~ '-' ' " . ''
. . .
WO9]/15~0~ ~,~'~,~!'1 ~ PCTtVS91/019~1
- 71 -
Receptor assav usin~ BQvine adrenal cortex preparation
Bovine adrenal cortex was selected as the
source of AII receptor. Weighed tissue (O.l g is
needed for iO0 assay tubes) was suspended in Tris.HCl
(50mM), p~ 7.7 buffer and homogenized. The
homogenate was centrifuged at 20,000 rpm for 15
minutes. Supernatant was discarded and pellets
resuspended in buffer CNa2~P04 (lOmM)-NaCl
(120mM)-disodium EDTA (5mM) containing phenylmethane
sulfonyl fluoride (PMSF~(O.lmM)~. (For screening of
compounds generally duplicates of tubes are used).
To the membrane preparation (0.5 ml) there was added
3~-angiotensin II (50 mM) (lO ~l), with or without
the test sample, and the mixture wa incubated:at
37C for l hour. The mi~ture was then diluted with
Tris buffer (4 ml) and filtered through a glass fiber
filter (GF/B Whatman 2.4" diameter). The filter was
soaked-in scintillation cocktail (lO ml) and counted
for radioactivity using Packard 2660 Tricarb liquid
scintillation counter. The inhibitory concentration
(IC50) of potential AII antagonist, which gives 50%
displacement o~ the total specifically bound ~'
3H-angiotensin II, was presented as a measure of the
efficacy of such compoundæ as AII antagonists.
The,potential antihypertensive:-effects of
the compounds described in the present invention may -~
be evaluated using the methodology described belo~: ~
Male Charles,~iver Sprague-Dawley-rats.(300-375 gm) ' ,
were anesthetized with methohexital (Brevital; 50 ~
mg/kg i.p.). The trachea was cànnulated--with PE 205
tubing. A stainless steel-pithing rod (l.5 mm thick,
'~
WO 91J15209 PcI/l)S91/0195i
2 ;~
- 72 -
150 mm long~ was inserted into the orbit of the right
eye and down the spinal column. The rats were
immediately placed on a Harvard Rodent Ventilator
(rate - 60 strokes per minute, volumn - 1.1 cc per
100 ~rams body weight). The right carotid artery was
ligated, both left and right vagal nerves were cut,
the left carotid artery was cannulated with PE 50
tubing for drug administration, and body temperature
was maintained at 37C by a thermostatically
controlled heating pad which received unput from a
rectal temperature probe. Atropine ( lmg/kg i.v.)
was then administered and 15 minutes later
propranolol (1 mg/kg i.v.). Thirty minutes later
angiotensin II or other agonists were administered
intravenously at 30-minute intervals and the Increase
in the diastolic blood pressure was recorded before
and after drug or vehicle administration.
Using the methodolo~y described above,
representative compounds of the invention were -
evaluated and were found to exhibit an activity of at
least IC50 ~ 50~M, thereby demonstrating and
confirming the utility of the compounds of the
invention as effective AII antagonists. -
Thus, the compounds of the invention are
useful in treating hypertension. They are also of
value in the management of acute and chronic : -
congestive heart failure, in the treatment of
secondary hyperaldosteronism, primary and secondar-;
pulmonary hypertension, renal failure such as
diabetic neph_opathy, glomerulonephritis, -
sclerederma, and the-like,irenal vascular -
hypertens~ ; left ventricular-dysfunction, diabetic
retinopathy, and in the management of vascular
W091/15209 2 ~ ~n ~ ~ pcT/ussl/olss
- 73 -
disorders such as migraine or Raynaud's disease. The
application of the compounds of this invention for
these and similar disorders ~ill be apparent to those
skilled in the art.
The compounds of this invention are also
useful to treat elevated intraocular pressure and can
be administered to patients in need of such treatment
with typical pharmaceutical formulations such as
tablets, capsules. injectables and the like as well
as topical ocular formulations in the form of
solutions, ointments, inserts, gels, and the like.
Pharmaceutical formulations prepared to treat
intraocular pressure would typically contain about
0.1% to 15% by weight, preferably-0.5% to 2% by
weight, of a compound of this invention.
In the management of hypertension and the -~
clinical conditions noted above the compounds of this
invention may be utilizcd in compositions such as
tablets, capsule3 or eli~irs for oral administration,
suppositories for rectal administration, ~terile
solutions or suspensions for parenteral or
intramusoular administration, and the like. The
compounds of this invention can be administered to
patients (animals and human3 in need of such
treatment in dosages that will provide optimal
pharmaceutical efficacy. -Although the dose will vary
from patient to patient, depending upon the nature
.-and severity of disease, the patient~s-.weight,--
special diets then being followed by a patient,~
concurrent medication and other:factors,-which-:those
skilled in the art will recognize the dosage range
will generally be about l to lO00 mg. per~ ~ ient per
day which can be administered in single or multiple
;
~-'`' ": '
""' ;" ~ '",-,",'~,i,'' ~" ,,~ ",",,,~",", ,"~
WO 91 / 15209 PCI /US9 1 ~01 951
2~
-- 74 --
doses. Perferably, the dosage ran~e will be about
2.5 to 250 mg. per patient per day; more preferably
about 2.5 to 75 mg. per patient per day. ;~
The compounds of this invention can also be
administered in combination with other antihyper-
tensives such as diuretic~, angiotensin convertin~
enzyme inhibitors, clacium channel blockers or
~-blockers. For example, the compounds of this
invention can be given in combination with such
compounds as amiloride, atenolol, bendroflu-
methiazide, chlorothalidone, chlorothiazide,
clonidine, cryptenamine acetates-and cryptenamine :
tannates, deserpidine, diazoxide, guanethidene
sulfate, hydralazine hydrochloride, hydrochloro- : .
thiazide, metolazone, metoprolol tartate, methy-
clothiazide, methyldopa, methyldopate hydrochloride, ;
minoxidil, pargyline hydrochloride, polythiazide,
prazosin, propranolol, ~a~olfi~ se~p~ntiD~,
rescinnamine, reserpine, sodium nitroprusside,
spironolactone, timolol maleate, thrichlormethiazide,
trimethophan camsylate, benzthiazide, quinethazone,
ticrynafan, triamterene, acetazolamide, amino-
phylline, cyclothiazide, ethacrynic acid, furosemide,
merethoxylline procaine, sodium ethacrynate, .:
captopril,-delapril hydrochloride,-enalapril,
enalaprilat, fosinopril sodium, lisinopril,
pentopril, quinapril hydrochloride, ramapril,
teprotide, zofenopril calcium~ diflusinal~ diltiazem.
felodipine,1 nicardipine,:nifedipine, niludipine,. ~:
nimodipine, nisoldipine, nitrendipine, and the-Iike,
aæ well as admixtures and combinations thereof.- .
,~ ,
~ , .: .
'
WO9l/15209 PCT/US91/01~51
2~ ? ~?, ~, ~
Typically, the individual daily dosages for
these combinations can range from about one-fifth of
the minimally recommended clinical dosages to the
maximum recommended levels for the entities when they
are given singly.
