Note: Descriptions are shown in the official language in which they were submitted.
20~0~7
The invention relates to the use of substituted pyrro-
lidineR, some of which are known, as medicaments, in
particular as antLmycotic agentR, to new active sub-
stances and to processes for their preparation.
Pyrrolidinecarboxylic acid derivatives are known from
many publications [cf., for example, J. Heterocycl. Chem.
12 ~3), 595-6; Liebiqs Ann. Chem. (6) 1073-88;
Tetrahedron 27 (1971), 4681-6~.
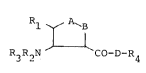
It has now been found that the substituted pyrrolidones
of th~ general formula (I)
R, A~B
~ (1),
R3R2N CO-D-R~
in which
A and B are always different and
represent a group of the formula -CHX5 or -NR6,
in which
R5 denotes hydrogen or straight-chain or branched
alkyl having up to 8 carbon atoms, which is
optionally substituted by halogen, hydroxyl,
phenyl or carboxyl or by straight-chain or
branched alkoxy or alkoxycarbonyl each having
Le A 28 558 - l -
2~80~67
up to 6 carbon atoms,
R6 has the meaning of R2 .indicated below and is
identical to or different from this, or denotes
methoxycarbonyl.
Rl represents hydrogen or ~traight-chain or branched
alkyl having up to 8 carbon atoms, which is option-
ally substituted l or 2 tLmes by identical or dif-
ferent substituents from the series comprising
halogen, hydroxyl, phenyl and carboxyl or by
straight-chain or branched alkoxy, acyl or alkoxy-
carbonyl each having up to 6 carbon atoms or by a
group of the formula -NR7R8,
in which
R7 and R8 are identical or different and denote
hydrogen, phenyl or straight-chain or branched
alkyl haviny up to 6 carbon atoms,
R2 represents hydro~en or
represents straight-chain or branched alkyl having
up to 8 carbon atoms, which is optionally substitut-
ed 1 or 2 times by identical or different sub-
stituents from the serie~ comprising hydroxyl and
formyl or by straiyht-chain or branched acyl having
up to 6 carbon atoms or by phenyl or benzoyl, each
of which i~ optionally substituted up to 2 times by
identical or different substituents from the series
comprising halogen, nitro and cyano, or by straight-
chain or branched alkyl or alkoxy each having up to
Le_A_28 558 - 2 ~
2080~67
6 carbon atoms,
or
represents straight-chain or branched acyl having up
to 8 carbon atoms,
or
represents tert. butyloxycarbonyl (boc) or benzoyl
which is optionally substituted as described above,
or
repxesents a group of the formula-SO2R9,
in which
R9 denotes straight-chain or branched alkyl having
up to 8 carbon atoms, or benzyl or phenyl,
where the latter are optionally substituted up
to 3 tLmes by idsntical or different sub-
stituents from the series comprising halogen,
hydroxyl, nitro, cyano, trifluoromethyl and
trifluoromethoxy or by straight-chain or
branched alkyl, alko~y or alkoxycarbonyl each
having up to 6 carbon atoms, carboxyl or by the
abovementioned group -NR7R8,
in which
R7 and R8 have the abovementioned meaning,
represents phenyl which is op~ionally substituted up
to 3 times by identical or different ~ubstituents
from the series comprising halogen, hydroxyl, nitro,
Le A 28 558 - 3 -
2 ~ 6 7
trifluoromethyl, trifluoromethoxy, straight-chain or
br~nched alkyl, acyl, and alkoxy or alkoxycar~onyl
each having up to 6 carbon atoms or by a group of
the formula -NR7R~ or -SozR9,
in which
R7, R8 and R9 have the abo~ementioned meaning,
~3 represents hydrogen or straight-chain or branched
alkyl having up to 8 carbon atoms, which i8 option-
ally substituted by phenyl,
or
R2 and R~ together represent the radical of the formula
=CHRl,
in which
R10 has the abovemen~ioned meaning of R5 and is
identical to or different from this,
D represents an oxygen or sulphur atom or the
group,
R4 represents hydrogen or straight-chain or branched
alkyl ha~ing up to 8 carbon atoms, or phenyl, where
the latter are optionally sub~tituted up to 3 times
by identical or different substituents from the
Le A 28 558 - 4 -
2~0gg7
group compri~ing hydroxyl, halogen, nitro, cyano,
car~oxyl, trifluoromethyl and trifluoromethoxy, by
straight-chain or branched alkoxy, in the case of
phenyl also by alkyl, acyl or alkoxycarbonyl each
having up to 6 carbon atoms or by a group of the
formula -NR7RB or -SO2R9,
in which
R7, R8 and R9 have the abovementioned meaning,
or for the case in which D repre ents the NH group
R4 represents the group of the formula -SO2R9,
in which
R9 has the abovementioned meaning,
surprisingly exhibit a potent antimicrobial action, in
particular potent antLmycotic action, against dermato-
phytes, budding fungi and biphasic fungi and are thus
suitable for use in the control of dexmatomycoses and
systemic mycoses.
The physiologically acceptable acid addition salts and
metal salt complexes of the compound~ of the general
formula ~I) and the r~cemic modifications, the antipodes,
the diastereomeric mix~ures and the individual isomers
are also preferred for this use.
Le A 28 558 5 -
20~0~1~7
Preferably used compounds of the general formula (I) are
those
in which
A and B are hlways different and
represent a group of the formula -CHRs or -NR6,
in which
R5 denotes hydrogen or straight-chain or branched
alkyl having up to 6 carbon atoms, which is
optionally substituted by halogen or hydroxyl,
or by straight-chain or branched alkoxy or
alkoxycarbonyl each having up to 4 carbon
atoms,
R6 has the meaning of R7 indicated below and is
identical to or different from this, or denotes
methoxycarbonyl.
