Note: Descriptions are shown in the official language in which they were submitted.
33046CA
~ Q ~
. ..
ETHERIFICATION PROCESS
This invention relates to the production of alkyl tertiary
alkyl ether compounds.
It i.s known that alkyl tertiary alkyl ether compounds can be
prepared by reacting primary or secondary alcohols with olefin compounds
having a double bond on a tertiary carbon atom in the presence of an
acidic ioni.c exchange resin catalyst. The particularly more common
etherification reactions are those that involve reacting methanol with
either isobutyl.ene or isoamylenes to form respectively methyl tertiary
buty] ether (MT~E) and methyl tertiary amyl ether (TAME). These
tertiary alkyl ether compolmds are particularly useful as octane
improvers for liquid fuels, especially gasoline. Also, because of the
low vapor pressure of these compounds, they are particularly useful for
reducing the vapor pressure of gasoline. Recent federal government
regulations have resulted in the requirement that motor gasoline be
reformulated to include greater concentration levels of oxygenate
compounds of which tertiary alkyl ether compounds have been found to be
especially suitable for assisting in compliance with these new federal
regulations.
While processes for the production of high octane tertiary
alkyl ethers have been known in the art, there still remains various
problems witb the known processes that have heretofore not been resolved
by those skilled in the art. In particular, because standard
33046CA
2 ~ 0 ~
-
etherification reactions are equilibrium type reactions, most
etherification processes do not provide economical means for obtaining
high olefin reaction conversions without incurring high energy and
capital costs to obtain such high olefin conversions. An additional
problem with known etherification processes is their inability to
alternatively produce either an MTBE product or a TAME product in
processes that give high tertiary olefin conversions without incurring
significant modifications in the process flow schemes and process
equipment. Another problem encountered by those skilled in the art of
etherification processing is the difficulty of removing the cyclopentene
compounds, which can be contained in etherification process feeds, along
with the ether product produced in the etherification reaction section
of the process. It is desirable to remove any cyclopentene charge to
the etherification process system concurrently with the ether product
because of its potential negative effect on downstream processes to
which the nonreactive compounds contained in the etherification process
feedstream are fed. Finally, as is generally the case for most process
technologies, it is desirable to have an etherification process that
provides for high purity product streams produced at low operating
costs.
It is therefore an object of this invention to provide an
etherification process that produces tertiary alkyl ethers at high
olefin conversion rates and low operating capital costs.
It is another object of this invention to provide an
etherification process that can be operated in dual production modes for
producing either MTBE or TAME, or both, and stil] maintain a high
tertiary olefin conversion across the process.
Yet another object of this invention is to provide an
etherification process that allows for the removal of a significant
amount of the cyclopentene compounds contained in the feed to such
process concurrently with the final tert alkyl ether product.
A still further object of this invention is to provide product
streams having high purities but at low operating costs.
33046CA
_ 3 ~ 3
The process of this invention includes passing an
etherification reactor effluent as a first feed to first separation
means for separating feeds into a first stream comprising a first ether
product and a second stream comprising primary alcohols and tertiary
olefins. The second stream is passed to a reaction zone containing
therein an acidic ion exchange resin and wherein the primary alcohols
and tertiary olefins of said second stream react to form a second ether
product contained in a reactor effluent stream. At least a portion of
the reactor effluent stream is passed to second separation means for
separating said reactor effluent stream into a third stream comprising
said second ether product and a fourth stream comprising hydrocarbons
and primary alcohols. The remaining portion of the reactor effluent
stream that is not passed to second separation means is passed as a
second feed to said first separation means.
Other objects, aspects and features of the present invention
will be evident from the following detailed description of the
invention, the claims and the drawings in which:
FIG. 1 is a schematic process flow diagram illustrating one
preferred embodiment of the invention having three sections which
include a first reaction section, a second reaction section, and an
a]cohol recovery section;
FIG. 2 is a schematic process flow diagram illustrating an
alternative embodiment of the second reaction section of the inventive
process; and
FIG. 3 is a schematic process flow diagram illustrating
another embodiment of the second reaction section of the inventive
process.
The inventive process has various unique features of which
none of the prior art etherification processes have. For instance, one
novel feature of this process is the inclusion of a second
etherification reaction zone inside the reflux loop of a fractionator
utilized to separate an etherification reaction product. The
significance of utilizing a second reaction zone in such a manner is
that it provides for an exceedingly high overall conversion of reactive
33046CA
4 n~a ~
olefin compounds acrosG the etherification process but at significantly
lower operating and capital costs than those of the prior art processes.
