Note: Descriptions are shown in the official language in which they were submitted.
~7US9 1 /02 8 ~
p~ ~3 i ~ 1992
,.~
LEAD-FREE MIRRORS AND
ENVIRON?~lENTALLY SAFE M~NUFACTURE THEREOF
--This Application is a continuation in part of
Application Serial No. 686,011, filed April 12,
1991, which is a continuation of Application
Serial No. 514,109, filed April 25, 1990.--
BACKGROUND OF THE INVENTIONPlate glass mirrors have long been made by wet
chemistry processes involving polluting chemicals.
One well known process involves forming, by means of
wet chemistry methods, a thin layer of tin oxide upon
a glass sheet, providing next a layer of metallic
silver to a thickness sufficient to render the layer
substantially opaque (and highly reflective when
viewed through the glass), overcoating the silver
layer with a layer of copper, and overcoating the
copper layer with a lead-based paint. When the silver
layer is not completely opaque, the copper layer may
impart a very slight copper tint to the mirrored
surface viewed through the glass. The copper layer
serves also as a sacrificial layer inasmuch as it will
react with oxygen and other contaminants with which i t
may come into contact and thus protect the silver
layer from becoming sulfided or oxidized. The
lead-based paint not only protects the back of the
mirror from being physically abraded by scratches or
the like, but the lead oxide pigment further
contributes to the sacrificial protection afforded t~.e
.TE SHEE~
I~A/US
PCT1U~ 9 ~ / 0 2 8 t l
,..i~ ioF~B1992 ~
-lA-
silver layer and copper layer.
The wet chemistry methods employed for making
mirrors as described above have many drawbacks, not
the least of which is pollution. The wet chemistry
E S~EE~
WO91/16197 PCT/US91/02811
20~ 2 -
methods involve the use of aqueous coating
compositions which may contain various highly
contaminating substances. Once a coating solution has
been largely exhausted, disposal of the solution poses
a substantial environmental problem. Further, the
paint that is employed, being of necessity lead-based
to provide corrosion protection to the silver layer,
may also lead to toxic results if the paint becomes
accidentally ingested or if lead or lead compounds
from discarded or rejected mirrors or wasted paint
leaches into landfills to contaminate water supplies.
SUMMARY OF THE INVENTION
We have found that mirrors of excellent quality
and high resistance to corrosion can be manufactured
through the use of substantially non-polluting
sputtering techniques and lead-free paints to avoid
the substantial environmental problems associated with
the wet chemistry mirror manufacturing methods of the
past.
In a preferred embodiment, we have found that the
amount of silver that is employed to manufacture a
mirror can be vastly reduced while maintaining the
desired reflectivity and mirror color. In another
preferred embodiment, a mirror is provided with
sputtered-on coatings and with an exterior polymeric
protective coating that is free of lead or lead
compounds and that exhibits tenacious adhesion to the
underlying sputtered-on layer to provide substantial
protection not only from chemical attack but from
scratching and other physical damage as well.
In general, the mirrors of the invention comprise
a transparent substrate such as float glass, and a
sputterea, transparent primer layer which is carried
on the glass surface and which may constitute a metal
salt, particularly an oxide or nitride such as an
WO91/16197 PCT/US91/02811
~ - 3 - 20~ 1 3 4 i
oxide of tin, titanium or zinc or nitrides of zinc or
titanium. Upon the primer layer is carried a
sputtered reflective metal layer of a bright
reflective metal such as silver, aluminum, palladium,
platinum or chromium in sufficient amount as to
provide the mirror with a transmittance in the range
of about 0-30%. In a layer spaced further from the
glass surface than the reflective layer is a barrier
layer comprising one or more metal compounds different
from the metal of the reflective layer and sufficient
to protect the reflective layer from contact with
contaminants such as water, oxygen, sulfiding gases
and the like.
