Note: Descriptions are shown in the official language in which they were submitted.
~3~
LITERAL A~D VERIFIED TRANSLATION OF PCT-APPLICATION NO.
PCT/EP91/00959 (AS AMENDED)
1 METHOD FOR THE UTILIZATION OF ORGANIC WASTES AND APPARATUS FOR
CARRYING OUT THE METHOD
The invention relates to a method for the utilization of organic
wastes consisting predominantly of macromolecules.
German Patent Publication DE-A-3,735,061 describes a method in
which partly organic wastes are burned with high percentage oxygen,
instead of with combustion air. Household garbage, special wastes
and the like were mentioned under the category of waste, as well
as the waste of shredders, which will be further referred to
separately.
The wastes are burned with oxygen in a suitable oven, the exhaust
gases are cooled in a waste heat boiler and then, in a suitable
gas cleaning plant, they are freed substantially from pollutants.
In this combustion process, carbon and/or carbon containi~g matter
are mainly combusted to carbon dioxide and water.
Since the nitrogen, which is present as a ballast when combusting
with air is lacklng here, the burning exhaust gases have only
small nitrogen contents, depending on the nitrogen levels in
the waste to be burned. Carbon dioxide is an unwanted combustion
by-product, since it adds to the so-called "greenhouse effectN.
Further, many methods are known, in which carbon monoxide is
hydrogenated to various hydrocarbon compounds of most varied
. . . .
2~8361~1
1 compositions, through the use of a suitable catalyst. The Fischer-
Tropsch benzine synthesis should be mentioned here:
nCO + 2nH2 ~ nCH2 + nH2O,
or methanol synthesis.
CO + 2H2 ' CH3OH
Carbon monoxide and water vapor can also be transformed into
various hydrocarbon compounds, with suitable mixing conditions
and catalysts (H. Kloebel and Fr. Engelhardt, Applied Chemistry
64, 1952, pp. 54-5~
mCO + nH2O = oCH2 + pC2H5OH + qco2
The hydrogenization of carbon dioxide with hydrogen is also pos-
sible (European Patent Publication 0,079,207):
mCO2 2 = oCH4 p 3 q
While usually, so-called synthesizing gases are used for the
synthesis of hydrocarbon compounds, however synthesis has also
been carried out with blast furnace gas and water gas, it can
be taken from the German Patent Publication DE-A-3,525,479 to
convert the CO2 that is generated by combusting gases, at a high
temperature with methane and water vapor into a gas made up mostly
of CO and H2, which is suitdble as a synthesizing gas for various
synthesis products.
.
The hydrogenization of carbon containing waste is already known
from ~Kunststoff Journal" 12/87, p. 3.
Tbe shredder wastes mentioned above will now be dealt with. Wastes
made of synthetic material are produced in large quantities during
.
.: ,
-
-.: : , :
.~
f ~3~
1 the shredding of used car bodies. These wastes make up about 3/4
of the so-called light fraction, which in turn make up 25% of the
entire weight of the car, that is, the synthetic material wastes
constitute about 12~ to 18% of the car's total weight. These wastes
are decreased by the removal of reusable parts, such as large com-
ponents. However, at least half of the waste still remains, because
these parts are so permanently fixed to the body that their removal
is not possible or would require too great an effort. These wastes
in the light fraction are available as shreds of a mixture of var-ious
synthetic materials, textiles, caoutchouc (rubber) and wood. There-
fore, they can no longer be economically separated and they cannot
be reused, for example, directly out of a melt, so that they must
now be stored at a waste dump. In addition, these wastes are so
contaminated with motor and hydraulic oils, that they also cannot
be burned, so that they must be disposed of as special waste at
a very high cost.
This mixture of organic waste materials, however, has a high heat
value of about 12.5 MJ/kg, since it is made up of, for the most
part, polymers produced from crude oil. Processing such mixtures
by melting has not led to useful, marketable products.
Methods have been suggested, however, to crack the high molecular
hydrogen ox~cunds into lower molecular gases and oils. These pro-
cesses are the pyrolysis, hydrolysis, and hydrogenization. The
latter process relates directly to the hydrogenization of coal.
The wastes must be cleaned and cdmminuted, in order to be mixed
to form a mash with used oils, which is then hydrogenized. The
hydrogenization, however, requires a costly preparation of the
wastes by manual sorting and grinding to a fine powder.
,. . ~ .,~ :
2~83~
1 In pyrolysis it has been found that the by-products such as chlorine
and sulfur, collect in the form of undesired, sometimes highly
toxic compounds in the pyrolysis oils. Therefore, pyrolysis today
is sensible only with chlorine-free wastes. Hydrolysis is only
suitable for a portion of the synthetic materials, namely for those
that were produced through polycondensation.
