Note: Descriptions are shown in the official language in which they were submitted.
W O 91/15~06 P ~ /GBg1/00~29
PHOSPHONOPEPTIDES ~ITH COLLA6ENASE INHIBITIHG ACTIYITY
~he presen. invention reiates to novel pAos?Ac_us
derivatives, processes fc- thei- prepara~ion an_ ~n~ r usc
5 in medicine. In particular, the ?resen~ nven-:or. -el2~ee
~o thei- use as inhibito-s o enzymes c_ ~he coliager.ase
family c' neutral metallop_oteases, fo- ~rea_~ng a-~h~
and othe_ dlseases.
10 The mammalian collagenase amily or enzymes comp-~ses -
numDe- c r p-oteases, exemp'_'ied by -.te S~ ' _' 2~ ~~V_ ;
~~_lagenase itse!', the s~-omeiyslns (also ~nowr, ze
p:oteoglycanases or transins), fibroblas; and
polymorphonuclear leucocyte gela;inases (also known 2S
15 collagen-IV-ases), and 'pùmp-l' (putative metalloprotezse
1, uterine metalloprotease). ~.embership of the mammal-an
collagenase family of proteases ~s evldent by possession
of a number of hiç~hly characteristic and experimcntally
ver fiable properties. (Goldberg e_ al., ~,. Bio!. Che.~r
20 261C, 6600, 1986; Whltham et al., Biochem. ~. 24C, 91-,
' 986; ~reathnach et al., Nucleic Acids Res., ~ 3~,
987; Ml ller et al., Biochem. ~.., 2~', 18" i9B&; '__i_e-
e~ al., J. Blol. Chem., 263, 6579, 1988; Murphy ~~ 21.
~iochem. J., 258, 463, 1989; Quan:in et al., ~iochelr,.
~ 28, 5327, 1989; B rkedal-Hansen, ~ :a Pa-~.c .,
" 445, 1988].
~he :ange c' therapeut c aDpi iC2~ ' ons c' :he inve^.__^^
de_-_iDed hereina',e- re'lec:s :he '~ ndamer._~1 -oi-
~0 c^ilagen as~d othe- ?:^teinaceo~s subs--a~es of th~
~ Llzgenase 'amily c er.zymes r ~nc -onne-- ve ~l_S__
m2_~ h-oushou: :r.e bod-y. Ap_!_ca.ions e~..e~
ca' n~erven:ions -. many -iseases anc ?henos~er.a
-.~rc_-~ n the des~~u_t_c~. _' cc!_ager. an- C-ha- cc.ne~
'~; tLSSU'` components, an~ 2iso no~ma_ ^ d:sc:^e:e- ::_s.i-
emodelsng~
WO91/15506 ~ ' PCT/GB91tO0529
- 2 -
In..ibitors of the collagenase family c- enzymes are
considered to provide userul t.ea,ments fo-:
arthritic diseases, such as rheumatoic and osteo-
a-thritis, so't tissue rheumatism, ?olychond- t s anc
tendonltis; bone resorption diseases, suc;- as
osteopo-osis, 2aget's dlsease, hy?er?a-athyroidism ar.~
cholesteatoma; the enhanced collagen. dest-uction tha~
occu-s in association wi.h diaDetes; the recessive class^s
of dystro?hic epidermolysis bullosa; ?erioàontal ciseas-
and related consequences of ginsival --oduc.ion o~
c_llagenase, or o. P~NL collacenase -e:ease ~ol low~ r.c
-e'l~lar infiltration .~ in-lamed inc:va, _?.cludir.s by
combating the greater suscepti~ili~y c diabetes patientC
to periodontal disease; cornezl ulce-a~ion, e.g. .hat
induced by alkali or other burns, by radiation, by vi~am r.
~ or retinoid deficiency; ulceration of the skin and
gastro-intestinal tract, and abnormal wound healing; post-
operative conditions, including colonic anastomosis, in
which collagenase levels are raised; cancer, where membe-s
of the coliagenase family of enzymes have been implicate~
-. ,he neovascularizat_on required to suppor' tumour
sr3wth and survival [P. Basset et ai. ~ature, 348, 699,
'990] in the tissue remodelling recui-e~ to accommoda~e
t:~e s-owing primary and secondary rumours, and in the
penetration Oc tumour cells th-ou-: tre basement memiDr2n.e
_- ~he vascula_ w2lls du-_ng me~2c~2sis, a.nd demyel r.-- -.~
^ seases c the centra: and pe-ip~.s-a_ ne-vous sys.emâ,
--.^lucing syndromes ln which myeli-, 'oss s the primar
?a-hological event and ~hose in w;-ich demyelina.ior.
'^1 ows axonal a_rophy. ~he degra~ati-r. c- myelin i.-
these ciseases, exe.m?li-ied ~y r.--:-i?le scle-osis, is
me~ia~e~ y ~embers of the ccllagenase -amily of enzir.es.
W091/15506 PCT/GB91/~29
~ f ~ r~ ~3
3 ~
As a particular example of the therapeutic value of
inhibitors of the collagenase family o enzymes such s
are disclosed in the present inventior., ch-onic ar.hri_ic
diseases leading to extensive loss of the collagen,
proteoglycan and elastin components of the cartilage, bGne
and tendons within the joints, should be amenable tG
treatment with inhibitors of the collagenases,
proteoglycanases (stromelysins) and gelatinases cu_rer..:;
.hought to be the major enzymes involved.
mhese enzymes have been detected in ex--ac-s o synov -:
and ca-~ilage tissue, and have also been ex~ensively
studied in tissue cultures of a wide _ange of connective
tissues. Apart from control of the biosyn~hesis,
secretion and activation of the enzymes, the most
important natural regulation of these enzymes in normal
and diseased states, is considered to be the endogenous
production of inhibitors such as the family of Tissue
Inhibitor of Metalloproteases (TIMPS), and alpha-2
macroglobulin. An imbalance between the local levels o
the proteo~ytic erzymes and natu_al inhibitors will alio~;
dest-~c_ion of c~nnective tissue componentC to oc~ -.
mhe compounds described in the presen_ invention, being
synthetic and low molecular weigh- inhibitGrs of this
'amiiy of enzymes, offer a therapeut cally useful way n
wA_ch 2 more normal o- non-~a-hoiogic2' b2iance be~weer.
nhibi~ion and enzymic activity can be res_ored: -Ae-i thus
ac~ to complement and supplement .he endosenous enzymc
r.hibi.ors. Indeec, because ~hese er.zymes usual:y ac.
or.ly wi~hin rest_icted pericellu_ar envircnments, be o;-
being inactivated by inhibitors ci-cu ating ir, the blcod
and present in most ir.flammato.y exuc2tes, tre lcw
molecula- weign_ inhibitors cisc osec he-e may be mcre
WO9l/15506 PCT/GB91/~2g
2.. ~
effective than endogenous protelnaceous nhibitors that
are excluded by their size f-om the iocal zed regions o-
connective tissue destruction.
S European ?atent Application 88310492.9 (3eecham Gro~-?)
dlscloses a class of phos?ho-us de_ivat ves having
activi_y as inhibitors of collagenase ana utillty in ~h-
treatment of rheumatoid a-thr:t s anc -elated d seases n
which collagenolytic actlvity is a cont- su~ing --2C.C-.
Novel st~ucturally related com?ounds :.ave now been
ciscove-ed, which are coilagenase inh ~1-o-s anc thus c~
potential utility in the treatment o_ diseases in which
collagenolytic activity and tissue -emodell ns is
implicated.
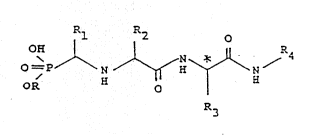
According to the present invention there is provided a
compound of general formula (I), or a sal., solvate or
hydrate thereof:
OR N /~H~\Ni
R3 (I)
i;l w..~ c;.,
~0
?. _s hyc-oger., C,_5 alkyl o- ^?._,na__y s__s___u_e
~en.zyl;
is hyc~oger, c- C,_~ alkyl;
W09l/l5~06 PCT/GB91/00529
- 5 - ~"..
R2 is C3_5 alkyl; and
R3 and R4 are joined together as -(CH2)?-X-(C~2)C- "ne~e ?
is an integer f-om 1 to 9, q is an integer 'rom 2 to 1^,
and the moiety -(CH2)?- is ad~acent t3 .he carbon ato,.
bearing R3 marked with an as~erisk in formula (I), an^ X
is -NR5- wAere R5 is selected f-om hyc-oge-., C,_6alkyl,
C2_6alkanoyl, Cl_6alkoxycarbonyl, aroyl, a-alkyi c-
aralkyloxycarbonyl ln each of which the a-yl moie~y ls
optionally subs~ituted.
'Jnless o~herwise s?eciflec, each alkyl g-^~-? ~S _-efe--b ,
a C1_8 g_ou?, mo-e ?referably Cl_6, ar.d may be a s_-a ~
chain or Dranchec`. ~n aryl moiety is ?referably pheny:.
Optional subs;ituents for an aryl moiety may be selec~ed
from OH, C1_6 alkyl, C1_6 alkoxy and halogen.
R is preferably hydrogen, methyl, ethyl or benzyl,
especially hydrogen.
Values fcr R1 _nclude hydrogen, methyl, e~hyl, soprc?yl
and n-bu_yl. As an alkyl crcup, R~ ~s ?-e~e-ably me-h : ^-
ethyl.
~2 is p-eferably a C4 alkyl s-cu?, such as ^.-bu~y ,
_sc-buty' c~ se^-~u~yl, espec ally iso-_u.y'.
Values fc- R3 ar.- R4 ~ogether ~nclude -(C..2)p-X-(Ci2)_-
where p and q have values such th2~ R3 and ~ fsrm pa-- c
an _1- or 13 to 6-membered azalactam s_~_~~ure, a..d .~. _s
-NR-- whe-e Rc s r.yd-ogen, methy:, benzil, --bu~_~.y-
-arbonyl c_ benzyloxycarbonyl.
WO91/15506 PCT/GB91/0052g
-- 6 --
Most preferred are compounds where R3 and R4 are joired
tosether as ~(CH2)p~X~(CH2)q~ wnere p is 4 and ~ is _ or p
is 3 and q is 6 or p is 4 and q is 6 or p is 4 and q i5 3
and X is -NR5- where R5 is hydrogen o- methyl.
The compounds of formula ( ) may fsrm salts wit;~ bzses
e.g. sodium hydroxide. The compounds of formula (I) have
a basic nitrogen atom and may form ac d addition sal~s
e.g. with hydrochloric acid. Such com?ounds form pa-~ o
the p_esent inventlon.
Where com?ounds of fo_mula (I), c- salts thereof, -o-.-,
solvates or hydrates, these also form an aspect cf the
invention.
The compounds of formula (I) have at least two, and may
have three or more asymmetric centres and therefore exist
in more than one stereoisomeric form. The invention
extends to all such forms and to mixtures thereof,
lr.cluding racemates, and diastereoisomeric mixtures.
--efe-re~ isomers are those having the S-conf guration a
~he chlral centre bea-ing R2 and ~he S-configuration a.
~he chiral centre bearing R3, marked with an asterisk in
_o-mula (I).
mhe compounds cf formula (~) or _hei- salts, solvates ^-
hyd-ates are preferably in pharmaceutically acceptabie
for~.. By pharmaceutically acceptable form is mean~, inte-
30 zli2, 0_ a pharmaceul cally acceptabie level o ?ur .yexc'usinc normal pharmaceuticai addi~ives such as d ~er.-s
ar.d carrlers, and including no mate-ial considered toxi~
2- no_mal dosage levels.
