Note: Descriptions are shown in the official language in which they were submitted.
HOE 91/H 027
The invention relates to a process for the preparation of
fullerenes. Fullerene is the name given to carbon clus-
ters having a molecular size of C28 to C9s, in particular
Cso to Coo. Heterofullerene is a carbon cluster in which
all or some of the carbon is replaced by boron and
nitrogen.
Fullerenes are solids and represent a third allotropic
form of carbon besides graphite and diamond, which
differs from the known forms by its molecular structure.
The prototype Cso was given the name buckmi.nsterfullerene
and has the shape of a football made up of twelve
pentagons (CS ring) and twenty hexagons (Cs ring).
Fullerenes are solids which can be sublimed and have
limited solubility in organic solvents, giving a red-
brown solution. .Although the preparation of macroscopic
amounts (0.1 - 1 g) of fullerenes was described for the
first time in 1990 by W. Kratschmer et al., Solid Cso: a
new form of carbon, Nature, Vol. 347 (1990), 354-358, a
wide range of applications (lubricants in the form of
miniaturized ballbearings, battery raw material due to
its redox behavior, photocatalysis due to excitation to
the triplet state of Cso by light of wavelength 450 nm,
diamond synthesis, occlusion compounds) has already
opened up. Metallic conductors and even superconductors
having superconductive transition temperatures of 18 K
(KaCso) and 28 K (Rb3Cso) can be obtained from the insulator
Cso by reaction with alkali metals, see Nature, Vol. 350
(1991), 320 and 600.
Furthermore, a wide range of applications has opened up
for fullerenes and heterofullerenes due to the great
variety of possible electrocyclic reactions, since Cso
behaves like an olefin.
The wide range. of applications of fullerenes and hetero-
fullerenes has triggered a high demand for the substances
themselves and also fox simple preparation processes.
- 2 -
The preparation of fullerene has until now been based on
noncontinuous electric-arc processes in which graphite is
vaporized by the method of quasi-contacting resistance
heating, Chem. Phys. Lett., Vol. 170 (1990) 167; Nature,
Vol. 347 (1990), 354. Other preparation processes fox
fullerene utilize the laser vaporization method, Nature,
Vol. 318 (1985), 162. Finally, Chem. Phys. Lett., Vol.
137 (1987), 306 describes the isolation of fullerene from
the soot of optimized flames.
The object of the present invention is to describe a
process fox the preparation of fullerene and hetero-
fullerene in which carbon is partly replaced by boron and
nitrogen, with increased space-time yield, which can be
extended to a continuous preparation process.
The object is achieved by vaporizing shaped carbon pieces
or shaped carbon pieces in contact with shaped boron
nitride pieces in an inert gas atmosphere under reduced
pressure in an inductive heating zone and depositing the
vaporized portions on a cold surface as fullerene-
containing soot and isolating fullerene or hetero-
fullerene in a purification step from the soot using a
solvent.
The process of the invention can furthermore comprise the
following optional steps of
a) using shaped carbon pieces made of graphite, iso-
statically compressed high-purity graphite, pyro-
lytic graphite or of glassy carbon obtained from
polymer precursors and containing residual hydrogen;
b) using thss shaped carbon piece and the shaped boron
nitride piece in the form of cylindrical tubes;
c) using cylindrical tubes having an inside diameter of
5 to 60 non and a wall thickness of 1 to 25 mm as the
_ 3 _
shaped piece;
d) using solid shaped carbon pieces;
e) using slotted, laminated or cone-shaped carbon
pieces;
fj operating under argon or helium at a pressure of 10
to 400 hPa, in particulear 25 to 200 hPa, as the
inert gas atmosphere;
g) arranging one or more insulating tubes around the
shaped carbon piece at a distance;
h) selecting a distance of 0.5 to 20 mm between the
shaped carbon piece and the insulating tubes;
i) using one or more boron nitride insulating tubes;
j) using one or more mufti-slotted graphite insulating
tubes;
,15 k) operating in a high-frequency furnace as the induc-
tive heating zone;
lj heating the shaped carbon piece to more than 2500°C,
in particular to 2600 to 3500°C;
m) depositing the fullerene-containing soot on a water-
cooled quartz glass tube as the cold surface;
n) extracting the fullerene or heterofullerene from the
soot with toluene as the solvent;
o) introducing a long shaped carbon piece continuously
into the inductive heating zone so that the
vaporized carbon is replenished.