A method of the initially described type has also become known
from the US-PS 3,984,288, wherein the wastes are first heated
and melted, and then transferred in this state from an extruder
into a special heated chamber for the decomposition. That method
requires a high apparatus expenditure and is therefore not economical.
Only one extruder is used therein to facilitate the continuous,
uniform delivery and to produce a molten mass from the synthetic
material wastes not yet sufficiently heated for the method and
to bring this into a decomposition pipe or so-called pyrolysis
chamber. It is expressly pointed out that the pyrolysis reaction
shall only begin in the decomposition pipe. That method, no doubt,
facilitates the pyrolysis but without eliminating the disadvantages
of pyrolysis.
Starting from this prior art, it is the object of the present inven-
tion to suggest a method of the above mentioned type, with which
synthetic waste materials can be utilized in a simple and environ-
mentally safe manner, or with which it is possible to produce or
to prepare for the simple production of valuable raw materials,
replacement materials or energy carriers. Further, an apparatus
for carrying out the method shall be suggested.
. .
2~83~
1 With reference to the method, the object is achieved, starting
from a method of the above described type, in that in a single
reaction chamber the wastes are heated to more than 150C, melted
and then decomposed to wax, oil, or gas by the addition of oxygen,
and/or hydrogen, and/or water, and/or air, and/or water vapor and/or
metals, or metal oxides. Hereby, the disadvantages and difficulties
of pyrolysis, of hydrolysis and of hydrogenization processes are
avoided. ~ather, the organic polymers are broken down or cracked
into lower molecular polymers, oligomers, and monomers, so that
liquid and/or gaseous constituents are formed, which can then be
further processed simply and as desired. All this is performed
in a single reaction chamber, which naturally must be constructed
in a manner suitable for this purpose, as may be taken from the
apparatus claims.
! The wastes can, in addition! be exposed to pressure and/or to a
shearing force. Hereby also, the necessary heating is produ~ed,
or at least a portion of the heat needed is generated. They are
hereby easily split or cracked into lower molecular molecules and
thereby liquified or gasified.
~20~ In addition to the heat supply, different reactive gases, such
as oxygen, or hydrogen or water vapor can be added individually
.
or together, simultaneously or in a chosen sequence for breaking
- down the polymers. The break-down of the polymers is hereby ac-
,
celerated. Furthermore, other materials can be added to the mixture
of synthetic materials and reactive gases, which catalytica~ly
accelera~te the molecular break-down, and which in addition, or
~ . -
alternatively, prevent the recombination of the radicals which
are~produced. These catalytic materials can be metals, metal
::
~ ~ compounds, for example, metal oxides.
_ 5 _
.. ,. ,: , ~ ...... , . . - . . . .
,;
2~8~
1 With the measures mentioned herein so far, the macromolecules of
the synthetic materials are split apart in the melt, that is to
say they are shortened and thereby are liquified at a suitable
pressure and temperature, and are possibly also completely or partially
gasified. As far as it is necessary, the molten mass can be
dehalogenized at the same time.
With mechanically polluted synthetic materials, a sufficient separ-
ation of non-liquified or non-liquifiable constituents can simul-
taneously take place with the liquification of the synthetic ma-
terials in a suitable apparatus. In many mixed synthetic materialwastes, non-meltable synthetic material components must also be
expected. These do not disturb the process, however, if more than
half are thermo-plastics. It is then necessary, however, to in-
crease the shearing force and possibly the oxygen content of the
gases which are added.
The above described method is based on the fact that the covalent
bonds of the carbon or the heteroatoms of the main chain of the
polymers become increasingly labile at about 400C. The molecular
bond characteristic of the synthetic materials breaks up under
such conditions, it can however, recombine if the radicals produced
are not saturated. For this reason, oxygen, possibly hydrogen,
and water vapor are added already as reactive gases to the molten
mass that is being formed.
Thereby, the degradation of the polymer molecules by oY.ygen is
the fastest working disintegration process for most polymers.
,; . , .
.. :, . - , : ' ,' '
2~3~
.
1 Water vapor has, among others, the special purpose of breaking
down the synthetic materials present, for example in the shredder
wastes, which were produced through polycondensation. In addition,
it also makes sense to add water to the wastes. The water can
also be added as a moistening of the product being treated. This
water changes to water vapor during the process, so that the extra
addition of water vapor is not necessary. The break down or decom-
position of the polymers can be further amplified, if metals, metal
compounds, such as metal oxides, which act as catalysts, are added
to the wastes, for example, by mixing them in drums before filling
them into the melting cylinder or by injecting them at the entrance
of the melting cylinder. Thus, for example, iron containing com-
pounds work catalytically at the splitting of polyvinyl chlorides
(PVC), and heavy-metal containing compounds, especially copper,
act correspondingly on polypropylene and other polyolefins.