WO9l/1~506 PCT/GB91/00529
- 7 - 2.,; ~ ~
The com?ounas o- formul2 (I) o- thei- salts, solvates G_
hycrates are preferably in substantially pure form.
A substantially pure Co-m will generally c~ntain at 'ezst
50~ by weight, preferably 75%, more ?re-erzDly 90% znA
sti_l more preferably 95% o- 99% or mo-e Oc .he com?ou..c
o -o-mul2 I o- lts szlt o- solvate.
Compounds of formul2 (I) or their salts, soivates o-
hyd-ates may be isolated as crystalllne sollds o- - -hc
fo-m Oc 'oams or gums.
A p-e e--ed ?ha-maceutlca!ly acceptable form is rhe
c.ystalline form.
The present invention provides the compounds of fo-mul2
~I) or pharmaceutically accepta~le salts, solvates or
hydrates thereof for use as active therapeutic agents,
particularly as agents for treatment of conditions in
which degradation of connective tissue and other
proteinaceous components of the body occurs, such as
.m.,usculo-skeletal disorders resul.ing from collaqenoly. c
ac~ v:-y, ?a-- cularly rheumatism anc/o- 2-thrltic
condi_ ons, and issue remodell ng.
Compounds cf fo.~ul2 (I) also h2ve potential uti i-y 1-
.he t-e2;ment o;~ cancer; for prevent r.c myelin degrad2~- C?.
r. _he cer.tral and peri?heral ne-vous sys-em; anc :n cthe-
condl~lons in wnich members cf the col'agenase family o
neu_-zl metallo?roteases have pztholoclcai or oth.e- .oles.
mhe ?-eser.~ .nvention also p.ov des a _-ocess fo- _he
pre?a-2tion cc a compound of fo-mula ( ) wnich ccmp-ises
~c?.ve-~ing a group R20 to hydrocen ~y cleaving c g-ou? ~r
~-5m C compound of fo-mul2 ( T ):
WO91/15506 PCT/GB9l/ ~ 29
~ f ~. ~. ';' ~
-- & --
Rl 2
OR2 ~ ~ N ~ R4
~3
(I-)
wherelr. R20 is alkyl, optionally substituted phenyl, o-
op~ionaily substituted benzyl and R21 is hydrogen, alkyl,
optionallv su_sti~uted ?henyl, or o?t onally subs~i~u-ed
benzyl and R., R2, R3 and R4 2: e as de~inec in for~ula
(I), and where necessary, converting R21 to hydrogen.
Cleavage Oc R20, and where necessary R21, may be carried
out in aqueous acid or alkali or using a trimethylsilyl
halide, preferably bromotrimethylsilane, in an inert
solvent, for example dichloromethane or acetonitrile.
Benzyl esters may alternatively be removed by
hydrogenolysis or other standard debenzylation procedures.
Phenyl ~esidues may be -emovec by hyd-ogenation over
_la.lnum cx.~e.
When both R20 and ~21 are alkyi, cleavage of R20 only, to
give tc a compound o fo-mula (I-~) in which R20 is
r.yd-ogen and R21 alkyl, which is 2 compound of formula (T)
-. whlch R is alky', may be c_--ied ou_ by treatmen. wi-h
excess a;kal_ under mild cond_~ions, ~or exam?le wit~
aqueous sodium hydroxide in a-. alcoholic solvent 2- _oom
tempe-a~u-e.
Si.milar y, wnere R20 is optic-._lly substituted ben~yl ar.c
R2, s alXyl, .he senzyl s-ou^ or.:y ~2y be cleaved by
WO91115~06 PCT/GB91/00529
9 2. . ~s.;3
hydrogenation to gi~e a compound of formula (II) in wnich
R20 is hydrogen and R2l is alkyl.
Cleavage of an R2l alkyl group may thereafter be carried
out as desc-ibed above to give a compound Oc formula (I)
in which R is hydrogen.
When R in a compound of formula (I) is hydrogen and R2l _n
a compound of formula (T') is not hydrogen, ~hen cleavage
of both R2i and R20 is conveniently effecced i?. a singie
reac~ion. ?referably R20 and R2l are both alky1, such ac
me~hyl or e~hyl, o- benzyl.
It will be appreciated that compounds of formula (II) i?,
which R2l is hydrogen are themselves compounds of the
invention of formula (I).
Compounds of formula (II) may be prepared by treating a
compound of formula (III):
Rl R2
O - P N ~
OR2l (III)
- -
in whl-h R1, R2, R20 and R2, a-e as de_ineA in formula
(__) (exce?. that R2i -5 no. :~), with. _ com?ound cf
formula (IV):
o
H2N Jl~
~ NHR4
R3
(;V)
WO91/15506 PCT/GB91/00529
2~
-- 10 --
ln which R3 and R4 together are as defined in fo-mulc ~
The reaction is preferably carried out in the presenc^ ^
a coupling agent, such as dicyclohexylcarbodiimide o-
l-ethyl-3-[3-(dimethylamino)propyl]carbodilmide
hydrochloride in the presence of l-hyd-oxybenzc~-iazo:-,
or using l,l'-carbonyldlimidazole, in ar. iner~ soiven~
such as dichloromethane or acetonitrile.
0 Selec~ive cleavage of the group R2l may then be ca--~-
~out usirs the procedures described above -o- tAe
?repara.ion of compounds of formula (T) ~0 sive com?c~ s
of formula (II) in which R2l is hydrogen.
The intermediate compounds of formula (-_I) may be
prepared by treating a compound of formula (V) or a sal.
thereof:
OR
O _ P NH 2
OR21
(V)
_n w..ic.. R', R20 and R2l are as c-fined in ormul2 (_~~),
with a com?ound of _ormula (VIA) o- (VI3) o- a sal~
~hereof:
''O R2 R2
Rl~ C02R12 o -- C02R12
(VIA~ (VIB)
WO9l/15~06 PCT/GB91/OOSZg
i _
in which R2 is as defined in formula (I), Rll is a leav~rc
sroup such as halogen, methanesulphonyioxy or
trirluoromethanesulphonyloxy and R12 is hydrogen o_ a
carboxyl protecting group, and thereafter removins an ?~ 2
carboxyl protecting group. ~referred method is .he
reaction of tV) with (VIA).
When a compound of formula (V}B) is used, the rec~
amination may be carried out by hydrogenatior. ove- a no-l-
metal catalyst such as palladium on ca-bon o- by -ea~~ c..
wi.h sodium cyanoborohydride at pH '6 ~o 7. Lowe- 2 lky `
alcohol solvents such as methanol anc eth2nol a-s s~
for both -eactions. These reactions ~ay be carriec ~u- ^.
the presence of molecular sieves.
A hydrogenation reaction is preferred but this process
precludes the use of compounds of formulae (V) and (V~3)
in which any of R20, R21 or R12 ~is benzyl. ~referably a
carboxyl protecting group is a methyl or ethyl ester.
Ester protecting groups may be removed under s~andarc
basic hydrolysis conditions using dilute base suc:- as '
~or~al a~ueous sodium hydroxide n me~hanol or aueous
~otassium hydroxide in 1,4-dioxane.
'~hen the compound of formula (V) is in the form c ~e
f-ee base, ~he compound of formula (VI3) is suitzbly a^.
a-keto este~ (R12 = alkyl).
When the compound of formula (V) is 2 salt, su-h as
hydrochloride salt, the compound of ormulc (VI3) s
suitably a salt of an a-keto ac ~ (~ 2 = r.), ~o; exam~'-
the sodium salt.
WO91/15506 PCT/GB91/ ~ 29
~f ~ ~?'~,~ - 12 -
The preparation of compounds of formula (III) ucing a
compound of formula (VIA) may be carried out unde-
standard alkylation conditions. A halogen leaving sroup
is preferably bro,mine and an oxygen-based leavin5 5~~? -5
preferably trifluoromethanesulphonyloxy.
Compounds of formula (III) may alternatively be ?.e?a-e^
by condensing a compound of formula (VII) c- a sal.
thereof:
H2N CO2~12
(V
in which R2 is as defined in formula (I) and R12 is a
carboxyl protecting group with an aldehyde, R1-CH0 in
which R1 is as defined in formula (I) and treating the
condensation product with an appropriate dialkyl or
trialkyi phosphite, for example dimethyl phosphite, and
thereafter removing the carboxyl protecting group. The
ca-boxyl group is conveniently protected as ar. alky' c~
benzyl ester whicA may be removed using star.darc,
hydrolysls or hydrogenation conditions.
As described above in connection with reduc~ive amin2tio.
cf -ompounds o- formula (VIB), where a benzvl ?rotec~ir.~
s-oup ~12 is removed by hydrosenation then ~20 and ?~2, are
_es~ricted to alkyl.
.lternatively, compounds of formula (I~) ir. whic:~ ?~2~ an_
~21 are alkyl or optionally substituted benzyl may be
?repare~ by the reaction of a co...pound o fo-mula (`jT;, )
WO 91/15506 PCr/GB91/00529
- 13 ~ ,~7~3
R2
H N ~ H ~ ~ ~ 4
o
R3
( V _ _ _ )
_n which ~2~ R3and R~ are as defined i-. formula ( ), -" th
2 compound of formula (IX):
\ 1
O ~ \ 11
OR21 (IX)
in which Rlis as defined in formula (;), R20 and R21 a_e
alkyl, optionally substituted ?henyl, c- op~ionai1y
substit_ted benzyl and Rll is a le2vins g-su? 2S ce~i~.e~
'o- fo-mula (VIA), in the presence of a base sucr. 2S
~rie~hylamine or Proton Sponge
(',8-~is(dimethylamino)-naphth21ene), ^- usins a-.hyd-ous
?otassium carbona~e in an alcohol-c solvent.
'~nere R,, is an oxygen-based leav-ng s-ou?, .or exam? e
.-ifluoromethanesulphonyloxy, whi.h ls ?referrec,
d-s?lacemer.~ o- the leaving grou- .s c~nver.ien~ly -2--' ec
OUt ir. -he presence of Proton Sponge -n an ~ner. scl-~Jer,-
such as acetonitrile o- dichlc-omerhane, ove_ a ?e-:c~- c
sever21 c,ays in the absence o 1 h~.
W091/15506 PCT/GB91/0052
A further alternative preparation of compounds o4 formul2
(III) may be carried out by reacting a compound o4 'or-.u_c
(IX) as hereinbefore defined with a com?ound of c-muiG
(VII) in which R~2 is a carboxyl protec ing g-ou?, us ~.
conditions as described for the reaction of compounds c-
40rmula (VIII) with compounds of fo-mula (IX), anc
thereafter removing the protec'ing grou? R12.
Suitable carboxyl protecting groups include zl~y', cenzy:,
t-ialkyisilyl and trialkylsilylethyl g-ou?s. A
trialkylsilyl protecting group, for example
~-imethylsilyl, is especially useful in tha~ - may be
readily incorporated, ln situ, for exam?le by addi.ion o
hexamethyldisilazane to the reactants in acetonit-ile in
the presence of triethylamine, and selectively removed ^.
aqueous methanol, without imposing any limitations on the
value of R20 and R2l. Other silylating agents include
trimethylsilyl chloride and
N,N-diethyltrimethylsilylamine.
