Note: Descriptions are shown in the official language in which they were submitted.
W091/173Q5 P~T/~Sg1/02933
t~
--1--
LIQUI~ COMPOSI~TON ANALY~ZE~ ~13 ~-~HOD
~CKGRO~D OF T~E INVEN~ION
Accurate knowledge of white and green liquor
composition is necessary for close con~rol Oc kraLt
pulping and recausticizing operations. Firs., if
changes in the green liquor composition ca~ be
monitored, feed forwaxd control of the lime feed rate in
the causticizing plant can be achieved. second,
compositional information of the white liquor can b~
used as feedback to compensake for varia~ions in the
lime quality or reactivity, and as feed forward
compens~tion for pulping.
In the past, white and green liquox
compositions have been determined by laboratory
titrations. However, both the analysis rate and the
accuracy of routine titrations are not sufficient to
take full advantage of modern control systems and
strategies.
The Wallin U.S. Patent 3,941,649 describes an
attempt to control the pulping time and pulping
temperature by taking a sample of the pulping liquor
after initial digestion has occurred. The pulping
sample is titrated to provide an alkaline content of the
liquor. From this alkaline content, the pulping
inte~sity expressed as -~-s ~actor is determined and used
to obtain the desired K~PPA number.
The ~ultman et al U.S. Patent 4,236,960
describes a proces~ for controlling the degree of
causticization of white liquor~ The process of the
Hultma~ et al pat~nt includes determining the sodium
carbona~e co~centration o~ green liquor fed to ~he
caus~iciza~ion, then de~ermining tha sodium carbonate
concentratio~ of white liquor resulting from the
WO9l/17305 P~T/US91/~2933
~r$~
-2-
causticization and thereby controlling the degree of
caus~icization within a predetermined range while taking
both sodium carbonate concentrations into account.
The Bertelsen U.S. Patent 4,536,2S3 describes
a process for controlling the properties of white liquor
by measuring the electric conductivity of the green
liquor before causticization in addition to me~suriny of
the conductivity of the white liquor. The conduc~ivity
of the green liquor is measured both before the slaker
lo and gradually as it passes through th~ slaker to
determine the reaction of the ~arbonate.
SUMMARY OF THE INVF~TION
The present inv~ntion includes a method for
determining the concentration of each of at least thre~
components intermixed in a homogeneous solution. For
example, in th~ case of th~ kraft pulping or
reca~-sticiæing operations, th¢ white or gre~n liquor
co~position includes three major components, sodiu~
hydroxide, sodium sulfidç, and sodium carbonate~
~he method includ~s identi~ying
characteristiGs of the co~ponent~ that are
quantitatively detectable in relation ~o the
concentration o~ th~ compon~nts. A mathematical
relationship is then developed between the concentration
o~ each o~ the com~onents and the d~tectable
characteris~ics using ~he characteristics as independent
variablas. The solutlon is then s~nsed usiny detectors
to cbtain quan~itative data for each of the
characteris~ics. The quanti~a~ive data is ~hen employed
in the math~mati~al relationship ~o obtain the
concentration o~ each Or the components.
The present lnven~ion also includes an
analyzer having de~ectors which ~ense the three
W091/17305 P~r/US91/02933
3--
components and provide quantifiable data to a co~puter
~or emplo~ment in the mathematical relationship that was
developed~ In one preferr~d mode, a sample is extracted
- from the process and analyzed by the detectors. In
another preferred mode, the process solution is
continuously passed by the detectors ~or analysis.
~ F:D~SCRIP~ON OF T~E DRA~tINÇS
Figure 1 is a sch2matic diagram of a liquid
analyzer of the present invention.
Fiyure 2 is a graphical ~iew of a ~ypi~al
response of the analyzer of Figure 1 to a kraft liquor.
Figure 3 is a graphical view illustrating
de~iations between titrated and predicted industrial
white liquor compositions using the analyzer o~ ~iqure
l.
Figure 4 is a graphical view o~ the deviations
between titrated and predicted industrial green llquor
compositions using the analyz~r o~ Figure l.
Figure 5 is a schematic diagram of an
lternative ~bodi~ent o~ the liquor analyzer of the
present invention.
Figure 6 is a graphical view of a comparison
between the titrated and predicted sodium sulfide
concent~ations for the analyzer of Figure 5 ~or white
liquor-~ype solutions..
Figur~ 7 is a graphical view of the comparison
b~tw~en tikrat~d and predic~ed sodium hydroxide
concentration~ for ~he analyzer ~g Figure 5 for white
liquor typ~ solution~.
Figur~ 8 is a ~raphical ~iew o~ the comparison
b~tween o the tltrated and predicted sodiu~ carbonate
conc~ntration~ ~or th~ analyzcr o~ Figure 5 rOr white
liquor-type solution3~
W~91/17305 PCr/USgltO~933
Figure 9 is a graphical view of the co~parison
between the titrated and predicted sodium sulfide
concen~rations for the analyz~r of Figure S for green
liquor-type solu~ions.
Figure 10 is a graphical view of ~he
comparison between the titrated and predicted sodiu~
hydroxide concentrations ~or the analyzer of Figure 5
for green liquor-type solutionsO
Figure 11 is a graphical view o~ the
comparison b~tween the titrated and predicted sodium
carbonate concentrations for the analyzer of Figure 5
for green liquor-type solutions.
Figure 12 is a diagra~matical view o~ one
exa~ple of a control system using the analyzer of the
pres~nt invention.