Depending on the construction of the apparatus suitable for carrying
out the method, the organic wastes can be processed continuously
or in batches. The use of a worm machine as a reaction chamber
is especially suitable, because all of the reaction phases can
be carried out in it, and during the process it can produce a shear-
ing force and pressure by itself, so that separate devices for
transporting or for further processing are not necessary. Hereby,
it is suitable to control during processing at least the hydrogen
content in the liquid and/or gaseous constituents and to influence
said content in the organic wastes in a desirable way by suitable
dosing. Hereby, the composition of the liquid or gaseous constituents
that are being produced, can be purposefully controlled. Prior
to the further processing or utilization o-f-these products, mechanical
pollutants are suitably removed from the molten mass.
.
'
.. . . . .
.~
2~83~
1 The components produced by the break down of the macromolecules
have a consistency, such that they can be transferred into or in-
jected into,without problems by conventional devices, conventional
containers, combustion chambers or reactors and the like. This
can also be advantageous to replace, at least partially, the ma-
terials used in conventional systems until now, for example, to
produce synthesis gas or other useable gases or also as a reduction
agent. According to the invention, it is also possible that syn-
thetic material wastes consisting predominantly of PVC can be
directly transformed into granular material using the method des-
cribed above, and can thus be further utilized for metallurgic
purposes. In this context, the production of titanium tetra-
chloride should be mentioned, for ex~mple. Normally, TiO2 or rutile
is mixed with coke and tar, gasified and calcined. Then it is
chlorinated at 800C, for example, in a shaft furnace or fluidized
bed. However at least a portion of the co~e and tar can be replaced,
for example, by the hydrocarbons from the PVC wastes and the chlori-
nation can be carried out as usual at an increased temperature,
with the complete or partial elimination of the usual chlorine
gas. The PVC-fraction that arises from the separation can be
shredded or formed into granules or a powder, and then mixed with
metal containing and/or metal compounds containing materials as
a~ at least partial replacement of carbon and chlorine containing
materials, and can then be converted in conventional metallurgic
processes.
The feed material to be processed should be made up of substantially
clean synthétic materials, whereby these materials are suitably
separated substantially from all contained foreign matter and then
coarsely comminuted. Herein, it is advantageous if the comminution
is to less than lOOcm2.
.
.:
2a83~
1 In the separation, it is especially advantageous if the fractions
are separated into non-synthetic material and synthetic material
with a fraction having a specific weight over approximately 1 g/cm3
and a fraction with a specific weight below approximately 1 g/cm3.
In this manner, on the one hand, non-synthetic materials are separated
from the synthetic materials, and through the separation of synthetic
materials by specific weight, it is possible to make a further
separation of the PVC and other synthetic materials. Fractions
separated in such a way can then be introduced advantageously to
separate processes which are suited to the special characteristics
of the separated synthetic materials. Particularly, the fraction
with a specific weight under 1 g/cm3 has the special advantage,
that it can easily be separated from foam material and then consists
only of unfilled synthetic materials of polyolefin polymers, which
have almost no toxic components, so that they can be converted
into a gas or oil rich in hydrogen which can be used as reduction
agent.
The processing of the synthetic materials described so far, makes
it possible to have the chlorine content andtor hydroc~rbon content
of the wastes available again for use, which takes place advanta-
geously in that the contents mentioned are used in metallurgic
reactions.
Thus, it i~ suggested on the one hand to break down said wastes
again, and it is further suggested that this break down does not
necessarily take place with a view to obtain raw materials for
the production of synthetic material, rather to provide raw materials
which can be utilized in other processes, for example, in metallurgic
reactions, to produce energy, among others.
,
:
2~3G~
1 It is also possible to heat a mixture of PVC wastes, metal- and/or
metal compound containing materials and other desired additional
materials, whereby the PVC wastes become plastified so that pellets
or the like are then produced from the entire mixture for later
utilization.
It is also possible to bring the PVC fraction in liquified or powder
form by means of a nozzle lance into a liquid metal bath and then
to use it there for the removal of contaminants. The PVC fraction
is advantageously brought into a reactor under increased tempèrature
for reaction with metal or metal compound containing materials.
Hereby, the PVC fraction can also be gasified first. It is also
possible to bring the gasified PVC fraction into a liquid metal
bath with the help of a nozzle lance.
