Note: Descriptions are shown in the official language in which they were submitted.
~O 91/16294 - 1 - PCT/EP91/00649
- Description 2 ~ 8 0 4 7 8
Process for catalys-t reco~ery
The present invention relat~s to ~ process for the
recovery of catalyst in the preparation of ether-car--
boxylic acids by catalytic oxidakion of the correspondingether alcohols with oxygen on suspended noble me~al
catalysts~
The catalytic oxidation of ether alcohols according ~o
the general equation
Pk/C
R(OC~32CH2 )nOCH2CH2(~
has long been known and i5 described for example in
German Patent 2,936,123 and European Patent 206,054~
However, as the molar mass of the radical R increases,
the separation and thus the complete recovery ancl recy-
cling of the noble metal catalyst becomes problematicO
Thus in German Patent 3,446,561, on ~age 4, a laborious
four-stage method is described to xestrict the losses of
noble metal. An essential processing disad~antage of this
procedure lies in the fact that the reaction mixture;
prior to the filtra~ion, i~ diluted with 1-10 times -tha
amount of acetone, which must -then be remov~d again by
distillation, purified and recycled. The noble metal
contents of 1-4 ppm, which can ultimat~ly be obtained in
the product, are also relatively high and lead to a
gradual decrease in the acti~ity of the catalyst. Over-
all, this entail~ a sexious sconomic disadvantage.
The object was therefore to develop an indus^trially and
economically acceptable process for ca~alyst recove~y.
The sub~ec~ of the presen~ i~venkion is a process for-the
recovery of catalysk in the prepara~ion of e~hsr-car-
boxylic acids by catalytic ox:idation using a susp~ndecl
catalyst, characterized in that the reaction mixture is
~O 91/16294 - 2 -2 ~ ~ O ~ ~J 3 PCT/F.P9ltoo649
~,..
. ..;
subjected to a cross-flow filtration.
Preferred ether-carboxylic acids are-those of the formul~
R--( OC~I2C~I2 ) nOC~I2COOH
in which R is a linear or branched alkyl radical of 1 to
S about 24 carbon atoms, an aryl radical, such as for
example a phenyl, naphthyl or biphenyl radical, addition~
ally an alkyl(Cl-C24)aryl radical, the aryl radical being
for example a phenyl, naph~hyl or biphenyl radical, or an
arylalkyl radical, f~r example a benzyl rzdical/ and n is
a number from 0 to about 24. The abovementioned aryl
radicals can be substituted.
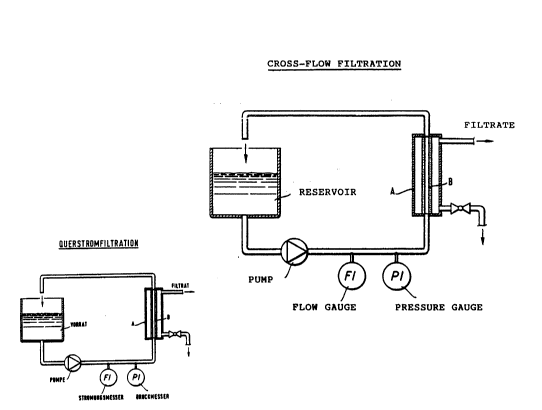
In the cross-flow filtration, the catalyst-con-taining
reaction mixture is pumped at a high overflow velocity
tangentially ~o the membrane surface through -~he filter
elemen-t, the filtrate being withdrawn perpendicular to
the direction o~ flow through the membrane layer, as
described in de~tail in ULLMANN's Encyclopedla of
Industrial Chemistry, 5th Editiont Vol. B2, p. 10-54.
Tu~e-shaped ilter element~ having a membrane layer on
the inner ~ide of ~he tubes are par~icularly suitc~hle.
Preferred membrane and support material is ~ -Al203 and
ZrO2; however, plastic and carbon elemen.ts can also be
used. Membrane and support can be composed of different
materials. The necessa~y pore sizes are expediently in
the ultrafiltration range, for example b~we~n abou~ 1
and about 200 nmO A suitable appara-tus is descr:;bed i~
th~ ex~mple (cf. in this con~ext also Figure 1 on
page 7).
The cro~s-flow 1Eil~ration according ~o thP inven~io:n can
be pe.rformed at tamperat~res from about ~0 -to c~bout 150~C~
It has proved ~o ~e advan~ageou~ to carry out ~he :Eilt
ration at temperatures from c~bout 50 -to c~bout 100C, :in
particular at tempera~ure~ ~rom abou~ 60 to abou-t 80C
W~ 91/1~294 - 3 - PC'~ P91/00~9
A pressure drop must of course prevail hetween the Eront
side and the rear side of the membrane, ~o that the
reaction solution can be moved -through.
A solubilizer used in the oxidation can -facilitate filt~
ration. Solubilizers without hydroxyl groups are suit~
able. Glycol ether~ without hydroxyl groups are particu~
larly suitable, in particular diethylene glycol dime-thyl
ether.
A brief hydrogen treatment after the oxidation lS
completed at the reaction temperature (about 50 to about
100C) has proved to be advantageous, -to precipi-ta-te an~
dissolved and colloidal noble metal traces, so -that
filter elements with somewhat larger pores can be used.
Apar-t from hydrogen Eormaldehyde, ~or example, can also
be used as reducing agent.
Surprisingly, the problems of filtration (blockage,
losses of noble metals) are solved by the process accord~
ing to the invention without the need for expenst~e
measures, such as for example clilution with a solventO
The example below ~erves to illustrate the proce~
according to the invention wi-thou-t restricting it
theretoO
Example
A reaction sol~tion Erom the catalytic o~ida-tion f
comprising 25 % by weight of ether carboxylic acids of
the formula R-(OCH2C~23nOC~2COO~ having linaar alkyl group~
R having a distribution of C12 to C14 and a mean value of
n = 4 and also 45 % by weight of diethylene ylycoL
dimethyl ether~ 25 % by weight of water and 5 ~ by weight
of a suspended commercial cataly~t (5 % by weigh-t o-
~platin~n on acti~ated charcoal) is txeated for 30 minutes
at 70C with hydrocJen in a bubbl~ column and then sub-
jec-ted to a cross-Elow Eil-tration.
~O 91/16294 2 ~ 8 ~ ~ 7 8 PCT~Epgl,00~49
The filter element is composed of a ZrO2 tube (diametQr:
7 mm, length: 250 mm), the inner side of ~hich is
composed o~ a membrane layer having pore sizes of 35 nm
( 1O-a m) . An apparatus is used, which corresponds to the
accompanyiny drawing (cf. Figure 1 on page 7)O The
reaction solution is pumped thxough ~he filter elemen~
(B) situated in a housing (A) at a linear flow velocity
of 5 m/s. A pressure P1 o~ 3 bar is established a~ a
temperature of 70C. A fil-trate flow of 2.5 l/h is
obtained. The catalyst is concentrated to a solids
content of approxLmately 30% by weightj this concentrate
is returned to the catalytic oxidation.
After separation of the diglycol dimethyl ether and ~ater
from the filtrate by distillation, the mixture of ether-
15 carboxylic acids is obtained as a clear, p~le producthaving a residual ~lantity o~ platinum of less than
0.5 ppm.
Comparison example
The reaction solution described in -the above ex~mple i5
repeatedly filtered on a suction fil-ter wi-th insertsd
filter paper (for quantita-tive analysis). The filter
paper must be changed frequently because of blockage. A
cloudy filtrate is obtained, which after work-up
according to the example yields a dark, cloudy produc-
~
having a platinum content of 36 ppm.