None of the prior art discloses the utilization of a second reaction
zone within the reflux loop of a fractionator overhead system for the
purpose of providing high tertiary olefin conversion and low energy and
capital costs.
The inventive process includes two separate reaction sections
and an alcohol recovery section. Each of the reaction sections has a
reactor vessel used to define a reaction zone containing therein a
suitable etherification catalyst for promoting or catalyzing an
etherification reaction between reactive tertiary olefin compounds and
primary or secondary alcohols. A feedstream comprising tertiary olefins
and primary alcohols is charged or fed to the first reaction zone of the
first reaction section of the process wherein it is contacted with the
etherification catalyst under suitable reaction conditions for promoting
the reaction of the tertiary olefins and alcohols contained in the
feedstream to produce an etherification reactor effluent or a first
reaction section effluent stream.
The first reaction section effluent stream is then passed to a
second reaction section wherein it is first separated by separation
means for separating the stream into a first ether product stream
containing the etherification reaction products and another stream
containing the nonreactive compounds, isoolefins and alcohols charged to
the first reaction section of the process but which remained
lmconverted. The stream containing the nonreactive compounds, and
unreacted isoolefins and alcohols is then contacted with an acidic ion
exchange resin catalyst, which is the same or substantially similar to
the catalyst used in the first reaction section and which is contained
in a reactor vessel defining a reaction zone, under suitable
etherification reaction conditions, to produce a second reaction section
reactor effluent stream. This second reaction section reactor effluent
stream is then divided into two streams with at least a portion of the
second reaction section reactor effluent stream going to second
separation means for separating the ether product produced in the second
33046CA
_ 5 ~ ~
reaction section from the other nonreactive compounds and unreacted
isoolefins and alcohols. The remaining portion of the second reaction
section reactor effluent stream not charged to second separation means
is fed to first separation means whereby the ether product contained
therein is separated from the nonreactive compounds, isoolefins and
alcohols.
The nonreactive compounds and unreacted isoolefins and
alcohols separated by second separation means is passed to an alcohol
recovery section whereby the alcohols are separated and recovered for
reuse from the nonreactive compounds charged to etherification process
system 10 and undesirable reaction by-products produced in the two
etherification reaction zones. The undesirable reaction by-products are
generally oxygenate compounds; and, in particular, the most prevalent
undesirable reaction by-product is dimethylether, which is a reaction
product produced by reacting two molecules of methanol. The
dimethylether compound is undesirable in that it has a high vapor
pressure that has a negative impact on the gasoline pool by raising its
overall vapor pressure. Therefore, it is desirable to remove the
dimethylether compounds from the gasoline pool and utilize it elsewhere,
for instance, in the fuel gas stream of a process plant. An additional
problem the reaction by-product dimethylether poses is its impact on
downstream alkylation processes. In the instances where dimethylethers
are fed to HF alkylation processes, to which the nonreactive olefin
compounds that pass through the etherification process are charged, acid
consumption in such processes is dramatically increased due to the
unwanted reactions with the dimethylethers in the feed. The inventive
process described herein provides an effective means for removing
dimethylethers from alkylation feedstreams and thus eliminating problems
associated wlth having such compounds contained within feeds to HF
alkylation processes.
The nonreactive compounds separated by the alcohol recovery
section can be passed downstream for further processing; and, in the
case where these compounds are predominantly hydrocarbons and, in
particular, olefin hydrocarbons, they are often charged to alkylation
2 0 ~ J 33046CA
-
processes wherein they are reacted with isoparaffins to produce high
octane alkylate compounds. It is important that the nonreactive
compound stream contain minimum concentration levels of cyclopentene due
to the negative impact that such a compound has on fl lkylation processes.
As will be further discussed herein, certain of the alternative
inventive embodiments of this invention provide for minimizing the
concentration levels of cyclopentene in the re~ected nonreactive
compounds that are passed downstream to alkylation processes. Also,
various other embodiments of the inventive process provide for a high
removal and recovery of the undesirable reaction by-products produced in
the etherification process, namely, dimethylether.