To the extent that the reflective layer is not
entirely opaque, the barrier layer includes a layer of
a bright metal such as stainless steel, aluminum, or
chromium, that is different from the metal of the
reflective layer and which cooperates with the silver
or other reflective metal in the reflective layer to
render the mirror totally opaque. The barrier layer
may in fact comprise two contiguous strata, one
stratum (nearer the glass surface) comprising
aluminum, stainless steel, chromium or other bright
metal to supplement the opacity of the reflective
layer, and the second stratum comprising a different
metal compound that provides good barrier properties,
such stratum being formed by sputtering of stainless
steel, titanium nitride, silicon nitride, silicon
dioxide or titanium dioxide. If a soft metal such as
aluminum or copper is employed in the barrier layer to
supplement the opacity of the reflective layer, then
it is desired that the barrier layer include the
second stratum to provide physical and chemical
protection to the reflective layer of the mirror.
~ f~ IPE'/"S
The outer layer of the mirror is an opaque
protective polymer layer such as a paint, this layer
preferably being free of lead contamination and being
applied from a water-based coating composition. In a
preferred embodiment, there is provided immediately
below the protective polymer layer a sputtered-on
metallic layer of a material such as zinc or zinc
oxide, and the polymer layer includes a polymer that
is tightly cross-linked and tenaciously adherred to
the porous layer. Included in the polymer layer
preferably is a pigment comprising one or more zinc
compounds such as zinc oxide, zinc phosphate and such
other zinc-derived compounds as may be needed to
improve the resistance of the mirror to sulfide
formation by restraining sulfide ions from coming into
contact with the silver or other reflective metal of
the reflective layer. The outer, sputtered-on layer
of, eg., zinc or zinc oxide and the adherrent polymer
layer provide the mirror with substantial resistance
to sulfiding and other corrosion.
DESCRIPTION OF THE DRAWING
Figure 1 is a schematic, cross-sectional view
showing the layers involved in a mirror of the prior
art;
Figure 2 is a schematic representation of a
mirror of the invention utilizing a homogeneous
barrier layer;
Figure 3 is a schematic, cross-sectional
representation of a mirror of the invention in which
the barrier layer is a composite layer; and
Figure 4 is a schematic, cross-sectional
representation of another modification of a mirror of
the invention.
DESCRIPTION OF THE PREFERRED EMBODIMENT
The metals and metal compositions employed in the
present invention are applied by a sputtering
TUTE SHEE~
A!US
091/161g7 PCTIUS91/0281
~_ -- 5 --
technique such as that described in U.S. patent
4,166,018 (Chapin). This technique, sometimes
referred to as a magne~ron sputtering technique,
involves the formation of a plasma wllich is
concentrated in a magnetic field and which serves to
eject metal atoms from an adjacent metal target, the
metal atoms ~eing deposited upon an adjacent surface
such as the surface of a glass pane. When sputtering
is done in an inert atmosphere, the metal alone is
deposited whereas if Sputtering is done in the
presence of oxygen or nitrogen, then the metal is
deposited as an oxide or, if the metal readily forms a
nitride, as a nitride, respectively. Magnetron
sputtering techniques and apparatuses are well known
in the field and need not be described further.
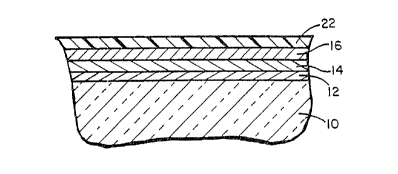
Figure 1 refers to a pricr art mirror which
contains a glass sheet as shown bearing seguential tin
oxide, silver, copper and paint layers. As noted,
paints employed in mirrors of the prior art of this
type generally were lead-~ased, and the tin oxide,
copper and silver layers were applied using wet
chemistry procedures.