Surprisingly, it has now been discovered, that it is also possible
to dehalogenize larger portions of PVC in the synthetic material
waste mixtures, in that the halogens are treated for a duration
of 10 seconds to 10 minutes at a temperature of 150~C to 350C,
in order to split off the chlorine or other halogens, possibly
together with hydrogen. These split off halogens can be regained
through condensation or by washing the gases that are produced
during this heat treatment. They can also be brought directly
into reactions with other materials. The toxic gases that are
split off during the heat treatment, so far as any are produced,
can be vacuumed off and supplied to a wet wash for separation.
In such a wet wash an aqueous solution of alkaline and/or earth
alkaline compounds are preferably used.
-- 10 -- -
r 2a~3~
1 It is also especially advantageous that the liquified and/or gasified
constituents can be supplied to an incineration, whereby the heat
produced by the incineration can be given off into the surroundings
or it can be utilized in other devices.
A further possibility according to the invention, for utilizing
the organic wastes lies therein, to burn a portion of the wastes
with oxygen, to liquify or gasify the other portion and to inject
the liquified and/or gasified constituents of this other portion
into the smoke gas stream produced by burning the first portion
and thereby to form a gas suitable for synthesis or a synthesis
gas itself. Especially that portion should be incinerated with
oxygen, which contains toxic materials or is not of the polyolefin
type. Herein, the oxygen content of the incineration medium should
be greater than 50%.
Fillers and fuels gases can also be worked in.
It is also possible to transform the gas mixture produced by the
injection, through the choice of a suitable catalyst and pressure
and temperature, into synthesis gas, fuel gas, reduction gas, or
other useful gases. This can take place advantageously, in that
the gas mixture produced by the injection is brought into existing
systems for producing such gases as a complete or partial replace-
ment for the normally used materials or also in addition to these
materials.
A system with one or more worm machines, in which the reaction
chambers are formed by the worm machines themselves, is especially
suited for carrying out the method of the invention. These worm
.
~.
. . .
2~83~
1 machines are constructed to be heatable, having openings for gas
removal and injection, and having a device connected to the worm
machine for further processing of at least a portion of the product
produced by the worm machine. Naturally, several worm machines
of the same or different construction can be connected to one another
and/or utilized.
Advantageously, a sorting apparatus and a comminuting apparatus
and a filling or packing-in apparatus are arranged upstream of
the worm machine or worm machines. The comminuting apparatus
can reduce the size of the waste pieces to a granular size suitable
for extruding. The sorting apparatus can be arranged upstream
of the comminuting apparatus or else between the comminuting ap-
paratus and the worm machine, depending on its purpose.
.
An incinerator can also be included in the system for urther proces-
sing the liquified and/or gasified wastes. ~y a suitable incinera-
tion, the wastes can be disposed of in an environmentally safe
way and the energy gained can be utilized for further processes.
The environmentally safe disposal lies especially therein, that
with the help of the smoke or flue gases and other constituents
brought into the smoke gases, further gases of a desired composition
are produced by the incineration. In this manner, the gases pro- -
duced by the incineration do not enter the environment, but are
used to produce useable gases. As far as excess heat is produced
during this incineration, it makes sense to use this heat in a
thermal prime mover, which advantageously is part of the system
described above for further processing. Additionally, or alterna-
tive}y, this excess heat can be regained by a heat exchanger for
further use. This heat exchanger is then also advantageously a
component part of the system for further-processing.
1 _
.,
..
,. , - - ,, ~ ~ ,
20~3~
1 In order that the possible remainder gases from the incineration
do not produce an environmental hazard, it is ~urther an advantage
if this system for further processing also includes a suitable gas
cleaning apparatus.
Due to the various processing possibilities, it is further especially
advantageous if the system for the further processing comprises
a mixing apparatus for admixing to the melt, organic materials
such as wood, straw, textiles, or paper. In order to produce spec-'
ific gases for a desired further use or for neutralizing, it is
useful, if the apparatus ~ the invention comprises a separator
for separating the desLred gases from the gas mixture.
In order to work with a substantial automated process, it is sug-
,gested according to the invention, that the plant comprises an
input feeder and sensors for ascertaining desired status data of
the final and intermediate products, and that furthermore, open
loop and closed loop controls are provided for the input feeder
which control,the input feeder in an open or closed loop in response
to the results measured by the sensors. In this way it is possible
to ,monitor the composltion of the melt and/or of the produced gases,
and the composition can be influenced by a respective admixing
of suitable ~synthetic material wastes.
In the following reference will be made to several example possi-
bilities that are opened up by the method of the invention, whereby
the organic polymers are first decomposed to form low molecular
hydrocarbons, which still can be polymers. These can then, for
example at very high temperatures, be converted to a synthesis
gas which is useful for recovering any hydrocarbons. This approach
- 13 -
.