An Rl2 alkyl carboxyl protectir.g sroup may be removed -y
base hyd-olysis, for example using so~ium hyd-oxide -.
aqueous methanol or potassium hyd~oxide in aqueous i,4-
dioxane.
T_ W' '' 1 be appreciated that where ~he carboxyl orctec~ -.
5-U? ~2 is alkyl, R20 and R21 m2y be alky , 2henyi c-
benzyl derivatives, but where Rl2 ls a benzyl grU?r -~2
and R21 are limited to alkyl and phenyl.
When compounds of formula (II-~) z-e prepared Dy this
-oute, __ is preferred that R20 and R2, are benzyl ar.d ~
s .- 4'uoromethanesulphonyloxy ^. he com~ound c_ fo-m_ -
~IX) and Rl2 is trimethylsily o- methyl n the ccmpour.-
~5 c' 40rmula (VII).
WO91/15506 PCT/GB91/00529
- 15 -
Compounds of formula (VIII) may be DreDared by t-ezti~.g -
compound of formula (VII):
R2
H2N CO2R12
(v_ _ )
n wAich R2 is as defined in formula (T), R12 _s hyc-_ce~.
and wherein the amino g-oup is optionally protec ec, ~
a compound of formula (IV) as hereinbe-^o~.e de inec, -. --.-
?resence o_ a coupling agent as here~nberorG -es^-ibed --
the preparation of compounds of formula (II) -rom
com?ounds of formulae (III) and (IV).
Compounds of formula (IX) may be prepared from hydroxy-
alkylphosphonate derivatives by conversion of the hydroxyi
group to the leaving group R11 by conventional methods.
For example, where R11 is trifluoromethanesulphonyloxy,
trifluo-omethanesulphonic anhydride may be added tO a
solution of the hydroxyalkylphosphonate in an ine-~
solvent such as dichloromethane, the -eacrion bein~
car-ied out at reduced tempe-ature unde- an .ne_t
atmosphere, according to the gene-a! method Oc E. Ve~e,s
G~ al., Journal of Organic Chemistry CA, 2165, (1585).
:-ya-oxyalkylphosphonate compounds may ir tu-n be ?-epc~_~
by -eac~ion G~ the corresponding ?hos?r.ite, o- exam?l
dibenzylphosphite, with an aldehyae ~1-rHO in which ~ -s
3G as aefined ln formula (I) according ~o the gene-a: me_hc_
o -. Texie--Boullet and A. ~ouca~1d, Synthesis, 51
(1982). Benzyl and alkyl phosph tes a-e e .he~
comme--ially available compounds ^~ c2n be p-e?arer ~o..
commercially available starting mate-izls by s~an-2-^
35 me~hods.
WO91/15~06 PCT/GB91/~K2g
2~
-- ~6
Intermediate compounds of formula (V) are elthe- knowr.
_ompounds or may be prepared from known aminoalkyl
phosphonic acid de-ivatives using standard _rocedures tc
introduce R20 and R2l as required.
~rotec~lon o_ the amir.e func~lon du_ing these reac~ior.C
may be necessary.
_ntrodu^.ion of ar. R20 or R2l methyl group m2y be effect--
bv re2c_ion with diazomethane in a suitable ine-t solve?.~.
Compounds o' fo-mula ~V) of flxed confisu-2_ion may be
?repared by the general method of R. Jacquier et al.,
~hospho-us and Sulfur 36, 73, (1988).
Compounds of formula ~IV) may be prepared by oxidising tn
primary alcohol function in a compound of formula (X):
Y
H NH H
Z-N-(CH2)p ~--N-(CH2)q-OH
o
(X)
~ne-el-. ? and q z-e as define- i-. formuia (_), Y is 2
-. _-ogen ?-otectlon g-oup, and Z is R5 to c~ve the
cor-espcnding aldehyde, followed 3Y remova~ OL- Z wr.e-e .
is zn zcyl g_oup; cyclisation znc reductior.; and
~:^e-eaf~e , as necessary, -emovi-.g the ni~-oqen p-o~ec~
-oup Y and lr.t--_onvertir.g R_.
WO91/15506 PCT/GB9l/00~29
Z~ 5.;~3
Suitable nitrogen protection groups include
t-butoxycarbonyl (BOC) and benzyloxyca-bonyl g-oups.
The oxida~ion may be carried out using pyridinium
chlorochromate, or under Swern oxidising conditions, CO-
example by trea~ment with dimethylsulphoxide and an acyl
halide followed by triethylamine, as desc_ibed by ~. S~e--
et al., J. Ors. Chem., 43, 2480 (1978). The cyc'isa~c?.
and reduc~ve amination s;ep may be e' ec.ed by c2.2_y_:-
nydrogenation ove- a suitable noble me.al catalys~, or
example palladium cn carbon, c- by reac~lon ~ SOG' U.-
cyanoborohydride or sodium boronydride. -. some cases
the yield of azalactams may be increased by carrying ou-
the reductlve amination step under acidic conditions.
Nitrogen protection groups may be removed by standard
methods. A t-butoxycarbonyl group may be removed by
treatment with trifluoroacetic acid at reduced
temperature. Where Z is a nitrogen protection group, i.
may be selected to undergo concomitant cleavage durins t~e
cyclisation reaction to give a compound in which R5 is
nyd-ogen. .~or example, when Z is a benzyloxycarbor.y'-
sroup, it will be readily removed by ca-aly.ic
hyd-ogenation.
Pn R5 hyd-ogen may be interconve-.ed t^ an R5 ~_,ziky_,
aralkyl or aryl sroup. The secondary amine gro~? n _h^
azalactam ring may be alkylated .o form an R5 aikyl g-_u~.
-o. example, the amine group may be met;nylated to ~orm a^.
~5 methyl group. The methyl2~ion ste? may be e'-ecred -y
ca~aly~ic hydrogenation over a suitable noble met21
catalys., for example palladium on carbon, in tr.e ?-ese-.-e
of aqueous formaldehyde. Othe- suita~le methyl2~-or.
_rocedures are desc-ibed by E. Askito-iu et al., Hel~.
WO91/15506 PCT/GB91/~K2g
2s.~
~8 -
Chim. Acta., 68, 750, (1985); _. Engler ~- al., :~el~r.
Chim. Acta., 68, 789, (1985); and M. _ennon et a'.. ...
Chem. Soc. (~erkin I), 622, (1975).
Compounds of formula (X) may be prepa-ec by re2c~ins z
compound of formula (XI):
H ~
z-N-(cH2)P ~ OH
(XI)
wherein p, Y and Z are as defined for formula (X~, with a
compound of formula (XII):
H2N~(CH2)q~OH (XII)
wherein c is as defined for formu a (X).
The reaction may be ca_ried OUt using standa-d procedures
for forming an amide from a carboxylic acid and an amine,
for example usin~ a couplins agen_ su_h 2S ~
ca-bony'diimid2zole, 1,3-dicyclohexylcar_od_i.mide o- :-~3-
dimethylaminopropyl)-3-ethylc2rbo~iimide.
Ccmpounds of formul2 (XI) are di-zmlnoalkanoic acic
de-ivatives. These are known com_ounds cr may be pre?a-e~
'rom known s;artinc materials by s~anda-d methods.
rOr e~ample the compound of ~ormu a ( J) in which R~ an^
~.a ~ogether a-e ~(C:.2)p~X~(CY.2)q- whe-e _ is ', c _s ~ a-.d
WO91/15~06 PCT/CB91/00529
2.~
_ l9 _
X is -N~.- is prepared from 2 compound of formula (XI)
derived f-om ornithine which is commerclal_y availab1e,
The compounds of formula (IV) in which R~ and R4 ~oge~he-
are ~(CY~2)p~X~(CH2)q~ where ? is 4, c is 3, 5 o- 6 anc X
is -NH- are prepared f-om a compound o- fo-mul2 (X-~ )
àerived from the amino acid lysine. The com?our._ c
-ormula (XI), derived from S-lysine, in wr.i-:r ~ is
.-butoxycarbonyl and Z is benzyloxyca-bony', ,
commercially available.
Similarly, the compound of 'ormula (T'~) ln wnicn ?.3 an- .
toge~her are -(CH2)p-X-(CH2)c- where p s _, a ls 8 a^;d X
is -NH- may be prepared from 2,3-diamino?ro?ionic 2CiC.
The compounds of formula (VII) are either known amino acid
derivatives or can be made from these derivatives by known
methods. Compounds of formula (VIA) and (VI3) are either
known compounds or may be prepared from known compounds by
known methods.
The inte-mediates of formulae (; ), (~ ~), znc ce--2'n
intermediates of formula (V) disclosed herei^. are novel
compounds and form an aspect of the presen~ lnven.ion 25
do the described processes fo- thei- ?repa~2tion.
~he-e obtainable, pharmaceutlcally 2cceptaDie sal-s c ~:ee
compounds of formula (I) may be .ormed conventi on 2 _ 1y - ';
reac~ion with the appropriate acid or ~ase. Solva-es m2:
be 'o-med by crystallizatlon from the apDr^~riatC sol-~e.r.-.
WO 91/15~i06 PCl~/GB91/0052g
2 ,. , ?~
-- 20 --
As men.ioned previously, the compounds of formul2 (I)
exist in more than one diastereoisomeric form. Where ~he
?rocesses of the invention produce mixtures thereof, ~he
individual isomers may be separated one from another by
chromatog-aphy e.g. H~LC.
Alternatively, separate diastereoisomeric compounds o
formula (I) can be obtained by usins stereoisomer-cally
pure starting materials or by separating desired isomers
of intermediates at any stage in the overall syntheti^
process, and converting these intermeciates to compouncs
of fo-mula (I).
It will be appreciated that wnere a single diastereoisome-
of a compound of formula (I) is prepared by more than oneprocess variant as hereinbefore described, each of which
allows a different chiral centre to be defined, it may be
possible to deduce the configuration at a chiral centre
which is not pre-determined using a particular process
variant.
~urthe~more, it will be appreciated that althous;~ ~he
absolute configuration at a particular chiral centre may
not be known, it is possible to characterise a given
diastereoisomer relatlve to its epimer by reference to the
~ -ec~ion in which the plane of ?olarised l ght is
-st2ted.
The present invention further provides a pharmaceutic21
composition, which comprises a compound o' formula ( ),^-
a ph2-m2ceu.ically acceptable sal. o- solvate thereo , anc
a pn2_maceutically acceptable car-ier.
WO91/15506 PCTIGB91/~29
- 21 - 7,., i~v~
A composition of this invention is useful in the treatmen_
of musculo-skeletal disorders, particularly arthri_i^
diseases and for modulation of tissue remodelling.
A composition of the invention also has potential utility
in the treatment of cancer; fo. preventing myelin
degradation in the central and peripheral nervous system;
and in other conditions in which members of the
collagenase family of neutral metallo?roteases have
pathological or other -oles.
A composition of the invention, whic;. may be prepared by
admixture, may contain a diluent, binder, filler,
disintegrant, flavouring agent, colouring agent, lubricant
or preservative in conventional manner. These
conventional excipients may be employed in conventional
manner, for example as in the preparation of compositions
of related peptide enzyme inhibitors, such as the ACE
inhibitor enalapril.