To illustrate these combinations, one of
the angiotensin II antagonist of this invention
effective clinically in the 2.5-250 milligrams per
day range can be ef~ectively combined at levels at :
the 0.5-250 milligrams per day range with the
following compounds at the indicated per day dose
range: hydrochlorothiazide ~15-200 mg) chloro-
thiazide (125-2000 mg), ethacrynic acid (15-200 mg),
amiloride (5-20 mg), furosemide (5-80 mg),
propranolol (20-480 mg), timolol maleate (5-60 mg),
methyldopa (65-2000 mg), felodipine (5-60 mg),
nifedipine 5-60 mg), and nitrendipine (5-60 mg). In
addition, triple drug combinations of hydrochloro-
thiazide (15-200 mg) plus amiloride (5-20 mg) plus
angiotensin II antagonist of this invention (3-200
mg) or hydrochlorothiazide (15-200 mg) plus timolol
maleate (5-60) plus an angiotensin II antagonist of
this invention (0.5-250 mg) or hydrochlorothiazide
(15-200 mg) and nifedipine (5-60 mg) plus an
angïotensin II antago~ist of this invention (0.5-250
mg) are effective combinations to control bllod
pressure in hypertensive patients.- Naturally, these :
dose ranges can be adjusted on a unit basis as -- - -
necessary to permit divided daily.dosage~and, as~
noted above, the dose will vary depending on the-: ,
nature~and sevêrity of the,disease, weight of ;~ -
patiènt, special diéts and other fàctors.
~ .
WO91/1520~ PCTtUS91/01951
76 -
Typically, these combinations can be
formulated into pharmaceutical compo~itions as
discussed below.
About l to lO0 mg. of compounds or mixture
of compounds o~ Formula I or a physiologically
acceptable salt is compounded with a physiologically
acceptable vehicle, carrier, excipient, binder, ". -~'
preservative, stabi~izer, flavor, etc., in a unit
dosage form as called for by accepted pharmaceutical '~
practice. The amount of active substance in these
compositions or preparations is such that a suitable
dosage in the range indicated is obtained. '
Illustrative of the adjuvants which can be -
incorporated in tablets, capsules and the like are
the following: a binder such as gum tragacanth,
acacia, corn starch or gelatin; an excipient sich as
microcrystalline cellulose; a disintegrating agent
such as corn starch, pregelatinized starch, alginic
acid and the like; a lubricant such as magnesium
stearate; a sweetening agent such as sucrose, lactose
or saccharin; a flavoring agent such as peppermint,
oil of ~integreen or cherry. When the dosage '
unitform is a capsule, it may contain, in addition to
materials of~the above type, a Iiquid carrier such as
fatty oil. 'Various other materials may be present as
coatings or to otherwise-modify the physical form of
the dosage unit; For instance', tablets may be coated
with shellac, sugar or both. A syrup or elixir m~-:
contain the~active compound,'''sucrose as a sweétëning
agent, methyl and~propyl'-parabens'as preservatives, a
dye and a flavoring such as cherry or orànge flavor.
~ .
. . .
"'" ' '', '., ' ' ' , ." ' ' :', '' '' ," ' .' . ' ,' , : . ': ' :~,
.. .. .
W O 91/15209 PC~r/US91/01951 ' n ?.i~
Sterile compositions for injection can be
formulated according to conventional pharmaceutical
practice by dissolving or suspending the active
substance in a vehicle such as water for injection, a
naturally occurring vegetable oil like sesame oil,
coconut oil, peanut oil, cottonseed oil, etc., or a
synthetic faffy vehicle like ethyl oleate or the - -
like. Buffers, preservatives, antioxidants and the
like can be incorporated as reguired.
The following examples illustrate the
preparation of the compounds of formula (I> and their
incorporation into pharmaceutical compositions and as
such are not to be considered as limiting the
invention set forth in the claimæ appended hereto.
All lH-NMR spectra were recorded on a Varian XL-300
Fourier transform spectrometer or on a Bruker 250 MHz
spectrometer. Chemical shifts are reported as (parts
per million) downfield from tetramethylsilane. Mass
spectra were obtained from the Merck and Co. mass
spectral facility in Rahway, N.J. Analytical TLC was
conducted on E. Merck precoated silica plates (0.25
mm in glass, Kieselgel 60 F254~ with W
visualization. All chromatography was conducted on -
E. Merck silica gel. All reactions were carried out
under an atmosphere of dry nitrogen under standard
conditions for those skilled in the art.-. --
. .
. .
,: ; . -; - ~- ,
,.
-
W091/15209 PCT/US91/01951
2;~,-7~.?.!~ ~
- 78 -
The useful central nervous system ~CNS) - -
activities of the compounds of this invention are ~ ~-
demonstrated and exemplified by the ensuing assays.
CQGNITIVE FuN~TIoN ASSAY
The efficacy of these compounds to enhance
cognitive function can be demonstrated in a rat
passive avoidance assay in which cholinomimetics such
as physostigmine and nootropic agents are known to be
active. In this assay, rats are trained to inhibit
their natural tendency to enter dark areas. The test
apparatus used consists of two chambers, one of which
is brightly illuminated and the other is dark. Rats
are placed in the illuminated chamber and the elapsed
time it takes for them to enter the darkened chamber .
is recorded. On entering the dark chamber, they
receive a brief electric shock to the feet. The test
animals are pretreated with 0.2 mg/kg of the
muscarinic antagonist scopolamine which disrupts
learning or are treated with scopolamine and the
compound which is to be-tested for possible reversal
o~ the scopolamine effect. Twenty-four~hours later,
the rats are retu-rned to-the illuminated chamber.
Upon-return to the illuminated chamber, normal young
rats who have been subjected to this training and who
have been treated only with control vehicle take -
longer to re-enter the dark chamber than test animals
who have been exposed to the apparatus but who have
not received a shock. Rats treated with scopolamine
be~ore training do not Rhow this hesitation when
tested 24 hours later. Efficaciou ~ est compounds can
~ ~ .
- .~
. . :. : ' ` ' . ` ' ' ', , , , `', ` : , , , , , ` ' : ', . ,
W O 91/15209 PC~r/US91/Ol9Sl
2~. ~ ?~
- 79 -
overcome the disruptive effect on learning which
scopolamine produces. Typically, compounds of this
invention should be efficacious in this passive
avoidance assay in the dose range of from about 0.1
mg/kg to about 100 mg/kg. .
ANXIOLYTI~ ASS~Y
The anxiolytic activity of the invention
compounds can be demonstrated in a conditioned
emotional response (CER) assay. Diazepam is a
clinically useful anxiolytic which is active in this
assay. In the CER protocol, male Sprague-Dawley rats '
(250-350 g)
are trained to press a lever on a variable int:erval
(VI) 60 second schedule for food reinforcement in a .'
standard operant chamber over weekly (five days per
week) training sessions. All animals then receive
daily 20 minute conditioning sessions, each session
partitioned into alternating 5 minute light (L) and 2
minute dark (D) periods in a ~ixed LlDlL2DZL3
sequence. During both periods (L or D), pressing a
lever delivers food pellets on a VI 60 second
schedule: in the dark (D), lever presses also elicit
mild footshock (0.8 mA, O.5 sec) o~ an independent
shock presentation schedule of VI 20 seconds. Lever
pressing is suppressed,during,the dark periods
reflecting the formation of a conditioned emotional
response (CER)., ,-,, , ,- , i- ,
~ -, Drug-.testing,;in this paradigm is carried out
under e~tinction conditions. During"extinction,
animals,learn;that:responding for-food in the dark is
no longer punished by,shock. Thereforel response - , -
rates gradually increase in the dark periods and
~- .
~ .