The alcohol recovery section can be any suitable process
section for separating solute components contained in solution, which in
the present process is preferably a primary alcohol, from the remaining
compounds of the solution, which are primarily hydrocarbons. Generally,
it is preferred for the alcohol recovery section to be of the type
involving conventional liquid-liquid extraction or solvent extraction
methods wherein a feed solution is intimately contacted, by use of
contacting means, with a solvent to produce an extract stream containing
the solvent rich in the alcohol solute and a raffinate stream. The
raffinate stream is lean in alcohol content and is essentially that
feedstream charged to contacting means but having a substantial
reduction in its alcohol content. The extract stream is passed to
separation means, which is preferably a conventional fractionator, that
separates the alcohol from the solvent. The separated alcohol can be
recycled and utilized as a reactant feed to the first reaction section
of the etherification process. The recovered solvent is recycled and
reused in contacting means for recovering alcohol from its feedstream.
The raffinate stream is passed to separation means for separatlng the
raffinate into an oxygenate rich stream and a hydrocarbon stream.
Conventional fractionation techniques can be used to separate the
hydrocarbons from the oxygenate compounds contained in the raffinate
stream. Any solvent having suitable properties for removing an alcohol
33046CA
7 h~ r'~
solute from a hydrocarbon and alcohol solution can be utilized in the
inventive process; however, the preferred solvent is water.
The feed to the first reaction section of the etherification
process, as earlier dèscribed, is a mixed stream comprising a stream of
primary or secondary alcohols and a stream having isoolefins and other
compounds that are nonreactive in the presence of an acidic ion exchange
resin catalyst at certain etherification reaction conditions.
Generally, the isoolefins include those hydrocarbons having 4 to 16
carbon atoms per molecule. Examples of such isoolefins include
isobutylene, isoamylene, isohexylene, isoheptylene, isooctylene,
isononylene, isodecylene, isoundecylene, isododecylene, isotridecylene,
isotetradecylene, isopentadecylene, and isohexadecylene, or mixtures of
two or more thereof.
The alcohols which may be charged or fed to the first
etherification reaction zone include the primary and secondary aliphatic
alcohols having from 1 to 12 carbon atoms, such as methanol, ethanol,
propanol, isopropanol, the primary and secondary butanols, pentanols,
hexanols, ethylene glycol, propylene glycol, butylene glycol, the
polyglycols, and glycerol, etc., or mixtures of two or more thereof.
The presently preferred reactants of the etherification
process are methanol and isobutylene and/or an amylene because they
respectively yield methyl tertiary butyl ether (MTBE) and methyl
tertiary amyl ether (TAME). Accordingly, it is currently preferred for
the isoolefins to be either predominantly isobutylene or predominantly
isoamylene compounds with the double bond on the tertiary carbon atom of
said isoamylene compounds, or both isobutylene and isoamylene, and the
alcohol predominantly methanol.
It is generally preferred for the isoolefin and the alcohol to
be passed through the etherification reaction zones in the presence of
diluents which do not have an adverse effect upon the etherification
reaction and which are nonreactive under the conditions of
etherification. Examples of suitable diluents include alkanes and
straight chain olefins. The feed to the reactors, excluding alcohol, is
~:~J Q~
-
generally diluted so as to include from about 2 to about 80 weight
percent isoolefin, preferably from about 10 to about 60 weight percent.
Any suitable molar ratio of alcohol to isoolefin in the
feedstream to the etherification reactor zones can be utilized in this
invention that will give the desired high tertiary olefin conversion
sought to be achieved by the process of this lnvention. Generally, the
molar ratio of alcohol to isoolefin in the feeds to the etherification
reaction zones will be in the range of from about 0.5:1 to about 4:1;
but, preferably, the molar ratio can range from about 0.8:1 to about
1.2:1. However, to achieve the highest conversion of the isoolefins in
the process feeds to the etherification reaction zones, it is most
preferable to have a molar ratio of alcohol to the isoolefin as close to
1:1 as is practically achievable.
Typical etherification reactions are well known in the art and
are not a critical aspect of this invention except in the case of the
second reaction zone where the operating pressure has an impact on the
energy utilization of first separation means. As will be demonstrated
by the examples herein, there is a positive benefit, undisclosed by the
art, from operating the second etherification reaction zone at lower
operating pressures in that it reduces the energy required to provide
the separation performed by first separation means. The temperature for
the etherification reaction zone and the space velocity for the feeds to
the etherification reaction zone can be selected as desired depending
upon the degree of olefin conversion sought; but, generally, they should
be such to provide the highest degree of olefin conversion that is
economically feasible. Generally, the temperature of the reaction zones
will range upwardly to about 150~C. Preferably, the etherification
reaction temperatures can range from about 30~C to about 120~C, and most
preferably, the temperature shall range from about 35~C to about 80~C.