R1 represents hydrogen or straight-chain or branched
alkyl having up to 6 carbon atoms, which is option-
ally substituted by halogen or hydroxyl, by
straight-chain or branched alkoxy, acyl or alkoxy~
carbonyl each having up to 4 carbon atoms or by a
group of the formula -~R7Ra,
in which
R7 and R8 are identical or different and denote
hydrogen or straight-chain or branched alkyl
having up to 4 carbon atoms,
Le A 28 558 - 6 -
2~0~67
R2 represents hydrogen or
represent~ straight-chain or ~ranched alkyl having
up ~o 6 carbon atom , which is optionally
substituted 1 or 2 tLme~ by identical or different
substituents from ~he Reries comprising hydroxyl and
formyl or by straight-ch~in or bxanched acyl having
up to 4 carbon atoms or by phenyl or benzoyl, each
of which is optionally sub~tituted by halogen, nitro
or cyano r or by straight-chain or branched alkyl or
alkoxy each having up to 4 carbon atoms,
or represents straight-chain or branched acyl having
up to 6 carbon atoms or repre~ents tert . butyl oxycar-
bonyl (boc) or benzoyl which is optionally substituted as
descri bed above, or
represent~ a group of the formula -SO2R9,
in which
R9 denotes straight-chain or branched alkyl having
up to 6 carbon atoms, phenyl or benzyl, where
the latter are optionally substituted up to 2
times by identical or different substituents
from the series comprising halogen, hydroxyl,
nitro, cyano, trifluoromethyl and trifluoro-
methoxy or by strai~ht-chain or branched alkyl
or alkoxy each having up to 4 carbon atoms or
by the abovementioned group of the formula
-NR'R6
in which
Le A 28 558 - 7 -
2~80~7
R7 and R3 have the abovementioned meaning,
represents phenyl which is optionally ~ubstituted up
to 2 times by identical or different groups from the
series comprising halogen, hydroxyl, nitro, tri-
fluoromethyl, trifluoromethoxy and straight-chain or
branched alkyl, acyl, alkoxy or alkoxycarbonyl each
having up to 4 carbon atoms or by a group of the
formula -NR7R3 or -SO2R9,
in which
R7, RR and R9 have the abovementioned meaning,
R3 represents hydrogen or straight-chain or branched
alkyl having up to 6 carbon atoms or benzyl,
or
R2 and R3 together represent the radical of the formula
=CHR~,
in which
R10 has the abovementioned meaning of Rs and
is identic~l to or different from this,
D repre~ent~ an oxygen or sulphur atom or the NH
group,
Le A 28 558 - 8 -
2~8~7
R~ represents hydrogen or straight-chain or branched
alkyl having up to 6 carbon atoms or phenyl, where
the lat~er are optionally substituted up to 2 tLmes
by identical or different substituents from ~he
S series comprising hydroxyl, halogen, nitro, cyano,
trifluoromethyl and trifluoromethoxy, by straight-
chain or branched alkoxy, acyl or alkoxycarbonyl
each having up ~o 4 carbon atoms or by a group of
the form~la -NR7Ra or -SO2R9,
in which
R7, R~ and R9 have the abovementioned meaning,
\
or for the case in which D represents the NH group,
R~ represents the group of the formula -SO2R9,
in which
R9 has the abovementioned meaning,
if appropriate in an isomexic form and their physiologi-
cally acceptable acid addition salts and metal salt
complexes in the control of dermato~ycoses and systemic
mycoses.
Particularly preferably used compounds of the general
formula (I) are those
Le A 28 558 - 9 -
2~80867
in which
A and B are always different and
repre ent a group of the formula -CHRs or -NR6,
in which
S R5 denote~ hydrogen or stxaight-chain or branched
alkyl having up to 4 carbon atoms,
R6 has the meaning of R2 indica~ed below and i5
identical to or different from this, or denotes
methoxycarbonyl.
R1 represents hydrogen or
represents ~traight-chain or branched alkyl having
up to 4 rarbon atoms,
R2 represents hydrogen or
represents straight-chain or branched alkyl having
up to 4 carbon atom~, which is optionally
substituted 1 or 2 times by identical or dif-'erent
phenyl, which can in turn be substituted by methoxy
or ethoxy, or
represents straight-chain or branched acyl having up
to 4 car~on atoms or tert. butyl oxycarbonyl, or
represents a group of the formula -SO2R9,
in which
R9 denote~ straight-chain or branched alkyl having
Le A 28 558 - 10 -
2 ~ 6 7
up to 4 carbon atoms, phenyl or benzyl, where
the latter are optionally substituted by
hydroxyl, fluorine, chlorine, bromine, nitro,
cyano, methyl, ethyl or methoxy,
R3 repre~ents hydrogen or
represents s~raight-chain or branched alkyl having
up to 4 carbon atoms or benzyl,
or
R2 and R3 together repre~ent the radical of the formula
=CHR',
in which
Rl has the abovementioned meaning of R5 and is
identical to or different from this,
D represents an oxygen or a sulphur atom or the
\
NH group,
R4 represents hydrogen or straight-chain or branched
alkyl having up to 4 carbon atoms or phenyl, where
the latter are optionally substituted by fluorine,
chlorine, bromine, nitro, cyano, methoxy or ethoxy
or by a group of the formula -NR7R8 or -SO2R9,
in which
Le A 28 5~58 - 11 -
2o~67
R7 and R~ are identical or different and denote
hydrogen, methyl or ethyl
and
R9 has the abovementioned meaning,
\
or in the case in which D represents the N~ group,
R~ represents the group of the formula -SO2R9,
in which
R9 has the abovementioned meaning,
if appropriate in an isomeric formt and their physiologi-
cally acceptable acid ad~ition salts and metal salt
complexes in ~he control of de.rmatomycoses and ~ystemic
mycoses.
Very particularly preferably used compounds of the
general formula (I) are those in which both substituents
-NR2R3 and -Co-D-R4 are present in the cis-position in the
control of dermatomycose6 and systemic mycoses.
The invention additionally relates to new active sub-
stances of the general formula (Ia)
Le A 28 558 - 12 -
~0367
Pl'~ `B'
l (Ia),
R3R2N CQ D'-R~'
in which
A' and B' are alway~ different and
represent the group of the formula -CHRs or -NR6,
in which
Rs' denotes hydrogen or straight-chain or
branched alkyl having up to 8 carbon
atoms, which i5 optionally subs~ituted by
halogen, hydroxyl, phenyl or carboxyl or
by straight-chain or branched alkoxy or
alkoxycarbonyl each having up to 6 carbon
atoms,
R6~ has the meaning of R2' indicated below and
is identical to or different from this,
or denotes metho~ycarbonyl.
15 Rl' represents hydrogen or straight chain or branched
alkyl having up ~o 8 carbon atoms, which i5 option-
ally substituted 1 or 2 times by identical or
different sub~tituents from the series comprising
halogen, hydroxyl r phenyl and carboxyl or by
traight-chain or branched alkoxy, acyl or alkoxy-
carbonyl each having up to 6 carbon atoms or by a
Le A 28 558 - 13 -
2 ~ 6 7
group of the formula -NR7R~,
in which
R7 and Ra are identical or different and denote
hydro~en, phenyl or straight-chain or branched alkyl
having up to 6 carbon atoms,
R2 represents hydrogen or
repres~nts ~traight-chain or branched alkyl having
up to 8 carbon atoms, -which is optionally
substltuted 1 or 2 time6 by identical or different
groups from the series comprising hydroxyl and
formyl or by straight-chain or branchad acyl having
up to 6 carbon atoms or by phenyl or benzoyl, each
of which i8 optionally substituted up to 2 time~ by
identical or different gubstituents from ~he series
compri~ing halogenr nitro and cyano, or by straight-
chain or branched alkyl or alkoxy each having up to
6 carbon atoms, or
represents straight-chain or branched acyl having up
to 8 carbon atoms,
or represents benzoyl which is optionally substi-
tuted as described above,
or represents a group of the formula -SO2R9',
in which
R9' denotes straight-chain or branched alkyl
having up to 8 carbon atoms, benzyl or
Le A 28 558 - 14 -
2 ~ 7
phenyl, where the latter are optionally
substituted up to 3 times by identical or
different substituents from the series
comprising halogen, hydroxyl, nitro,
cyano, ~rifluoromethyl and trifluorometh-
oxy or by ~traight-chain or branched
alkyl, alkoxy or alkoxycarbonyl each
having up to 6 carbon atoms, carboxyl or
by the abovementioned group -NR7R3,
in which
R7 and R8 have the abovementioned meaning/
repre ents phenyl which is optionally substituted up
to 3 times by identical or different substituents
from the serie~ compri~ing halogen, hydroxyl, nitro~
trifluoromethyl, trifluoromethoxy and straight-chain
or branched alkyl, acyl, alkoxy or alkoxycarbonyl
each having up to 6 carbon atom~ or by a group of
the formula -NR7R3 or -So2R3,
in which
R7, R~ and R~ have the abovementioned meaning,
R3 represants hydrogen or straight-chain or branched
alkyl having up to 8 carbon atoms, which is option-
ally substituted by phenyl,
Le A 28 558 - 15 -
or
R2 and R3 to~ether represent the radical of the formula
=CHRl'
in which
R10' ha the abovementioned meaning of R5' and
ic identical to or different from thi~,
D' represents an oxygen or ulphur ato~ or the
group,
R4 represents hydrogen or straight-chain or branched
alkyl having up to 8 carbon atoms or phenyl, where
~he latter are optionally sub~tituted up to 3 tLmes
by identical or differen~ substituents from the
series comprising hydroxyl, halogen, nitro, cy~no,
carboxyl, trifluoromethyl and trifluoromethoxy, by
straight-chain or branched alkoxy, in the case of
phenyl also by alkyl, acyl or ~lkoxycarbonyl each
having up to 6 carbon atoms or by a group of the
formula -NR7R8 or -SO2R9,
in which
R7't Ra' and R9' have the abovementione~ meaning,
or for the case in which D represents the N~ group,
Le A 28 558 - 16 -
_
2~o~67
R4 represents the group of the formula -SO2R9,
in which
R9 ha~ the abovemen~ioned meaning,
with the proviso that if A' represents the -CH2- group,
B' represents the -NR6 group, D' represents oxygen and
R'/ R2, R3 and R~ represent hydrogen, R6 must not
repre~ent hydrogen,
and in the case in which R2 repre6ents the tert-butyl
group and R3 represents hydrogen, R6' must no~ repre~ent
the acetyl group,
and if B' represents the -CH2- group, R1, R2 and R3
represent hydrogen, D' represents oxygen, R~ represents
ethyl and A~ represent~ the -NR6 group, R6 mus~ not
represent hydrogen or benzoyl.