- . . : -
20836~
1 is especially advantageous if the waste materials to be processed
contain toxic ingredients such as oils containing PCBs, dioxin,
and furanes, different aromatic compounds and so forth. Such hydro-
carbon compounds are very stable and decompose only at temperatures
well above 1200C. Further, some carbon companions are considered
to be directly responsible for the formation of highly toxic dioxin,
if the combustion or processing temperatures remain too low. As
far as a combustion P12 is involved here, the temperatures in the
reactor should be 1200C and higher.
The synthesis gases that are produced are the direct basis for
the production of hydrocarbon compounds. Conventionally, these
are produced almost exclusively of natural gas and crude oil. They
are, however, also produced of coal or other hydrocarbon compounds.
Thus, heretofore natural gas, crude oil, and coal have been used
to produce synthesi~ gas. According to the method of the invention,
however, synthetic material wastes are used in place of the con-
ventionally used raw materials. For this purpose, clean synthetic
material wastes, for example from synthetic material production,
or prepared wastes from other sources can be used. However, the
use of contaminated synthetic material wastes is also possible.
-; In this way, for example, the light fraction which arises when
shredding automobiles, the so-called shredder wastes can be used,
whereby it is naturally useful to first screen out the nonpolymer
components, mostly minerals, such as glass shards and stone. A
further difference to known processes lies therein, that the material
fed into the process is melted down by a simple warming without
the exclusion of air, whereby the macromolecules of the synthetic
materials can be broken down and shortened by processing with reactive
gases, prefera~ly with oxygen, but also with hydrogen and water
,
- 14 -
.
. .. .
:. . ,.: . ..
. . .. - , :
; . -
~.
, .. . .
(- 2a~3~
1 vapor, either alone or in combination, simultaneously with or after
melting, and thereby, under suitable pressure and temperature,
be liquified to a point so that they can be injected by conventional
nozzles or burners or the like into the reactors to produce the
desired synthesis gases or gases for other applications. To achieve
this, one may proceed as follows:
The synthetic material parts which come, for example, from a shredder
for used cars, are comminuted to about the size of a palm and are
processed in accordance with the extent to which they are intermixed
with non-thermoplastic synthetic material components, in systems
with higher shearing forces and simultaneously higher oxygen content.
The prepared wastes are melted down.
For this purpose, a worm extruder is used (see Fig. 2), such as
a worm kneader rotating in the same direction. Thereby, the sup-
plied synthetic materials shreds can be brought together very early
that is, already in the feeding funnel, with the reactive materials
that start the intended break down of the polymer molecules,for
this the funnel is flooded with this gas or gas mixture. To make
the feeding of the synthetic material shreds easier, filling devices
or stuffers can be arranged.
The worms which may be provided with one or more kneading, shearing,
and stowing steps, have the task of continually advancing the feed-in
materials; of melting and breaking down under the supportive action
of the shearing force, with the help of which the molecule chains
are mechanically ripped apart, and the possibly added reactive
agents; of building up a pressure great enough for the injection,
in combination with the mixing-in of the reactive materials and,
if applicable, the pulverizing propellant.
:: . ............... .
: :, . .
- ~
..
2~83~
1 The fed-in polymer material is heated in the worm to approximately
400C or even higher. Under the influence of high temperatures,
shearing forces, oxygen, hydrogen, air, water, and possibly other
additives, the molecules are broken down and neutralized, so that
a thin liquid molten mass is formed due to the now short molecules.
In the last third of the worm gear, the molten mass that has been
prepared in this manner, flows into a decompression stage provided
on the worm gear and formed by suitable worm gear elements, into
which the heated reactive gases are injected. In the last zone
of the worm gear, which is formed as a mixing zone, these reactive
materials are evenly distributed into the molten mass and the mixture
is condensed at a pressure between 20 and 100 bar. A cascade-like
arrangement of extruders or pumps around a mixing chamber is, however,
also a possibility.
The molten mass can then be brought into the reaction chamber through
an apertured plate or through a rotating disk or another suitable
device, and can then be dispersed into fine droplets through a
nozzle. The molten mass can be divided into thin strands especially
through an apertured plate, so that the high pressure gases in
the molten mass disperse the mass into fine droplets as soon as
they enter the low pressure reactor. Other known nozzle~ or burners
can, however, also be used to inject the liquified synthetic material
into the reactor.
The injected molten droplets should partially be combusted, so
that the necessary high temperatures of at least about 1200C are
obtained. In this way a portion of the injected polymer molten
mass is used as combustion fuel, the rest is transformed into the
~ desired synthesis gas.
,.