Referring now to Figure 2, the transparent
substrate 10 such as glass and particularly plate
glass is first su~jected to sputtering in an
oxygen/argon or nitrogen/argon atmosphere using a
"target" of a metal such as tin, titanium or zinc to
provide a tin oxide, titanium dioxide, zinc oxide or
zinc nitride primer layer 12. A tin oxide primer
layer having a thickness in the range of 5 - 75
Angstroms would be appropriate. Upon the primer layer
is sputt~rea the layer 14 of a ~rlght, reflective
metal such as silver, aluminum, palladium, platinum or
chromium in sufficient amount as to provide that layer
WO91/16197 ~ PCT/US91/02811
7~ 6 -
with a transmittance in the range of 0-30%. The
bright, reflective metal preferably is silver. Figure
2 illustrates the mirror in which the silver or other
bright metal layer 12 provides a transmittanee of
approximately 0%; that is, in which the bright layer
is essentially opaque. Sputtered silver layers having
thicknesses above about 525 Angstroms and preferably
in the range of 525 to 625 Angstroms provide
appropriate substantially opaque layers. Preferably,
as shown in Figure 3, the silver or other bright metal
layer 14 is not opaque, but rather has a transmittance
in the range of approximately 1-30%, preferably
l-lO~. Sputtered silver layers having thicknesses
less than about 525 Angstroms and preferably in the
range of 450 - 525 Angstroms generally exhibit some
transmittance. In general, the transmittance of a
sputtered silver layer decreases to about 10% or below
as its thickness exceeds about 400 - 450 Angstroms,
and decreases to substantially zero as the thickness
reaches the 500 - 550 Angstrom range. A relatively
large amount of silver is required to reduce the
transmissivity of the reflective layer from several
percent to zero. The silver or other bright metal is
sputtered from a metal target in an atmosphere of
argon or other inert gas.
In the embodiment of Figure 2, in which the
bright, reflective layer 14 has substantially zero
transmittance, that layer is overcoated with a
protective barrier layer 16, this layer comprising one
or more metals or metal alloys that are different from
the metal of the reflective layer, the barrier layer
being sufficient in thickness and properties to
protect the underlying bright metalllc layer 14 from
contact with contaminants such as water, oxygen,
sulfiding gases and the like. The barrier layer
PC77lrs 9 1 / 02 811
IPE~./US i & '.B 1S92
desirably contains one or more of the following
sputtered materials: stainless steel, aluminum,
copper, chromium, titanium nitride, zinc oxide,
silicon nitride, titanium dioxide and silicon
dioxide.
The barrier layer 16 preferably ranges in
thickness from about 100 to about 500 Angstroms; when
made of successive layers of stainless steel and
titanium nitride, a stainless steel thickness of 310
to 410 Angstroms and a titanium nitride thickness of
150 - 250 Angstroms provides acceptable results.
Referring to Figure 3, in which the bright
reflective layer 14 is not completely opaque but has a
light transmittance in the range of about 1-30% and
preferably 1-10%, the protective layer 16 may be a
composite layer made of several strata of which one,
designated 18 in Figure 3, is deposited directly upon
the bright reflective layer 14 and comprises a bright,
reflective metal different from the metal employed in
layer 14. Stratum 18 thus may be aluminum, stainless
steel, copper or chromium, or for that matter, silver
if the reflective layer metal is not silver, and the
stratum 18 is applied to a thickness sufficient to
render the layers 14 and 18 together substantially
completely opaque, that is, with approximately zero
transmissivity. For example, the layer 14, if made of
silver metal, may have a thickness of approximately
S00 plus or minus about 50 Angstroms to provide a
transmissivity of about 1-10%; upon this surface then
may be coated the stratum 18 of e.g., stainless steel
to a thickness of about 300 plus or minus about 50
Angstroms to render the combined layers 14 and 18
substant~ally opaque. The remaining portion of the
barrier layer 16, represented as stratum 20, may in
this example be titanium nitride sputtered to a
E S~EET
~/US
P~U~91/0281
I~A/VS 1 8 FEB 1992
....
-8- ~ ,t~
thickness on the order of about 200 plus or minus 50
Angstroms and functions, together with the layer 18,
as a barrier layer 16 as described above.