In a pre~erred emhodiment, the present
invention includ~s ~n on-line au~omatic liquor analyzer
for a kraft pulp-paper mill application. Timely
knowledge of liquor co~position is n~cessary ~or close
control o~ the digesting and recovery operations in a
pulping prccess. ~lthou~h the procsss described herein
is a kra~t (alkali based) proc~ss, the analyzer of ~he
present invention ~ay very well be used in other
proceC~ such as a sulfite process.
` In a kra~t pulping op~ration, the three
p~imary co~ponents of th~ liquor include sodiu~
hydroxide, sodium sulfide, and so~iu~ carbonate. The
present invention provides a non-invasive t~pe o~
meAsureme~t o~ the green liquor (the liquor exiting the
re~overy furnace) or the white liquor (th~ liquor
exiting th~ causticizer) or green, whita, or weak liquor
solutions in other par-ts of the proc~ss can also be
WO91/17305 PCT/US91/02933
~r'~
--5--
measured. Detectors ar2 chosen for sensing a
characteristic of each of the components. For example,
in one of the preferred embodiment~, W absorption at
25~ nm was used to detac~ sodium sulfide which
hydrolyz~s into sodium hydrosulfide in kraft liquors.
Conductivity and refractive index were used to detect
sodium hydroxide, sodium sul~ide, and sodium car~onate
in differing proportions.
The present invention also includes a process
for obtaining the concentrations of components of a
process solution by initially identifying the
characteristics of the components that are
quantitatively detectable in relation to the
concentration o~ the component. A mathematical
lS technique such as re~ression analysis is used to develop
a mathemakical relationship between the relative
concentration of the components and the detectable
chara~teristics. For example, eq~ations are developed
using the detectable oharacteristics as independznt
variables. A sa~pl~ o~ the solution is then analyzed to
obtain quantitative data for each o~ ~he detecta~le
characteristics. That quantitative data is then
employed in the mathematlcal relationship developed
pr~vio~sly to obtain the concentration of sach ~f the
co~ponents in the sampl
Using the analyzer of the present invention,
whit~ or gr~en l~quor is drawn without interrupting the
proc~ss or contaminating the process soiution. Since a
ampl~ an be taken at any time and an analysis done
quickly, ~or example in les~ than three minutes, th~
present invention provide~ ~or close monitor.ing of the
proc~ss that was pr~viously not po~sible.
WO91/17305 P~ /US~1/02933
-6- ~'~J~
The analyzer of the present inventio~ can be
used in at least one og two preferred modes. In a ~irst
mode, a sa~ple is extracted from the process and
analyzed. In a second mode, the proces~ solution is
continuously pa~sed by the detectors.
EX~RACTIv~ SAMPLE~ANALYZER
ANI~I~YZE~ - D~SIGN
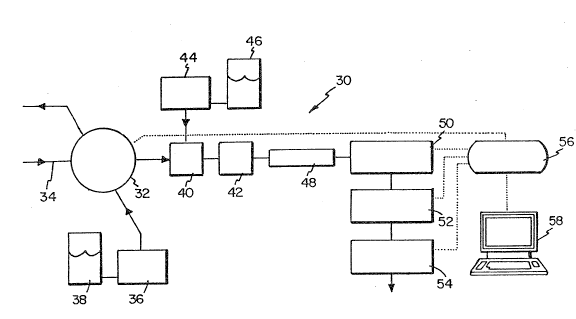
The extractive sample analyzer 30 is
illustrated in Figure 1. The analyzer 30 includes a
Valco EC6W 6-port sa~ple injection valve wit~ electric
actuator 32 for extracting a sample from a sample stream
at 34 fro~ the process of-the present invention. A
Wat~rs S10 HPLC pump 36 is usEd o pump wa~er 38 into
the valve 32. A Waters zero dead volume ~ee 40 is
disposed upstream from a Waters high pressure gradient
mix~r 42. A Waters 510 HP~C pump 44 pumps ~ater 46
through the tee 40. A Waters column heater 4R is.
dispos~d downstr~am ~rom the mixer for maintaining a
selected temperature of the extracted sample. Located
downstream ~rom the h~ater are a WatQrs 481 varia~le
wavelength W spectrophotom~ter 50, a W~ters 430
enhanced conductivity detec~or 52, and a Wa ers 410
di~erential refracto~e~er 54. Data fro~ the three
d~t~ctors 50, 52, and 54 is collected by a Keithley 570
d~ta acqui~ition system 56 and a Zenith 248
roco~uter 58.
Th~ operation of the analyz~r proceeds as
follows. A liquor stream is run t~rough the extrac~ive
valve 32 and a very small sample (5 microliters) is
captured i~ a constant volume loop in ~he valve 32. The
sampl~ i~ flushed. from the valve 32 by a stream o~
dis~llled, d~gassed water 38 provided by pump 36~ The
flowing sample is dilut~d by additional distilled,
W091/17305 PCT/US91/02933
-7~ 3~" ~j!~
degassed water ~6 en~ering through the ~ee 40. The
resulting sample is mixed thoroughly in the gradient
mixer 42. The mixed sample is heated to a unifor~
temperature by passing through the column heater 48.
The sample then flows through the W spectrophotometer
50, conductivity detector 52, and differential
refractometer S4. The responses from the detectors are
sent to the computer 5~ by the data acquisition system
56 where the responses are integrated over time. The
areas are calculated by the computer in units of volt-
sec X 10. The conc2ntrations of sodiu~ hydroxide,
sodium sulfide, and sodium carbonate in the liquor are
then calculated by correlations with the detector
response areas.