The operating pressure of the etherification reaction zones are
generally selected to ensure that the feedstreams or charges to the
reaction zones and the product streams from the reaction zones remain in
the liquid phase during the etherification reaction. Typical pressures
are in the range of from about 30 psig to about 300 psig, but as earlier
~ .i 33046CA
noted, it has been determined that if it is feasible to operate the
second etherification reaction zone at operating pressures below 20
psig, significant reductions in the energy consumption in operating
first separation means can be achieved thus making the novel process
described herein much more economical to operate than the prior art
processes. However, in most circumstances, the etherification reactions
should be conducted in the liquid phase. Generally, the liquid hourly
space velocity (LHSV) of feed to the etherification reactors will be in
the range of from about 1 hour~l to about 20 hours~l; but, preferably,
the LHSV can be in the range of from about 2 hours~l to about 10
hours~l. Most preferably, the LHSV can be in the range of from 3
hours~l to 5 hours~l.
The etherification reaction is that which selectively reacts
tertiary olefins with alcohol, which is preferably methanol, to form a
tertiary ether compound. The etherification reaction is an equilibrium
type reaction that can be represented as follows:
tertiary olefin + alcohol ' alkyl tertiary alkyl ether
Kl
Due to the values and temperature dependencies of the equilibrium
constants of the aforementioned reaction, the equilibrium condition
which favors the formation of the tertiary ether product is a low
reactor temperature condition; but, in any event, because the
etherification reaction is an equilibrium type reaction, the percent
conversion of the tertiary olefin contained in a reaction zone to an
ether product is thermodynamically limited. It has been surprisingly
found that it is possible to increase tertiary olefin conversion by
carrying out the etherification reaction process in two reaction stages
with the second reaction stage following a separation step, which
necessarily follows a first reaction stage, and with the second reaction
stage being placed within the reflux loop of a fractionator that serves
as the separation step.
~ 33046CA
By utilizing the novel etherification process features flnd
improvements, high tertiary olefin conversions can be achieved. For
instance, in the case of an MTBE production mode process, the
isobutylene conversion across the process can exceed about 96 weight
percent. Preferably, however, the isobutylene conversion exceeds about
9~ weight percent, and most preferably, the isobutylene conversion can
exceed 99 weight percent. As for the case of the TAME producton mode
process, the isoamylene conversion is not as high as that of isobutylene
conversion due to the different reaction kinetics and thermodynamic
relationships. However, exceedingly high isoamylene conversions are
obtainable by use of the inventive process with conversions exceeding
about 88 weight percent; but preferably, exceeding about 90 weight
percent. The most preferred isoamylene conversion achievable from use
of the novel and inventive process described herein is 92 weight
percent.
The acid ion exchange catalysts utilized in the etherification
reaction zones of the present invention are relatively high molecular
weight carbonaceous material containing at least one S03H functional
group. These catalysts are exemplified by the sulfonated coals
("Zeo-Karb H*", "Nalcite X*" and "Nalcite AX*") produced by the
treatment of bituminous coals with sulfuric acid and commercially
marketed as zeolitic water softeners or base exchangers. These
materials are usually available in a neutralized form and in this case
must be activated to the hydrogen form by treatment with a strong
mineral acid such as hydrochloric acid and water washed to remove
sodium and chloride ions prior to use. The sulfonated resin type
catalysts are preferred for use in the present invention. These
catalysts include the reaction products of phenolformaldehyde resins
with sulfuric acid ("Amberlite IR-l*", "Amberlite IR-100*" and
"Nalcite NX*"). Also useful are the sulfonated resinous polymers of
coumarone-indene with cyclopentadiene, sulfonated polymers of
coumarone-indene with cyclopentadiene, and furfural and sulfonated
polymers of cyclopentadiene with furfural. The most preferred cationic
exchange resins are strongly acidic exchange resins consisting
essentially of sulfonated polystyrene resin. These resins are
manufactured and sold commercially under various trade names such as
* trademark
~ ~,
~ 33046CA
11
"Dowex 50~", "Nalcite HCR*" and "Amberlyst 15*". As commercially
obtained, they have solvent contents of about 50 percent and can be
used as is or the solvent can be removed first. The resin particle
size is not particularly critical and therefore is chosen in accordance
with the manipulative advantages associated with any particular size.