Applied over the barrier layer 16 in the
embodiments of Figures 2 and 3 is a protective
polymeric film 22. The protective film desirably is
free of lead, and may be any of several known types of
coatings including epoxy resins, urethanes, alkyds,
and the like. In one embodiment, a powdered epoxy
resin that is capable of melting and fusing when
heated is applied to the outer surface of the mirror,
for example, over barrier layer 16. The glass sheet
may then be passed under heat lamps to heat the epoxy
resin to its fusing point, whereupon the epoxy resin
melts and fuses into a hard solid coating. A paint or
other resinous coating may be applied to the outer
surface of the mirror by spraying, roller coating or
other means as may be desired. The polymeric layer 22
desirably has a dry thickness in the range of 0.5-4.0
mils, and preferably in the range of 1.5-1.8 mils.
In the most preferred embodiment, the paint
includes a curable polymer system desirably comprising
a crosslinkable polymer such an acrylic resin and a
suitable curing agent therefor such an an aminoplast
(eg., a melamine resin) or phenoplast resin, the
polymer system being supplied as an aqueous solution
or suspension. Acrylic resins are preferred, and are
capable of cross-linking at moderate temperatures to
form extremely hard, protective films. Epoxy polymer
systems and acrylic systems, and particularly the
latter, are preferred because of the hard,
scratch-resistent films that are produced. Moreover,
these films are highly resistant to hydrolysis and
resist degradation even in warm, humid environments.
The polymer coating systems desirably are
provided as aqueous coating compositions, that is, as
-~ S~EET
S
WO91/16197 PC~/US91/02811
2~8~3~1
.. , g
solutions or suspension in water, the composition
being desirably free or nearly free of organic
solvents. As mentioned earlier, the polymer coatings
desirably are free of lead or lead compounds.
It has been further found that great adhesion
between the outer sputtered on coating and the paint
can be obtained by over-coating the sputtered-on film
with the paint from an aqueous coating composition.
Increased adhesion of the polymer layer (as measured
by a "tape" test described below) is particularly
evident when the outer sputtered on layer is zinc or
particularly a zinc compound such as zinc oxide, and
the polymer ingredient of the paint is an acrylic that
is heat-curable to form an extremely hard surface, the
acrylic being applied from an aqueous solution or
dispersion. Without being bound by the following
explanation, it appears that the coating composition
is carried into intimate contact with the metal or
metal oxide (eg., zinc or zinc oxide) surface, and
appears to actually penetrate that surface. When the
coating is cured, (ie., when it has been crosslinked
by application of heat, curing agents, catalysts or
the like), an exceedingly strong physical or
mechanical bond is formed between the polymeric
coating and the underlying sputtered film. Thus, the
final sputtered-on layer desirably has a surface that
is readily wetted by the aqueous paint composition and
with which the polymer can form a strong mechanical
bond.
The cured paint films derived from the aqueous
paint compositions employed as described above are
characterized as being highly resistant to hydrolysis
and resistant to softening in most household cleaning
chemicals such as dilute ammonium hydroxide, vinegar
and the like. As noted above, the films also have
WO91/16197 PCT/US91/02811
~ ~ Y ;~ 1 0
quite hard surfaces, and preferably are rated 2H or
greater in pencil hardness. Heat-curable polymeric
systems employing acrylic polymers, that is, polymers
resulting from the copolymerization of acrylic and/or
methacrylic acid and other acrylic monomers, are known
in the field of paint chemistry and need not be
described further.
Adhesion of the protective polymeric layer to the
sputtered-on film stack can be measured, at least on a
comparative basis, by a test in which the cured
polymeric film is scored using a diamond or other
hard, sharp object, sets of parallel score lines
crossing one another to define a diamond shaped
pattern in the polymeric layer. A length of adhesive
tape (3M Company's Scotch brand Magic Mending tape is
appropriate) is then pressed into intimate adhesive
contact with the scored surface. A free end of the
tape is then stripped away rapidly from the polymeric
surface at right angles to the coated glass sheet.
When an epoxy-based paint is coated upon a protective
titanium nitride layer (eg., layer 20 in figure 3),
small "diamonds" of paint can be lifted from the
titanium nitride surface. When a sputtered coating
having a zinc or zinc oxide outer surface is employed
and is overcoated with an aqueous acrylic resin
coating, the adhesion between the polymer coating and
the sputtered on film is so great that the tape strips
away none of the polymer coating in the tape adhesion
test, and in fact the adhesive of the tape is
transferred to the polymer coating.