Wo91/15209 PCT/US91/01951 '-
~d ~ .; Y. .t
- 80 - '
animals treated with an anxiolytic drug show a more
rapid increase in response rate than vehicle treated '
animals. Compounds of this invention should be ''
efficacious in this test procedure in the range of ':'
from about O.l mg/kg to about lOO mg/kg.
DEPRESSION ASSAY
.~.
The antidepressant activity of the compounds
of this invention can be demonstrated in a tail
suspension test using'mice. A clinically useful
antidepressant which serves as a positive control in
this assay is desipramine. The method is based on
the observations that a mouse suspended by the tail
shows alternate periods of agitation and immo~ility
and that antidepressants modify the balance between
these two forms of behavior in favor of agitation.
Periods of immobility in a 5 minute test period are
recorded using a keypad linked to a microcomputer
which allows the experimenter to assign to each
animal an identity code and to measure latency,
duration and frequency of immobile periods.
Compounds of this invention should be efficacious in '
this test procedure in the range of from abou~ O.l
mg/kg to about lOO m~/kg.~
~ SCHIZOPHRENIA ASSAY
. . - - ................................................... ~ : .
The anti'dopaminergic activitv of the
:- compounds of.Xhis invention can b'e demonstrated in an
apomorphine-induced~sterotypy model:.- A'clinically
useful antip'sychotic d'rug that is;used' ~ po'sitive ~ -
control-in this asæay is haloperidol."' The assay
method is based upon the obser~ation that stimulation
, ~ . .
::
,, . ,: . .. . .,,;; ,-. , . . . : ~ ., ., :, . , : ~ . . .
WO91/15209 ~ PCT/US91/01951
- 81 --
of the dopaminergic system in rats produces stereo-
typed motor behavior. There is a strong correlation
between the effectiveness of classical neuroleptic
drugs to block apomorphine-induced stereotypy and to
prevent schizophrenic symptoms. Stereotyped behavior
induced by apomorphine, with and without pretreatment
with test compounds, is recorded using a keypad
linked to a microcomputer. Compounds of the inven-
tion should be efficacious in this assay in the range
of from about O.l mg/kg to about lO0 mg/kg.
In the treatment of the clinical conditions
noted above, the compounds of this invention may be
utilized in compositions such as tablets, capsules or
elixirs for oral administration, suppositories for
rectal administration, sterile solutions or snspen-
sions for parenteral or intramuscular administration,
and the like. The compounds of this invention can be `
administered to patients (animals and human) in need
of such treatment in dosages that will provide
optimal pharmaceutical efficacy. Although the dose
will vary from patient to patient depending upon the
nature and severity of disease, the patient's ` -
weight, special diets then being followed by a
patient,-concurrent medication, and other factors
which those skilled in the art will recognize, the
dosage range will generally be about 5 to 6000 mg.
per patient per day which can be administered in
single or multiple doses. Perferably, the dosage
range will be about lO to 4000 mg. per patient per
day; more pr~ferably about 20 to 2000 mg. per patient
per day.
In order to obtain ma~imal enhancement of
cognitive function, the compounds of this invention
.... - .. - - . - -.. - .,;. - . . , - . .. - j.. - ., . , .. , - .. , .. . - .. . ... . . . .
: . - :- . .: . ~i, " ,,, ", , " " , ,,, ~ :
: ~ . ~. . . . . . . ..... . . . .
WO91/15209 PCr/US91/01951
2~t5~ 82 -
may be combined with other cognition-enhancing -~
agents. These include acetylcholinesterase inhibitors
such as heptylphysostigmine and tetrahydroacridine
~THA; tacrine~, muscarinic agonists such as
oxotremorine, inhibitors of angiotensin-converting
enzyme such as octylramipril, captopril, ceranapril,
enalapril, lisinopril, fosinopril and zofenopril,
centrally-acting calcium channel blockers and as
nimodipine, and nootropic agents such as piracetam.
In order to achieve optimal anxiolytic
activity, the compounds of this invention may be
combined with other anxiolytic agents such as
alprazolam, lorazepam, diazepam, and busipirone.
In order to achieve optimal antidepressant
activity, combinations of the compounds o~ this
invention with other antidepressants are of use.
These include tricyclic antidepressants such as
nortriptyline, amitryptyline and trazodone, and
monoamine oxidase inhibitors such as tranylcypromine.
In order to obtain maximal antipsychotic
activity, the compounds of this invention may be
combined with other antipsychotic agents such as
promethazine, fluphenazine and haloperidol.
. .
'' ' : . -
.. . . .
.. . . .
:~ .
' ` ~.
Wog~ 209 PCT/US9 1/01951
- 83 -
PREPARATION OF INTERMEDIATES
2-t-~UTOXYCARBQNYL-4'-METHYLBIPHENYL
To a solution of p-bromotoluene (30g) in
dry ether (150 ml) at -78C,. a solution of t-BuLi in
pentane (1.7M) (210 ml) was added slowly over a
period of 1.5 hr using a dropping funnel. The bath
was then removed and the mixture was stirred at room
temperature for an additional 2 hours. The content
of the flask was then added slowly (using a cannula)
at room temperature to a premixed solution of ZnC12
in ether (lM, 180 ml) and dry THF (360 ml). The
mixture was stirred for 2 hr at.that temperature and
then the slurry was added (using a cannula) to a
solution of 2-t-butoxycarbonyl iodobenzene (35.6 g)
and NiCl~(Ph3P)2 (2.1 g) in dry THF (360 ml). The
mixture, after stirring at room temperature overnight
(18 hr), was poured slowly under stirring into -
ice-cold 0.5N HCl (1500 ml). The organic layer was
separated, and the aqueous phase was extracted with
ether (3 X 300 ml). The combined organic layer was
washed with water, brine and then dried over MgS04. -
Removal of the solvent gave the crude product as an
oil (32 g). The materi-al was purified on a
. silica-gel flash column using ethyl acetate-hexane
12) to give the titled compound as an oll (24 g,
76%). lH NMR..(CDC13): ~ 1.24 (s,9H) 2.42 (s,3H),
7.2-7.8 (m,8H-); FAB-~S: m/e 269(M+H~
..~
' :.
. ~:, :: :
. . ~ . ' ' ~ ~ ' . ' ' , ' i .
' i~ . .,, '; `i`,: '.~ :
WO91/15209 PCT/US91/0l951
~r ~
~,~, ~..,
- 84 -
4-BROMOMETHYL-2'-t-BUTOXYCARBONYL-BIPHENYL
To a solution of 2-t-butoxycarbonyl-h'-
methylbiphenyl (25.3 g, 95 mmol) in CC14 (200 ml)
were added freshly opened N-bromosuccinimide (17.6 g,
0.099 mole) and dibenzoyl peroxide ~2.28 g, 0.0094
moles). The mixture was refluxed for 4 hours, cooled
to room temperature and filtered. The filtrate was
washed with sat. NaHS03 (lx50 ml), sat. NaHC03 (lx50
ml), water (lx50 ml), sat. NaCl (lx50 ml) and dried
over MgS04. The solution was filtered and
concentrated in vacuo. The residue was dissolved in
100 ml of hot hexane. Crystallization gradually took
place as the solution cooled. The flask was finally
cooled to -20C and the precipitate recovered by
filtration. The solid was washed with ice cold
hexanes and dried in vacuo to give 27 g (88%~ of a
white solid. l~_NMR (CDC13):1.23 (s, 9H), 4.53 (s,
2H), 7.2-7.5 (m, 7H), 7.68 (d, lH).