The compounds of the general formulae (I) and (Ia)
according to the invention can also be present in the
form of their salts. In general, salts with organic or
inorganic bases or acids may be mentioned here.
The acids which can be added preferably include hydro-
halic acids, such as, for example, hydrochloric acid and
hydrobromic acid, in particular hydrochloric acid, and
also phosphoric acid, nitric acid, sulphuric acid, mono-
and bifunctional carboxylic acids and hydroxy carboxylic
acids, such as, for example, acetic acid, maleic acid,
malonic acid, oxalic acid, gluconic acid, succinic acid,
Le A 28 558 - 17 -
2~0g67
fumaric acid, tartaric acid, citric acid, salicylic acid,
sorbic acid and lactic acid as well as sulphonic acids,
such as, for example, p-toluene ulphonic acid, 1,5-
naphthalenedisulphonic acid or camphorsulphonic acid.
Physiologically acceptable salts can also be metal or
ammonium salts of the compounds according to the
invention which have a free carboxyl group. Those
particularly preferred are, for example, sodium,
pota4sium, magnesium or calcium 8alt3, and smmonium salts
which are derived from ammonia, or organic amines such
as, for example, ethylamine, di- or triethylamine, di- or
triethanolamine,dicyclohexylamine,dimethylaminoethanol,
arginine, lysine or ethylenediamine.
The compoundfi of the general formulae (I) and (Ia~
according to the invention can exist in stereoisomeric
forms, for example either as image and mirror Lmage
(enantiomers) t or which do not behave as Lmage and mirror
image (diasteromers), or are present as a diasteromer
mixture or as pure cis- or trans-isomers. The invention
relates both to the antipodes, racemic modifications and
diasteromer mixtures and to the pure isomers. The racemic
modificationP~, like the diasteromers, can also be
~eparated in a known manner into the stereoisomerically
uniform constituents [cf. E.L. Eliel, Stereochemistry of
Carbon Compounds, McGraw Hill, 1962]. Separation into the
stereoisomerically uniform compounds is carried out, for
example, by means of a chromatographic resolution of
diastereomeric esters and amides or on optically active
Le A 28 558 - 18 -
20~6~
phases. In addition, crystallisation of diastereomeric
salts is possible.
The compounds of the general formula (I) according to the
invention and the new compounds of the general formula
S (Ia) and their acid addition salts and metal Qalt
complexes have antimicrobial actions, in particular
potent antimycotic actions. They have a very broad
spectrum of antimycotic action, in particular against
dermatophytes and budding fungi as well as biphasic
fungi, for example against Candida specie~ such as
Candida albicans, Epidermophyton specieC such as ~pider-
mophyton floccosum, Aspergillus species such as
Aspergillus niger and Aspergillus fumigatus, Trichophyton
spe~ies such as Trichophyton mentagrophytes, Microsporon
species such as Microsporon felineum and Torulopsi
species, such as Torulopsis glabrata. The enumeration of
these microorganisms in no way represents a restriction
of the microorganisms which can be controlled, but is of
only illustrative character.
Indica~ion e~amples which may be mentioned in human
medicine are, for example:
Dermatomycoses and systemic mycoses caused by
Trichophyton mentagrophytes and other Trichophy~on
species, Microsporon species and Epidermophyton
floccosum, budding fungi and biphasic fungi as well as
mould fungi.
In addition, a process for the preparation of the
Le A 28 558 - 19 -
2~8~57
compounds of the general formulae (I) and (Ia) ha~ been
found characteri4ed in that
[A] in the case in which A and A' each correspondingly
represent the -NR6 or -NR6 group and ~ or B'
correspondingly represent the -CHRs or -CHRs group,
compounds of the general formula (II)
E
R N
3~ ~ R,I (Il),
W ' Co2-R,2
in which
W represents a group of the formula ~N- or the N3-group,
R1l and R13 correspondingly encompass the particular scope
of meaning of R1~R1 and R5/R5,
E represent~ a Cl-C6-alkoxycarbonyl radical,
G represents an amino-protective group, preferably
benzyl,
and
Rl2 represents Cl-C6-alkyl,
are first converted according to customary methods by
removal of the protective group G, i n the case in which W
represents the -NH-group, and in the case of the N3-group
by reaction with triphenylphosphonoxide/H20 into the compounds
of the general formula (III)
Le A 28 558 - 20 -
20~8fi7
~ H-R,l (In~,
H2N C02-R~2
in which
R1l, R12, Rl3 and E have the abovemen~ioned meaning,
and then reacted with activated carboxylic acid deriva-
tive , in the pre~ence of a base, to give the compound~
of the general formula (IV)
~ (I~,
HN C2 R~2
L
in which
E, Rll, Rl2 and Rl3 have the abovementioned meaning
and
L represents a Cl-C6-alkoxycarbonyl radical,
and in a last step both the amine and the acid function
are libera~ed by reaction with HBr in water, ~imulta-
neously with elimination of the radicals ~, L and R~2,
or compounds of the general formula (V~
Le A_28 558 - 21 -
2~Q~'67
Rl3 CH-R,l
(V3,
CO2^~l2
~N
M
in which
E, Rl1, Rl2 and Rl3 have the abovementioned meaning
and
M has the abovementioned meaning of R2 with the
exception of hydrogen, but preferably represents a
(Cl-C6)-acyl radical, such as acetyl, or the
-CH(C6H4-OCH3) 2 radical,
are reduced to the corresponding pyrrolidine compounds in
inert solvents by hydrogenation with hydrogen in the
presence of a catalyst or with hydride~
or
rB] in the case in which A and A' correspondingly
represent the -CHR5 or -CHR5 group and B and B' corres-
pondingly represent the -NR6 or -NR5 yroup,
compounds of the general formula (VI)
Le A 28 558 - 22 -
2 ~ 7
~11
Rl3 ~ C~N E (Vl),
C)~ C2R12
in which
E, R1l, R12 and R13 have the abovementioned meaning,
are converted with amineC of the formula (VII)
NH~-R~4 (V~,
in which
Rl4 represents (C2-C6)-alkyl or benzyl, preferably
benzyl,
into the corresponding enaminoesters of the general
- formula (VIII)
R11
R CH
13 ~ E (YIII),
Rl4HN C2R12
in which
E, Rlll Rl2, Rl3 and Rl~ have the abovementioned meaning,
Le A 28 558 - 23 -
2Q~0~7
the double bond i8 reduced wlth hydrogen in the presence
of a cataly3t, and in a last step the radical Rl~ i8
likewise removed with hydrogen in the presence of a
catalyst
S or
compounds of the general formula (VI) are directly
reductively aminated by reaction with ammonia or ammonium
salts and then with the action of hydrogen in the pres-
ence of a catalyst, or with cyanoborohydride, in the
presence of acids in inert solvents,
and
if R2/R3 = H, the respective protective groups in each of
the abovementioned steps are removed accordinq to custom-
ary conditions,
and in the case of ~he acids [(I), (Ia) D=O, R4=H] the
corresponding esters are optionally hydrolysed,
and in the case of the other definition~ mentioned above
for D, D', R4 and R4, likewise derivatised by customary
methods, such as, for example, amidation, sulphonation or
~ulphoamidation, if appropriate in the presence of
auxiliaries such a ca~aly~ts and dehydrating agents,
starting from the corresponding carboxylic acids, if
appropriate with prior activation.