A composition of the invention may be adapted for oral,
to?ical, rectal or parente-al administ-ation but oral
administration is preferred. Parente-al compositions m~y
be administered intravenously, in~ramuscularly, intra-
arricularly, intradermally, subcutaneously or in-o the
cerebro-spinal fluid.
Preferably, a pharmaceutical composition o the inventior.
is in unit dosage form and in a form ada?ted fo; use in
~he medical or veterinarial fields. For e.Yample, such
prepara.ions may be in a pack fc-m accompanied by w-it.e-.
or printed instructions for use as an agen~ in the
treatment or prophylaY.is of any of tne disorders mentior.ec
above.
WO91/15506 PCT/GB91/00529
- 22 -
The suitable dosage range for the compounds of the
invention may vary from compound to compound and may
depend on the condition to be treated. It will also
depend, inter alia, upon the relation of potency to
absorbability and the mode of administ ation chosen.
The compound or composition of the invention may be
formulated for administration by any route, the p-efe--ed
route depending upon the disorder for which treatment s
required, and is prefe-ably in unit dosage form or in a
form that a human patient may administe- to himseif in a
single dosage.
Compositions may, for example, be in the form of table-s,
capsules, sachets, vials, powders, granules, lozenges,
reconstitutable powders, or liquid preparations, for
example solutions or suspensions, or suppositories.
The compositions, for example those suitable for oral
administration, may contain conventional excipients such
as binding agents, for examplé sy-up, acacia, gelatin,
sorbitol, tragacanth, or polyvinylpyr-olidone; fille-s,
for example lactose, sugar, maize-starch, calcium
phosphate, sorbitol or glycine; tableting lubricants, __
example magnesium stearate; disintegrants, for example
s~arch, polyvinylpyrrolidone, sodium s.arch glycollate c~
mic-ocrystalline cellulose; o_ pharmaceutically accep~aîle
wetting agents such as sodium lauryl sulphate.
Solid compositions may be obtained by conventional me~hs_s
of blending, filling, tableting or the like. Repeated
~lending operations may be used to distribute the act V5
agent throughout those compositions employing large
quant ~ies of Cille-s. ~hen the composition is in the
WO91/15506 PCT/GB9l/~2g
i.'V'~
- 23 -
form of a tablet, powder, or lozenge, any c2~rier suita~1e
for formulating solid pharmaceutical compositions may be
used, examples being magnesium stearate, starch, slucose,
lactose, sucrose, rice flour and chalk. Tablets may De
coated according to methods well known in normal
pharmaceutical practice, in particula- with an enteric
coating. The composition may also be in .he form or ar.
ingestible capsule, for example of gelatin containing ~he
compound, if desired with a carrier or other excipients.
For example, in a hard gelatin capsule containing the
required amount of a compound of the invention in the orr.,
of a powder or granulate in intimate mixture wi~h a
lubricant, such as magnesium stearate, a filler, such as
microcrystalline cellulose, and a disintegrant, such as
sodium starch glycollate.
Compositions for oral administration as liquids may be in
the form of, for example, emulsions, syrups, or elixirs,
or may be presented as a dry product for reconstitution
with water or other suitable vehicle before use. Such
liquid compositions may contain conventional additives
such as suspending agents, for example sorbitol, syru?,
methyl cellulose, gelatin, hyd-oxyethylcellulose,
carboxymethylcellulose, aluminium stearate gel,
hydrogenated edible fats; emulsifying agents, for example
lecithln, sorbitan monooleate, o- acacia; aqueous or non-
aqueous vehicles, which include edible oils, for example
almond oil, fractionated coconut oil, oily esters, for
example esters of glycerine, or p-opylene glycol, or ethyl
alcohol, glycerine, water or normal saline; preserva~ives,
for example methyl or propyl p-hydroxybenzoate or sorbic
acid; and if desired conventional flavourina or colouring
agents.
WO91/15506 PCT~GB91/ ~ 29
,~ ~c ~ - 24 -
The compounds of this invention may also be administered
by a non-oral route. In accordance with routine
pharmaceutical procedure, the compositions may be
formulated, for example for rectal administration as a
suppository or for parenteral administration in an
injectable form. For injection, for example by int~a-
articular injection or by injection into the cerebro-
spinal fluid or via other routes which will gain access to
sites of demyelination, such as by intramuscular,
intradermal or subcutaneous injection, as freely soluble
solutions or as poorly dispersed depot stores, the
compounds of the invention may be presented in an aqueous
or non-aqueous solution, suspension or emulsion in a
pharmaceutically acceptable liquid, e.g. sterile pyrogen-
free water or a parenterally acceptable oil or a mixtureof liquids, which may contain bacteriostatic agents, anti-
oxidants or other preservatives, buffers or solutes to
render the solution isotonic with the blood, thickening
agents, suspending agents or other pharmaceutically
acceptable additives. Such forms will be presented in
sterile unit dose form such as ampoules or disposable
injection devices or in multi-dose forms such as 2 bot_le
from which the appropriate dose may be withdrawn or a
solid form or concentrate which can be used to prepare an
injectable formulation.
For topical and percutaneous administration, the
preparations may also be presented as an ointment, cream,
lotion, gel, spray, aerosol, wash, skin paint or patch.
~0
A unit dose for treating diseases in which enzymes o the
collagenase family are involved will generally contain
'rom lO to lO00 ms and preferably will contain from lO to
500 mg, in particular lO, 50, lO0, 150, 200, 250, 300,
350, 400, 450 Gr 500 mg. The composition may be
WO 91/15506 PCr/GB91/00529
- 25 -
administered one or more times a day, or example 2, 3 Gr
4 tlmes daily, so that the total daily dose for a 70 :~c
adult will normally be in the range 10 to 3000 mg. Su-h a
dosage corresponds to approximately 0.15 to 50 mg/kg pe-
day. Alternatively, n particular fo- injec_ion, the uni~
dose will contain f-om 2 to 200 mg of a compound af ~he
invention and be administered in multi?les, if desirec, t^
give the desired daily dose.
The present invention additionally provides ~ me-hod o-
treating cor.ditions ir. which degradat_on o connec-ive
tissue and other proteinaceous componen~s c- the body
occurs, such as rheumatism and/or arthritic conditions in
mammals, such as humans, which comprises administering tc
the mammal in need of such treatment an ef'ective amount
of a compound of formula (I) or a pharmaceutically
acceptable salt thereof.
The present invention also provides the use of a compound
of formula (I) or a pharmaceutically acceptable salt
thereof, for the manufacture of a medicamer.t for use in
the t~eatment o' cond_tions in which degradation o'
connective tissue and othe- proteinaceous components o
the body occurs such as rheumatism and/or a-thriti-
conditions.
'.he 'cllowing Descriptions and Exampies i; ustra~e ~hepreparation of compounds of ~he inven.ior.. -.11
temperatures are expressed in C.
WO91/15506 PCT/GB91/~K29
2s ~ ~ ~n~a - 26 -
DescriDtion 1
Dibenzvl (1-hydroxvPro~vl)phosPhonate (D1)
(PhCH20~P ~ OH
C2H5
The general method of F. Texier-Boullet and A. -oucauc
[Synthesis, 1982, 916] was employed. A mixture cf
dibenzyl phosphite (31.13 ml, 0.14 mole) and
propionaldehyde (10.21 ml, 1 equiv.) was stirred at room
temperature and basic alumina (70g) added in one portion
After standing overnight at room temperature chloroform
was added and the alumina collected and washed with
chloroform. The filtrate was evaporated to dryness and
the resulting clear oil chromatographed on silica gel
- (600g) with gradient elution (ether - 5% methanol/ether).
The title compound was obtained as a clear oil which
solidified on standing (27.82g, 64%). A sample was
recrystallized from ether/pentane to give a white
crystalline solid, m.p. 81-82C.
~ound C,64.09; H,6.71. C17H2l04~1 requires C,63-~q;
~:,6.61%.
~ (CDCl3) 1.04(3H,t,J=7Hz), 1.6-1.95(2H,m), 2.27(1:-, srs),
3.8(1~., 2 overlapping triplets,J=5 and lOHz),
4.g7-5.18~4H,m), 7.34(lOH,s).
WO91/15506 PCT/GB9l/~K29
- 27 - ~r~ ,8
Descri~tion 2
Dibenzvl ((1-trifluoromethanesul~honvioxv)~ro~vl) Dn,oS-
phonate (D2)
.S CF~
C2H5 0
The title compound was prepared by the general method o-
E. Vedejs et al. ~J. Org. Chem. 1985, 50(12), 2165]. A
solution of dibenzyl (1-hydroxypropyl)phos-phonate (D1)
(24.97g, 0.078 mole) in methylene chloride (180 ml) was
cooled to ~50C under N2. 2,6-Lutidine (11.12 ml, 0.095
mole) was added followed by trifluoro- methanesulphonic
anhydride (15.1 ml, 0.0898 mole) keeping the temperature
-50C. The mixture was allowed to warm slowly to 0C and
then taken into cold ether. The sol~,ion was subjected ~o
a rapid aqueous work-up by washing the o.ganic layer w _h
ice-cold water, dilute hydrochlo-ic acid (x2) and finall
brine. The organic layer was dr ed (anhydrous MgSO~) ar.
evaporated to d-yness to give the t ~`e compound- 2s a
pinkish orange oil (33.77g, S6%) whic î was used w zhou_
furthe r purification.
~ (CDCl3) 1.08(3H,t,J=7Hz), i.88(2H,r.), 4.94(1H,2
overlapping triplets,J=5.5 ar.d 7:-.z), -..88-5.22(4H,m) an-
7.35(10H,m).
WO9l/15506 PCT/GB91/00529
- 28 -
Descri~tion 3
N-(1-~R)-Dibenzyloxy~hosphinvl~ropyl)-(S)-leucine (D3A~
and N-(1-(S)-Dibenz~loxY~hos~hinvlpr3~vl)-(S)-leu-in.e
(D3B)
CH3
C2H5 ~ CH3
(PhCH20~p N CO2H
O H
Method A
rollowing the general method of US 4808741 for the
preparation of leucine trimethylsilyl ester a mixture of
15 (S)-leucine (1.15g, 0.0088 mole), hexamethyldisilazane
(1.75 ml), and triethylamine (1.38 ml) in acetonitrile
(13.5 ml) was heated at reflux for a total of 4h.
Dibenzyl ((1-trifluoromethanesulphonyloxy)propyl)-
phosphonate (D2) (4.5g, 0.01 mole) was then added and the
mixture maintained at 40-42C for 48h. The reaction can
also be car-ied out at ambien~ tempe-at~-e. After coc'ir.
the mixture was filtered, washed with methanol and the
filtrate evaporated to dryness. The residue was taken u?
in chloroform and washed with dilute HCl (x2) and finaily
wate-. The chloroform layer was dried (anhydrous Na2S~A),
_ilte-ed and evaporated to dryness to sive an orange cummy
scl c (3.67g). The crude p_oduc~ W25 triturated wi~h .he
minimum volume of ether/pentane to g ve a white
c-yst211ine sGlid which after collec_ion, washinc wi . a
1 ~tle cold ether/pentane and d-y nc save the ti~ e
compound, R,S isome- (D3A) (0.47g, 11%), ~.p. 112-:15-.
WO9l/15506 PCT/CB91/~529
2s, ~ 3~
- 2~ -
Observed Desorption CI (NH3) MH+ 434. C23~32N05P reGuire~
M 433.