2-CYAN0-4'-MET~YLBIPHENYL
To a solution of p-bromotoluene (30 g) in
dry ether (150 ml) at -78C, a solution of t-BuLi in
pentane (1.7 M) (210 ml) was added slowly o~er a
period of 1.5 hr-,-using a dropping funnel~. The bath
was -then removed and the mixture was stirred at room
temperature for an additional 2 hr. The contents of
the flask was then added slowly (using a cannula) at
room temperature to a.premi~ed solution ^f ZnC12 i~
ether (lM) (180 ml) and dry THF (3~0 ml). The
mixture was s~irred for 2h at that temperature and
then the slurry was added (using a cannula) to a
solution of 2-bromobenzonitrile (21.3 g) and i~.
W091/1~209 PCT/US91/01951 ''
2 ~..
- 85 -
NiCl2(Ph3P)2 (2.l g) in dry THF (300 ml). The
mixture, after stirring at room temperature overnight
(18h), was poured slowly under stirring into ice-cold
lN HCl (1500 ml). The organic layer was separated,
and the agueous phase was extracted with ether (3 X
300 ml). The combined organic layer was washed with
water, brine and then dried over MgS04. Removal of
the solvent gave the crude product as a semisolid
mass (34 g). The material was purified on a ~'
silica-gel flash column using ethyl acetate-hexane
(l:12) to give the desired nitrile as a low-melting , ':'
solid (28 g, 88%). lH NMR (CDGl3): 2.42 (s, 3H), ~'
7.2-7.8 (m, 8H); FAB-MS: m/e 194 (M++l). :
TRIM~I~YLSTANNYL A~IDE : ~:
To a concentrated solution of NaN3 (1.2 kg, '',
18.5 moles) in water (3 L), a solution of ,~,
trimethyltin chloride (600 g, 3 moles) in dioxane ~
(400 ml) was added in three portions under vigorous ;-~,'
stirring. A precipitate formed instantaneously. The
mixture, after stirring overnight at room
temperature, was filtered. The residue was washed
with water and dried under suction and then in vacuo ~,
over P205. Yield 541 g (88a/o)~ mp-l20-l22C. ~ -
5- r 2-(4'-M~T~YLBIP~ENYL~lTETRAZOLE
To a solution of 2-cyano 4'-methylbiphenyl '-
(390 g, 2.02 moles) in toluene ~2.3 L) was added- '~
trimethyltin azide (525 g, 2.55 moles) at,r.t. The
mixture was refl,uxed for-24,h.,cooled to r.t.,
.; filtered, washed with,t,oluene and sucked-dry -in~a
funnel. The-precipitate was resuspended in toluene -~
. . .
:
.
,~ ~ " '' ';' -:
~: ` . .
WO 91/15209 PCI/US91/01951
Z~ ?~
(3.5 L) and THF (250 mL) was added. Anhydrous HCl '~
was bubbled in at a moderate rate at r.t. to give a
clear solution (45 min). Addition of HCl gas was
continued for another 20 min. with stirring whereupon
a white precipitate formed. The reaction mixture was
stirred over night. The solid product was filtered, '~
washed with toluene followed with ether and then
dried under vacuum. This produced 250 g (53% yield
of the tetrazole. m.p. 152-154C; l~-NMR (CDCl3):2.40 ;~
(s, 3H), 7.19 (dd, lH), 7.55 (m, 2H), 8.25 (dd, lH).
N-TRIpHEN~LMET~yL-5-~2-(4l-METE~LBIp~ENyL)] ~ETRAZOLE
To a cloudy solution of 5-~2-(4~-methylbi-
phenyl)]tetrazole (250 g (1.06 mole) in CH2C12 (4 L)
was added triphenylmethylchloride (310 g l.ll mole) ~ '
:
at r.t. The reaction mixtur~e was stirred and '
triethylamine (190 mL, 138 g, 1.36 mole) was added -
portionwise. After addition, the mixture was stirred
at reflux for 90 min. The solution was cooled to
r~.t.~, washed with water (2xl~L) and dried over MgS04,
`fi~ltered;;th~rough a silica gel plug and concentrated
on the~rotovap;to a~solid. This was cry~tallized ''
from toluene`to-~give the~product as an off-white ' ' :
solid~(425 g,~84%),~m.p. 166-168 C; lE- ~ (CDC13):
2.~28~<s,~3~ 6.9-7.05 (m, lOH), 7.2-7.5 (m, 12H),
YL~ET~YL-5-t2-<4'-BROMOMETHYLBIPHENYi)~
T~TRAZOLE
;To~a~-solution~of~:N-triphenylmethyl-5-'-' , '
2 ~ 4'-methylbipheny'1)3~tetrazole (425-g, O.89-moles)
in~14~4.0:~L)'~werè~added~N-br'omsuccinimide (159 g,
WO91/15209 2~'J ~S~ PC~/US91tO1951
0.89 mole) and dibenzoyl peroxide (22 g, 0.089
moles). The mixture was refluxed for 2 hours, cooled
to room temperature and filtered. The filtrate was
concentrated in vacuo to give a thick oil. The
addition of ether (2.0 L) to this oil resulted in a
clear solution. Crystallization, followed by
filtration, gave a white solid (367 g, 74%). m.p.
137-139.5C; lH-NMiR (CDC13): 4.38 (s, 2H), 6.9~8.0
(m, 23H).
ETHYL 3-OXOHEPTANOATE
A two liter, three neck, round bottom flask ~-; -
equipped with a mechanical stirrer was charged with
50 g ethyl hydrogen malonate, 875 mL dry THF, and a
few milligrams of l,10-phenanthroline as indi:cator,
under dry nitrogen. The stirred mixture was cooled
to -78OC. To this was added 308 mL 2.5 M
n-butyllithium in hexanes over 30 minutes until a
brown color persisted several minutes. The mixture
was warmed to 0C for -30 minutes then was cooled to
-78C again. To this was added 22.5 mL valeroyl
chloride in Z5 mL TXF over 15 minutes. The mixture
was then warmed to room temperature, stirred 15
minutes, and acidified with ~150 mL 5% ~Cl. The
mixture was extracted 3 times with ether. The
combined organic material was washed twice with -
saturated NaHC03 solution and twice with brine, dried
over Na2SO4, stripped of solvent in-vacuo. and
distilled at-~15 Torr with the title compound:-
- ;distilling a~ 80-83C. -The title compound-was~
isolated as a--clear oil, 21.4 g, 66% yield. Rf 0;30
i ~ % EtOAc/hexane,- visualized by W and~-ninhydrin
.. .. . - ..
., : .,
' : '
WO 91/1~209 PCr/US91/01951
2~ ?.~ ~
stain; lH-NMR ~250 MHz, CDC13): ~ 4.19 (q, 2H), 3.44
(s, 2H), 2.54 (3 line m, 2~), 1.59 (m, 2~), 1.30 (m,
2H), 1.28 (t, 3H), 0.90 (t, 3H).