`' ` . ' ~
2~36~
1 Besides the especially high temperature in the reactor, which serves
to destroy all dangerous hydrocarbon compounds, it is of special
importance that the polymer molecules are substantially broken
down during the treatment in the extruder by the addition of oxygen,
hydrogen or water vapor, said materials being added also in combi-
nation, so that the molten mass, even before entering the mixing
zone at the end of the worm, has a viscosity as low as possible,
so that the mixing-in of the reactive materials is simplified,
and so that said mass can be easily ripped into simple droplets
upon exiting into the low pressure chamber provided by the reactor.
The early contact with oxygen and possibly with hydrogen, has the
further important task of preventing a recombination of the polymer
fractions and radicals that were formed by the saturization of
the radicals and the free valences of the molecular fractions by
the atoms of the reactive gases.
It can be advantageous for the continuously operating system, with
reference to a uniform composition of the synthesis gases, to con-
tinuously control the molten mass with regard to the relationship
of carbon to hydrogen, that is the hydrogen content, and to auto-
matically conform this content to the synthetic material supply.The sensor that supplies these data is advantageously mounted in
a by-pass and comprises, for example, an infrared detector that
detects the critical molecules. This control simultaneously provides
the possibility that the addition of a determined amount of a chemical
or other components to absorb undesired constituents can also be
regulated by a suitable regulator.
If the synthetic material wastes to be processed contain mechanical
contaminants, then the extruder is equipped with an apparatus, such
- 17 -
83~0~
1 as a conventionally known molten mass filter, in order to separate
the liquified synthetic material from the solid materials. Further
processing of the synthetlc material that has been comminuted,
liquified, and injected into the reactor follows now in a known
manner. Fig. 2 is a schematic drawing of the arrangement of a ~"
suitable system and the steps of the process. Fig. 2 is self-
explanatory and and needs to additional comment.
Process Example Embodiment
The experiment described below shows one possible sequence of
the process.
The light fraction of the shredder wastes which have been coarsely
cleaned of non-organic wastes through a sieve, originate from
an automobile shredder system from the processing of, as already
stated, 106 passenger automobiles.
The following Table 1 gives information about the origins or the
shredder wastes.
T e Number Percent %
YP
VW/Audi 33 31
Opel 23 22
Daimler 9enz 11 10
Ford 9 8.5
Renault/Peugeot/Citroen 15 14
Fiat 9 8
BMW 3 3
Mazda/Honda 2 2
~ord USA 1
--- lD6 100
- lB -
2a~3~
1 Table 1
The polymer wastes were obtained by winnowing behind the hammer
mill. They comprise various synthetic materials (compare Table 2).
Weight Percent of
Material ~ame Abbreviation the Auto Body %
Polyurethane PUR 42
Polypropylene PP 24.5
Polyethylene PE 11.3
Acrylonitrile/Butadiene-
Styrene-Copolymer ABS 11.0
Polyvinyl chloride PVC 6.0
10 Non-neutralized polyester UP-GV 2.5
Polyphenylene oxide (modif.) PPO 2.1
Polyamide PA 0.4
Polyacetal POM 0.3
100 C
Table 2: Weight percent of the synthetic materials in the autobody
type PORSCHE 928 is without elastomers, textiles and paints.
Most of the polymer wastes are chip-like fragments of synthetic
material components. Since the diameter of these fragments was
too large for the experimental worm gear machine used in this
experiment, they were additionally reduced to fragmentary granules
of less than 3 mm in diameter by a granulator.
The extruder - a Werner and Pfleiderer ZSK - double worm gear
laboratory ~neader ZSX 30 with a worm gear diameter of 30 mm -
was equipped with a funnel from which the fragments were pu}led
into the worm gear in a free-flowing manner. Manual help was
not necessary. The funnel was flooded with oxygen.
,
:
-- 19 --
~,
-
2~83~
1 The extruder was equipped with plug-in type worms having a length
36 x D. These were provided with conveying elements on the first
10 x D of their length. This conveying zone was closed by two
shearing elements with a length of 2 x D, followed by two further
conveying-kneading zones, each zone having a length of 2 x D for
the conveying element and 1 x D for the kneading element. Down-
stream of the two zones is a radial choke with a length of 1 x D.
Conveying elements 5 x D having a double gear pitch are arranged
behind the radial choke. Since in this zone a low pressure pre-
vails, the injection of oxygen and water vapor took place here.
The remaining 11 x D of the worm gear was provided with conveying
elements with a simple gear pitch and with mixing elements, in
order to be able to build up the necessary pressure and to dis-
tribute the reactive gases throughout the molten mass. The cylinder
of the worm gear was closed off with a perforated plate provided
with bore holes 2 mm in diameter. A normal sieve plate with three
fine sieve layers was superimposed, preferably to cause a pressure
build-up in the molten mass. The perforated plate fed into the
' reactor chamber.