Le A 28 558 - 24 -
2~0867
The proces~es according to the invention can be
illustrated by way of example by the following reaction
5C heme:
Le A 28 558 - 25 -
c6H5 2 0 g O g 6 7
HN ~, COC2H~ H2Pd H2N ~ COC~H5
COlCH3 HCl COzCH3
C02CHl
K2CO3 HN ~ COC2H5 3r I H20 HzN ~ ~ COzH
COlCH3 H
[A~
H2N C02C2H, AcHN CO2C2H5 AcHN CO2C2H5
~J Ac O ~ H2/Pl ~
COlC2H5 CO2CzH5 ClH50H CO2ClH5
[A]
o CO2C2H5 HO CO2C2Us N3 CO2C2Hs
N:~H4 ~n~ Zn(N3)2 \~/
N N P(C6H5~3 N
DEAD
CH3 CH3 CH3
H2N CO2C2Hs NH2 COOH
P(c6Hs)3lH2o \~/HCI or \~/
CH3 CH3
B]
l.)C6Hs-CHz~~H2
2.)Pt / C
,~ 3.) Pd / C ~H2
N 1 CO2C2H5 ~ CO2H
CO2CH3 1.) NH4 + Cl~ C02CH3
2.) H2 / Pd or cyanoborohyclride
Le A 28 558 - 26 -
2~0~67
Amino-protective groupq in the contex~ of the invention
are the customary amino-protecti~re groups used in peptide
chemistry .
These preferably include: benzyloxycarbonyl, 3,4-di-
methoxybenzyloxycarbonyl, 3, 5-dimethoxybenzyloxycarbonyl,
2, 4-dimethoxybenzyloxycarbonyl, 4-methoxybenzyloxy-
carbonyl, 4-nitrobenzyloxycarbonyl, 2-nitrobenzyloxy-
carbonyl, 2-nitro-4, 5-dimethoxybenzyloxycarbonyl,
methoxycarbonyl, ethoxyca~tionyl, propoxycarbonyl, iso-
propoxycarbonyl, butoxycarbonyl, isobutoxycarbonyl,
tert.-butoxycarbonyl, allyloxycarbonyl, vinyloxycarbonyl,
2-nitrobenzyloxycarbonyl, 3, 4, 5-trimethoxybenzyloxy-
carbonyl, cyclohexylcarbonyl, 1, l-dimethyl-
ethoxycarbonyl, adamantylcarbonyl, phthaloyl, 2, 2, 2-
trichloroethoxycarbonyl, 2, 2, 2-trichloro-tert . -butoxy-
carbonyl, menthyloxycarbonyl, phenoxycarbonyl, 4-nitro-
phenoxycarbonyl, fluorenyl-9-methoxycarbonyl, formyl,
acetyl, propionyl, pivaloyl, 2-chloroacetyl, 2-bromo-
acetyl, 2, 2, 2-trif luoroacetyl, 2, 2, 2-trichloroacetyl,
2 0 benzoyl, 4-chlorobenzoyl, 4-bromobenzoyl, 4-nitrobenzoyl,
phthalimido, isovaleroyl or benzyloxymethylene, 4-nitro-
benzyl, 2, 4-dinitrobenzyl or 4-nitrophenyl .
Suitable solvents for all steps of processes [A] a~d ~B]
are in general water and all inert organic solvents which
2 5 do not change under the reaction conditions . These
preferably include alcohols such as methanol, ethanol,
propanol and isopropanol, ethers such as diethyl ether,
dioxane, tetrahydrofuran, glycol monomethyl ether or
glycol dimethyl ether, or amides sllch as
~0 dimethylformamide, dimethylacetamide or
hexamethylphosphoric triamide, or glacial acetic acid,
dimethyl sulphoxide, acetonitrile or pyridine. Suitable
Le A 28 558 - 27 -
2~0~7
ba~es are organic amines (trialkyl (C1-C5)amines) such as,
for example, thiethylamine, or heterocycles such as
pyridine, methylpiperidine, piperidine or morpholine.
Triethylamine i8 preferr0d.
The reaction tQmperatures can be varied within a rela-
tively wide range. In general, the reaction is carried
out between -78~C and +150C, preferably between -lO~C
and +100C, in particular at the boiling point of the
respective solvent.
The rsaction~ can be carried out at normal pressure, but
also at elevated or reduced pres~ure (for example O.5 to
3 bar). In general, the reactions are carried out at
normal pressure.
When carrying out proces variants [A] and [B] according
to the invention, any desired ratio of the substances
participating in the reaction can bs used. In general,
however, the reaction is carried out with molar amounts
of the reactants. The substances according ~o the inven-
tion are preferably isolated and purified by distilling
off the 301vent in vacuo and recrystallising the re~idue,
which may only be obtained in crystalline form after ice-
cooling, from a suitable solvent. In ~ome cases, it may
be necessary to purify the compound~ according to the
invention by chromatography.
The amino-protective groups are removed in a manner known
per se [cf. Th. Greene, "Protective Group~ in Organic
Le A ~8 558 - 28 -
2~0~67
Synthesi~", 1. Aufl. J. Wiley & Sons, New York 1981 and
Houben Weyl "Methoden der organischen Chemie", Volume
XVtl and 1] (~ethod~ of Organic Chemistry).
The hydrogenations (reductions, removal of protective
groups) are in general carried out in one of the above-
mentioned ~olvent~, ~uch as alcohols, for example meth-
anol, ethanol or propanol, in the presence of a noble
metal catalyst such as platinum, palladium, palladium on
animal carbon or Raney nickel, in the case of the double
bond of the compound of the general formula (V) prefer~
ably with H2/platinum, in the case of the reductiYe
amination of the compounds of the general formula (VI)
and the enaminoesters of the general form~lla (VIII)
preferably with H2/palladium.