ANALYZER OP_R~T~QN
Experi~ents were carried out with aqueous
solutions containing sodium hydroxide, sodium sulfide,
and sodium carbonste, th~ three major components in ~ost
kraft pulp mill liquors. The exp~riments were divided
into two groups, white liquor typ~ solutions and green
liquor solutions. The white liquor solutions containing
between 60 and 120 g/1 o~ NaOH, 10 to 40 g/l Na2S, and
O to 40 g/l of Na2C03, all expressed as Na20 ~qui~alentsO
The gre~n liguor solutions contained between 60 and 120
g/l o~ Na2C03, 0 to 40 g/l of NaO~, and O to 40 g/1 Na2S,
all expre ~ed as Na20 @quivalents.
The test solutions were prepared from
concentr ted stock solutions of the individual
compounds. The ~tock solutions wer~ prepar~d in
distill~d, dQga~ed water, u~ing rea~ent qrade
chsmicals. The~e solutions wer~ kept ~ightly capped and
the runs w~r~ ~ad~ within 10 days o~ stock solution
preparation. The concentrations o~ th~ stock solutions
W091~17305 PCr/US91/OZ933
~8~ 7
were determined by titration with HCe. The solution
den~ities were deter~ined by weighing known volumes.
The concentration and density of each solution were
periodically checked and no changes were detected during
S the course o~ the experiments.
The test solutions were prepared by mixing
speci~ic masses of the stock solutions and water, if
necessary, to produce the desired ~oncentration of each
component. The solutions were injected into the
analyzer 30 imm~diately ~fter preparation to minimize
any compositional changes du~ to sulfidè oxidation,
carbo~ate formation, or evaporation.
The operating param~texs ~or the analyzer
components are listed in Table 1. The inputs ~o the
data acquisition -~yst~m w~re carri~d by twisted,
shielded pairs, and filtered by first-order RC ~ilters
with a 207 K ohm r~sistor and a 10 yF non-polar
capacitor.
T~LE 1
Operating Para~ters for ~he Ex~racti~e Sample
~hite and Green Liquor Analyzer Components
Pu~p~: Sample flow = 0.1 ~l/min
Dilution flow = 5.0 ~l/min
H2ater: Temperature = 30~0 C
UV detector: ~avelength - 254 nm
~ime consta~t = 1 sec.
Ou~pu~ ~ lV / ~g
Condu~tivity: Output ~ 2V / ~S
detQctor Temperatt~e control on
Re~racto~e~er: Ti~e con~tant ~ 1 sac.
S~nsi~ivity ~ 64
Scale ~actor - ~0
Temperature ~ 32.0 C
WO91~17305 P~l/U~91/02g~3
_9_ ~ ,~'~J~
~ fter the sample injection, the detector
responses were recorded for five minutes by the data
acquisition system. A sample response is illustrated in
Figure 2. The response shows a dead time of just over
one minute, where the sample zone was traveling from the
injection valve to the tee. The analysis was run for
over a minute after the responses returned to the
baseline although this additional time is not necessary.
Thus, the minimum analysis time per sample is
approximat~ly three minutes.
AN~YZER_~LI_RATION
For white liquor ~ype solutions, 21
experi~ental runs were made to establish correlations
~or t~e individual components. The concentration of
each co~pon~nt and the deteotor re~ponse areas w2re
recorded ~or each run. The data was analyzed in two
ways. First, the concentrations were taken as the
independent variables to give an indication of how the
liquor component concentrations a~fect the deteotor
responses.
Stepwise multiple linear regression was used
to determine the best co~bination of factors to describe
the det~ctor output. The results for white liquor
indicat~:
R~ ~ ~.5~59 O~ ~ 5.g751 S + ~.9~313 C ~ 4.4202 (l)
W 2 19. 0756 S - 0.~3ll s2 1 0.1586 OH + ll.lB99 (2)
CO = l5.9066 OH - 0.0120 oH2 ~ 10.5559 S (3)
~ 7.2305 C ~ 50.0796
where
RI = di~ferential re~ractome~er re~ponse
W = W sp~ctrophotome~er response
CO = conductivity detector respon~
OH - ~odium hydroxide concsntra~ion (g/l Na~O)
S = sodium sulZide concentra~ion (g/l Na20)
C ~ s~dium carbonat~ concentration (g/l Na20)
WO 91~17305 P~/U.591tO2~33
~10- z~ ~7
The standard deviations for the predic~ions are:
RI = 1.28, W = 3.97, and Co = 4.74 area units. The
coefficients of varia~ion ~or each detector response
are: ~I = 0.23%, W = 1.05~, and C0 = 0.32%. These
values are very close to the rep atability deviations of
each detector respon~e as determined by multiple tests
of the same solution. The repeatability limits are
approximately: RI - +1, W = ~3, and C0 = +4 units.
This analysis in~icates that the error in the
predicted responses is pri~arily due to random errors
introduc2d by the analyzeE, including injection volume
differemces, carri~r flow fluctuations, detector
respo~se variations, and data acquisition noise. This
conclusion is verified by regression residual analysis
which show~ no residual pattern with respect to the
solution concentrations or test order.