,1
The extruder cylinder was heated to have a rising temperature
` in the first half and then heated to 420C in the second half.
The throughput or mass flow was up to 60 kg/h at 500 r.p.m. Oxygen
in a quantity of up to 50 kg/h preheated to 250C and hot steam
were pressed into the molten mass at 20 x D in the decompression
.
zone upstream of the mixing zone. The extruder was flanged onto
a combustion chamber set to atmospheric pressure and serving as
a reactor, so that the perforated plate extended into the reaction
chamber. The reactor could be heated by electrical heating bands
:
- 20 - - -
~ .
2~3~
1 for starting combustion. In addition, a gas burner was connected,
which ignited the gas synthetic material droplet mixture entering
from the extruder.
In order to measure the exhaust gases, these were caught in bags
and analyzed by conventional methods. Depending on the tempera-
ture of the reaction chamber, a gas mixture comprising low molecular
hydrocarbons, CO, hydrogen and up to 10% carbon-dioxide was produced.
The gas composition could be varied substantially by varying the
amounts of oxygen and water vapor fedinto the mixing chamber.
During injection of these gases, their temperature was held con-
stant at 250C.
In case the gasification of certain synthetic materials was not
yet sufficient downstream of the extruder, the still liquid synthe-
tic materials were not injected directly into the combustion cham-
ber or reactor. Rather, these liquids were injected into a super-
imposed upstream arranged gasifying chamber which is heated and
in which the gasification takes place.
Not only synthesis gas or fuel gas can be produced of the liquified
synthetic materials, as shown thus far. Rather, also reduction
gas can be produced which is suitable, for example, for the pro-
duction of spongy iron.
It makes sense in all these utilizations, to use the synthetic
materials that have been liquified or gasified in the manner des-
cribed, not only in systems that have been built exclusively for
these materials. Rather, they can be utilized in already existing
- 21 -
2083B~
1 systems to partially or completely replace the reactive materials
usually used therein. They can also be utilized in addition to
these conventional materials.
The liquified synthetic materials can also, as mentioned, be formed
into granules or into powder by spraying. Such granules or powder
are raw materials which are easily dosed for producing synthesis
gas and/or other useable hydrocarbons and gases. They can also
be used as combustibles or fuel and as a reducing agent. EspeciaIly
for this purpose the characteristic of the synthetia material
in question is important, namely to be able to gasiy practically
without residue at an increased temperature. With a granule size
of 2mm in diameter, gasification occurs in less than 0.5 second
at 1000C.
If the synthetic material produced contains a PVC fraction or
is made up entirely Gf PVC, special difficulties arise in the
conventional technology. PVC is considered to be one of the least
"friendly-to-recycling" synthetic materials. In the conventional
methods of waste removal of PVC, the chlorine in the PVC and other
~toxic gaseous components that are produced, cause special diffi-
culties. These difficulties are even more problematic, sincePVC is an unusually widespread mass-produced synthetic r rial,
which can hardly be done without, as a result of which it occurs
in all synthetic waste material mixtures. In addition, severe
corrosion damage due to hydrochloric acid used to occur in the
combustion plants, mainly in the waste-heat boilers.
The method according to the invention provides a possibility for
the removal of chlorine containing PVC wastes, whereby our experiments
-
2083~
1 have shown that the content of remaining chlorine was less than
0.3~ in the produced waxes or oils. Such oils or waxes would
be officially approved as a heating oil.
It is known, that PVC begins to give off chlorine and hydrogen
at relatively low temperatures of about 100C, so that a hydro-
chloric acid is produced in its status nascendi. Surprisingly,
it has been found that, in spite of the so-called stabilization
of the PVC above 200C, and especially 250C, a very rapid and
complete splitting off of the chlorine takes place. This splitting
off is practically completed within normal dwell times of a few
minutes at 300C. Further, it appears that when this heating
procedure occurs under substantially air-free conditions, only
this splitting off occurs on the polymers, so that the gases pro-
duced are made up almost exclusively of hydrochloric acid. In
addition, above 200C the splitting off increases in speed, so
that it becomes unnecessary to separate the PVC wastes. Dienes
remain as remnants that can be completely decomposed and broken
down at higher temperatures.