3-OXOHEPTANENITRILE
To a mechanically stirred solution of 14.3
g MgS04-dried cyanoacetic acid and ~100 mg
l,10-phenanthroline in 500 mL T~F at -780C was added
60 mL 2.5 M n-butyllithium in hexanes (~one half of
the total). The indicator color persisted at this
point. The solution was warmed to -5 to +5C after
which the indicator color disappeared. Another 55 mL
2.5 M n-butyllithium in hexanes was added until the
indicator color again persisted. The mixture was
cooled to -78C then 10.0 mL valeroyl chloride in 10
mL THF was added over 3 minutes. After 10 minutes
the now yellow solution was allowed to warm to room
temperature and stir for 1 hour. The mixture was
poured into a solution of 50 mL concentrated HCl in
300 mL water. The mixture was extracted 3 times with
ether. The combined organic material was washed
twice with saturated NaHC03 solution then once with
brine. The washes were back extracted with ether and
the back extracts were washed with brine. The back
extracts were combined with the other organic
material and then were dried over MgS04. The organic
material was stripped of solvent in vacuo then was
distilled at ~l Torr with the title compound
distilling at-87-91C. The title compound was
isolated.;as a.clear oil,.6.32 g,--60% yield.:-To-this
: material there was added 1% by~weight BHT to prevent
polymerization. The material was also kept ^
refrigerated at 0C under nitrogen. Rf 0.18 in 20%
~ ` .
. ~.
. ^ - .
j:
WO91/15209 ~ '~` PCT/US91/01951
.
.
- 89 -
EtOAc/hexane, visualized by ninhydrin stain, lH-NMR .
(300 MHz, CDCl3): ~ 3.46 (s, 2H), 2.62 (3 line m,
2H), 1.61 (m, 2H), 1.35 (m, 2H), 0.92 (t, J=7.3Hz,
3H); 13C-NMR (75.4 M~Iz, CDCl3): ~ 197.6, 113.8,
41.9, 31.9, 25.3, 22.0, 13.7. -;
ETHYL 2-[(2'-(N-TRIPHENYLMETHYL-TETRAZOL-5-YL)~IPHEN-
4-YL~METHYLl-3-OXOHEPTANOATE .:
To a solution of 370 mg ethyl 3-oxoheptan- -~
oate in lO mL DMSO was added 86 mg 60% Na~ in oil.
After two minutes 600 mg N-triphenylmethyl-5-[2-(41-
bromomethylbiphenyl)]tetrazole was added all at once .
to the solution. After 20 minutes the solution was .. ~:
poured into brine and extracted 3 time~ with ether.
The organic material was dried over MgSO4, stripped .
of solvent in vacuo, and MPLC'd in 10% EtOAc/hexane. .
The title compound was isolated as a white foam. Rf
0.30 in 20% EtOAc/hexane, visualized by W and
ammonium molybdate/ceric sulfate stain; dialkylated
material was observed at Rf 0.21; l~-NMR (300 MHz, .
CDCl3): ~ 8.l9 (4 line m, lH), 7.57 (ll line m, 2H), :
7.4Q (4 line m, lH), 7.19 (m, 4~), 4.17 (4 line m, `.:
2H), 3.79 (3 line m,. lH), 3.19 (d, J=8.3 Ez, 2~),
- 2.66-2.32 (m, 2H),- 1.54 (m, 2H), 1.28 (m, 2H), 1.24
(3 line m, 3H),-0.88 (3 line m, 3H). Spectrum
recorded after detritylation in MeO~/HCl. .. ~
Z-[(2'-(N-TRIPHENYLMETHYL-ThTRAZOL-5-YL~BIPHEN-4-
YL)M~THYLl-3-OXO~EPTANENITRILF
. To a solytion of 225 mg 3-oxoheptanenitrile
in lO mL DMSO was added 144.-mg 60% NaH in oil. After
two minutes 500 ~g~:N-triphenylmethyl-5 ~2-(4'-
: ;:
.
WO 91/15209 PCI/US9i/019~1
2 ~ ~ ~ 7
- 90 -
bromomethylbiphenyl)]tetrazole was added all at once
to the solution. After ~0 minutes the solution was
poured into brine and extracted 3 times with ether.
The organic material was dried over MgS04, stripped
of solvent in vacuQ, and MPLC~d in 15% EtOAc/hexane.
The title compound was isolated as a white foam, 125
mg, 23% yield. Rf 0.23 in 20% EtOAc/hexane,
visualized by W and ammonium molybdate/ceric sulfate
stain; lH-NMR (300 MHz, CDCl3): ~ 7.93 (m, lH), 7.47
(10 line m, 2H), 7.40-7.20 (m, lOH), 7.04 (m, 4H),
6.90 (m, 6E), 3.44 (X of ABX, lH), 3.03 (AB of ABX,
JAB=13.8 Hz, JAX=8.6 Hz, J~X=5 3 Hz, ~v=43.5 ~z, 2H),
2.59 (sym. 12 line m, 2H), 1.55 (m, 2H), 1.28 (m,
2H), 0.88 (t, J=7.3 Hz, 3H).
~ B~pLE 1
Ethvl 2-r(2'-(N-triphenylme~thvl-tetrazol-5-y~ hen-
4-vl)methyl]-3-(trifluoromethanesulfonato)-2~Z)-
heptenoate
The title compound is prepared by
dissolving 1.0 equivalent of ethyl 2-[2'~(N-triphenyl-
methyl-tetrazol-5-yl)biphen-4-yl)methyl]-3-oxoheptan-
oate in.THF so that the solution is ~0.1-0.3 M. To
this is added 1.3 equivalents of sodium hydride.
After 10 minutes a room temperature, 1.2 equivalents
of trifluoromethanesulfonic anhydride is added. When
the reaction is complete the mixture is neutralized
with ~saturated NaHC03 solution-and extracted with
ether. The combined organic matërial:is-dried over
-` MgS04, strip~ed of solvent in vacuo,- and MPLC~d to
--give .the title compound.
WO91/15209 P~ S91/01~51
~` .,
-- 91 -- ,
_AMPLE~2
6-Butvl-2-methvl-5- r ( 2'-(N-triphenvlmethvl-tet~azol-5-
vl)biphen-4-vl~methvllpyrimidin-4(3H~-one
The title compound may be prepared by
dissolving 1-5 equivalents of acetamidine
hydrochloride and an equal mole-equivalent of sodium
methoxide or sodium acetate in DMF. To this mixture
is added 1.0 equivalent of ethyl 2-C(2'-(N-triphenyl-
methyl-tetrazol-5-yl)biphen-4-yl)methyl]-3-
~trifluoromethanesulfonato)-2(Z)-heptenoate. Enough
DMF is used so the the solution is ~0.1-1.0 M in the
heptenoate compound. The mixture is either stired at
RT or is heated to reflux, until complete. The
mixture is poured into brine and extracted with
ether. The combined organic material is dried over
MgSO4, stripped of solvent in vacuo, and MPLC'd to
give the title compound.
EXAMPLE 3
6-Butyl-2-methvl-5-r(2'-(tetrazol-5-yl~iphen-4-yl)-
methyllpyrimidin-4-(3H~-one
The title compound may be prepared by
dissolving 6-butyl-2-methyl-5-[(21-(N-triphenylmethyl-
tetrazol-5-yl)biphen-4-yl)methyl]pyrimidin-4(3~)-one
in methanol and adding excess co~centrated ~Cl and
stirring for 10-30 minutes. An indicator quantity of
phenolphthalein is added followed by 10% NaO~ .
solution until pink. Excess acetic acid~is added .~n~!
the -mixture is-extracted three times with e~her.
The combined organic material is dried-over.MgSO4,
-,stripped of solvent in vacuo, and MPLC'd to give the
title compound.