Generally mesh sizes of 10 to 50 U.S. Sieve Series are preferred. The
reaction may be carried out in either a stirred slurry reactor or in a
fixed bed continuous flow reactor. The catalyst concentration in a
stirred slurry reactor should be sufficient to provide the desired
catalytic effect. Generally catalyst concentration should be 0.5 to 50
percent (dry basis) by weight of the reactor contents with from 1 to 25
percent being the preferred range.
Acid ion exchange resins, such as Rohm & Haas Amberlyst 15*
and Dow Chemical Dowex M-31*, are currently the most preferred
catalysts for the etherification.
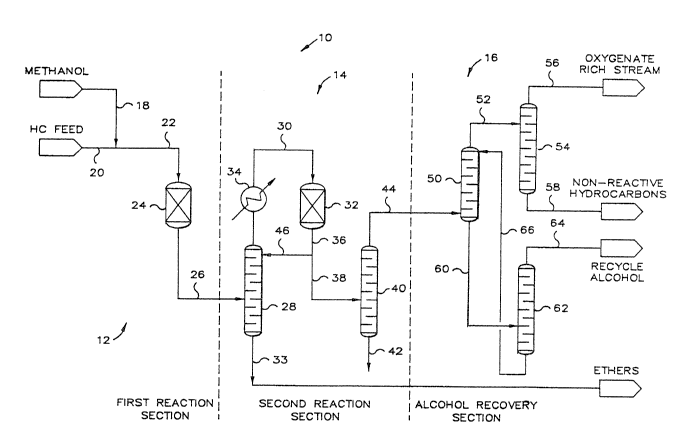
Now referring to FIG. 1, there is provided a schematic
representation of etherification process system 10 having a first
reaction section 12, a second reaction section 14, and an alcohol
recovery section 16.
An alcohol feedstream, which preferably contains methanol, is
charged to etherication process system 10 via conduit 18. A hydrocarbon
feedstream, containing the reactive isoolefins of either isobutylene or
isoamylene, or both, and a nonreactive diluent, is charged via conduit
20 to etherification process system 10. The two streams passing through
conduits 18 and 20 are mixed together prior to passing by way of conduit
22 to first etherification reactor vessel 24, which defines a first
etherification reaction zone wherein is contained an acidic ion exchange
resin catalyst as described herein. The first etherification reaction
zone is operated under suitable etherification reaction conditions so as
to react at least a portion of the tertiary olefins with the alcohols
contained in the feeds-tream to first etherification reactor vessel 24 to
produce a first etherification reactor effluent.
* trademark
~, ~
33046CA
12
-
The first etherification reactor effluent passes via conduit
26 to first separation means 28 for separation of its feeds into a first
stream comprising the ether product produced from the reactions that
take place in first etherification reactor vessel 24 and a second stream
containing unreacted alcohols, unreacted tertiary olefins and at least a
substantial amount of the compounds contained in the incoming
hydrocarbon feedstream that are nonreactive under the etherification
reaction conditions at which first etherification reactor vessel 24
operates. First separation means 28 can be any equipment or process
which suitably can separate ether compounds from a stream comprising
primary alcohols and hydrocarbon compounds, but it is preferred that
first separation means 28 be a typical conventional distillation column
that defines a separation zone and which can comprise a rectifying zone
and a stripping zone. In the novel process described herein, first
separation means 28, or in the preferred case, first distillation column
or first fractionator 28, will separate the first etherification reactor
effluent into an overhead stream containing primary alcohols and
hydrocarbons that passes as an overhead stream via conduit 30 to second
etherification reactor vessel 32 and a bottoms stream containing a first
ether product that is conveyed from fractionator 28 via conduit 33.
Second etherification reactor vessel 32 defines a second
etherification reaction zone wherein is contained an acidic ion exchange
resin catalyst identical to the type utilized in the first
etherification reaction zone. Interposed in conduit 30 is heat
exchanger 34 defining a heat transfer zone utilized for removing heat
energy from the overhead stream leaving first separation means 28. The
second etherification reactor effluent stream leaves second
etherification reactor vessel 32 via conduit 36. At least a portion of
the second etherification reactor effluent stream passes by way of
conduit 38 to second separation means 40. Second separation means 40
can be any suitable means for separating the at least a portion of said
second etherification reactor effluent stream into a third stream
comprising the ether product produced by the reaction of tertiary
olefins with primary alcohols in second etherification reactor vesse] 32
13 ~ ~ 33046CA
_.
and another stream comprising hydrocarbons, primary alcohols and any
by-products produced in the previous two etherification reaction zones.