For th~ purpose of con~entration prediction,
a ~ore useful regression involves the use of detector
respons~s as independent variables. In this way, the
solution composition could be calculated directly from
the detector responses. Stepwis~ multiple linear
regression produces the following equations:
0~ = O.il63 C0 ~ 4.721X10-6 Co2 ~ 0.1882 RI (4)
25- 0.~2067 W - 1.210
S ~ 0.~2955 UV + 6.487XlOs w2 - 2.882Xl07 C0~ (5)
+ ~.113
1~ æ 0~36484 P~I ~ 0~107~? C0 ~ 2.923X10 6 Co2 (6
- 0~02529 W ~ 6.354X10 5 w2 ~ 1.614
The standard devlation~ for ~he predic:a~ions are: OH -
0.35, ~ s 0.37, and c - 0.60. Equatic~ns (4, s, and 6)
re~ul~ in an approximate 90% con~i~enc~ lnterv~l o~ ~0.5
g/l for bo~h sodium hydroxidQ and ~odiu~ sul~idQ, and
+0.8 g/l tor sodium carbonate.
WO91/17305 PC~/US91/02933
These correl tions were entered into the
computPr data acquisition system in order to calculate
component concentrations for subsequent trials. A set
of four additional samples were run as a test set to
check the prediction capability. The predictions were
also compared wi~h values obtained by manual titration
of the samples. The results are shown in Table 2. ~he
results verify the error limits as predicted by thP
regression analysis.
TABLE 2
Comparison of Extractive Sample Analyzer Results
with Titration Results for Synthetic
White Liquor Solutions
Test Component Actual Analyzer Titration
No. Conc. Conc. Conc.
.......... ~ _ . .
Sodium Hydroxide 79.9 79.9 79.2
1 Sodiu~ Sul~ide27.5~7.8 27.4
Sodium Carbonate 32.8 32.7 32.9
~ , . . .
Sodium Hydroxide 56.7 s6.7 56.7
2 Sodi~m Sulfide13.813.9 13.4
Sodium Carbonate 30.9 30.8 30.9
Sodium Hydroxide 40.4 40.1 40.4
- 3 Sodium Sulfide20.B20.8 20.3
Sodium Carbonate 23.7 23.6 23.9
Sodiu~ Hydroxide 98.5 99.l 99.0
4 Sodium Sulfide4l.l~l.5 40.O
Sodium Carbonate 0.0 0.0 0.5
_ _ .
NOTE: all concentrations in g/l as Na2O
A similar set of ~xperiments was run ~or green
liquor type solutions. In this case, 23 experiments
~091~17305 PCT/US~1/02933
-12- ~
were run to establish correlations. The r~gression
using concentrations as independent variables yielded
tAe following equations:
RI = 4.869 OH 6.873X103 oH2 + 50857 S (7)
~ 4.910 C + 3.821
W = 20.483 S - 0.1519 s2 + 0.1576 C - 9 533 (8)
CO = 13.680 OH + 10.266 S ~ 6.813 C (9)
+ 3.080X103 ~ 134.665
.
The standard deviations for the predictions are: RI =
1.?1, UV = 4.30, and CO = 3.32 units. The coefficients
of variation are: RI = 0.27%, W = 1.28~, and CO =
0.28%. These errors are again close to the analy2er
repeatability error. It should be noted tAat equations
(7, 8, and 9) are not exactly the same as eqUatiQnS (1,
2, and 3) for white liquor but are very similar. Much
of the observed di~ferences can be attributed to
interactions between the liquox components which can
cause nonlinearities in detector responses both white
and green liquor samples.
The regression with detector responses as the
independent variables produces:
OH - O. 1483 CO - 0.2219 RI - 0.01069 W (~0)
- 8.050X10-6 w2 - 15.5~3
S = 0.04006 UV = 4.9~3X10-5 w2 (11)
- 1~893X10~ RI2 + 0.6781
C = 0.4180 RI o 0.1415 CO - 0.03506 W (12)
5.538XlOs w2 + ~2.10~
The standard deviations for these predictions are: OH
~ 0.35, S - 0.37, and C = 0.54. ~his gives approximate
90% con~idence intervals o~ ~0.5 g/l for sodium
hydroxide and sodium sulfide and ~0.7 g/l ~or so~ium
carbonate. These prediction errors are similar ~o those
WO~1/17305 PCT/US91/02933
.
-13-
for white liquor, as would be expected since the basic
equations are very similar.
Analyses were conducted on four additional
samples as a test set to check these correlations. The
samples were also manually titrated to compare with the
predictions. The results are shown in Table 3. As with
the white liquor, the results are excellent, with the
analyzer error less than the predicted error ~ounds.
TABLE 3
Comparison of Extractive Sample Analyzer Results
with Titration Results for Synthetic
Green L~quor Solutions
Test Co~ponent Actual Analyzer Titration
No. Conc. Conc. Conc.
Sodium Hydroxide21.4 21.4 21.S
1 Sodium Sulfide 10.4 10.1 10.2
Sodium Carbonate115.9 116.1 115.4
__ _
Sodium Hydroxide30.0 29.9 30.2
2 Sodium Sulfide 0.O O.3 0.O
Sodium Carbonate78.9 78.4 78.9
.
Sod~um Hydroxide 9.9 9.9 9.8
3 Sodium Sulfide 24.9 25.1 24.7
Sodiu~ Carbonate86.3 86.2 ~6.6
Sadiu~ Hydroxide21.7 22.0 21~5
4 Sodium Sulfide 15.3 15.3 ~5.2
Scdium Carbonate94.2 94.6 94.3
NOTE: all concentrations in gJl as Na20
The results show that the analyzer works well
for white and green liquor type samples prepared from
pure chemicals. It is expected that the responses would
W091tl7305 PCT/USg1/02933
-14- 2~
cha~ge if other compounds were present in the liquor.