In the reductive amination or hydrogenation of the
compoundq of the general formulae (Y) and (VI) it is also
pos~ible to employ hydrides, such as, for example,
complex borohydrides or aluminium hydrides. Preferably,
in these reactions sodium borohydride or sodium cyano-
borohydride are employed. In this case the reaction is ingeneral carried out in a temperature range from -10C to
+30C, preferably at room temperature.
The catalyst~ used are in general acids. These preferably
include inor~anic acids such as, for example, hydro-
chloric acid or sulphuric acid, or organic sulphonic orcarboxylic acids such as, for example, methanesulphonic
. acid, ethanesulphonic acid, benzenesulphonic acid,
toluenesulphonic acid, acetic acid or propionic acid.
Le A 28 558 - 29 -
2~0~67
The hydrogenations can be carried out at normal, elevated
or reduced pressure (for example 0.5-5 bar).
The catalysts and base~ are in general employed in an
amount from 0 mol to lQ mol, preferably from 1.5 mol to
3.5 mol, in each case relative to 1 mol of the compounds
of the general formulae (IV~, (V), (VI) and ~VIII).
The acid is in general employed in an amount from 2 mol
to 30 mol, preferably from 5 mol ~o 15 mol, in each case
relative to 1 mol of the compound~ of the general for-
mulae (IV), (V), (VI) and (VIII).
The hydrolysis of the carboxylic acid esterC is carried
out by cu3tomary methods by treating the esters in inert
solvents with customary bases, it being possible to
convert the salts initially formed into the free car-
lS boxylic acids by treatment with acid.
The hydrolysis of the carboxyl~c acid esters can also be
carried out with one of the abovementioned acidR.
Suitable bases for the hydrolysis are the customary
inorganic bases. These preferably include alkali metal
hydroxides or alkaline earth metal hydroxides such as,
for example, sodium hydroxide, potassium hydroxide or
barium hydroxide, or alkali metal carbonates such as
sodium carbonate or potassium carbonate or sodium
hydrogen carbonate, or alkali metal alkoxides Quch as
sodium ethoxide, sodium methoxide, potassium ethoxide,
Le A 28 558 - 30 -
potafislum methoxide or potas~ium tert-butoxide. Sodium
hydroxide or potassium hydroxide are particularly prefer-
ably employed.
Suitable solvents for the hydrolysis are water or the
5 organic ~olvents customary for hydrolysis. These prefer-
ably include alcohols such a~ methanol, ethanol,
propanol, i~opropanol or butanol, or etherc such as
tetrahydrofuran or dioxane, or dimethylformamide or
dLmethyl sulphoxide. Alcohols such as methanol, ethanol,
propanol or i~opxopanol are particularly preferably used.
It is al~o possible to employ mixtures of the solvents
mentioned.
The hydrolysis i8 in general ~arried out in a temperature
range from 0C to +100C, preferably from +20C to +80C.
In general, the hydroly6i~ i8 carried out at normal
pressure. However, it is al~o possible to work at reduced
pressure or at elevated pressure ~for example from 0.5 to
5 bar).
When carrying out the hydrolysis~ the base or the acid is
in general employed in an amount from 1 to 3 mol, prefer-
ably from 1 to 1.5 mol, relative to 1 mol of the ester.
Molar amount~ of the reactant are particularly prefer-
ably u~ed.
When carrying out the reaction, in the fir~t step the
~alts of the compounds according to the invention are
Le A 28 558 - 31 -
20~67
formed as intermediates which can be isolated. The acids
according to the invention are obtained by treating the
~alts with customary inorganic acids. rhese preferably
include mineral acids ~uch a~, for example, hydrochloric
acid, hydrobromic ~cid, sulphuric acid or phosphoric
acid. It ha~ proved advantageous in the preparation of
the carboxylic acids to acidify the basic reaction
mixture from the hydrolysis in a second step without
isolation of the 8alt5. The acid~ can then be i~olated in
a customary manner.
As an example of the abovementioned derivatisation
possibilities, amidation and sulphonation or sulpho-
amidation will be illu~trated here.
Amidation is in general carried out in inert solvents in
the presence of a base and of a dehydrating agent.
Suitable solvents in this case are ~nert organic solvents
which do not change under the reaction conditions. These
include halogenohydrocarbons such as dichloromethane,
trichloromethane,tetrachloxomathane,1,2-dichloroethane,
trichloroethane, tetrachloroethane, 1,2-dichloroethylene
or trichloroethylene r hydrocarbon~ such as ben~ene,
xylene, toluene, hexane, cyclohexane or mineral oil
fractions, nitromethane, dimethylformamide, acetonitrile
or hexamethylpho~phoric triamide. It is also possible to
employ mixtures of the 801vent8. Dichloromethane is
particularly preferred.
Le A 28 558 - 32 -
2 ~ 7
Suitable base~ for the amidation are the customary basic
compounds. The e preferably include alkali metal and
alkaline earth metal hydroxides such as lithium
hydroxide, sodium hydroxide, potas~ium hydroxide or
barium hydroxide, alkali metal hydrides such a~ sodium
hydride, alkali metal or alkaline earth metal carbonates
3uch as sodium carbonate or potassium carbonate, or
alkali metal alkoxides such as, for example, ~odium
methoxide or ethoxide, potassium methoxide or ethoxide or
potassium tert-butoxide, or organic amines such as
benzyltrimethylzmmonium hydroxide, tetrabutylammonium
hydroxide, pyridine, triethylamine or N-methylpiperidine.
The amidation is in general carried out in a temperature
range from 0C to 150C, preferably at 25C~ to 40C.
The amidation is in general carried out at normal
pressure. However, it i~ al o possible to carry out the
proce~s at reduced pressure or at elevated pressure (for
example in a range from 0.5 to 5 bar).
Suitable dehydrating reagents are carbodiimides such as,
for example, diisopropylcarbodiLmide, dicyclohexyl-
carbodiimide or N-(3-dimethylaminopropyl)-N'-ethyl-
carbodiimide hydrochloride or carbonyl compounds such as
carbonyldiimidazole or 1,2-oxazolium compounds such as 2-
ethyl-5-phenyl-1,2-oxazolium-3-sulphonate or propane-
pho~phonic anhydride or isobutyl chloroformate or benzo-
triazolyloxy-trîs-(dimethylamino)phosphonium hexafluoro-
phosphate or diphenyl pho~phoramidate or methanesulfonyl
Le A 28 558 - 33 -
~0~0~7
chloride, if appropriate in the presence of bases ~uch as
triethylamine or N-ethylmorpholine or N-methylpiperidine
or dicyclohexylcarbodiLmide and N~hydroxysuccinimide [cf.
J.C. Sheehan, S.L. Ledis, J. Am. Chem. Soc. 95, 875
(1973); F.E. Frerman et al., J. Biol. Chem. 225, 507
(1982) and N.B. Benoton, K. Rluroda, Int. Pept. Prot.
Res. I3, 403 (1979), I7, 187 (1981)].
The sulphonation or ~ulphoamidation i8 carried out in the
abovementioned inert solvents, if appropriate using the
bases and dehydrating agents likewise mentioned above.
