Note: Descriptions are shown in the official language in which they were submitted.
2082~73
FIELD OF THE INVENTION
The invention relates to a process and apparatus
to remove sodium sulphate from an aqueous solution of sodium
chlorate, sodium chloride, and sodium sulfate.
BACKGROTJND OF THE INVENTION
Sodium chlorate is widely used to produce chlorine
dioxide gas for use in pulp bleaching. Sodium chlorate is
produced by electrochemical apparatus which electrolize a
brine of sodium chloride in an aqueous solution.
Sodium chloride salt is commonly available as rock
salt with several impurities including sulfate ions. When ~ i -
salt containing sulfate impurities is used in a brine
solution to produce sodium chlorate, sulfates may enter the
electrolytic system and introduce serious detrimental
consequences. As a result, conventional production methods
include means to reduce the sulfate concentration to an
acceptable level. The present invention relates to an
improved method and apparatus for removing the sulfate ion - -
in the form of sodium sulfate precipitate.
United States patent No. 4,702,805 to Burkell et
al. issued October 27, 1987, describes a method of
production of sodium chlorate which includes a secondary
crystallization procedure to crystallize a sodium sulfate
.. .
sodium chlorate admixture in order to reduce the sulfate
concentration to an acceptable level. Other conventional
2082~73
methods include a liquor purge which is less advantageous
due to loss of valuable components in the mother liquor.
Conventional processes to produce sodium chlorate
include the following steps in general. Water and sodium
chloride in the form of rock salt are mixed to form an
electrolyte. The electrolyte may be contaminated with
various impurities, such as sulfate ion, calcium and ~ ~-
magnesium. The sodium chloride brine may be mixed with
sodium hydroxide and sodium carbonate to precipitate calcium
carbonate and magnesium hydroxide, which are then removed by
filtration. The remaining calcium and magnesium ions are
reduced in concentration through an ion exchange column.
The purified brine containing sulfate ion contaminants is pH ;
adjusted (mixed with acid) and a portion of the chloride is '
converted to chlorate in an electrolytic cell. The
electrolized liquor flows into a primary crystallizer
operating under vacuum wherein the chlorate is partially
crystallized. The major portion of the chlorate depleted
mother liquor of reduced chlorate concentration is recycled
in the process, however, a minor portion is removed and
forwarded to a secondary crystallizer. In such conventional
processes as described in Burkell, the minor portion of
mother liquid is further cooled to a temperature wherein
dual crystallization of sodium sulfate and sodium chlorate
admixture is effected. The spent liquor from the secondary
crystallization is also recycled. The crystalline admixture
2~82473
is removed and fresh brine is continually fed to make up the
electrolyte.
As a result of the secondary crystallization and
conventional processes, the sulfate ion concentration in the
electrolyte is maintained at an acceptable level. The
admixture of sodium sulfate and sodium chlorate as described
in Burkell are blended with the pure chlorate crystals
obtained from the primary crystallization process to produce
a blended product having an acceptably low concentration of
sodium sulfate.
The advantage of removing sodium sulfate in
conventional systems is that it prevents sulfate ions from
building up in the electrolytic system and increasing to a
concentration that adversely effects the electrolytic power
consumption and avoids problems due to localized
precipitation in the electrolytic cells. A disadvantage of
the process described in Burkell, of significance, is the
inability to separate sodium sulfate as a contaminant from
the sodium chlorate produced. Although, for many
applications, a low concentration of sodium sulfate
contaminant in the sodium chlorate crystals is acceptable, a
more pure product would be desirable to increase
environmental acceptability and efficiency of the pulp
bleaching process.
The term "mother liquor", as used herein, refers
to an aqueous solution of sodium chlorate and sodium
~ ~ 2~82473
chloride. Sodium sulfate is considered to be a contaminant,
not an integral part of the mother liquor by definition.