[]D20 = -23.09 (c=0.97 MeOH).
.~ound: C,63.73; H,7.42i N,3.23. C23H32NOsP requires
C,63.73; H,7.44; N,3.23%.
(CDC13): 0.89 (6:i,t), 1.03 (3H,t), 1.25-2.0 (5r.,m), 2.7-.
(lH,m), 3.28 (2H, br s), 3.73 (lH, br t), 4.9-5.15 (4.,
m), 7.35 (lOY., s).
~he o~her isome-, N-~l-(S)-dibenzyioxyphos?r.inylp~opy'!-
(S)-ieucine (D3B), can be obtained by preparative :~ ~
usinc a Hamilton P~P-l column, 300 x 7.Omm, 264~ with c
40:60 acetonitrile:water eluent mixture and a flow r2te c
4.0 ml/min. Under these conditions the R,S isomer (D3~)
elutes first with a retention time of 34.6 min and the S,S
isomer (D3B) is well separated at 42.7 min.
For the isomer ~D3B):
Observed FAB (M+H)+ 434. C23H32Ns~ requireS M 433.
(CDC'3): 0.88 (6H,cd), 0.98 (3H,t), 1.4 (l..,m), .52-:.^
(4H,m), 2.72 (lH,m), 3.38 (lH,m), 4.9-5.15 (4H,m), 7.32
(lOH,s).
The S,S isomer (D3B) on coupling with (S)-amino 2C' -
deriv2~ives leaàs to the S,S,S, series.
Me-hod B
~0
. miY.tu-e of (S)-leucine methyl ester r.ydrochloriGe
(0.543g; 0.003 mole), dibenzyl (l-trifluoromethane-
suiphonyioxy)propyl)phosphonate (D2) (1.35g; 0.003 mc e)
anc annydrous potassium carbonate (1.0g) in me~hano:
~5 (2 ml) was heated a- 50C, wi~h stir-ins, ~or 4 ho~~s cnd
then left at room temperature overnigh~.
WO91/15506 PCT/GB91/0052~
2~ 30 -
The reaction mixture was evaporated to dryness ir V2CUC,
and dissolved in chloroform (5 ml) and filtered. The
filtrate, and washings, were combined and chromatographed
on silica gel 60 (50g) using ethyl acetate-pentane (l:'~
as the eluent, to afford a mixture of N-(l-(R)-dibenzyl-
oxyphosphinylpropyl)-(S)-leucine methyl ester and
N-(l-(S)-dibenzyloxyphosphinylpropyl)-(S)-leucine me.hyl
ester as an oil (O.SSg). The esters can be sep2rated
into individual diastereomers by column chromatograpr.v o,.
silica gel with initially 50% diethyl ether/pentane as
eluent, rising to 100% diethyl ether.
The above mixture of esters (l.lg, 0.0025 mole) in
methanol (4.0 ml) was treated with a solution of sodium
hydroxide (O.llg; 0.00275 mole) in water (1.5 ml), and the
solution was stirred at room temperature overnight. It
was evaporated to one third volume, ln vacuo, taken in
water and extracted with ether. The aqueous fraction was
acidified with citric acid to pH 3-4 and then extracted
(5x) with chloroform. The chloroform fraction was dried
(Na2S04) and evaporated to dryness in vacuo to give a
mixture o~ the title compounds ~D3A) and (D3B) as an oil
.hat slowly solidified.
Trituration of the product with ether gave
N-(l-(R)-dibenzyl-oxyphosphinylpropyl)-(S)-leucine (D3A)
(0.34g) as a white crystalline solid, identical to the
?rdUc- obtained by Method A.
Alternatively, the single isomer can be hydrolysed
separately. For example N-(l-(S)-dibenzyloxy-
phosphinylpropyl)-(S)-leucine methyl ester on hydrolys s
by the aoove method gave N-(l-(S)-dibenzyloxy-
phospninylpropyl)-(S)-leucine (D33), m.p. 71-73C.
WO91/15~06 PCT/GB91/~29
- 31 -
DescriDtion 4
N-tert-Butoxycarbonvl-N -benzYloxvcarbonvl-(S)-lvsine-(5-
hvdroxv)~entvlamide (D4)
H O
~ ~ OH
H C N ~ OCH~Ph
0
To a solution of N~-tert-butoxycarbonyl-Nf-benzy1-
oxycarbonyl-(S)-lysine (7.8g, 21 mmol) in anhydrous
dichloromethane (150 ml) maintained at 0C was added
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (4.3g, 22.5 mmol) and 1-hydroxybenzotriazole
(3.6g, 26.5 mmole). The mixture was stirred for 0.5h at
0C, 5-aminopentan-1-ol ~2.3g, 22.5 mmol) added and
stirring continued at room temperature. After 3h the
mixture was washed with saturated aqueous NaHCO3 (60 ml),
dried over anhydrous magnesium sulphate and evaporated _
vacuo to afford a viscous oil. Purification by flash
chromatography ~(CHC13:MeOH) (20:1) v/v] gave the title
compound (D4) as a clear oil (8.01g).
Observed (M+H)+ 466. C24H39O6N3 requires M 465.
WO91/15506 PCT/GB91/00529
2. . ~.~ ~3
- 32 -
Description 5
N-tert-Butoxycarbonvl-N~-benzyloxvcarbonvl-(S)-
lvsine-(4-formYl)butvlamide (D5)
3 CH3
0
To a s~irred solution of oxalyl chloride (1.47g, 12 r~,o:)
in anhydrous dichloromethane (40 ml) maintained unde- 2n
atmosphere of nitrogen at -60C was added dimethyl-
sulphoxide (1.21g, 15 mmol) dropwise, such that thetemperature remained below -50C. The mixture was left
stirring at -60C for 15 minutes, alcohol ~D4) (3.6g,
7.7 mmol) diluted in anhydrous dichloromethane (10 ml) was
added, and allowed to warm up to -25C over lh. The
mixture was then cooled down to -60C, triethylamine
(4.7g, 46 mmol) added slowly such that the internal
temperature remained below -50C. On completion of
addition, the mixture was gradually warmed up to room
temperature, washed with water (30 ml) and sat. aq. NaC
(30 ml). The aqueous washes were bac~ extracted with
dichloromethane (2x30 ml) and the combined organic
-ractions were dried over anhydrous magnesium sulph2 - 2..5
evaporated in vacuo to yield a v scous clear oil.
Purification by flash chromatography
[(EtOAc:MeOH)(20:1)v/v] afforded the .itle compound (~)
as an oil (2.8g).
ed (M H) 464- C24H376N3 _eq~ s ~ 463-
WO91/15506 PCT/GB91/0052g
2 ~
- 33 -
Descri~tion 6
(S)-3-(N-tert-Butoxvcarbonvl)amino-8-(N-benzvloxv-
carbonvl)-1,8-diazacvclotridecan-2-one (D6)
s
H3C~ o ~ N ~
1 0 O10CH2Ph
~ethod A
The aldehyde (D5) (1.8g, 3.88 mmol) was dissolved ir.
ethanol (180 ml) and hydrogenated over 5% palladium on
charcoal (200 mg) at atmospheric pressure and 35C for
72h. The suspension was filtered through Kieselguhr and
evaporated in vacuo to give crude 3-[N-tert-butoxy-
carbonyl]amino-(S)-1,8-diazacyclotridecan-2-one. The
crude amine was dissolved in a mixed solvent system of
tetrahydrofuran/water, (6:20 ml) v/v cooled to 0C and
treated with benzylchloroformate (0.66g, 3.88 mmol) anc
excess sodium carbonate to maintain a pH between 10 and
11. The mixture was left stirring at room temperature
overnigAt, washed with ethyl acetate ~3x25 ml), and t~.e
combined organic fractions dried over anhydrous magnesium
suiphate and evaporated in vacuo to arford z clear G' 1 .
Durification by flash chromatography
[(EtOAc:MeOH)(20:1)v/v] yielded the tl~le compounc (~6) z_
a white solid (0.2g).
Observed M+ 447. C24H3~O5N3 requires M 447.
W091/15506 PCT/GBgl/00~29
vB
Method 3
The aldehyde (D5) was hydrogenated at about 100 psi ove-
5% palladium on charcoal in methanol, and then in acidic
methanol to afford crude (5)-3-[N-tert-
butoxycarbonyl]amino-1,8-diazacyclot-idecan-2-one.
The amine was treated with benzylchloroformate and
purified as described in Method A to yield the identic21
title compound (D6).
Desc-i~tion 7
(S)-3-Amino-8-~N-benzvloxvcarbonvll-1,8-diazacvclo-
tridecan-2-one, trifluoroacetate salt (D7)
H 0
TFA.H~N .~ N
~ N ~
OCH2Ph
A cooled (0C) solution of the lactam (D6) (0.19g,
0.42 mmol) in dichloromethane (5 ml) was treated with
trifluoroacetic acid (2 ml). After 0.5h the solvent was
evaporated under reduced pressure, the residue diluted
witA cichloromethane (15 ml) and washed with sat. 2q. NaCl
(10 ml). The organic fraction was dried over annydrous
m2gnesium sulphate and evaporated ln vacuo to sive c-ude
title compound (D7) as an oil. This was used as such
without further purification.
WO91/15~06 PCT/GB91/00~29
- 35
Descri~tion 8
(S)-3-~N-~N-(R)-(1-Phos~honoProPvl)-(S)-leucYlllamino-
8-(N-benzvloxvcarbonyl)-1,8-diazacYclotridecan-2-one,
dibenzvl ester tD8)
CH3
~- CH3
C2Hs ~ H
~hCH2O)2P~ ~ N ~ ~
OCH,Ph
A solution of N-(1-(R)-dibenzyloxyphosphinylpropyl)-
(S)-leucine (D3A) (0.194g, 0.45 mmol) in anhydrous
dichloromethane (20 ml) maintained at 0C was treated with
1-~3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (0.085g, 0.45 mmol) and 1-hydroxy-
benzotriazole ~0.079g, 0.58 mmol). After stirring for
0.5h the reaction mixture was sequentially treated with
lactam ~D7) ~0.15g) and N,N-diisopropyl-ethylamine ~0.12g,
0.89 mmol). Stirring was continued for 18h at room
temperature, then the mixture was washed with sa~. 2C.
NaHCO3 ~2x20 ml), and sat. aq. NaCl (2x20 ml). The
aqueous washes were back-extracted with dichloromethane
and the combined organic frac~ions dried over anhydrous
magnesium sulphate, and evaporated in vacuo to yie!d an
oil. On purification by flash chromatography (2% methanc:
in chloroform), the title compound (D8) was obtained zs z
clear oil ~0.110g).
Observed (M+H)+ 763. C42H59N4O7? requires ~ 762.
WO91/15506 PCT/GB91/~29
2. ~ ~v~
- 36 -
(CDCl3): 0.96(6H,t), 1.2(3H,t), 1.25-1.93(18H,m),
2.70(lH,m), 2.90(lH,m), 3.18(2H,m), 3.39(2Y.,dt),
3.62(2H,m), 4.23(lH,m), 4.92-5.12(6H,m), 6.0(1H,brs),
7.28-7.42(15H,m).