The sulphonation and sulphoamidation are in general
carried out at normal pressure. However, i~ i8 also
possible to carry out the proce~es at reduced presRure
or elevated pressure (for example in a range from 0.5 to
5 bar).
The sulphonation and the sulphoamidation are in general
carried out in a temperature range from 0C to +150aC,
preferably from +25C to ~40C.
For the amidation, the commercially available amine~ ~nd
their derivative~ known from the literature are in
general suitable [cf. ~ouben-Weyl, ~Methoden der
organischen Chemie" (Methods of organic chemistry), Vol.
XI/1 and XI/2].
The sulphonation and zulphoamidation are in general also
carried out with the customary sulphonic acid~ and their
Le A 28 558 - 34 -
2 ~ 7
activated derivatives [cf. Houben~Weyl, "Methoden der
organischen Chemie~ (Method~ of organic chemistry), Vol
IX, p. 407 et ~eq.; Beilstein 11, 26].
The esterification of the acid~ i8 carried out by a
customary method by reacting the acids, if appropriate in
one of the abovementioned solvents, with the appropriate
alcohols in the presence of a catalyst. Preferably, this
alcohol is al~o employed as the 801vent.
Catalysts which can be employed are inorganic acids, such
as, for example, sulphuric acid or inorganic acid
chlorides, such a~, for example, thionyl chloride.
In general, 0.01 to 1, preferably 0.05 to 0.5, mol of
- cataly~t are employed, relative to 1 mol of reactant.
Both the esterification and the amidation can optionally
proceed via activated stages of the carboxylic acids,
such as, for example, acid halide~, which can be prepared
from the corresponding acid by reaction with thionyl
chloride, pho~phoru~ trichloride, phosphorus penta-
chloride, phosphorus tribromide or oxalyl chloride.
The acid addition 8alt8 of the compounds of the formulae
(I) and (Ia) can be obtained in a simple manner by
cu~tomary salt formation methods, for example by
dissolving a compound of the formula (I) or (Ia) in a
suitable solvent and adding the acid, for example
hydrochloric acid, and isolated in a known manner, for
Le A 28 558 - 35 -
2 ~ 6 7
example by filtering off, and if appropriate purified by
washing with an inert organic solvent.
The compounds of the general formulae (II), (III), (IVl
and (V) are known in ~ome caces [cf. Acta Chemica
Scandinavica Vol. 35 (1981), 473-479] or are new and can
then be prepared, however, in an analogy to those method~
published in the literature reference cited above.
The compounds of the general formula (VI) are likewi~e
known in some cases [cf. J. Am. Chem. Soc. Vol. 86, 1964,
5293-5298] or are new and can then be prepared by the
proce6ses mentioned therein.
The amine~ of the general formula (VII) are known ~cf.,
for example, Beil tein 12, 1013].
The enaminoesters of the general formula (~III) are known
in some cases or are new and can then be prepared, for
example, by the process described in [B].
The above preparation processes are only giYen for
clarification. The preparation of the compounds of the
general formulae (I) and (Ia) according to the invention
is not restric~ed to the e processes, and any
modification of the~e processes can be used in the s~me
manner for th~ preparation.
The compound~ of the general fonmulae (I) and (Ia)
according to the invention and their acid addition salts
Le A 28 558 - 36 -
2~0~67
have antLmicrobial actions, in particular potent
antimycotic action~. They have a very broad spectrum of
antLmycotic action, in particular against dermatophytes
and budding fungi as well as biphasic fungi, for example
against Candida species such as Candida albicans,
Epidermophyton species such as Epidermophyton floccosum,
Aspergillus species such as Aspergillu~ niger and
Aspergillus fumigatus, Trichophyton species such as
Trichophyton mentagrophytes, Micro~poron species ~uch a~
Microsporon felineum and Torulop~is ~pecie~, such as
Torulops i8 glabrata. The enumeration of these
microorganisms in no way represents a restriction of the
microorganisms which can bs controlled, but is of only
illustrative character.
Indication examples which may be mentioned in human
medicine are, for example:
Dermatomycoses and systemic mycoses caused by
Trichophyton mentagrophytes and other Trichophy~on
species, Microsporon specie~ and Epidermophyton
floccosum, budding fungi and biphasic fungi as well as
mould fungi.
Indication axeas in veterinary medicine which may be
mentioned are, for example:
All dermatomycoses and systemic mycoses, in particular
those which are caused by the abovementioned pathogens.
The new active substance~ can be converted in a known
manner into the customary formulations, such as tablets,
Le A 28 558 - 37 -
coated tablets, pill6, granules, aerosols, syrups,
emulsions, suspensions and solutions, using inert, non-
toxic, pharmaceutically suitable excipients or solvents.
In this case the therapeutically active compound should
in each case be present in a concentration of about 0.5
to 90% by weight of the total mixture, i.e. in amounts
which are sufficient in order to achieve the dosage range
indicated.
The formulations are prepared, for example, by extending
the active substances with solvents and/or excipients, if
appropriate using emulsifiers and/or dispersants, where,
for example, in the case of the use of water as a diluent
organic solvents can optionally be used as auxiliary
solvents.
Administration is carried out in a customary manner,
preferably orally or parenterally, in particular perlin-
gually or intravenously.
In the case of parenteral use, solutions of the active
substance can be employed using suitable liquid
2Q excipients.
In general, it has proved advantageous on intravenous
administration to administer amount~ of about 0.001 to
10 mgtkg, preferably about 0.01 to 5 mg/kg, of body
weight to achieve effective results, and on oral ~dminis-
tration the dosage is about 0.01 to 25 mg/kg, preferably
0.1 to 10 mg/kg, of body weight.
Le A 28 558 - 38 -
23~8~ ~Q~6 7
In spite of this it may sometimes be nece~sary to deviate
from the amounts mentioned, in particular depending on
the body weight or the type of application route, on
individual behaviour towards the medicament, the manner
of its formulation and the time or interval at which
administration takes place. Thus, in some cases it may be
adequate to manage with less than the abovementioned
minimum amount, while in other caRes the upper limit
mentioned must ~e exceeded. In the case o~ the adminis-
tration of relatively large amounts, it may be advisableto divide these into several individual doses over the
course of the day.
The invention also extends to a commercial
package containing, as active pharmaceutical ingredient,
a compound of general formula (I) or (Ia), together with
instructions for its use as an antimicrobial agent.
Le A 28 558 - 39 -
2~8~7
Startinq Compounds
Example I
Methyl l-methoxycarbonyl-4-(4/4~-dimethoxybenzhydryl)
amino-3,4-dehydropyrrolidine-3 carboxylate
(~13C~ ~ ; CH - Ntl ~ C02CH3
CO2CH3
The preparation is carried out by a standard method (see
Act. Chem. Scand. Vol. 35. 1981, 473-479) from methyl 1-
methoxycarbonyl-4-oxopyIrolidine-3-carboxylate.
-
Example II
Ethyl 4-(4,4'-dimethoxybenzhydryl)amino-1-methoxycarbo-
nyl-3,4-dihydropyrrolidine-3-carboxylate
CO~Me
N ~ .- =
~=<
CO~Et NHCH( ~ OMe)2
Example II is prepared starting from ethyll-methoxycarbo-
nyl-4-oxopyrrolidine-3-carboxylate and 4,4'-aimethoxy-
benzhydrylamine (see Example I).