The aqueous polymer coatings of the invention
also desirably contain pigment, and it has been found
that the choice of pigment is lmportant in providing
mirrors of the invention with further and better
resistance to sulfiding. Pigments comprising one or
WO91/1~197 PCT/US91/02811
25~8l3,~,
more zinc compounds give particularly good
anti-sulfiding properties. The zinc compounds
employed in the pigment may include zinc oxide and
other water-insoluble zinc compounds, particularly the
various water-insoluble zinc salts such as zinc
phosphate, the latter being particularly preferred.
The zinc compounds desirably provide at least about 5%
and preferably at least about 8% by weight (as
elemental zinc) based on the solids of the paint
compositions.
The anti-sulfiding properties conferred by the
zinc-based pigments thus mentioned appear to be
particularly beneficial when the underlying final
sputtered-on layer is zinc or a zinc-based compound
such as zinc oxide. This coating can be sputtered
from a zinc cathode in an atmosphere containing oxygen
to form the oxide directly, or metallic zinc can be
sputtered on in a nitrogen atmosphere or an atmosphere
of a relatively non-reactive or inert gas such as
argon, the zinc oxidizing readily upon exposure to air.
Water-based polymeric coating compositions, such
as the acrylic composition referred to above, can be
coated, dried and cured quickly. The acrylic-based
coating compositions of the invention, utilizing
zinc-based pigments, are slightly more viscous than
water, and may be coated upon upwardly facing,
sputter-coated faces of horizontally moving glass
sheets using curtain coating procedures that are known
in the coating art. Curtain coating involves the
formation of a downwardly moving liquid curtain of an
appropriate coating composition, the curtain being of
substantially uniform thickness throughout its width
and the curtain itself being deposited downwardly upon
the horizontally moving surface of the coated glass
panes.
7'-' ~7~ - 12 - PCT/US91/0~811
The wet coating thus applied may first be
subjected to a high volume flow of warm, dry air to
flash off a large portion of the water and to render
the coating self-supporting and substantially dry to
the touch, following which the coated pane may be
cured in, eg., a forced air oven at about 350~ F for
typically a period of 3-5 minutes, the polymer
undergoing substantially complete curing. Heat curing
may be accomplished in one or more elongated forced
air ovens through which the panes pass horizontally.
The resulting panes may be cooled down rapidly by
means of ambient air flow and, if desired, a warm
water quench. Cured polymer film thicknesses of up to
4.0 mils or above can be employed. The thickness of
the cured paint film desirably is not greater than
about l.8 mils and preferably is in the range of about
l.5-l.8 mils, the reduced thickness enabling the
aqueous polymeric composition to be applied and cured
more rapidly on the sputter coated s11rf~ce of the
glass pane, and this thickness further provides
substantial protection to the underlying film.
In a preferred enbodiment, the polymeric
overcoating is applied in the form of two layers, the
first comprising a pigmented layer as described above
and the second being a clear coat, that is, a
pigment-free layer. The pigmented and pigmented-free
polymeric layers may be applied one over the other
using the curtain coating techniques described above,
and the second layer may be placed upon the first
layer when the first layer is yet wet. Some physical
intermixing occurs between the wet layers to improve
adhesion therebetween, and the outer clear coat
provides substantial protection against inadvertent
scratching of the resulting product. Typical coating
thicknesses of the cured pigmented layer may be in the
WO91/16197 PCT/US91/02811
2Q8~3~ ~
- 13 -
range of l.0 mils and the clear coat may be in the
range of about 0.5 mils in thickness.
Referring now to Figure 4, the transparent glass
substrate l0 contains a primer layer 12 of eg., tin
oxide, a reflective layer 14 of eg., silver, and a
barrier layer 16, the barrier layer containing a first
film or stratum 18 of stainless steel or the like to
supplement the opacity provided by the silver layer
14, if needed. The second barrier layer stratum 20,
of titanium nitride or the like, is then provided.