'': -
WO91/15209 PcT/ussl/ol951
2~ 7~ 92 -
EXAMPLE 4
6-Butyl-2-methyl-5-r(2'-(tetrazol-5-yl)~iphen-4-yl)
methvl~4-trifluoromethan~sulfonatopyrimidine
To a solution of 1.0 equivalents of
6-butyl~2-methyl-5-[(2'-(tetrazol-5-yl)biphen-4-yl~
methyl]pyrimidine-4(3H)-one and 5.0 equivalents of
2,4,6-collidine in methylene chloride at 0C is added
2.4 equivalents of trifluoromethanesulfonic
anhydride. When the reaction is complete, the
mixture is washed twice with saturated CuS04
solution, dried over MgS04, and stripped of ~olvent
in vacuo.
EXA~PL~ 5
6-Butyl-4-cya~o-2-methyl-~-[(2'-(tetr~zol-5-yl)biph~n-
4-vl)methvllpvr mi~in~
A solution of CuCN and 6-Butyl-2-methyl-5-
[(2'-(tetrazol-5-yl)biphen-4-yl)methyl]-4-trifluoromet
hanesulfonatopyrimidi~e in pyridine is heated to give
the title compound with standard brine workup.
EXAMPLE 6
6-Butyl 4-carboxv-2-~.ç~hyl-5- r (2'-(tetrazol-5-yl)bi- -
~hen-4-yl)methvllpyrimidine -- ~-
-- ~~ Heating 6-Butyl-4-cyano 2-methyl-5-[(2'-
(tetrazol-5-yl)biphen-4-yl)methyl]pyrimidine with
several equivalents of sodium hydroxide and excess
hydrogen peroxide-in methanol provides the
corresponding primary-amide after~workup. --Treatment
of this mater~al.~with concentrated~HCl with warming
-~following by an-neutralization and extractive workup
gives th ~title compound. ~ ~~
.
- :, ~ :. -~. ,: . , ,. :, . :. . .;... . .. . . .. . ...
WO~1/15209 Pcr/us9l/ol9
~ 3~
- 93 -
EXAMPLE 7
6-Butvl-4-methoxvcarbonyl-2-methyl-5-r(2'(tetrazol-5-
yl)biphen-4-vl)methyllpyrimidin
Step A: Ethyl 2-[(4-iodophenyl)methyl]-3-oxohep-
tanoate
To a solution of 3.77 g (43.8 ~mol) ethyl
3-oxoheptanoate in 200 mL DMS0 was added 1.75 g ~43.8
mmol) 65% NaH in oil. After 10 minutes, 6.5 g (21.9
mmol) a-bromo-4-iodotoluene was added. The mixture
was allowed to stir for 2 hours. It was then poured
into an ice/brine mixture and extracted 3 times with ~-
ether. The combined organic material was washed with -
brine, dried over MgS04, stripped of solvent in
vacuo, then Still flash chromatographed in 40alO
CH2C12/hexane to give 2.46 g of the title compound,
29% yield. Rf 0.23 in 40% CH2C12/hexane, visualized
by UV and ninhydrin stain;
l~-NMR (300 M~z, CDC13): ~ 7.58 (m,ZH), 6.93 (m,
2H), 4.14 ~m,2~), 3.72 (3 line m, lH), 3.09 (m, 2H),
2.44 (12 line m, 2H), 1.50 (m, 2H), 1.22 (m, 2H),
1.21 (3 line m, 3H), 0.86 (3 line m, 3H).
,
Step B: 6-Butyl-2-methyl-5-[(4-iodophenyl)methyl]
. pvrimidine-4(3H)-one
A solution of 2.46 g (6.33 mmol) ethyl
2-[(4-iodophenyl)methyl]-3-oxoheptanoate, 6.0 g (63.3 .
mmol) acetamidine hydrochloride, 5.2 g (63.3 mmol~ .
sodium acetate. and 84-mg (0.381 mmol) 2~6-di-tert-
butyl-4-methylphenol in.30 mL-DMF was heated to 153C
for 12 hours.~-.The cooled reaction mixture wa~ poured
'~ ' ~,
. ~:
.
: .
.:
'
WO 9 l / 1 5209 PCI /US9 l /o 1 95 1
.6 ~ L 94 _
into brine and extracted 3 times with ether. The
combined organic material was washed with brine,
dried over MgS04, stripped of solvent in vacuo, then
was medium pressure chromatographed on silica gel
using 1/65/34 AcOH/EtOAc/hexane to give 485 mg of the
title compound, 20% yield. Unreacted ethyl 2-[(4-
iodophenyl)methyl]-3-oxoheptanoate was recycled. Rf
0.25 in 1/65/34 AcOH/EtOAc/hexane, visualized by W
and ninhydrin stain;
lH-NMR (300 MHz, CDC13~: ~ 7.56 (m, 2H), 6.98 (m,
2H), 3.83 (s, 2H), 2.54 (3 line m, 2H), 2.38 (s, 3H),
1.52 (m, 2H), 1.43 (m, 2H), 0.89 (3 line m, 3H).
Step C: 6-Butyl-4-cyano-2-methyl-5-[(4-iodophenyl)
methvl~pyrimidine
A solution of 485 mg (1.27 mmol) 6-butyl-2-
methyl-5-~(4-iodophenyl)methyl]pyrimidine-4~3H)-one
in 7 mL POC13 was refluxed for 60 minutes. After
cooling to room temperature, excess POC13 was
stripped off in vacuo~ The crude product was
partitioned between CH2C12 and a mixture of brine and
NaHC03. The organic layer was removed and the
aqueous layer was extracted three more times with
- CH2Cl~. The combined~organic material was dried over
MgS04, stripped of solvent in vacuQ, then was medium
pressure chromatographed on silica gel using 10%
EtOAc/hexane to give 380 mg of 6-butyl-4-chloro-2-
methyl-5-[(4-iodophenyl)methyl3pvrimidine, 75%-yiel~
. - :a . To a solution of 380 mg (O.947 mmol)
-~6-butyl-4-chl~ro-2-methyl-5-[(4-iodophenyl)methyl]-
pyrimidine in 10-mL acetone were~added 0.119 mL 57%
HI <0.900 mmol) a~d 8Sl mg (5.68 mmol) NaI. The
.. . .
~'.
Wo91/15209 ~ ~ PCT/US9l/019~1
- 95 -
mixture was warmed to 40C for 2.5 hours. As the
reaction proceeds, NaCl can be seen precipitating out
of solution. The mixture was diluted with brine and --
saturated NaHC03 solution. The mixture was extracted
3 times with ether. The combined organic material
was dried over MgS04 and decolorized with activated
charcoal, was stripped of solvent in vacuo, then was
medium pres~ure chromatographed on silica gel using
10% EtOAc/hexane to give 440 mg of 6-butyl-4-iodo-2-
methyl-5-[(4-iodophenyl)methyl]pyrimidine, 94% yield.
A mixture of 800 mg (8.94 mmol) CuCN and 10
mL pyridine was heated to llO~C until all of the CuCN
went into solution ( 7 minutes~. To this mixture was
added a solution of 440 mg (0.894 mmol) 6-butyl-4-
iodo-2-methyl-5-[(4-iodophenyl)methyl]pyrimidi:ne in 3
mL pyridine. After 5 minutes, the reacton mixture
was cooled to room temperature, diluted with CH2C12,
filtered through powdered cellulose flock, stripped
of solvent ~a vacuo, and was medium pressure -
chromatographed on silica gel using 13% EtOAc/hexane
to give 306 mg of the title compound, 87% yield. Rf . .