In ind~1strial white and green liquor, there could be
trace amounts o~ sodium sulfate, sodium sul~ite, sodium
thiosulfate~ and polysulfide sulfur. The detector
response to these impurities was tested by lnjecting
solutions containing ~hese rontaminants into the
analyzer. The results are shown in Table 4. The data
indicates that in each case the presence of impurities
will add area to each of the detector respo.nses of a
pure liquor. Thus, ~or industrial use, the analyzer
must be calibrated to accommodate the concentration of
impurities in the liquor. In all cases with kra~t
liquors, the impurities will be present in only minor
a~ounts and will be present at nearly constant levels.
TA3LE 4
Effect of ~hite and Green Liquor Impurities on
Extractive Sampl~ Analyzer Detector Responses
~0
Compound Concentration W RI Cond
(g/l as Na20) Area Area ~rea
~ Na2S203 10.5 42.7 69.7 90.7
Na2S3 9.6 5.3 53.0 73.5
Na2S~4 lO~0 0.2 4603 82.1
5~ CIl~_o~lC~L~L
Kr~t Liquors
Analyses were per~ormed on various white and
green liquors obtained fro~ kraft mills. The object of
the tests was to deter~ine the ef~ect of actual mill
liquor impurities on the analyzer results. Ten white
and green liquor samples wexe analyzed.
The results ~rom the industrial white liquor
analysis are ~hown in Figure 3. The results indi~ate
that each componen~ is generally overpredicted when
. .
WO91/17305 PCT/US91/02933
15-- 2
using ~he correlations developed for pure white liquor.
The sodium hydroxide estimate is the least affected by
the impurities. The observed deviations ~re reasonable
consid~ring the types of impurities which may be
present. Some thiosulfates and polysulfides may be
present which would contribute to the absorbance at 254
nm. This would result i~ overprediction o~ sodium
sulfide. Sodium sulfate and sulfite would basically
appear to the detectors as sodium carbonate. Sodium
sulfate and sulfide are species with low conductivity
contribution, but significant refractive index
contribution. The average deviations for the industxial
white liquors are shown in Table 5.
TABLE S
Analysis ~rrors for Industrial ~hite Liquors
Using the Extractive Sample Liquor Analyzer
~ .
Component Avg. rror Std. Deviation
Sodium Hydroxide 0.14 g~l 0.50 g/l
Sodi~m Sulfide l.07 g/l l.lO g/l
Sodium Carbona~.e 6.21 g/l 2.77 g/l
. ~ . ... . _ . . _ . _
The results from the industrial green liquor
analyses are shown in Figure 4. The results are ~imilar
to the industrial white liquor analysis. The sodiu~
carbonate i5 always overpredicted. This is again caused
by the influence of impurities having small
contribu~ions to solution conduc~ivity. The sodium
sulfide error is small~r, and the sodium hydro~id~ error
is larg~r than that o~ the white liquor. Th~ average
deviations for industrial green liquors are shown in
Table 6.
WO91/17305 PCT/USg1/02933
-16- 2r ~ 7
TABLE 6
Analysis Errors for Industrial Green Liquor
Using the Extractive Sample Liquor Analyzer
Component Avg. Error Std. Deviation
lOSodium Hydroxide 1.23 g/l O.S7 g/l
Sodium Sulfide 0.61 g/l 0.~6 g/l
Sodium Carbonate 5.81 g/l 2.1~ g/l
15IN-SITU ANALYZER
Analyzer Desian
A schematic diagram of the in-situ analyzer 60
is illustrated in Figure 5. The analyæer 60 includes a
Rosemou~t Model 222 Toroidal Conductivity Sensor with a
y 20 Model ~054T Toroidal Conductivity Analyzer/Transmitter
62, a Micro Motion Model D25 Mass Flow M~ter with a
Micro Motion DMS Liquid Densitometer 64, and a Rosemount
Model 340A Selective Ion Sensor with a Model 1033
Selective Ion AnalyzPr/Transmitter with a Phoenix
Silver/Sulfide Ion Electrode 66. Temperature data was
transmitted throuyh a Ros mount Series 78S platinum RTD
with a ~odel 444 Temperature Transmitter 82. Data
acquisition was a~complished with a Keithley 570 data
acquisition ~ystem 68 and a Ze~ith 248 microcomputer 70.
Th~ conductivlty sensor ~2, the densitometer 64 and the
sul~lde ~lectrode 66 are disposed serially along a
~ypass conduit 72 that prQvides a ~tream of liquor from
a vessel 80. The bypass stream 72 is maintain~d a~ a
uniform temperature by a heater 74 with temperature
control 76. A pump 78 provides the mode o~ ~orce for
circula~ing the bypass stream 72.
The analyzer operation involves pumping the
liquor through the various sensors and processing the
WO91/17305 PC~/U$91/02933
-17~ ~J.'.1,,~
sensor data to calculate liquor composition.