The outer sputtered on layer, designated 24 in Figure
4, may be zinc or a zinc compound such as the oxide,
and the thickness of this layer may vary widely.
Thicknesses in the range of about 50 Angstroms to l000
Angstroms or more can be used, and thicknesses of
about l00 Angstroms to about 900 Angstroms are
preferred. If zinc is deposited as the metal in a
sputtering operation, and the metallic surface is then
exposed to the air, oxidation of the metallic film
occurs to form an approximately stoichiometric ZnO
surface, the atomic ratio of oxygen to zinc decreasing
beneath the surface. Upon this layer is deposited a
first pigmented polymeric coating 26, followed by a
second, unpigmented "clear coat" 28.
The following illustrative examples will serve to
explain the invention in greater detail. The
"measured thicknesses" reported in the examples were
measured by X-ray photoelectron spectroscopy ("XPS")
using a Perkin-Elmer Model 5500 XPS spectrophotometer.
Example I:
Glass panes, suitably cleaned, were subjected to
magnetron sputtering procedures from a series of
target cathodes. The amount o~ each material that was
thus sputter coated was controlled by varying the
number of cathodes beneath which the glass panes were
WO91/16197 PCT/US91/02811
~ - 14 -
passed during the coating operation. Directly upon
the glass surface was deposited a layer of tin oxide
from a tin cathode operating in an oxygen-argon
environment, the tin oxide layer being deposited to an
estimated approximate thickness of about 50
Angstroms.
The glass panes were then overcoated with silver
metal from a silver cathode operating in an argon
atmosphere, the silver layer being deposited to a
measured thickness of approximately 500 Angstroms to
provide a reflective layer having a transmittance of
approximately 2~. Sputtered on top of the silver
layer was a stainless steel (316) layer to a measured
thickness of approximately 310 Angstroms, the
transmissivity of the silver and stainless steel
layers being essentially zero. Over the stainless
steel layer was sputter coated a layer of titanium
nitride from a titanium metal electrode operating in a
nitrogen environment. The titanium nitride protective
layer was applied to a measured thickness of
approximately 210 Angstroms. Finally, over the
titanium nitride layer was coated a commercial
lead-free, pigmented alkyd-based paint to a coating
weight of 8 grams per square foot, that is, to a
thickness of l-2 mils.
Example II:
Glass panes, cleaned and coated with a primer
layer of tin oxide as in Example I, were then
overcoated with silver metal sputtered from a silver
cathode in an argon atmosphere to a measured thickness
of approximately 570 Angstroms. The silver layer
exhibited a transmissivity of zero percent. Sputtered
on top o~ ~he silver layer were sequential layers of
stainless steel and titanium nitride, as in Example I,
to measured thicknesses of approximately 360 Angstroms
WO 91/16197 ~ 3 q 1
.
and 210 Angstroms, respectively. Finally, the mirror
was overcoated with a lead-free, pigmented,
alkyd-based paint as in Example I.
The mirrors resulting from Examples I and II each
exhibited excellent reflectivity. Each mirror was
tested by subjecting a freshly cut edge of the mirror
to a 20% salt spray for 2000 hours to determine the
weathering resistance of the mirror. Neither mirror
showed any evidence of "black edge", that is, silver
corrosion from the edges. Black edge is a common
problem in the mirror industry. There also was no
evidence of massive pin holes or oxidation of the
metal coating due to improper mirror backing. The
occasional small pin holes were well within mirror
specifications. Ammonia tests were also performed. A
freshly cut edge of each mirror was subjected to a 15%
ammonia vapor solution for 24 hours without failure,
indicating excellent resistance, failure being judged
the same as with the above salt spray. Finally, a
bevel was ground into a freshly cut edge of each
mirror, and the bevelled edge was subjected to the
salt spray and ammonia tests described above.
Similarly excellent results were obtained.