0.29 in 15% EtOAc/hexane, visualized by W and
ammonium molybdate/ceric sulfate stain;
lH-NMR (300 M~z, CDC13): ~ 7.63 (m, 2H), 6.84 (m, ~ -
2H), 4.16 (s, 2E), 2.73 (s, 3K), 2.68 (3 line m, 2H),
1.53 (m, 2E), 1.31 (m, 2H), 0.87 (3 line m, 3H).
.. - . : .:
; ,
.. . . . .
: :
~
~ '
: .
', '
; ' : . .. :, i : . ~. ' . ' ' ,. ' . .
W091/15209 PCr/US9l/Ol9~l
~fff` f'`'~
O'v `~
- 96 -
Step D: 6-Butyl-4-methoxycarbonyl-2-methyl-5-
r ~ 4-iodophenvl)methvll pyr imi d ine
To a solution of 306 mg 6-butyl-4-cyano-2-
methyl-5-[(4-iodophenyl)methyl]pyrimidine in 6 mL
methanol was added O.885 mL (~7.81 mmol) 30~/0 X202 and
0.937 mL f~2.34 mmol) 2.5 N NaOH. The mixture was
allowed to stir for 1 hour at room temperature. To
the mixture was added ~l mL HOAc. Solvent was
removed in va~uo. The crude material (409 mg) was
dissolved in ~20 mL methanol. To this wa~ added 4.0
g Amberlyst-15~. The mixture was heated to 60 for
18 hours. After cooling to room temperature, ~5 mL
pyridine was added. After stirring for 1 hour, the
mixture was filtered through powdered cellulose
floc~, stripped of solvent in vacuo, stripped from
toluene to remove remaining pyridine, then was medium
pressure chromatographed on silica gel using 25%
EtOActhexane to give 152 mg of the title compound,
46% yield. Rf 0.21 in 30% EtOAc/hexane, visualized
-by UV and ammonium molybdate/ceric sulfate stain;
lH-NMR (300 MHz, CDC13); f~ 7.58 (m, 2HO, 6.81 (m,
2H), 4.12 (s, 2~), 3.87 (s, 3H), 2.75 (s, 3H), 2.67
(3 line m, 2H), 1.53 (m, 2~), 1.31 (m, 2~), 0.86 (3
line m, 3X).
Step E: 6-Butyl-4-methoxycarbonyl-2-methyl-5-
[(2'-tetraæol-5-yl)biphen-4-yl)methyl]-
pvrimidine _
To -25C solution f2f 278 mg (0.717 mmol~
5~phenyl-2-triphenylmethyltetrazole in 4 mL THF was
added dropwise a solution of 1.7 M tert-butyllithium
in pentane until a faint ~ d color persisted (drying
, .
.' ' :'
-
O 91~15209 PCr/US~1/019r !--~, ~ ~9 ~
- 97 - ....
process). Then 0.420 mL (0.717 mmol) of the 1.7 M
tert-butyllithium in pentane was added. After 2
minutes the solution was blood red. After 25
minutes, the organolithium salt was precipitating
from the THF. At this time, 0.358 mL (0.358 mmol)
1.0 M ZnC12 in ether was added. The color changed
from blood red to medium yellow and the precipitate
went back into solution. The mixutre was warmed to
room temperature. To the solution was added 13 mg
(O.018 mmol) (Ph3P)2PdC12 followed by 152 mg (0.358 -
mmol) 6-butyl-4-methoxycarbonyl-2-methyl-5-~(4- ~r .
iodophenyl)methyl]pyrimidine. The mixture was
refluxed for 3.25 hours then cooled to room
temperature, diluted with water and brine, and was
extracted 3 times with ether. The combined o~ganic
material was dried over MgSO4, stripped of solvent in
vacuo, then was medium pressure chromatographed on
silican gel using 30% EtOAc/hexane to give 146 mg of
the trityl protected title compound, 60% yield. Rf
0.18 in 30% EtOAc/hexane, visualized by W and ~,
ammonium molybdate/ceric sulfate stain.
A solution of 146 mg of the trityl
protected title compound in 4/1/1 AcOHlE2O/CH2C12 was
~tirred for 6 hours at room temperature.- The mixture
was diiuted with brine and extracted 3 times with
CH2C12. The-combined organic material was dried over
MgSO4, stripped of solvent ~B Yacuo, stripped from
toluene, then was Still flash chromatographed in
1/13/86 NH40H/MeOHtCH2C12 to give 9Z mg of the title
compound, 97/O yield. Rf 0.18 in 1/50/49 AcOH/EtOAc/
hexane; Rf 0.2Z in 1/13/86 NH4OH/MeOH/CH2C12,
visualized by W ; a: ~ :
, :.
WO91/15209 PCT/US91/0l9~l
,
2.
- 98 -
lH-NMR (300 MHz, CDC13): ~ 8.10 (m, lH), 7.57 (m,
2H), 7.37 (m, lH), 7.04 (m, 4E), 4.24 (s, 2H), 3.79
~s, 3H), 2.67 (3 line m, 2H), 2.53 (s, 3H), 1.58 (m,
2H), 1.34 (m, 2H), 0.88 (3 line m, 3H); MS (FAB) m/e
443 (M~l).
~ XAMPLE 8
6-Butyl-4-carboxy-2-methyl-5-[~2~ etrazol-~-yl)-
biphen-4-vl~methyllpvrimidine
To a solution of 68.4 mg (0.155 mmol)
6-butyl-4-methoxycarbonyl-2-methyl-5-[(2'-(tetrazol-
5-yl)biphen-4-yl)methyl]pyrimidine in 5 mL methanol
was added ~0.300 mL 10% NaOH. After stirring at room ~ :
temperature for 2.5 hours, the mixture was acidified
with ~0.500 mL HOAc. Volatiles were removed ~n vacuo. --
The crude material was redissolved in methanol along
with a couple of drops of TFA, then was HPLC'd using
the following conditions: Rainin Dynamax~ C-18
column, 25 ~ 2.14 cm w/Guard Column; gradient of
acetonitrile in water 5 to 100% over 40 minutes at 5
mL/minute; gradient held at 82% for 7 minutes. A .-
yield of 66.1 mg (100%) of the title compound was
obtained. Rf 0.14 in 1/30/69 N~40~/MeOH/CH2C12,
visualized by- W ; ~~-
H-MMR (300 MHz, CDC13): ~~9.7-8.8 (v br s, 2H), 7.97
(m,- lH), 7.52 (m, 2H), 7.37 (m, lH), 7.00 (br s, 4H),
4.51 (br s, 2H), 2.86 (3 line m, 2H), 2.75 (br s,
3E), 1.-62 (m, 2H), 1.36 (m, 2H), 0.88 (3 line m. 3H~:
MS(FAB) m/e 429 (M+l).
: . . - . . ~ . .:
WO91/1~209 ~ ~J~!~ ~ PCT/USgl/01
_ 99 _
EXAMPLE 9
6-Butvl-6-methyl-3-r(2'-(tetrazol-S-yl)biphen-4-yl)-
methyl~-4H-pyrido[l.2-alpvrimidine-4-one
Step A: 2-Butyl-3-[(4-iodophenyl)methyl]-6-methyl-
4H-pvridorl.2-a~pyrimidin-4-one
A mixture of 1.59 g (4.09 mmol) ethyl 2-[(4-
iodophenyl)methyl]-3-oxoheptanoate, 487 mg (4.50
mmol~ 2-amino-5-methylpyridine, and 3 g PPA was
heated to 160~C for 1 hour. The mixture was cooled -
to room temperature then NH40H was added with
cooling. When all of the PPA had reacted, the
mixture was extracted e times with ether. The
cominbed organic material was dried over MgS04,
stripped of solvent in vaCuo, then Still flash
chromatographed in 10/S0/40 EtOAc/CH2C12/hexane to
give 177 mg of the title compound, 10% yield. Rf
0.21 in 10/50/40 EtOAc/C~2C12/hexane, visualized by
UV;
lH-NMR (300 MHz, CDCl3): ~ 8.80 (s, lH), 7.55 (m,
2H), 7.51 (s, 2H), 7.02 (m, 2H), 4.05 (s, 2H), 2.72
(3 line m, 2H), 2.40 (s, 3H), 1.61 (m, 2H), 1.39 (m,
2H), 0.91 (3 line m, 3H).