Le A 28 558 - 40 -
2 ~ 6 7
Example III
Ethyl 1-methylpyrrolidine-3-on-4-carboxy~ate
Me
O CO2Et
The compound is prepared by a Dieckmann cyclisation of
N-(~-ethoxycarbonylethyl)-N-(ethoxycarbonylmethyl)methyl-
amine following a well known literature procedure.
Ex mple IV
Ethyl 1-methoxycarbonylpyrrolidin-3-one-2-carboxylate
,~NCO2Me
O CO2Et
The preparation follows a ~ell known literature procedu-
re.
Example V
Ethyl 4-~4,4'-dimethoxybenzhydryl)amino-1-methyl-3,4-de-
hydropyrrolidine-3-carboxylate
Me
~ N ~
CO2Et NHCH( ~ OMe)2
A solution of 21.5 g (0.125 mol) ethyl 1-methylpyrroli-
din-3-on-4-carboxylate, 30.3 g (0.125 mol bis(p-methoxy-
phenyl)methylamine and 0,4 g p-toluenesulfonic acid in
400 ml benzene is refluxed or. a Dean-Stark trap for 16 h.
Concentration of the reaction mixture yields 49.5 g
(100 % of theory~ of an orange oil.
Le A 28 558 - 41 -
.. .. . , ..... , .. , . .. ~, _, .. .. .... ..... .. .. .. .... .. .... .. . ... . . .. . . . . .. .. . . .....
2 ~ 5 7
H-NMR (200 MHz, CDCl3):
= 1.24 (t, 3H); 2.38 (s, 3EI); 3.45 - 3.60 (m, 4H);
3.77 (s, 6H); 4.13 (q, 2H); 5.92 (d, MS (Cl, iso-
butane):
m/e: 396 [M~]
Example VI
Ethyl 4-hydroxy-1-methoxycarbonylpyrrolidine-3-carboxyla-
te
CO~Me
OH (~O2Et
10 g (46.5 mmol~ ethyl 1-methoxycarbonylpyrrolidin-3-one-
4-carboxylate in 100 ml ethanol were treated with 0.95 g
(25 mmol) sodium borohydride at O~C for 20 min. The
reaction mixture was acidified with sulfuric acid,
diluted with water and extracted with several portions
l 5 diethylether. After drying concentration of the combined
ether layers yielded 4.7 g (47 % of theory) colorless
oil.
MS (Cl, isobutane):
m/e: 217 [M~]
Le A 28 558 - 42 -
.. . ... .... , .; ~ ..... . . . . . ... . ..... .. ................ .... . . . . . .. . . . . . . .
2~80~7
Preparation Examples
Example 1
Methyl l-methoxycarbonyl-4-(4,4'-dimethoxybenzhydryl)-
aminopyrrolidine-3-carboxylate
(H3C ~ HC - NH ~ CO2CH3
CO2CH3
6 g (14 mmol~ of methyl l-methoxycarbonyl-4-(4,4~-
dimethoxybenzhydryl)-amino-3,4-dehydropyrrolidine-3-
carboxylate are reduced wi~h 0.7 g (14 mmol) of sodium
cyanoborohydride for 12 h in 90 ml of ethanol at room
temperature and pH 3-4. The residue obtained after
concentration i taken up in ethyl acetate, washed with
1 N ~odium hydrogen carbonate solutio~ and water and
dried. After drying and concentration, the oil obtained
is chromatographed on Florisil with petroleum ethar/ethyl
acetate (l:l). 2.3 g (38 ~ of theory~ of a pale, highly
lS viscous oil are obtained.
H-NMR (200 MHz, CDC13):
= 2.95-3.60 (m, 6H); 3.69 ( ,3H~; 3.70 (s,3H); 3.78
(s,3H); 3.80 (s,3H); 4.75 (s,br,lH); 6.78-~.90 and 7.20
7.30 (~,8H)-
Le A 28 558 - 43 -
2~a867
Example 2
Methyl l-methoxycarbonyl-4-aminopyrrolidine-3-carboxylate
H3CO2C NH2
N
CO2CH3
A solution of 2.3 g (5 mmol) of methyl l-methoxycarbonyl-
4-(4,4'-dimethoxybenzhydryl)-aminopyrrolidine-3-
carboxylate and 5 ml of glacial acetic acid/water (1:13
i8 stirred at lOOPC for 5 min. After cooling, the mixture
is diluted with about 50 ml of water and extracted twice
with diethyl ether, and the aqueous phase is then
concentrated to about 15-20 ml. At 0C~ the aqueous phase
is brought to p~ 10 with conc. an~nonia solu~ion and after
saturating with sodium chloride extr2cted several times
with diethyl ether. After drying and concentration,
O.35 g (35 ~ of theory) of a slightly yellow oil are
obtained.
MS (CI, isobutane):
m/e: 202[M~]
Le A 28 558 - 44 -
2 ~ 6 7
Example 3
Ethyl 4-(4,4'-dimelhoxybenzhydryl)amino-1-methoxycarbor-ylpytrolidinc-3-carb-
oxylate
CO2Me
CO2Et NHCH~ OMe)2
10 g (22.7 mrnol) ethyl 4-(4,4'-dirnethoxybenzhydryl)amino-1-methoxycarbonyl-3,4-
dehydropyrrolidine-3-carboxylate are reduced with 1.43 g (22.7 rnrnol) sodium
cyanoborohydride for 24 h in 150 rnl ethanol at room temperature and pH 34. The residue
obtained after concentration is taken up in ethyl acetate, washed with sodium hydrogen
carbonate solution and water and dried. The oil obtained after concentration is
chromatographed on Florisil with petroleum ether/ethyl acetate (1:1). 4.2 g (41.9 % of
theory), viscous oil.
MS (CI, isobutane):
mle: 442[M~]
Example 4
Ethyl 4-benzylamino-1-methoxycarbonylpyrrolidine-3-carboxylate
CO2Me
- CO2Et NH~3
96 g (0.316 mol) of ethyl 4-berl7ylarnino-1-methoxycarbonyl-3,4-dehydropyrrolidine-3-
carboxylate are reduced with 22.8 g (0.362 mol) of sodium cyanoboroh-dride for 24 h in
1.6 1 of ethanol at room temperahlre and pH 3-4. After concentration of the reaction
2 0 mixture the residue is taken up in ethyl acetate, washed once with l ,~ sodium hydroge
carbonate solution, once with water and dried. After drying and concentration, the oil
obtained is chromatographed on Florisil with petroleum ether/ethyl acetate (1:2). 81 g (83.8
% of theory)of a yellowish, viscous oil are obtained.