Descri~tion 9
Na-tert-3utoxvcarbonvl-N~-benzvloxvcarbonvl-(S)-ornithine-
(6-hydroxv)hexvlamide (D9)
3 ~ ~rN ~ N ~ OH
3 ~ ~rr OCH2Ph
H
A solution of Na-tert-butoxycarbonyl-N -benzyloxycarbonyl-
(S)-ornithine (12g, 0.033 mol) in anhydrous
dichloromethane (200 ml) maintained at 0C, was treated
sequentially with 1-(3-dimethylaminopropyl)-3-
ethylcarbodiimide hydrochloride (7.5g, 0.039 mol), and -
hydzoxybenzotriazole (5.3g, 0.039 mol). The solution wzs
stirred at 0C for 1 h, treated with 6-aminohexan-1-ol
(4.6 g, 0.039 mol) and left stirring overnight at room
temperature. The mixture was then washed with saturated
aqueous NaHC03, dried over anhydrous magnesium sulpha~e,
and evaporated in vacuo to afford a viscous oii.
Purification by flash chromatography [(CHCl3:MeOH)
(20:1)v/v] gave the title compound (D9) as a clear oil
(9.lg).
observed (M+H) 466- C24H395N3 reqUires _
WO91/15506 PCT/G891/00529
~r ~ ~ ~?~
- 37 -
(CDC13): 1.25-1.4(5H,m), 1.4(9H,s), 1.42-1.62(7~,m),
1.78(1H,m), 3.1-3.35(4H,m), 3.6 (2H,t), 4.12(1H,m), _ 08
(2H, br s), 5.31(lH,t), 5.42(lH,d), 6.73(lH,brs),
7.3(5H,m).
DescriPtion 10
N~-tert-Butox~carbonvl-N~-benzvloxvcar~onvl-(S)-o-n ---ne-
(5-formYl)Pentvlamide (D10)
H3C~ o ~ N
H
The alcohol ~D9) (6.5g, 13.9 mmol) dissolved in
dichloromethane (50 ml) was added to a vigorously stirred
mixture of pyridinium chlorochromate (9g, 41.8 mmol) and
4A molecular sieves (20g) in dichloromethane (200 ml).
Additional portions of pyridinium chlorochromate (4g) were
added after 30 minutes and 45 minutes. After lh tota
reaction time, the mixture was poured into ether (200 ml)
and the reaction flask rinsed with ether (3xlO0 ml). The
2~ combined organic fractions were filtered through
kieselguhr, and concentrated in vacuo to afford a ye__ow
oil. Purification by flash chromatography r(EtoAc:M~e5:~)
(49:1)v/v then (20:1)v/v~ yielded the title compou.~ (310)
as a viscous oil (3.5g).
Observed (M+H)+ 464- C24H37N36 reqUires _
WO91tlSS06 PCT/GB91/ ~ 2g
2. ~.~ - 38 -
Descri~tion 11
(S)-3-(N-tert-Butoxvcarbonvl)amino-7-(N-
benzvloxvcarbonvl)-1,7-diazacvclotridecan-2-one (Di
H3C~ o ~N
OCH~Ph
The aldehyde (D10) (2.5g) in methanol (300 ml) was .-ez.ec
with 5% palladium on charcoal (2.5g). The suspension was
hydrogenated at 100 psi and ambient temperature for 48r.,
treated with 2.5M aqueous hydrochloric acid (2 ml) and
hydrogenation continued at the said pressure for a further
24h. The suspension was filtered through kieselguhr and
evaporated in vacuo to give crude 3-~N-tert-butoxy-
carbonyl)amino-~S)-1,7-diazacyclotridecan-2-one. The
crude amine was dissolved in a mixed solvent system O r
tetrahydrofuran/water, (10:40 ml) v/v cooled to 0C and
treated with benzylchloroformate (0.92g) and excess sod_Lm
carbonate to maintain a pH between 10 and 11. The mix.ure
was left stirring at room temperature for 4h, solve~t
partly evaporated in vacuo and the residue extracted with
dichloromethane (3xS0 ml). The combined organic -^-ac~icns
were dzied over anhydrous magnesium sulphate and
evaporated in vacuo to afford z clear oil. Purific2~~0r.
by flash chromatography [(EtOAc:MeOH) (50:1) v/v! yiei-ec
the title compound (D11) as a whi~e solid (0.27g).
Observed M+ 447. C24H37O5N3 requ -es M 447.
WO91tlS~06 PCT/GB91/~29
- 39 - 2
Description 12
(S)-3-Amino-7-tN-benzvloxYcarbonvll-1,7-
diazacvclotridecan-2-one, trifluoroacetate salt (~12
H O
TFA. H ~ N
~
~OCH2Ph
A cooled (0C) solution of the lactam (D11) (O.i3S) -.
dichloromethane (5 ml) was treated with trifluoroace.ic
acid (3 ml). After lh the solvent was evaporated unde-
reduced pressure, to afford crude title compound (D12) asan oil. This was used as such without further
purification.
Description 13
(5)-3-rN-~N-(R)-(1-PhosPhono~roPvl)-(S)-leucvlllamino-7-
(N-benzvloxYcarbonvl)-1,7-diazacvclot-idecan-2-one,
dibenzvl ester (D13)
CH3
~ CH3
C2H5~H O
OCH,Ph
A solution of N-(l-(R)-dibenzyloxyphosphinylpropyl)~
leucine (D3A) (0.115g) in anhydrous cichloromethane
(20 ml) maintained at 0C was treated with 1-(3-
WO91/15506 PCT/GB91t00529
~ . ., " ~
- 40 -
dimethylaminopropyl)-3-ethylcarbodiimide hydrochlori~e
(0.OS6g) and l-hydroxybenzotriazole (0.036g). Afte-
stirring for 0.Sh the reaction mixture was sequentially
treated with the crude lactam (D12), N,N-diisopropyl-
ethylamine (0.07Sg) and stirring continued at roomtemperature for 4h. The mix.ure was washed with wa.e-
(20 ml) and sat. aq. NaHCO3 (2x20 ml). The aqueo~s washes
were back-extracted with dichloromethane and the cor_ine~
organic f.actions dried over anhydrous magnesium sulphate
and evaporated in vacuo to yield an o_l. On puri'ic2.ion
by flash chromatography (2% methanol n chlorofor~.), the
title compound (D13) was obtained as a white foam
(0.125g).
Obser~ed (M+H)+ 763. C42H59N4O7P requires ~ 762.
DescriPtion 14
N-tert-ButoxvcarbonYl-N~-benzYloxYcarbonYl-(s)-lysine-(5
hvdroxv)hexYlamide (D14)
H O
H3C ~ O H H OH
25 CH3 N ~ OCH2Ph
A solution of N-tert-butoxyca-bonyl-N-benzyloxyca--onyl-
(S)-lysine (15.8g, 0.042 mol) in anhydrous dichlorome~hane
(200 ml) maintained at 0C, was treated sequentially wi_h
.-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride (9.96g, 0.051 mol) and l-hydroxy-
benzotriazole (7.0g, 0.051 mol). The solution was s_ir-ed
WO 91/15~;06 PCr/GB91/0052g
~ 41 _ ~r~?~
at 0C for lh, treated with 6-aminohexan-1-ol (4.75, 0.04
mol), and left stir-ing overnight a. -oom temperatu-e.
The mixture was then washed with saturated aqueous NaY.CO~,
dried over anhydrous magnesium sulphate, and evaporated
S vacuo to afford a viscous oil. ~urification by flash
chromatography [(C:-C13:MeOH) (20:1) v/v] gave .he title
compounc (D19) as a clear oil (16g).
Observed (M+U)+ 480 C2s~4lN3c6 re~~eS M~ 479
Desc-i~tion '5
N~-tert-Butoxvcarbonvl-N-benzvloxvcarbonvl-(S)-lvsine-(--
formvl)~entvlamide (D15)
H O
H3C~ O~N ~ N ~ OH
3 N ~ OCH2Ph
A stir~ed solution of dimethyl sulphoxide (3.63g, 0. 046
mol) in anhydrous dichloromethane (100 ml) maintained at
-60C, was treated with oxalylchloride (2.58g, 0.0198 mol~
diluted in dichloromethane (10 ml) at such a -ate, so as
to ensure temperature remained below -50C. Afte-
sti-rinS fo- 20 minu~es, the clccho~ (Dl4) (6.35g, C.0:
mol) dissolved in dicAloromethane (50 ml) was added
dropwise ove- 5 mins. T~.e mixtu-e was stir-ed at -60C
for 15 mins, warmec UD to -35~, stirred fo- z fu.the- ~
mins then cooled down to -60C. ~he solution was trea.e^
with triethylamine (8g, 0.08 mol), warmed u- to room
WO91/15506 PCT/GB~1/~529
- 42 -
temperature, washed with water (2x100 ml), d-ied over
anhydrous magnesium sulphate and solvent evaporated undor
reduced pressure to afford a viscous oil. Purificztion bv
flash chromatography [(~tOAc:MeOH) (30:1) v/v] gave .;~e
title compound (D15) as an oil (5g).
Observed (M+H)+ 478. C25H39N36 -equires M 477-
Descri~tion 16
(S)-3-(N-tert-3utoxvca-bonvl)amino-?-(~-
benzvloxvcarbonvl)-1,8-diazacyclotetraaecan-2-one (~
H O
X `~ .~ N
H3C CH3O H N J
OCH2Ph
The aldehyde (DlS) (5.0g) in methanol (450 ml) was treated
with 5~ palladium on charcoal (S.Sg). The suspension was
ryd-ogenated at 140 psi and ambient tempera~ure fo- 48h,
treated with 2.5M aqueous hydrochlor c acid (3 ml) anc
hydrogenation continued at the said pressure for 2 fu-~:-e-
24h. The suspension was filtered through Kieselguhr ar.-
eva~orated in vacuo to give crude (S)-3-(N-tert-butcxy-
ca-bonyl)amino-1,8-diazacyclotetradecar.-2-one. T~.e c-~d_
amine was dissolved in a mixed soivent system of
tetrahydrofuran/water, (10:40 ml) v/v cooled to 0C an-
treated with benzylchloroformate (2.85g) and excess sod _-
ca-Donate to maintain a pH between 10 and 11. The mix~ur-
was ieft stirring at room tempera.ure for 4h, soiven.
WO91/15506 PCT/GB91/~K29
3 Z~, '^J~j~
-
partially evaporated in vacuo and the residue extracte~
with dichloromethane (3x100 ml). The organic 'racticn w2c
dried over anhydrous magnesium sulphate and evaporated
vacuo to afford a clear oil. Purification by flash
chromatography [(EtOAc:MeOH) (50:1) v/v] yielded the ti'l G
compound (D16) as a white solid (2.0g) m.p. 131.5-134.0~,
Observed M+ 461. C25H3gN305 requires _ 461.
Descri~tion 17
(S)-3-Amino-8-~N-benzvloxvcarbonvll-1,8-
diazacvclotetradecan-2-one, trifluoroacetate salt (D17)
H O
TFA.H,N .~ N
N ~
OCH2Ph
A cooled (0C) solution of the lactam (D16) (0.175g) in
dichlcromethane (Sml) was treated with trifluoroacetic
acid (3 ml). After lh the solvent was evaporated unde-
reduced pressure, to afford crude title compound (D17) as
an oil. This was used as such without further
?U~i,ication.