Step B: 2-Butyl-6-methyl-3-~(2'-(tetrazol-5-yl)-
biphen-4-yl)methyl3-4H-pyridotl,2-a]- -
pyrimidin-4-one
~ -To -25C solution of 318 mg (0.819 mmol) r`_ ,
phenyl-2-triphenylmethyltetra~Ie in S mL THF was
added dropwiso a solution of 1.7 M .~Q_~-butyllithium -
in pentane until:a faint red color.persisted (drying
:'
.
WO91/15209 PCT/US91/01951
- 100 -
process). Then 0.482 mL (0.819 mmol) of the 1.7 M
tert-butyllithium in pentane was added. After 2
minutes the solution was blood red. After 30
minutes, the organolithium salt was precipitating -
from the THF. At this time, 0.410 mL (0.410 mmol)
1.0 M ZnC12 in ether was added. The color changed
from blood red to medium yellow and the precipitate
went back into solution. The mixture was warmed to
room temperature. To the solution was added 14 mg
(0.020 mmol) (Ph3P)2PhC12 ~ollowed by 177 mg (0.410
mmol) 2-butyl-3-[(4-iodophenyl)methyl]-6-methyl-4H-
pyrimidin-4-one. The mixture was refluxed for 4
hours then was cooled to room temperature, diluted
with water and brine, and was extracted 3 times with
ether. The combined organic material was dried over
MgS04, stripped of solvent ~ vacuo, then was medium
pressure chromatographed on silica gel using 40%
EtOAc/hexane to give 159.4 mg of the trityl protected
title compound, 56% yield. Rf 0.18 in 40%
EtOAc/hexane, visualized by W and ammonium molybdate/
ceric sulfate stain.
To a solution of 159.4 mg of the trityl
protected title compound in 5 mL methanol was added
10 drops concentrated HCl. After 30 minutes, an
indicaor ~uantity of phenolphthalein was added and
the mixture was basified with 10% NaOH then
reacidified with HOAc. Ether was added and the
mixture was dried over M~SO4~ stripped of solven in
vacuo,:then was:Still flash chromato~raphed--in
l/12/87 NH40H/MeOH/CH2C12:to give 95.7--mg-of the
-title compound as.its ammonium~salt, 89% yield. Rf
0.22 in 1/13/86 N~40H/MeOX/CH2C12, visualized by W YQ~
.
. . ..
:: '
- ; ' ' i . ' ~ :. ; ; . i
WO 91/15209 PCI/US9l/01951
"~
- 101 -
1H-NMR (300 MHZ~ CDC13): ~ 8.78 (s, lT~, 8.13 (m,
lH), 7.53 (m, 4H), 7.37 (m, lH), 7 .17 (m, 2H), 7 . 06
(m, 2H), ~6.1-5.0 (v br s, 4H~, 4.06 (s, 2H), 2.75 (3 ....
line m, 2H), 2.39 (s, 3H), 1.67 (m, 2H), 1.41 (m, ~. :
2H), 0.92 (3 line m, 3H); MS (FAB) m/3 451 (M+l).
EXAMPLE 10 : ::
Typical Pharmaceutical Compositions Containing a
ÇQmPOUnd of the Invention . .
A: Dry Filled Capsules Containing 50 mg of Active .:
Ingredient Per Capsule
: ::
Ing~edient. Amount per capsule (mg) ;.:.
6-butyl-4-carboxy-2- 50
methyl-5-[(2'-(tetraæol-
5-yl)biphen-4-yl)methyl]-
pyrimidine
' . ' .
Lactose 149
Magnesium stearate
Capsule ~size No. 1) 200 . .
The 6-butyl-4-carboxy-2-methyl-5-[(2'- .~.
(tetrazol-5-yl)biphen-4-yl)methyl]pyrimidine
can be reduced to a No; 60-powder and the lactose
and magnesium stearate can then be passed through a
No. 60 blotting:cloth onto the powder. The combine~
ingredients can-then be mixed.for about 10 minutes
and filled-:into aANo. l dry gelatin.:capsule.: :
.. . . .. .. ..
: .
. i ~ .
I' . ' ., ' ' '' '' :' ` ',' ' ' . '' I ''`,. . ' ' ' ' ' ' '. " :' : ' ' . '
WO91/15209 PCl/US91/01951
?~
- 102 -
B: Tablet
A typical tablet would contain 6-butyl-4-
carboxy-2-methyl-5-[(2'-(tetrazo]-5-yl)biphen-4-yl)-
meth~l]pyrimidine (25 mg), pregelatinized starch USP
(82 mg), microcrystaline cellulose ~82 mg) and
magnesium stearate (1 mg).
. .
C: Combinat_Qn Tablet
A typical combination tablet would contain,
for example, 6-butyl-4-carboxy-2-methyl-5-[(2'-
(tetrazol-5-yl)biphen-4-yl)methyl]pyrimidine a
diuretic such as hydrochlorothiazide and consist of
hydrochlorothiazide (50 mg) pregelatinized stareh USP
(82 mg), microcrystalline cellulose (82 mg) and
magnesium stearate ~1 mg).
D: S~pposi~ry
Typical suppository formulations for rectal
administration can contain 6-butyl-4-carboxy-2-methyl-
S-[(2'- (tetrazol-5-yl)biphen-4-yl)methyl]pyrimidine
(0.08-1.0 mg), disodium calcium edetate (0.25-0.5
mg), and polyethylene glycol (775-1600 mg). Other
suppository ~ormulations can be made by substituting,
for example, butylated hydroxytoluene (0.34-0.08 mg)
for the disodium calcium edetate and a hydrogenated
vegetable oil (675-1400 mg) such as Suppocire L,
Wecobee FS, Wecobee M, Witepsols, and-the-likej for
the polyethylene glycol. Further, these suppository ~-
formulations can also include another active
.
~ .
, ~
.. . I . . , , .. . ~ .. .... . . -
WO91/15209 P~T/US91/01951
- 103 -
ingredient such as another antihypertensive and/or a
diuretic and/or an angiotensin converting enzyme
and/or a calcium channel blocker in pharmaceutically
effective amounts as described, for example, in C
above.
E: Injection
A typical injectible formulation would
contain 6-butyl-4-carboxy-2-methyl-5-~(2'-(tetrazol-
5-yl)biphen-4-yl)methyl]pyrimidine, sodium phosphate
dibasic anhydrous (11.4 mg) benzylalcohol (O.Ol ml)
and water for injection (l.0 ml). Such an injectible
formulation can also include a pharmaceutically
effective amount of another active ingredient such as
another antihypertensive and/or a diuretic and/or an
angiotensin converting enzyme inhibitor and/or a
calcium channel blocker.
' ' ' ~ . . ' '~.~ . ; ' .; ' . ' '