_ 4 _ ~~~ ~~~~_
To optimize the process according to the invention, it is
desired to bring the power input through the inductor of
the high-frequency generator into line with 'the removal
of heat via the cooling water of the cold surface. The
yield of fullerene or heterofullerene is increased in
particular by arranging a sing:Le or multiple insulating
tube made of pyrolytic boron nitride around the shaped
carbon piece, because this results in an increase in
temperature on the shaped carbon piece and a decrease in
temperature on the cold surface. The additional effect of
the insulating tube could be due to the longer and hotter
reaction zone, in which gas phase deposition of
fullerenes on soot particles may possibly take place, or
else a chimney effect may occur. However, soot deposits
on the inner surface of the insulating tube are free from
fullerenes.
The intimate contact of the shaped carbon piece with a
shaped boron nitride piece results in a process for the
preparation of heterofullerenes by co-sublimation.
The high-purity graphite type EK 966 and the pyrolytic
graphite used were obtained from Ringsdorff, Bonn. The
glassy carbon was purchased from Sigri, Meitingen. A
high-frequency furnace from Hiattinger IG, f'reiburg im
Breisgau, and a high-frequency generator of the 15/400
type were used. Owing to the good heat conduction, the
vaporization of solid shaped graphite pieces requires an
increased power input. This heat conduction may be
reduced by cutting slots or lamellae into the graphite.
However, it is better to vaporize mechanically stable
glass carbon tubes, the heat conduction of which is less
than that of graphite tubes.
The temperature of the shaped piece is measured by a
pyrometer. If boron nitride tubes are used, slots or
holes are made for this purpose by milling.
CA 02082775 2002-04-25
26632-6
To increase the yield, the apparatus is filled
with rare gas and then evacuated to 10-3 hPa three times
before the heating process. After the last evacuation, the
apparatus is then heated at 900°C in vacuo and thereafter
5 under a stream of rare gas at atmospheric pressure.
It has been found that helium at about 150 hPa
gives higher yields of fullerene and heterofullerene than
argon. At helium pressures of less than 100 hPa, the
amounts of fullerene formed are barely noticeable. In an
argon atmosphere, undesired bright white gas discharges
often take place, as a result of which the formation of
fullerene and heterofullerene is then reduced.
The general preparation of fullerene is
illustrated in more detail with reference to the 2 attached
Figures:
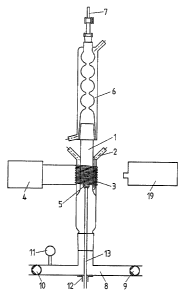
In quartz glass tube 1 equipped with water cooling
2, the inductive heating zone 5 is built up by means of
inductor 3 in combination with high-frequency generator 4.
A cooler 6 equipped with ventilation 7 is arranged on quartz
glass tube 1. A T-piece 8 containing throttle valves 9 and
10, bosshead 12 and manometer 11 is arranged on the bottom
of quartz glass tube 1. Throttle valve 9 reduces the rare
gas flow; throttle valve 10 is connected to a vacuum pump
and regulates the pressure in quartz glass tube 1 downstream
from manometer 11. A copper tube 13, through which cooling
water is passed and which allows reintroduction of longer
shaped carbon pieces into the inductive heating zone 5, goes
through bosshead 12. The temperature of the shaped carbon
piece is measured by pyrometer 19.
CA 02082775 2002-04-25
26632-6
5a
The arrangement of the shaped carbon piece is
shown in detail in Figure 2.
A copper block 14 is mounted on the upper end of
the copper tube 13 as a support. The shaped carbon piece 15
- 6 -
is positioned on a support disc 16 which in turn rests on
a support tube 17. The support tube 17 is supported by
the copper block 14. The insulating tube 18, which can
also be designed as a double-tube, is also supported by
the copper block 14.
The following special structural components produce high
yields of fullerene and heterofullerene:
1 Quartz glass tube diameter (inside): 50 mm.
Quartz glass tube diameter (outside): 70 mm.
3 Inductor: 10 windings of 6 mm Cu pipe, .
Height g0 ~
Inside diameter 71 mm.
4 High-frequency generator: 500 kHz,
Power output on terminals: 15 kW.
15 Shaped carbon piece:
Graphite tube, height: 25 mm,
Outside diameter; 22.5 mm,
Wall thickness: 2 mm.
16 Boron nitride support disc.
17 Boron nitride support tube:
Outside diameter: 25 mm,
Wall thickness: 1 mm.
18 Boron nitride insulating tube:
Outside diameter: 40 mm,
Wall thickness: 1 mm,
Height: 80 mm.
18 Additional boron nitride double insulating
tube:
Outside diameter: 29 mm,
Wall thickness: 0.5 mm,
Height: 40 mm.
15 Shaped carbon piece in contact with
a
shaped boron nitride piece:
Solid glass carbon tube or graphite
tube
Height: 25 mm,
Outside diameter: 27.5 nun,
7
Boron nitride tube
In side diameter: 28 mm,
Wall thic~Cness : 0 . S mm,
He~.ght: 40 mm.