Therefore, it is desirable to produce sodium
chlorate free of sodium sulfate contaminants, especially
where only minor modification of existing procedures and
equipment need be implemented.
SUM~ARY OF THE INVENTION ~;
The invention overcomes the disadvantages of the
prior art in a novel manner in the provision a of a process
for selectively precipitating crystalline sodium sulphate
from a mother liquor, the mother liquor being an aqueous
solution of sodium chlorate and sodium chloride and
aontaining dissolved sodium sulphate. The process includes
diluting the mother liquor with demineralized water thus ~ -
forming a diluted liquor. The diluted liquor is then
chilled to a temperature Tx thereby forming a sodium
sulphate crystalline precipitant, where Tx is in the range
between the saturation temperature of sodium chlorate in the
diluted liquor Tc and the saturation temperature of sodium
sulphate in the diluted liquor Ts. The sodium sulphate
crystalline precipitant is separated, through settling for
example, from a resultant clarified liquor.
Further according to the invention is provided an
apparatus for carrying out the above described process for
selectively precipitating sodium sulphate from a mother
- 4 -
2082~73
liquor. The apparatus includes dilution means having an
inlet communicating with a mother liquid input conduit, and
with a water input conduit, and having an outlet
communicating with a diluted liquor conduit. A chilling ;
unit has an inlet communicating with the diluted liquor
conduit and an outlet communicating with a chilled liquor
conduit. The chilling unit is capable of chilling the
diluted liquor to temperature Tx. A settling clarifier tank
has an inlet communicating with the chilled diluted liquor
conduit, a lower outlet communicating with a crystalline
sodium sulphate slurry conduit, and an upper outlet ~
communicating with a clarified liquor conduit. ~ ;
Further aspects of the invention will become ~ ~
apparent upon review of the following detailed description. ;
: '~ .
BRIEF DESCRIPTION OF THE DRAWINGS
In order that the invention may be readily
understood, a preferred embodiment of the invention will be
described by way of example with reference to the
accompanying drawings in which:
Figure 1 is a graph showing the relationship
between the solubility of sodium chlorate, temperature, and
relative concentrations of sodium chlorate to sodium
chloride in mother liquor;
Figure 2 is a graph showing the sodium sulphate
saturation curve relating temperature and dissolved sodium
sulphate in diluted liquor; and
2082~73
Figure 3 is a schematic representation of a
process apparatus according to the invention.
DETAILED_DESCRIP~ION OF PREFE~RED EMBODIMENTS
OF THE INVENTION
The invention proposes a method and apparatus to
remove sodium sulfate from a mother liquor. The mother ~-
liquor is an aqueous solution saturated with sodium chlorate
and containing varying concentrations of sodium chloride.
In general the process in a preferred embodiment follows
conventional sodium chlorate production methods in taking a
slip stream of mother liquor from the output of a primary
crystallization of chlorate crystals system. The novel
process involves diluting the mother liquor with just enough
demineralized water to reduce the saturation temperature of
sodium chlorate in the diluted liquor to near the "low
temperature boundary of the ice field". The term "ice
field" pertains to the area of Figure 1 indicated. This
area graphically represents any composition of sodium
chlorate, sodium chloride, and water where reducing the
temperature of the solution will cause the solution to
freeze before any of the chlorate or chloride will
precipitate out.
This ice field boundary is at approximately -20~C
in the relevant range as shown in Fig. 1. The dilution
reduces the saturation temperature of sodium sulfate in the
diluted liquor only to nominally below 0~C. As a result
- 6 -
2~8~73
chilling the diluted liquor to between 0~C and -20~c results
in precipitation of sodium sulfate as a decahydrate. The
total operable range of temperature is 0~C to -20~C, however
-15~C represents the preferred temperature in operation
determined by experiment.