It is preferable, however, that second separation means 40 be a
conventional distillation column or fractionator which defines a
separation zone. In the use of the preferred distillation equipment,
the bottoms product from second separation means 40 will comprise ether
compounds produced in second etherification reactor vessel 32 and passes
from second separation means 40 by way of conduit 42. The overhead
stream from second separation means 40 will comprise unreacted
hydrocarbons, primary alcohols and undesirable reaction by-products that
pass by way of conduit 44 to alcohol recovery section 16 whereby the
alcohol compounds are separated from the unreacted hydrocarbons and
other undesirable reaction by-products such as dimethylether.
The remaining portion of the second etherification reactor
effluent not fed or charged to second separation means 40 is fed to
first separation means 28 by way of conduit 46. In the case where first
separation means 28 is in the form of a conventional distillation
column, the remaining portion of the second reactor product can be fed
at any location along the column, but it is preferable to utilize the
remaining portion of the second etherification reactor effluent as a
reflux stream. It has been found that an essential feature of this
invention is that the second etherification reactor vessel 32 should be
interposed within the reflux loop of first fractionator 28 for this
invention to provide the benefits of high tertiary olefin conversion
rates and low energy consumption. Therefore, while this invention is
broad enough to encompass the use of the remaining portion of the second
etherification reactor effluent as a feed anywhere along a distillation
column that is utili~ed as first separation means 28, it is highly
preferable for the remaining portion of the second etherification
reactor effluent to be utilized as a reflux to first fractionator 28.
The overhead stream from second separation means 40 passes to
contacting means 50 for contacting an extraction solvent or solvent with
the overhead stream charged to contacting means 50 by way of conduit 44.
Contacting means 50 can be any suitable piece of equipment for
z ~ 33046CA
_ 14
contacting a solvent with a feed solution containing a solute, which in
the instant case is alcohol, and preferably, contacting means 50 will be
a contacting vessel defining a contacting zone and can be equipped with
either trays or packing for assisting in the intimate contacting of the
solution and solvent. Contacting means 50 produces a raffinate stream,
which is substantially free of alcohol, and an extract stream comprising
a solvent rich in alcohols. The raffinate stream is removed as an
overhead stream from contacting means 50 and passes by way of conduit 52
to third separating means 54 for separating the raffinate stream into a
stream comprising oxygenates, which are primarily dimethylethers, and a
stream comprising hydrocarbons. Third separating means 54 can be any
equipment suitable for separating a raffinate stream comprising
oxygenate compounds and hydrocarbon compounds; but, preferably, third
separating means 54 is a distillation column or fractionator that
defines a separation zone. The overhead stream from third separating
means 54 passes downstream by way of conduit 56 and the bottoms stream
from third separating means 54 passes downstream by way of conduit 58.
The extract stream from contacting means 50 is a solvent utilized for
recovering the primary alcohols from the feedstream charged to
contacting means 50 which is rich in primary alcohols. The extract
stream, comprising the solvent rich in primary alcohols, passes by way
of conduit 60 to fourth separating means 62 for separating the extract
solvent rich in primary alcohols into an alcohol stream and a stream of
recovered solvent lean in primary alcohols. Fourth separating means 62
can be any equipment suitable for separating the primary alcohols from
the solvent that is charged to it and will preferably be a conventional
distillation column or fractionator defining a separation zone. The
overhead from fourth separating means 62 is the separated alcohol and
passes by way of conduit 64 from fourth separating means 62. The
recovered solvent, which is lean in primary alcohols, is recycled back
to contacting means 50 by way of conduit 66 and is utilized as the
solvent for contacting means 50.
Another embodiment of the invention is depicted in FIG. 2,
which shows second reaction section 100 having a somewhat different flow
~ 33046CA
-
scheme from that i]lustrated in FIG. 1 for second reaction section 14.
The feature distinguishing second reaction section 100 from second
reaction section 14 is that the bottom stream from second separation
means 40, instead of passing downstream to either storage or further
processing, is recycled via conduit 102 to first separation means 28 as
a feed to the separation zone defined by separation means 28. Utilizing
this bottom stream as a feed to first separation means 28 provides
various significant advantages over other alternative flow schemes in
that it assists in removing a substantial portion of the cyclopentene
from etherification process system 10 that enters it by way of the
hydrocarbon feed charged to first reaction section 12. This is a
desirable advantage in that by removing the cyclopentene at this stage
of the process, it is prevented from passing with the raffinate stream
of alcohol recovery section 16. Cyclopentene is an undesirable compound
for downstream alkylation processes; and, if it is not removed prior to
being passed with the raffinate stream, it ends up in the hydrocarbon
stream that passes by way of conduit 58 to downstream processing. An
additional advantage of the embodiment depicted in FIG. 2 is that the
bottoms stream from second separation means 40 can be passed as a hot
stream to first separation means 28 and can be utilized to provide
additional reboil heat that may be required for fractionation. In other
words when distillation methods are utilized for first separation means
28 and second separation means 40, the utilization of the bottom stream
from second separation means 40 as a feed to first separation means 28
provides for heat integration of the two distillation columns thereby
lowering overall energy costs associated with the production of ether
compounds by novel etherification process system 10.