WO9ltlS506 PCT/GB91/00529
~f~ ~?~
- 44 -
Descri~tion 18
(S)-3-~N-tN-(R)-(1-PhosPhono~ro~vl)-(S)-leucvlllamino-8-
(N-benzvlox~carbonvl)-1,8-diazacyclotetradecan-2-one,
dibenzvl ester (D18)
CH3
~ CH3
C~H5 ~ H
(PhCH20)2P"`~ N ~ ~
OCH,Ph
A solution of N-(1-(R)-dibenzyloxyphosphinylpropyl)-(S)-
leucine (D3A) (0.133g) in anhydrous dichloromethane
(10 ml) maintained at 0C was treated with 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
(0.065g) and 1-hydroxybenzotriazole (0.046g). After
stirring for 0.5h the reaction mixture was sequentially
treated with the crude lactam (D17) and N,N-
diisopropylethylamine (0.lg) and stirring continued a~
room temperature for 4h. The mixture was washed with
water (10 ml) and sat.aq. NaHCO3 (2x10 ml). The aqueous
washes were bac~-extracted with dichloromethane and ~he
combined organic fractions dried ove- anhydrous magnesium
sulphate and evaporated in vacuo to yield an oil. Or
pur_'_cation by flash chromatography (2% methanol ir.
chloroCorm)~ the ti_le compound (D18) was obtainec 2S c
white foam (170 mg).
Ooserved (M+H) 777- c43H6lN4o7~ rec ~res M 77~-
W09ltl5506 PCT/GB91/~K29
_ 45 _ 2~ .~ ~,~,,~,~
Descri~tion 19
(S)-3-(N-tert-Butoxvcarbonyl)amino-8-(N-methvl)-1 8-
diazacyclotridecan-2-one (D19)
H3C~ o ~ N ~=
CH3
A solution of diazalactam (D6) (0.35g) in methanol (20 .;1)
was treated with 5% palladium on charcoal (0.2g) and
hydrogenated for 24h at atmospheric pressure and room
temperature. The suspension was filtered through
kieselguhr, and the filtrate diluted with methanol to a
total volume of 50 ml. The solution was treated with 5%
palladium on charcoal (0.3g) followed by 40% aqueous
formaldehyde (1 ml), and the resulting suspension
hydrogenated for 48h at a pressure of 100 psi. The
suspension was filtered through Kieselguhr, solvent
evaporated in vacuo, to yield an oil which cn exposu~
diethyl ether solidified. Pu-ifi-ation by flash
chromatography [(CHCl3:MeOH:NH3) (90:9:0.S) v/v] afforded
the title compound (Dl9) as 2 pale yellow solid (G.i4g).
m.?. 135-137C.
Observed M~ 327. C~7H33N303 -equ res M 32,.
WO91/15506 PCT/GB91/~K2~
2.~ a
- 95 -
DescriPtion 20
_
(5)-3-Amino-8-~N-methYll-1,8-diazacYclotridecan-2-one,
trifluoroacetate salt (D20)
s
H
TFA.H~N- ~ N
H
CH3
A cooled solution of the diazalactam (D19) (0.175g) in
dichloromethane (5 ml) was treated dropwise with
trifluoroacetic acid (3 ml). After lh the solvent was
evaporated under reduced pressure to afford crude title
compound ~D20) as an oil. This was used as such without
further purification.
Description 21
(S)-3-rN-~N-(R)-(1-~hosphono~ropvl)-(S~-leucvlllamino-8-
(N-methvl)-1,8-diazacvclotridecar.-2-o~.e, dibenzvl es~e-
(D21)
CH3
~ CH3
C2Hs ~ H
PhCH20)2P ~ o ~=?
CH3
solu~ion o- the N-(1-(R)-dibenzyloY.yphosphinylpropyl)-
(S)-leucine ~D3A) (0.133g) in an..ydrous dichloromethane
(10 ml) maintained at 0C was t-eated with 1-(3-
WO91/15506 PCT/GB91/00~29
- ~7 -
dimethylaminopropyl)-3-ethylcarbodiimide hydrochlo_iae
~0.065g) and l-hydroxybenzotriazole (0.046g). The mix~u e
was allowed to stir for 0.Sh at room temperature, ther.
treated with amine (D20) dissolved in dichloromethane
(10 ml), followed by N,N-diisopropylethylamine (0.ig).
After stirring for 18h, the mixture was washed wi-.. S2..
aqueous NaCl (2x10 ml), dried over anhydrous magnesiu-..
sulphate and solvent evaporated in vacuo to afforc an oil.
Purification by flash chromatography (2% methanol in
chloroform) gave the title compound (D21) as a wh te ~ozm
(0.17g).
Observed (M+H) T 643- C35~55N405~ requires M 642-
Descri3tion 22
N-tert-Butoxvcarbonvl-NF-benzvloxvcarbonYl-(S)-lvsine-(3-
hYdroxv)~ro~Ylamide (D22)
H C H
3 CH3O ~ ~ ~CH Ph
A sti--ed solution of Na-rer~-outoxycarbonyl-~E-
benzyloxycarbonyl-(S)-lysine (17.2g) maintained a_ 0~ n
anhydrous dichloromethane (250 ml) was t-eated wi-;n 1-(~-
dimethylaminopropyl)-3-ethylca-bo~iimide hydrochlo.ide
(7.5g) and l-hydroxybenzot~iazole (5.3g). The so: t o~.
was sti-red a; 0C for lh, treated with 3-aminopro?an-
~(4.0&g) dissolved in dichloromethane (50 ml~ and le _
WO91/15506 PCT/GB9l/~K29
2 . , . .~
- 48 -
stirring for 18h at room temperature. The solution wzs
then washed with sat. aqueous NaHCO3, dried over anhyd-ous
magnesium sulphate, and evaporated in vacuo to afford a
viscous oil. Purification by flash chromatography
[(EtOAc:MeOH) (49:1) v/v] gave the title compound ~D22) 2S
a clea- oil (15.2g).
Observed (M+H)+ 438- C22H35N36 re~uires M 437-
Descri~tion 23
Na-tert-3utoxvcarbonvl-N-benzvloxvcarsonvl-(S)-lvsine-l2-
formvl)ethvlamide (D23)
H C ~ ~ ~ N ~H o
CH3 N ~ OCH2Ph
o
The title compound (D23) was prepared rollowing the
procedure as described for the sy-.thesis of Na-te~~-
butoxyca-bonyl-N-benzyloxycarbonyl-(S)-lysine-(4-
formyl)butylamide (D5) (yield: 59~).
Observed (M+H) 436. C22~33N3O6 _equ_ _s -
WO91/15506 PCT/GB91/00~29
- 45 -
Desc i~tion 24
(S)-3-(N-tert-Butoxvcarbonvl)amino-a-(N-
benzvlox~carbonvl)-1,8-diazacvcloundecan-2-one (D24)
C ~ ~r ~ N
~ ~ OCH2Ph
The ti.le com?ound (D2~.) was prepa-ed following the
procedure described for the synthesis of (S)-3-(N-te-t-
butoxycarbonyl)amino-7-(N-benzyloxy-carbony1)-1,7-
diazacyclotridecan-2-one (Dll) (Vielc 11%), m.p. 172-
174C.
Description 25
20 (5)-3-Amino-8-~N-benzvloxvcarbonvll-1,8-diazacvcloundecan-
2-one, trifluoroacetate salt (D25)
H O
TFA.H N ~ N--
H
~/ ~r OCH2Ph
The tltle compound (D25) was pre_ared following the
?-ocedure described f^- the synt~.esis of (S)-3-amino- -!~;-
benzyloxycarbonyl~-1,/-~iazacycl^-;__cecar.-2-one,
trifluoroacetate sal. (D12), and usec wi~hout
purification.
WO91/15506 PCT/GB91/~K29
- 50 -
Descri~tion 26
(S)-3-rN-~N-(R)-(1-Phos~hono~ro~vl)-(S)-leucvlllaminc-~-
(N-benzvloxycarbonvl)-1,8-diazacvcloundecan-2-one,
dibenzvl ester (D26)
CH3
C2Hs ~ H
(PhCH O)P""~ N ~ N ~ N
\~ r OCH2Ph
The title compound (D26) was prepared following the
procedure described for the synthesis of (S)-3-[N-[N-(~)-
(1-phosphonopropyl)-(S)-leucyl]]amlno-7-(N-
benzyloxycarbonyl)-1,7-diazacyclotridecan-2-one, dibenzyl
ester (D13). (Yield: 44%).
Observed FAB [M-(PhCH2O)2PO] 473. C40H55N407P requi e
M 734.
Des- ~ 2tion 27
(S)-3-rN-rN-(S)-(l-Phos~hono~roPvl)-(S)-leucvlllamino-R-
2~ (N-benzYloxvcarbonvl)-1,8-diazacvclot-idecan-2-one,
~lbenzvl ester (D27)
CH3
C2Hs ~ H
~hCH,O)2P 1 N ~ ~
OCH2Ph
WO91/15506 PCT/GB91/0052~
-- 51 --
A solution of N~ S)-dibenzyloxyphosphinylpropyl)-(5)-
leucine (D3B) (0.27g) in anhydrous dichloromethane (10 -,.')
maintained at 0C was t-eated with 1-~3-dimethylamino-
propyl)-3-ethylcarbodiimide hydrochloride (0.125g) ar.d 1-
hydroxybenzotriazole (0.088g). After stirring for 0.5hthe reac ion mixture was sequentially treated with che
lactam ~-ifluoroacetate salt (D7) (0.75 mmol) and N,N-
diisopropylethylamine (0.16g). Stirring was continued -_-
18h at -oom temperature, then the mixtu-e was washea w_~h
sat.aq. NaHCO3 (2x15 ml), and sat. aq. NaCl (2xlS ml).
The organic fraction was dried over anhydrous magneslum
sulphate, and eva?orated in vacuo to yield an oil. On
purific2tion by flash chromatography (2% methanol in
chloroform)~ the title compound tD27) was obtained as a
clear oil (0.31g).
Observed (M+H)+ 763. C42H5907N4P requires M 762.
Description 28
(s)-3-rN-rN-(s)--(l-phosphonopropvl)-(s)-leucvlllamin
(N-benz~;loxvcarbonYl)-1,8-diazacvclotetradecan-2-one,
dLbenzvl ester (D28)
CH3
,C2Hs ~ H 3
~hCH2O)2p l ~ ~ ~
OCH2Ph
A solu.ion of N-(1-(S)-dibenzyloxyphos?hinylpropyl~-(â) -
leucine (D3B) (0.091g) in anhydrous dichloromethane
(10 ml) maintained a; 0C was treated with 1-~3-
WO91/15506 PCT/GB91/00529
c- ~3
- 52 -
dimethylaminopropyl)-3-ethylcarbodiimide hydrochlo_ de
(0.04g) and l-hydroxybenzotriazole (0.028g). Afte-
stirring for 0.5h the reaction mixture was sequentizlly
treated with lactam trifluoroacetate salt (D17) (0.23
mmol) and N,N-diisopropylethylamine (0.054g). Sti-- ng
was con~inued for 18h at room temperature, then tAe
mixture was washed with sat. aq. NaCl (2x10 ml). ~he
organic fraction was dried over anhyd-ous magnesium
sulphate, and evaporated in ~acuo to yield an oil.
0 Purif cation by flash chromatography (2% methanol
-hloroform) afforded the title compound as an o~l
(O . 105S) .