Importantly, the process does not result in
precipitation of sodium chlorate crystals. Therefore, the
invention represents an improvement on conventional methods
which rely upon the co-crystallization of sodium sulfate and
sodium chlorate crystals. As described above, this co-
crystallization is less than optimal in that the preferred
method provided by the invention separates sodium sulfate
and sodium chlorate formation.
Referring to Figure 1, the composition of aqueous
solutions saturated with sodium chlorate and containing
various concentrations of sodium chloride, and the influence
of temperature of such solutions is illustrated. The upper
boundary limitations represent the sodium chloride
saturation temperatures in degrees celsius, whereas the
lower boundary represents the "ice field" at approximately
-20~C.
Where the invention is used in the production of
sodium chlorate, the normal operating temperature of the
mother liquor is 35 to 40~C and the initial concentrations
of sodium chlorate, sodium cloride, and sodium sulfate in
the mother liquor are 40 to 42 wt%, 8.25 wt~ and no greater
2082473
';,
than 2.5 wt% respectively. This mother liquor condition is
represented in Figure 1 by a point designated as A.
Dilution of the mother liquor with water results in
concentrations graphically illustrated as a water dilution
locus in the form of a sloping line from point A to the
intersection of the co-ordinate axes of the graph in Figure
1. Intersecting with this water dilution locus are shown
three solubility curves, namely at temperatures 0~C, -15~C
and the boundary of the ice field, approximately -20~C.
These intersecting points are respectively designated as B,
C and D for reference.
Referring to Figure 2, the sodium sulfate
saturation curve is shown. In a process where sodium
chlorate is produced from sodium chloride, sodium sulfate is
present as a contaminant in relatively low concentrations in
the mother liquor, in general no greater than 2.5%. As
graphically illustrated in Figure 2 when the mother liquor
is chilled below 10~C sodium sulfate begins to precipitate
out of the solution until equilibrium in the solution at its
saturation point is achieved, as illustrated in Figure 2.
For example, if the mother liquor is chilled to 0~C, as
shown in Figure 2, the concentration of sodium sulfate would
approximate 0.95 wt%. Chilling the mother liquor to -10~C
would result in a concentration of sodium sulfate at
approximately 0.45 wt%. -
- 8 -
2082~73
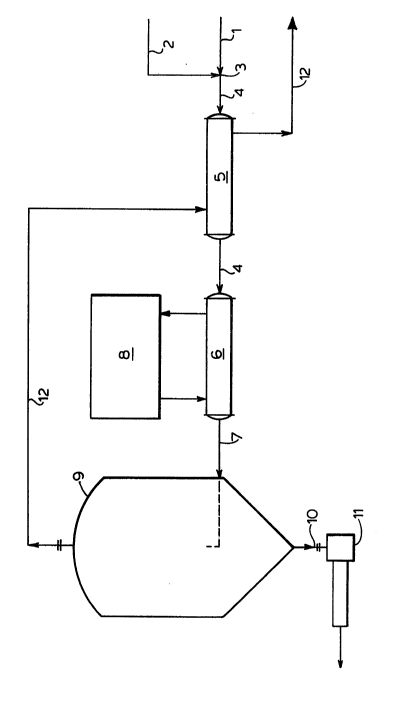
With reference to Figure 3, the process and
apparatus proposed by the invention for selectively
precipitating sodium sulfate from a mother liquor will be
described below. The mother liquor is an aqueous solution
of sodium chlorate and sodium chloride and containing
dissolved sodium sulfate which is conducted along the mother
liquor input conduit 1. Water, preferably demineralized
water, flows through a water input conduit 2 and is mixed
with the mother liquor with conventional dilution means 3.
The resulting diluted liquor is conveyed via diluted liquor
conduit 4. It will be understood that any suitable
conventional dilution means may be used, such as an in-line
mixer, a mixing tank and impeller, or a simple T-joint as
illustrated.