Example III
Glass panes, cleaned and coated with a sputter
coated primer layer of tin oxide as in Example I, are
then provided with a silver metal coating, a stainless
steel coating and a titanium nitride coating, all as
described in Example II. Upon the titanium nitride
coating may be sputtered coated a film of zinc from a
zinc metal electrode operating in a nitrogen
atmosphere, this coating being applied to a thickness
of approximately 900 Angstroms. After exposure of the
film to ambient air, the film was found in one such
run to have an outer surface that was approximately
WO91/16197 PCT/US91/02811
16 - .
stoichiometric zinc oxide, the atomic ratio of oxygen
to zinc decreasing below the stoichiometric amount at
depths of several hundred Angstroms into the film
surface.
Once the panes have emerged from the magnetron
sputter coating apparatus, the panes travel on a
conveyor beneath a coating apparatus which applies to
the upper, sputter coated surfaces of the panes a
predetermined layer of uniform thickness of an
aqueous, lead-free, pigmented, acrylic paint
composition. Although the paint may be applied via
several coating methods, it is preferred that the
paint be caused to flow downwardly in the form of a
"curtain" upon the upper surface of the moving glass
panes. The paint, at approximately 50% solids, is
deposited at a wet thickness of approximately 2 mils.
To quickly dry the wet paint film, the film is
subjected to a high volumetric flow rate of dry, warm
air to quickly remove water from the film and to
provide the film with a semi-hard surface; at this
point, the film may yet be soft enough to retain
fingerprints. The continuously moving glass panes are
subjected to the high volume flow of warm air for
approximate one minute, following which the panes pass
through one or more ovens capable of heating the panes
and the paint to a temperature in which the polymeric
component of the paint rapidly cross-links and becomes
extremely hard and scratch resistent. For example,
the panes may be exposed to hot air at 350~ F for a
period of 3-5 minutes, during which the temperature of
the glass is raised to 280~ F.
The temperature of the panes emerging from the
curing ovens may be rapidly reauced by treating the
panes, particularly the glass side thereof, with warm
water and a gentle flow of tempered air, the
~,J/~r5,o1 jo281
IP~ .3 1 8 FEB l992
.. -17-
temperature of the glass rapidly being reduced to 80~
F or below. The final paint thickness may be on the
order of 1 mil.
The aqueous pigmented paint composition thus
described may have the following composition:
Quantity
Inqredient Parts by Weiqht
water reduced acrylic thermosetting
resin 71 % so l ids
(Rhone-Poulenc resin CMD-9012) 195.74
triethylamine 17.38
water 217.25
butyl cellosolve EB (Union Carbide) 43.45
isopropyl alcohol, 91% 26.07
amorphous precipitated silica
(Degussa, OK-412) 17.38
titanium dioxide 108.625
silicone defoamer (Patcote 520) 0.261
hydrophobic amorphous fumed silica
(Degussa, Aerosil R-972) 1.043
organo nitrogen zinc salt
(Henkel, Alcophor 827) 8.69
zinc phosphate (Heubach, Heucophos ZPO) 86.90
carbon black 4.345
barium sulfate 173.80
methylated melamine
(Monsanto, Resimene 717, 84~) 99.066
TOTAL 1000 parts
If desired, the pigmented base coat thus
described may be provided with a non-pigmented
overcoat desirably utilizing the same or similar
$~,3~ J~E SHEET
IPEA/US
WO91/16197 PCT/US91/02811
A~ - 18 -
acrylic resin capable of cross linking to form a hard,
scratch-resistant surface. The non-pigmented paint
may have the following composition:
Quantity
Ingredient Parts by Weight
water reduced acrylic thermosetting
resin (Rhone-Poulenc CMD-9012, 71~)368.01
triethylamine 34.632
water 432.90
diethylene glycol monobutyl ether30.303
methylalkyl polysiloxane (Byk-Chemie,
Byk-325) 1.039
silicone defoamer (Patcote 520) .649
methylated melamine (Monsanto,
Resimene 717, 84%) 129.870
polyethylene wax (Micro Powders,
MPP-620VF wax) 2.597
TOTAL -Inno parls
While a preferred embodiment of the present
invention has been described, it should be understood
that various changes, adaptations and modifications
may be made therein without departing from the spirit
of the invention and the scope of the appended claims.