ANALYZER OPERATION
Experiments were carried out with aqueous
solutions containing sodium hydroxide, sodium sul~ide,
and sodium carbonate. The experiments were divided into
two groups, white liquor type solu~ions and green liquor
type solutLon The white liquor solukions contained
between 50 and l00 g/l of NaOH, 50 to 40 g/l of Na2S,
and 0 to 25 g/l of NazCo3, all expressed as Na2O
e~uiYalents. The green liquor solutions contained
between 65 and 105 g/l of Na2CO3, 0 to 30 g/l o~ NaOH,
and 5 to 35 g/l o~ Na2S, all expressed as NazO
equivalents.
The solutions were prepared from reagent grade
chemicals in distilled water and were used immediatPly
after preparation. A liquor sample was taken from the
vessel 74 and titrated ir. duplicate using HCe before
each experiment was begun. The vessel 80 was capped and
the contents heated sequentially to 70, 80, and 90C.
2 0 This temperature range was chosen because it is the
typical temperature range in which white and green
liquors are transported throughout a pulp mill. The
liguor was held at each temperature until all of th~
sensor responses had stabi1ized. In each case, the
25 sul~ide electrode had the slowest, and thus limiting,
recpo~se time. Sensor data wa~ recorded at ten second
intervals throughout each experiment as averages of ten
consec~tive readings. The da~a acquisition rate was
3.33 ~Z.
The proc~ss trans~itters were configured to
provide good signal resolution over the expected range
of liguor concentrations. Each transmitter ou~put was
connected to the data acquisition system as a 4-20 mA
current loop. R load resistor o~ ~50 ohms was used ~o
WO91/17305 ~CrJUS91/0~33
-18-
convert the signal into a voltage of 1-5 V. The
transmitter ranges and maximum signal resolutions are
shown in Table 7.
TABLE 7
In~Situ Liquor Analyzer Sensor Configurations
and Maximum Signal Resolutions
S~nsor . Range Max. Resolution
.
Temperature 0-210 C 0.13 C
Conductivity 0-1000 mS/cm 0.61 mS/cm
Density 950-1150 g/l 0.12 g/l
Sulfide Ion 730-~80 mV 0.09 mV
. ~
~NALYZER_CALIBRATION
~O For white liquor type sslutions, 15
experimental runs were made to establish correlations
for the individual components~ The data consisted o~
the concentration of each component and the sensor
readings at each temperature level.
The procedure for reducing the data into
corr21ations involved two steps. First, the tempera~ure
effect on the detec or responces was de~ermined. This
allowed the final rsgression to be made on temperature
co~pensated data. This approach was chosen based on the
eventual ~i~ld application of the analyzer. In ~he
~i~ld, ~he te~perature compensation could possibly be
performed prior to data trans~ission.
Examination of the white liquor data indica~es
that the temperature e~fec~ on both density and
conductivity i5 approximately linear over the range 70-
90C. The values at 80C were used as the re~erence
values. The da~ at o~her tempera~ures were adjusted to
the reference. The linear slope relating denslty
divided by re~erence density to ~emperature tooX on
,
W~91/17305 PCT/US9lJ02933
,7
--19--
values between -4.6X10-4 and -5.4X104Cl. The slope was
found to be a linear function of the reference density.
The regression equation is:
Dr a DT t13)
[m Dr + n]~T - 80) + 1
where
t = temperature (C)
DT = density at temperature T (g/1)
D, = density at reference temperature (g/1)
m = 3.9752Xl07
15 n = -9.3618X10-4
This relationship can be expressed as a
quadratic ~quation in Dr. Thus, Dr can be solved for
explicitly using the quadratic formula:
Dr = -b ~ ~(b2-4ac) (14)
where 2a
25 a = m (T ~ 80)
b = n (T - 80) ~ l
C = ~ DT
The conductivity divided by reference
30 conductivity data exhibited slopes between 8.6X103 to
11.5X103C1 which were also a linear function of the
reference density. Since the reference density can be
calculated by equa~ion (14), the regression equation is:
C, ~ CT, _ (15)
[m Dr + n~ (T ~ 80j ~ 1
where0
T = te~perature ( C )
CT = conductivity at temperature T (mS/cm)
C, = conductivity at reference temperature (mS/cm)
m = 2 . 9106X10~S
n = -2 . 2055X10~2
The liquor tempera~ure i not a signi~icant
factor in 'che sulfid~ electrode response for the white
liquor solutions in the range of 70-90C. T}~e da~a
WO91/17305 PCT/US91/02933
-2 O~ 3~ f~7
indicat~s that there is a small deviation ~f the
electrode response within this region, but no trend was
observed with temperature.
The co~plete set of data adjusted o the
reference temperature was analyzed using stepwi e
multiple linear regression to obtain best regression
equations for the component concen rations. The
relationship between sulfide concen~ration and sul~ide
electrode voltage is logarithmic:
V = V0 + B ln(X) (16)
where
V = ~ulfide electrode voltage (m~)
V0 = reference potential (mV)
B - electrode slop (mV/decade)
X = sulfide activity (M)
The activity coefficient relating the activity
and the concentration is dependent upon the total ionic
strength o~ the liquor b~ing measured. This indicat~s
that additional terms involving the other components in
the liquor ~ay be required to adequately fit the
electrode response to measured sulfide concentration.