Observed (M+H)+ 777- C43H61N47P reCuires _
Descri~tion 29
(S)-3-~N-~N-(R)-(l-Phosphonoethyl)-(S)-leucvlllamino-8-~N-
benzvloxvcarbonvl)-1,8-diazacvclotridecan-2-one, dibenzvl
ester (D29A) and (S)-3-~N-~N-(S)-~ hosPhonoethvl~-(S)-
leucyl~lamino-8-(N-benzvloxvcarbonyl)-1,8-
diazacyclot-idecan-2-one, dibenzYl es er (D29B)
CH3
~ CH3
CH3 ~ H
(phcH2o)2p H ~ ~
OCH2Ph
A solutlon o' the isomeric mixture o- acids (D29A) 2nd
(~29B) (0.28g) in anhydrous dichlorome_hane (20 ml)
malr.tained at 0C was treated with 1-(3-
dimethylaminopropyl)-3-ethylc2rbodiimide hydrochls- c_
~0. 37g) and l-hydroxyberzotriazole (^.097g). A'-e-
WO 91/15506 PCr/CB91/00529
- 53 -
stirring for 0.5h the reaction mixture was sequentially
treated with lactam (D7) (0.072 mmol) and N,N-
diisopropylethylamine (0.185g). Stirring was continued
for 18h at room temperature, then the mixture was washed
with sat. aq. NaCl (20 ml). The organic fraction was
d-ied over anhydrous magnesium ~ul?hate, and evaporz~ed _-
vacuo to yield an oil. On purification by 'lash
chromatog-aphy (2% methanol in chloroform), .he t ~le
compounc (D29) was obtained as oil (0.32g).
1 0
Observed rA3 ~M+H)+ 749. C~iH57N4O7? -equ res ~. 7.~.
Desc-i~tion 30
N-((R)-Dibenzvloxv~hosPhinvlethvl)-(S)-leucine (D30A) an-
N-((S)-l-dibenzvloxvphosphinylethyl)-(S)-leucine (D30B)
CH3
, CH3 ~ CH3
20(phcH2o)2p N CO2H
The title mixture of diastereoisomers (D30) was preparec
analogously to the method in Desc-iption 3, Method ~, as _
white solid.
Observed FAB (M+H) 420. C22H30NO52 -equi-es M ~ ,.
WO91/15506 PCT/GB91/~52~
2 . ~ 54 -
Exam le 1
(S)-3-~N-~N-((R)-l-PhosPhono~ropvl)-(S)-leucvlllam no-
1,8-diazac~clotridecan-2-one (E1)
CH3
C2H5 ~LHclI~o
O H O
H
A solu~_on of phosphonic diester (D8) (0.11g) in e~hano
(30 ml) was hydrogenated over 10% palladium on charcoal a~
atmospheric pressure for 24h. The solution was filtered
through ~ieselguhr and solvent evaporated in vacuo. The
residue was triturated with diethyl ether (5 ml) to give
the title compound as a white solid, 0.057g, m.p.
178.5-180.5C.
Observed FAB (M+H)+ 449- C20H415N~P requireS M 448-
(CD30D): 0.96(6H,t), 1.1(3H,t), 1.25-2.0(17H,m),
2.66(iH,m), 3.05(5H,m), 3.3 (lH,m overlapping with MeOD),
3.58(1:-,m), 4.38(lH,m).
WO91/15506 PCT/GB9l/~29
2 ~
- 55 -
Exam~le 2
(S)-3-rN-tN-((~)-1-Phos~honoDropvl)-(S)-leucvl1lamino-1,7-
diazacyclotridecan-2-one (E2)
5CH3
HH~ p~ ~ N
O H
H
A solution of phosphonic diester (D13) (0.09g) in methan~:
(25 ml) was hydrogenated over 5~ palladium on cha-coal a_
atmospheric pressure for 24h. The solution was filterec
through kieselguhr and solvent evaporated in vacuo. .ne
residue was triturated with diethyl ether (5 ml) to give
the title compound (E2) as a white solid ~0.038g).
Observed ~M+H)+ 449. C20H41O5N4P requires _ 448.
(CDC13): 0.96(6H,d), 1.2(3H,t), 1.35-1.9~17H,m),
2.49(1H,m), 2.9(1H,m), 2.98(3H,m),3.12(1H,m), 3.42(1r.,m),
3.7(1:',t), 4.33(lH,t).
WO91/15506 PCT/GB91/~52
~f ~ ~ ~ 56
Example 3
(S)-3-~N-~N-((R)-1-Phos~honoPropvl)-(S)-leucvlllamino-:,8-
diazacvclotetradecan-2-one (E3)
CH3
HO~ ~ N ~ N
O H ~ H N
H
A solu~_on of phosphonic dieste~ (D18) (0.09g) in me~hzno
(20 ml) was hydrogenated over 5% palladium on charcoz:
(0.08g) at atmospheric pressure for 24h. The solutior. w2S
filtered through kieselguhr and solvent evaporated ~n
vacuo. The residue was triturated with diethyl ether
(5 ml) to give the title compound (E33 as a white solid
(0.05g).
Observed FAB (M+H)+ 463. C21H43N405 requ
8 (CD30D): 0.98(6H,t), 1.12(3H,t), 1.3-2.1(19H,m),
2.7-3.25(6H,m), 3.72(lH,m), 4.45(lH,m), 4.55(1H,m).
W091/15506 PCT/GB91/~K29
- 57 - Z'. ~ ~7
Exam~le 4
~5)-3-~N-[N-((R)-1-Phosphonopro~vl)-(a)-leucyll1am~no-~-
(N-meth~,~1)-1,8-diazacvclotridecan-2-c~.e (~4)
CH3
HO`P"` ~N ~ N
O H
CH3
A solution of phosphonic diester (D2 ) (0.09g) _n me-nano_
(15 ml) was hydrogenated over 5% pallzdium on charcoa a.
atmospheric pressure for 24h. The solution was ~lteres
through kieselguhr and the solvent evaporated in vac~o.
The residue was triturated with diethyl ether (5 ml) to
give the title compound (E4) as a whi~e solid (0.051g).
Observed (M+H)+ 463- C21H43N405P reauires M 462-
(CD30D): 0.98(6H,t), 1.1~3H,t), 1.4-2.1(17H,m),
2.7(1H,m), 2.88(3H,s), 3.Q5-3.4(6H,m), 3.6S(l:',m),
4.4(lH,m).
WO91tl5~06 PCT/GB91/~K29
2 ~
- 58 -
Exam~le 5
(s)-3-rN-[N-((R)-l-~hos~honopro~yl)-(s)-leucvlllamino-l~8
diazacvcloundecan-2-one, hYdrochloride salt (E5)
CH3
H0 ~H5 ~ H 3
" H 0 ~ N`HHCl
A solution of phosphonic diester (D26) (O.C7g) i.. e.hz?.~:
(10 ml) was treated with 5% palladium on charcoal (0.06c)
and hydrogenated for 18h at atmospheric pressure. The
suspension was filtered through kieselguhr, and tne
solvent evaporated in vacuo to give a pale brown foam.
The foam was dissolved in ~thanol, and treated with lM
ethereal HCl to afford the title compound (E5) as a white
solid.
(CD30D): 1.0(6H,t), 1.18(3H,t), 1.58(2H,m),
1.7-2.1(llH,m), 2.9-3.2(6H,m), 3.6(1~.,m), 4.45(lr.,~),
~.55(1H,m).
Exam~le 6
(s)-3-rN-rN-((s)~ hos~hono~o~vi)-(s)-leucvlll2~ir
dlazacvclotr decan-2-one (E6)
CH3
HHo~p J ~ ~ Y ~ ~ \
WO91tl~506 PCT/CBgl/00~2g
2~ r ~?. ~
- 59 -
A solution of phosphonic diester (D27) (0.2gg) in metnanol
(40 ml) was hydrogenated over 5% palladium on charcoa
(0.25g) at atmospheric pressure for 24h. The solutior, was
filtered through kieselguhr and solvent evaporated ;r.
vacuo. The residue was triturated with diethyl ethe-
(5 ml) to give the title compound (E6) as a white sol-d
(0.16g).
Observed FAB (M+H)+ 449 C20H41N45? recuires M 448.
(CD30D): 1.0(6H,dd), 1.1(3H,t), 1.4-2.0(17H,m),
2.45(lH,m), 2.85-3.2(SH,m), 3.38(1H,m), 3.58(lH,~),
4.49(lH,t).
ExamPle 7
(S)-3-~N-~N-((S)-1-PhosPhonoProPyl)-(S)-leucYlllamino-1,8-
diazacYclotetradecan-2-one (E7)
CH3
HO-~ P 1N~O ~N. /~
O
A solution of phosphonic dieste- (D28) (0.085g) in
methanol (20 ml) was hydrogenated over 5% palladium on
charcoal (0.08g) at atmospheric pressure for 24h. ~he
solution was filtered through kieselguhr and solver.~
evaporated in vacuo. The residue was ~- turated wi-h
diethyl ether (S ml) to give the tit e compound (~7) as _
white solid (0.045g).
Observed F.~B (M+H)+ 463. C21H43~.~4O5? -e~uires M 462.
WO 91/15506 PCI-/GB91/00529
r ,~ ,~
~ 60 --
(CD30D): 0.98(6H,m~, 1.12(3H,t), 1.3-2.0(19H,m),
2.85-3.25(6H,m), 3.65(1H,m), 4.12(1H,m), 4.49(1H,dd).
Example 8
s
(S)-3-~N-rN-((R)~ hos~honoethvi)-(S)-leucvlllamino--,8-
diazacvclotridecan-2-one (E8A) and (S)-3-~N-~N-((S)- -
phos~honoethyl)-(S)-leucvlllamino-1,8-diaz2c~clot- ~ecan-
2-one (E8B)
CH3
CH3 ~H
HO- P 1N~
H
A solution of phosphonic diester (D29) (0.26g) in methanol
(30 ml) was hydrogenated over 5% palladium on charcoal
(0.2g) at atmospheric pressure for 24h. The solution was
filtered through Kieselguhr and solvent evaporated ln
vacuo. The residue was triturated with diethyl ether
(5 ml) to give the title compound (E8) as a white sol d as
a mixture of diastereoisomers.
Observed FAB (M+H)+ 435. C19~39N4Os~ requires M 434-
WO91/15506 PCT/GBg1/00529
r, :,J . r-s . ~ ~
- 61 -
COLLAGENASE INHIBITOR ASSAY
The test is performed essentially as in Cawston and
Barrett, Anal. Biochem. 99, 340-345 (1979). Compounds
for testing are dissolved in methanol by sonication and
added to collagenase (purified from culture supernat2n~s
from the human lung fibroblast cell line, ~I-38) n
buffer. After a 5 min pre-incubation at 37C, the ass2y
tubes are cooled to 4C and 3H-acetyla.ed rat skln type
collagen is added. The assay tubes a-e in~ubated at 37C
overnight. The 3H-collagen forms insoluble îib--ls,
which are the substrate for the enzyme.
To terminate the assay, the assay tubes are spun at 12000
rpm for 15 minutes. Undigested 3H-collagen is pelleted,
while digested 3H-collagen is found as soluble peptides in
the supernatant. A sample of the supernatant is taken
for liquid scintillation counting.
The activity of collagenase inhibitors (IC50: 50%
inhibitory concentration) is expressed as that
concentration of compound that inhibits a known (standard)
concent-ation of enzyme by 50%.
The compounds of Examples El-E7 had IC50 values ~ the
-ange 6 x l0 8 _ 3 x l0 6M.