MS (CI, isobutane):
2 5 rn/e: 306[M~]
Le A 28 558 - 45 -
fi 7
Exam~lc S
Ethyl 4-(4,4`-dimetiloxy~enzhydryl)amino-1-mclllylpyrrolidinc-3-carboxylate
Me
S~
CO2Et NHCEI(~ OMe)2
10 g (25.3 snmol) of ethyl 4-(4,4'-dimethoxybenzhydryl)arnino-1-methyl-3,4-
dehydropyrrolidine-3-carboxylate are reduced with 1.76 g ~28 rnrnol) of sodium
cyanoborohydride for 24 h in 100 ml of ethanol at room temperature and pH 34. The
residue obtau~ed after concentration is taken up in ethyl acetate, washed with 1 N sodium
hydrogen carbonate solution and water and dried. After drying and concentration, the oil
obtained is chromatographed on Florisil with petroleurn ether/ ethyl acetate (1
Exarnple 6
Ethyl 4-tert-butyloxycarbonylarnino-1-methoxycarbonylpyrrolidine-3-carboxylate
CO2Me
~0
CO2Et NH ot~u
-
13.8 g (50 rnmol) ethyl 4-arr~ino-1-methoxycarbonylpyrrolidine-3-carbo~;ylate in 70 ml of
1,4-dioxane are treated with 14.4 rnl (102 rnmol~ triethylarnine and 13.9 ml (60.5 mrnol~
1 5 di-tert-butyldicarbonate at room temperature for 16 h. The reaction n~ix~lre is poored onto
250 ml cold water and 100 rnl ethyl acetate, acidified with potassium hydrogen sulfate
solution and extracted with more ethyl acetate. The orgaruc layers are ~ 2shed with sodium
hydrogen carbonate solution, dried and concentrated to yield 6.9 g (45. / % of theory) of
a slightly yellow oil.
'H-NMR (200 MHz, CDCI3):
o = 1.26 (t, 3H); 1.53 (s, 9H~; 2.98 - 3.81 (m, 6H~; 3.70 (s, 3H); 4.15 (q, 2H); 4.93 (d,
IH).
Le A 28 558 - 46
2~08~
Examplc 7
4-tert-Butyloxycarbonyla~ino-l-mcthoxycarbonylpyrrolidine-3-carboxylic acid
CO2Me
CO2H NHBoc
6.9 g ~22 mmol) ethyl 4-tert-butyloxycarbonylamino-l-methoxycarbonylpyrrolidine-3-
carboxylate in 30 ml methanol, 20 ml tetrahydrofurane and 10 ml water are stirred with
1.5 g (62.5 rnrnol) lithium hydroxide for 16 h at room ternperature. The rni~ture is acidified
with potassium hydrogen sulfate solution at 0C and extracted with ethyl acetate. The
organic layers are washed with water, dried with magnesium sulfate and concentraled to
give 3.6 g (57 % of theory) of a pale yellow oil.
lH-NMR (200 MHz, DMSO-d~):
S = 1.40 (s, 9H); 2.85 - 4.2 (sev. m, 6H); 3.59 (s, 3H); 6.05 (d, lH).
MS (CI, isobutane):
m/e: 288[M~]
Example 8
4-Amino-l-methoxycarbonylpyrrolidine-3-carboxylic acid, hydrochloride
CO2Me
NH2 CO2H
HCI
A solution of 1.5 g (5 2 mmol) 4-tert-butyloxycarbonylamino-l-
methoxycarbonylpyrro1idine-3-Carboxylic acid in 10 ml 1,4-dioxane is treated with lO n71
of a saturated solution of hydrogen chIoride ir~ 1,4-dioxane for 4 h. Concentration yields
l.lS g (98.5 % of theory) hygroscopic material.
'H-NMR (200 MHz, D20):
o = 3.32 - 3.99 (m, 6H); 3.71 (s,3H).
MS (Cl, isobutane):
rn/e: 188[M~]
Le A 28 558 - 47 -
~o~7
Examelc 9
Ethyl 4-(N-benzyl-N-tert-butoxycarbonylamino)- I -methoxycarbonylpyrrolidine-3-
carboxyla~e
~O2~C
CO2Et N
O~O~u
20 g (65.4 mrnol) ethyl 4-benzylamino-l-metho~ycarbonylpyrrolidine-3-carbo~ylatedissolved in 200 ml 1,4-dioxane are treated with 6.6 g (65.5 mmol) triethylamine, 18.5 g
(85 mrnol) di-tert-'outyldicarbonate and 2 g dirnethylarninopyridine at room temperature for
30 h. After addition of 1 1 water and 300 ml ethyl acetate the pH is adjusted to 3 - 4 with
hydrochloric acid and the aqueous layer is extracted with more ethyl acetate. The combined
1 0 organic layers are washed with sodium hydrogen carbonate solution and brine, dried and
concentrated. 25.4 g (95.7 % of theory~ of a pale yello~v oil.
'H-NMR (200 MHz, CDCI3): ~
o = 1.24 (t, 3H); l.S0 (bs, 9H); 3.38 - 4.2 (m, 6H); 3.7û (s,3H~; 4.14 (q, 2H); 4.37 (m
2H); 7.15 - 7.4 (m, SH).
1 5 Example 10
Ethyl 4-amino-1-methoxycarbonylpyrrolidine-3-carboxylate
CO2Me
NH2 CO2Et
A solution of lS.0 g (S0 Irunoi) of Ethyl 4-benzylamino-1-methoxycarborlylpyrrolidine-3-
carboxylate in S00 rnl ethanol, S00 ml ~vater and 250 ml 0.1 N h~drochloric acid is
hydrogenated at 23C for 20 hours (10 bar H" Pd/C). Filtration and concentration yields
7.6 g (79.2 % of theory) of a highly viscous oil.
'H-NMR (200 MHz, CDCI3):
o = 1.28 (t, 3H); 3.32 - 4.05 (n~, 6H); 3.71 (s, 3H); 4.18 (q, 2H); 6.03 (bs. 2H).
Le A 28 558 - 48 -
- -- -- . _ _ . _ . .... . .. ~ . _ , _
Example 11 2 ~ 8 0 ~ 6 7
4-~enzylamino-1-me~lloxycarbonylpyrrolidine-3-carboxylic acid
CO2Mc
CO2 H N H~--~3
17 g (56 mmol) ethyl 4-benzylamino-1-methoxycarbonylpyrrolidine-3-carboxylate and 4.8
g (200 mmol) li~hiurn hydroxide dissolved in S0 ml tetrahydrofurane, 10 ml methanol and
10 rnl water are stirred at room temperature for 23 h. The reaction mixture is neutralized
by adding about 155 ml 1 N potassium hydrogen sulfate solution and thoroughly extracted
with ethyl ether. Drying and concentration yields 5.7 g (36.6 ~ of theory) of a viscous oil.
'H-NMR (200 MHz, CDCl3):
10 o = 2.61 (m, lH); 3.30 - 3.85 (m, 6H); 3.72 (s,3H); 4.25 - 4.41 (m, 2H); 7.20 - 7.40 )m,
SH).
Example 12
4-Aminopyrrolidine-3-carboxylic acid dihydrobromide
2HBr
CO2H NH2
=
15 1.2 g (4.17 mrrlol) 4-tert-butyloxycarbonylamino-l-methoxycarbonylpyrrolidirle-3-
carboxylic acid dissolved in 10 ml acetic acid were treated with 5 ml h~ drogenbrornide in
acetic acid at 60 C for 2 h. After cooling to roomtempera~r the precipitate ~as fîltered
off. 1.1 g (90 % of theory) of a pinc, highly hygroscopic powder were obla~ned.
IH-NMR (200 MHz, D20):
20 o = 3.47 - 4.03 (m, 5H), 4.29 - 4.50 (rn, lH).
MS (Cl, isobutane):
m/e: 130 [M+]
Le A 28 558 - 49 -
6 7
Example 13
Ethyl 4-benzylamino- 1-methoxycarbonyl-3,4-dehydropyrrolidine-3-carboxylate
CO2Me
CO2Et NH~
The synthesis is carried out according to the preparation of example I fiom ethyl 1-
metho~ycarbonyl4-oxopyrrolidine-3-carbo~ylate and benzylamine.
Le A 28 558 - 50 -