The ~est reqression equations for sulfide concentration
in the white liquor composition range are:
ln(S) = -~0.2719 t ~.7718X102 V + 9.5084Xl03 Cr
- 6.8623Xl06 Cr2 (17)
whe~e
S = sodium sulfide conc~n~ra~ion (g/l Na20)
V a sul~ide electrode voltage (-m~)
Cr ~ re~erence conductivity (mS/cm)
and
ln(S) ~ --39.18~3 + 4.6sl8xl0 2 V ~ 8.3142Xlo 3 Cr
-- 6.0181X10 6 Cr2 (1~)
WO~1/1730~ PCT/US91/02933
-21- ~r'~
Equation (17) is the best fit for liquor only at 800C,
equation (18) is best for the te~perature range 70-90oc.
These correlations indica~e that the basic
relationship between electrode voltage and sul~ide
concentration is exponential with some correction for
ionic strength effects. The choice of conductivity for
ionic strength correction was made by xamination of
residuals obtained by fitting only ~he ~lec~rode
voltage. The co~ductivity showed a clear trend with the
residuals. The sodium hydroxide ion concentration also
showed a trend, but it is not a measured variable. No
other correction term, including sodium hydroxide,
provided a significant regression improvement after
conductivity was included.
The prediction ability of the sulfide
electrode is shown in ~igure 6. The error at R0C
expressed as a 90% confidence interval is approximately
~2.3 g/l, while that over 70-90C is nearly +3 g/l.
Both errors are substantial.
The regr~ssions involving sodium hydroxide and
sodium carbonate were carried out for three di~ferent
cases. In the first case, the sodium sulfide
concentration was assumed to be known with accuracy
corresponding to that of the liquor titration. The
second case involved regression of the data at 80C
u~ing equation (17) ~or sul~ide prediction. The third
case was a regression of all data using equation ~183
for sul~ide prediction. The equation form de~ermined by
stepwise regression to be optimum was:
o~ or CO3 - a ~ b Df ~ C ~r + d Dr (19)
Th~ regression coe~icients for both ~aOH and
Na2CO3 in all ~hree cases are shown in Table ~. The
. ..
WO91/173G5 P-,~/US91~Z933
-22-
coefficients are of the same order of magnitude for each
case, reflecting the reasonable fit of the sulfide data
as compared to the known values. The accuracy of the
predictions as 90% con~idence interYals for each
compon~nt are shown for each case in Table 9.
TABLE 8
Regr2ssion Coefficients for NaOH and Na2CO3
~rediction Using the In-sitll Liquor Analyzer
Sodium Hydroxide
Case a b c d e f
Xlo~2 Xl03 Xl0-4
. ~
1 2851.68 -5.2877 ~3.184S 2.4756 1~3644 -5.7230
2 2310.31 -4.287~ -9.0360 2.0341 1.728g ~6.2667
3 2459.77 -4.5606 -7.0792 2.1521 1.6000 -~.9363
Sodium Carbonate
25 Case a b c d e f
x~o-2 Xl03 Xl04 XlO1
1 -2546.~3 4.1842 -~.4066 -1.6334 -7.314~ -2.6597
2 -2595.0~ 4.2732 -3.1885 --1.6707 -6.9820 -~.7991
3 -2733.89 4.5340 -4.3062 -1.7902 -6.1359 -2.70~7
TA~LE 9
Prediction Errors (g/e) for ~ite Liquor
Using th~ ~n-situ Liquor Analyzer
Case NaOH Na2S Na2CO3
. _ _ _ _ _ _ _ _ _ _ n _ _ _ _ _
1 0.58 - 0.51
2 0.97 2.30 0.71
3 1.61 2.9~ 1.17
WO91/1730S PCr/US91~2933
.J, ~ ,b ~J ~7
-23-
It is clear that the prediction ability is
excell~nt if the sulfide concen~ation i5 known. The
errors in this case are significantly less than l g/l
over the entire temperature range. The large error in
sulfide pre~iction clearly degrades the other
predictions. Simulation was used to examin~ the ef~ect
of a smaller sulfide error on the prediction of sodium
hydroxide and sodium carbonate. ~he sulfide error was
represented by a normal distribution N(0,0.25). This
distribution produoes a sulfide error of approximately
l g/l at 95% confidence. Trials using the correlations
obtain2d Por case l indicate that the errors in NaOH and
Na2CO3 prediction would not be inflatsd sisni~ica~tly at
this level of sulfide error. Thus, if a more accurate
detector for sulfide detection was available, the in-
situ analyzer should perform as well as the extractive
sample analyzer with white liquor. ~he results for all
three cases are shown in Fi~ures 7 and 8.
A similar set of experiments was run for green
liquor type ~olutions. In this case, 12 experimen~s
w~re run to establish correlations.
- The procedure for data regression parallels
that of th2 white liquor. Examination of the data
indicated that the temperakure effect on both density
25 a~d conductivity was linear ~nd the slope was dependent
on tAa re~rence den~ity. The equation for density
te~perature compensation is:
D - -b + ~b2-4ac) t20)
r _ 2a
where
a = In (T - 89)
b - n (T - 80) ~ 1
m - 1, 027~Xl0'6
n = 1. 6784X10 3
W091/17305 PC~/US91/02933
-~4~ 7
The equation for conductivity temperature
compensation is:
Cr = T (21)
where
T = Temperature (C)
C~ = conductivity at ~emperature T (mS/cm)
C, = conductivity at reference temperature (mS/cm)
m = 1.479~X10-s
n = -5.862lX10-3
The sulfide electrode response was regressed as an
exponential correlation at 80C, and the best fit
equation is:
ln(S) = -21.1551 ~ 3.6220Xl02 V - 5.8771X103 Dr (22)
where
s = sodium sulfide concentration (g/l Na20)
V = sulfid~ electrode ~oltage (-mV)
D, = refer~nce density (mS/cm)
Equation (22) is similar ~o equation fl7) for
white liquor. However, the ionic strength correction in
this case is density rather ~han conductivity. This
dependence reflects the ef~ect of sodium carbonate, the
primary green liquor component, on density elevation
rather than conductivity elevation. The standard
deviation o~ the prediction with equation (22) is 2.54
g/l, re~ulting in a 90~ confidence interval of ~4.60 g/l
for sodiu~ ~ulfide.