Another embodiment of the invention is illustrated in FIG. 3
where a third type of second etherification reaction section 110 is
depicted. Second reaction section 110 is similar to second reaction
section 14 with several significant differences. The first difference
between the second reaction sections is that essentially all the reactor
effluent leaving second etherification reactor vessel 32 passes to
second separation means 40 with none of the reactor effluent passing to
~ .t ~ ~ ~P, ~ 33046CA
16
first separation means 28. Additionally, the bottoms product from
second separation means 40 passes by way of conduits 42 and 112 to first
separation means 28 and is utilized as reflux in the case where first
separation means 28 is a conventional fractionator. Interposed in
conduit 112 is heat exchanger 114 used to cool the bottom stream from
second separation means 40 prior to feeding said stream to first
separation means 28 as a reflux. Heat exchanger 114 defines a heat
transfer zone for the indirect transfer of heat energy from the bottom
stream to a cooling medium.
The following examples are presented in further illustration
of the invention.
Example I
The following calculated example is to illustrate the benefits
achievable from the novel process as illustrated in FIG. 1 when compared
with a similar process, but one which does not have the improvements
incorporated in the invention of this process, disclosed in U.S. Patent
3,979,461. The comparative process is different from the process of
this invention in that it does not have a second reaction zone contained
in the reflux loop of the first distillation tower of a two reactor
stage etherification process. Table I shows the feedstream composition
to the TAME etherification process and Table II provides pertinent
process information for the two processes including such information as
the tertiary olefin conversion across the process and energy
requirements for the separation towers of each process. Also provided
in Tables III and IV are data for the processes in the MTBE production
mode. Table III shows the feedstream composition utilized for the
inventive and comparative processes in the MTBE production mode. Table
IV provides pertinent process information which includes tertiary olefin
conversion and process energy requirements for the inventive process and
for the comparative process.
17 ~ ~ î( 33046CA
-
Table I
TAME Production Mode Feed Composition
Feed Wt. %
2-Methyl-l-Butene 6.12
2-Methyl-2-Butene 32.08
Isopentane 18.07
l-Pentene 2.80
cis-2-Pentene 36.03
Isoprene 4.90
100 . 00
Table II
Calculated Results for TAME Process
Inventive Comparison
Process Process
Isoamylene Conversion (wt. %) 9Z.45 82.51
TAME Product Purity (vol.% TAME) 96.67 94.99
Raffinate (wppm TAME) <1 <1
(wppm heavy alcohol) 5 3
Column #l
Reboiler (MM BTU/Hr) 31.59 23.94
Condenser (MM BTU/Hr) 35.51 24.22
Reboiler (~F) 262 264
Condenser (~F) 159 146
Reboiler (psia) 50 55
Condenser (psia) 45 45
Diameter (Ft.) 9 7-5
Theoretical trays 15 30
Column #2
Reboiler (MM BTU/Hr) 7.41 8.78
Condenser (MM BTU/Hr) 8.41 10.41
Reboiler (~F) 221 237
Condenser (~F) 110 110
Reboiler (psia) 35 35
Condenser (psia) 20 20
Diameter (Ft.) 4.5 5
Theoretical trays 30 30
33046CA
4= ' P,i
Table III
MTB~ Production Mode Feed Composition
Feed Wt. %
propane 10.27
normal butane 10.02
isobutane 25.59
isobutylene 13.91
cis-2-butene 23.33
l-butene 11.54
isopentane 5.34
100 . 00
33046CA
19 2~8~
-
Table IV
Calculated Results for MTBE Process
Inventive Comparison
Process Process
IC4 Conversion (wt. %) 99.23 99.48
MTBE Product Purity (vol.% MTBE Cs free) 96.82 87.51
Raffinate (wppm MTBE) 9 5
(wppm heavy alcohol) <1 <1
Column #l
Reboiler (MM BTU/Hr) 7.43 8.29
Condenser (MM BTU/Hr) 8.34 8.43
Reboiler (~F) 281 281
Condenser (~F) 161 142
Reboiler (psia) 145 155
Condenser (psia) 140 140
Diameter (Ft.) 4 4.5
Theoretical trays 15 30
Column #2
Reboiler (MM BTU/Hr) 8.48 8.14
Condenser (MM BTU/Hr) 8.57 8.31
Reboiler (~F) 217 181
Condenser (~F) 110 110