As shown in Figure 3 after the step of diluting
the mother liquor with demineralized water to form a diluted
liquor, the diluted liquor is pre-chilled through a heat
exchanger 5. It will be apparent to those skilled in the
art that the heat exchanger is not an essential component,
however, in a preferred embodiment the use of a heat
exchanger results in cost savings in respect of chilling the
diluted liquor. The operation of the heat exchanger 5 will
be explained in detail below.
The next step in the process is to chill the
diluted liquor to a temperature Tx. A chilling unit 6 has
an inlet communicating with the diluted liquor conduit 4 and
'
2~2~73
.~.,.
an outlet communicating with a chilled liquor conduit 7.
The chilling unit 6 has an associated refrigeration unit 8
of conventional design.
The chilling unit 6 is capable of chilling the
diluted liquor to a temperature Tx. Tx is in the range
between the saturation temperature of sodium chlorate in the
diluted liquor (referred to hereinafter as Tc) and the
saturation temperature of sodium sulfate in the diluted
liquor (Ts).
Chilling the diluted liquor to a temperature Tx
results in the formation of sodium sulfate crystalline
precipitant in the chilled liquor.
In order to separate the sodium sulfate
crystalline precipitant from the resultant clarified liquor,
the chilled diluted liquor is conveyed to a clarifier tank 9
having an inlet communicating with the chilled diluted
liquor conduit 7 in its mid portion. Through conventional
settling processes the precipitant is conveyed through a
lower outlet communicating with a crystalline sodium
sulphate slurry conduit 10 and is pumped to further
processing with a positive displacement pump 11.
Through an upper outlet communicating with a
clarified liquor conduit 12, the resultant clarified liquor
is withdrawn and returned to processing of the mother liquor
through a return conduit via the heat exchanger 5. The
diluted liquor is pre-chilled in the heat exchanger 5 which
-- 10 --
2082~73
.~ .
comprises a counter-flow heat exchanger thermodynamically
communicating between the clarified liquor conduit 12 and
the diluted liquor conduit 4.
Energy consumption may be reduced in the chilling
of the diluted liquor as a result of using the heat
exchanger to pre-chill the diluted liquor with the clarified
liquor to a temperature greater than Tx. Therefore, the
chilling unit 6 need only further reduce the temperature to
Tx. Referring to Figures 1 and 2 therefore, an example of
the process will be described. Diluting of the mother
liquor is graphically illustrated in Figure 1 resulting in
the change in concentrations from point A to point C. As a
result the saturation temper~ture of sodium chlorate in the
diluted liquor is reduced to Tc equalling -15~C.
Referring to Figure 2 the same dilution of the
mother liquor reduces the concentration of sodium sulfate to
approximately 0.95 wt%. From the sodium sulfate saturation
curve it can be seen that this concentration of sodium
sulfate results in a ~aturation temperature of sodium ~ :
sulfate in the diluted liquor Ts equalling nominally less
than 0~C. :~
Therefore, if the diluted liquor is chilled by the
heat exchanger 5 and chilling unit 6, to a temperature Tx, ;
in this example between Ts 0~C and Tc -15~C, sodium sulfate ~ :
crystalline precipitant will be formed in the chilled
diluted liquor.
- 1 1 -
r r
2082~ 73
':,
Since the saturation temperature Ts of sodium
chlorate in this example is -15~C, the disadvantage o~
conventional methods involving co-crystallization of sodium
sulfate and chlorate crystals together is avoided.
It will be understood that, although various
features of the invention have been described with respect
to one or another of the embodiments of the invention, the
various features and embodiments of the invention may be
combined or used in conjunction with other features and
embodiments of the invention as described and illustrated
herein.
Although this disclosure has described and
illustrated certain preferred embodiments of the invention,
it is to be understood that the invention is not restricted
to these particular embodiments. Rather, the invention ' .
includes all embodiments which are functional or mechanical ..
equivalents of the specific embodiments and features that
have been described and illustrated herein.
j
., ~, ..
: ~ ... ...
- 12 -