The regressions ~or sodium hydroxide and
sodium carbonate were carried out ~or ~wo cases. Firs~,
the sulfide conc~ntration was assumed to be known with
titration accuracy. Second, the dat~ at 80C was used
with equation (2Z) ~or sul~lde prediction. Equation
(19) was ~ound to best represent the sensor response to
the gr~en liquor solutions. The reqression coefficients
WO91/17305 PCr/US91/02933
-25- 2~
for both NaOH and Na2CO3 in both cases are shown in
Ta~le l0. The accuracy of the predictions as 90%
confidence intervals for each compcnent are shown for
each case in Table ll.
TABLE l0
Regression Coe~ficients for NaOH and NazCO3
Prediction Using the In-situ Liquor Analyzer
on Green Liquor
Sodium Hydroxide
Case a b c d e f
Xl01 Xl0-3 Xl0-5 Xl01
l2608.g0 -4.6430 l.3885 2.0328 7.~224 -6.8430
22056.60 -3.7289 2.2~89 l.6464 -6.0929 -6.14~5
~
Sodium Carbonate
Case a b c d e f
xlo~2 Xl0'4 XlOs xlo~l
l-1738.50 2q533l 9.8034 -7.7130 -7.6584 ~2.0~2
2-1958.50 2.9015 -7.0751 -9.2~09 -l 7 09~3 -2.7331
TABLE ll
Pxediction Errors for Green Liquor
Using the In-situ Liquor Analyzer
Case NaO~ Na2S Na2C~3
~
l 0.~6 -- 0.61
2 2.70 4.60 0.94
As with white liquor, the prediction ability
is excellent if the sul~ide concentra~ion is known.
WO~1/17305 PCT/US~1/02933
-26- 2~ 7
However, due to the presently available sulfide
electrode, the sulfide predictions adversely ~ffect the
NaOH and Na2CO3 predictions when the correlation is
used. If the sodium sul~ide measurement could be made
S with aocuracy of +l.0 g/l or better, then sodium
hydroxide and sodium carbonate m~asurements would be
within 90% confidenc~ intervals of +0.7 g/l.
Mathematical manipulation of sodium sulfide data shows
this to be trueO The Na2S prediction results are shown
in Figure 9. The results ~or NaOH and Na2CO3 are shown
in Figures l0 and ll.
S~A~,Y
The novel extractive sample liquor analyzer o~
the present invention has the ability to analyze kraft
white and green liquor samples for sodium hydroxide,
sodium sulfide, and sodium carbonate concentrations with
accuracy comparable to titration. The in-situ liquor
analyzer also has comparable accuracy if a reliable
sulfide electrode that can withstand the continuous
hostile en~ironment is developed. The design o~ both
types of analyzers permits handling of other pulp mill
liquors based on the same components such as soda, soda-
AQ, ~eutral and alkaline sulfit~, and controlled alkali
ssmi-chemical liquors. It will be further understood
th~t the analyzer of this invention may be used in
processes other than paper pulping processes.
The liquor analyzer o~ the pres~nt invention
is suitabl~ for use in both eed forward and feed back
control ~yste~s. Many types of control systems can be
configured to help control ~he concentra~ion o~ the
green liquor in a kraft paper proc~ss. One simple
control sys~em is illustrated in Figure l~. C~ntrol ~or
a causticizer 80 includes analysis of the green li~uor
stream 82 entering the caustic~zer and the liquor stream
WO91/17305 PCr/USgl/0~933
-27~ 't~
~4 exiting the causticizer~ The addition of lime 86 is
re~ulated by a computer control system 88 which uses the
data received from detectors 90.
The minimum analysis time for the extractive
sample liquor analyzer is approximately three minutes.
The 90% confidence intervals for white and green liquors
are approximately +0.5 g/l f or sodium hydroxide and
sodium sulfide, and ~0.8 g/l for sodium carbona~e
expressed as equivalents of Na20.
The analyzer o~ this invention has featur~s
which make it advantageous as compared to current types
of analysis. One advantage is speed. The time for
analysis is approximately three minutes. This is
signi~icantly faster than automatic titrators or ion
lS chromatography~ Another advantage is that a minimal
amount o~ maintenance is required. There is no
sensitive chromatography column that must be
periodically regenerated or replaced. There are no
chemical reagents required that must be prepared and
standardized. ~he accuracy of analysis is also good
over a wide range of operating conditions.
- The in-situ liquor analyzer, speci~ically the
conductivity and density portion, has the advantages of
co~tinuous liquor monitoring and simplicity of design.
The question of temperature compensation has been
an~wered, and the accuracy could be comparable to that
o~ the extractive sample analyzer once a s~lfide
electrode is developed to withstand the harsh
en~ironment for an extended period of time.
Although the present inv~ntion has been
described with re~er~nce to preferred embodiments,
workers sk~lled in the art will ~cognize that changes
may be made in ~orm and detail without departing from
the spirit and scope of the invention.