Reboiler (psia) 100 100
Condenser (psia) 85 85
Diameter (Ft.) 4 4
Theoretical trays 30 30
As can be seen from the data presented in Table II, a
significant improvement in the isoamylene conversion is achievable by
the inventive process with the isoamylene conversion improving from 82.5
wt. percent to 92.5 wt. percent. This almost 10 wt. percent improvement
in isoamylene conversion is an unexpected result from utilizing a second
etherification reactor within the reflux loop of a distillation tower of
an etherification process. Generally, those skilled in the art would
expect that there would be no significant difference between placing an
etherification reactor in the reflux loop of the first fractionator of
the process as opposed to placing the reactor outside the reflux loop of
33046CA
~ ~ r~
such process. The data presented for the MTBE production mode case
demonstrate that the inventive process can be utilized in an MTBE
production mode as well as a TAME production mode without any
significant negative impact upon the process, yields and tertiary olefin
conversions. Due to the ability of the inventive process to operate in
both a TAME production mode and MTBE production mode, one of the
objectives of the inventive process is additionally achieved.
Example II
This calculated example illustrates some of the benefits that
are achievable from alternative embodiments of the inventive process.
Table V provides calculated process data for the inventive process in
the TAME production mode for both a base or primary embodiment of the
invention and a second embodiment of the invention as depicted in
FIG. 2. The calculated data presented in Table V is based on a feed
composition similar, but not identical, to that expressed in Table I
above with the primary differences in the compositions being in the
non-reactive components.
~ r~ 33046CA
21
Table V
Calculated Process Data for TAME Production
Mode and Alternative Embodiment
PrimaryAlternative
EmbodimentEmbodiment
TAME Yield
Based on TANE Balance 91.89 92.08
Steam Requirement
40 psig (M #/Hr) 44.2 62.23
150 psig (M #/Hr) 0 0
TAME Product
Purity (Vol. %) 90.4 88.9
RVP
C 5 Product
vppm TAME 10 0
vppm tertiary amyl alcohol 22 0
Vol. % cyclopentene 3.67 1.04
Mol. % cyclopentene 4.36 1.25
Column 1 BTM RVP 3 4
Column 2 BTM RVP 8.5 12
Heating Duties (MM BTU/Hr)
Column 1 Preheater 0 0
Column 1 Reboiler 32.65 34.44
Reactor 2 Preheater 3.76* 4.00*
Column 2 Preheater 0 0
Column 2 Reboiler 7.94 22.75
Temperatures (~F)
Column 1 Bottom 228 214
Column 1 Condenser 106 107
Column 2 Bottom 173 153
Column 2 Condenser 106 104
Pressures (psia)
Column 1 Bottom 30 30
Column 1 Condenser 20 20
Column 2 Bottom 30 30
Column 2 Condenser 20 20
Estimated Sizes
33046CA
Column 1 Diameter (Ft.) 9.5 9.5
Column 2 Diameter (Ft.) 4.5 8.0
*Duty supplied via heat exchange with process streams.
The data presented in Table V illustrates that there are some
benefits from using the alternative process, as earlier mentioned, of
reducing the amount of cyclopentene in the raffinate product. This is
demonstrated by the data under the heading of ~'Cs Product". The Cs
product stream is that in which the cyclopentenes are eventually
discharged unless earlier removed from the feedstream to the alcohol
recovery section of the process. As earlier indicated herein, the
presence of large concentrations of cyclopentenes in a feedstream can
have a negative impact on downstream processing, and it can be desirable
to remove cyclopentenes along with the ether product stream of the
inventive process prior to any non-ether compounds being charged to the
alcohol recovery section. The data demonstrate that the alternative
process is very effective at removing cyclopentenes from the system as
shown by the volume percent cyclopentenes in the Cs product being
significantly reduced under the alternative process.
Reasonable variations and modifications are possible within
the scope of the foregoing disclosure, drawings and appended claims.