,
To utilize wastes with a PVC fraction or pure PVC wastes, one
; 20 can however also proceed as follows; PVC has a relatively large
specific weight for a synthetic material, and it can therefore
be easily separated, for example, by floatation, from other syn-
thetic material wastes, so that a waste material exclusively com-
prising PVC is available for further utilization. Next, if it
is needed, the product can be cleaned of possibly clinging dirt
by washing. A comminution must only be undertaken, insofar as
lt is necessary for the preparation for the process. The prepared
PVC waste can now be further utilized in the following manner
in the reactions to be carrled out:
' :, . :.'.
- ~
. , , - - .
.~
2o836~l
1 ~1. The PVC waste is ground down cold, or is otherwise substantially
comminuted, so that a granule like product is produced. This
granule like product can now be mixed alone or in combination
with otherwise customary additives, with metal and/or metal com-
pound containing materials, and then brought to reaction in the
course of the usual processes. The chlorine that is liberated
by the break down at increasing temperatures reacts in the same
way as the chlorine in other chlorine containing materials or
like gaseous C12. Here hydrocarbons of the PVC simultaneously
serve as a reducing agent so that these hydrocarbons can either
completely or partially replace the carbon which is normally added.
The addition of the granule like product is carried out by simple
admixing or the mixture of PVC and metal and/or metal compound
containing materials is heated, so that pellets or the like can
be produced by the early softening of the PVC. As an example
of the above mentioned processing, the production of titanium
tetrachloride which was described above could be mentioned.
2. In order to refine liquid metals, PVC wastes, for example,
in the form of shreds or as a granule like product are pressed
into the liquid metal with the help of a suitable means, whereby
certain metals are preferably bound by the freed chlorine and
they can then be removed as salts from the molten metal. The
removal of tin from molten lead is given as an example here.
. ,
3. PVC wastes can also be gasified in a suitable apparatus, such
as a rotary kiln, without first being comminuted. The gas that
is produced is led into a reaction chamber, where it is brought
into a reaction with the metal or metal compound containing materials.
24 -
.
,. . . .
, '., ~ ,
2083~
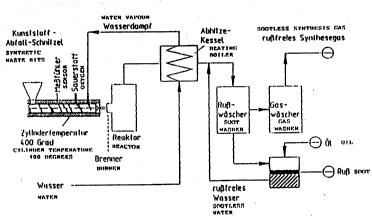
1 As Fig. 1 shows, th~ method of the present invention is also excel-
lently suited for performance in very small systems. It is often
the case that the yield of such organic wastes, preferably synthetic
material wastes, is small or that its transportation to large
central systems is uneconomical. With the method of the present
invention, however, even small amounts of organic wastes, especially
clean, mixed synthetic material wastes, can be sensibly and economi-
cally utilized, whereby the wastes are comminuted to a granule
size of less than 100 mm, preferably less than 50mm. Then, the
wastes can be separated according to a conventional gravitational
separation process into fractions of varying specific weights.
~ Depending on the composition of the wastes, several different
; fractions can be gained, for example:
a) metal, glass, stones;
b) PVC, PTFE, filled synthetic materials, as well as further
synthetic materials with a specific weight of over 1 g/cm3;
c) unfilled polyolefins.
t~ .
In this presentation, the specific weight of the metals decreases
toward the polyolefins. Separation of the halogen containing
synthetic materials, such as PVC, from the other synthetic materials
can be advantageous if a problem-free, direct burning in a combustion
system or a thermal power plant, such as a combustion engine or
a gas turbine, is desired.
The organic fractions are supplied to one or more extruders, which
; can be arranged in parallel to one another or in series, whereby
insofar as the synthetic materials contain halogens, most commonly
chlorine such as is the case in PVC, these can be split off at
a rel~tively low temperatuFe of 300C in a short time duration
, ~
''
` 2~3~
1 in one of the first stages of the extrusion system. The synthetic
material wastes are then heated to higher temperatures of over
350C, whereby they are broken down into low molecular molecules
and liquified or gasified under the influence of heat, of a possible
shearing action, and the possible addition of air or water, or
water vapor, and possible metal oxides that are mixed or injected,
or under the influence of other catalytic materials that would
accelerate the break down of the synthetic material molecules.
It is also possible, to filter the molten mass by conventional
means in the extruder or extruders, in order to separate out the
fine-grained mineral component or non-molten components.
Depending on the composition and consistency of the molecular
fragments that finally emerge from the extruder or extruders,
the fragments can now be incinerated, or if they are clean enough,
they can be supplied to a thermal power plant, such as a combustion
engine or a gas turbine. Insofar as they solidify at room temper-
ature, they can be transported away or used directly in granulated
form or foamed up in a comminuted form, alone or mixed with other
materials, such as wood or straw, in loose granular or powder
form or pressed into briquettes or another form.
.
If the products contain toxins which are freed during incineration,
then a gas purification is provided. Preferably, a gas wash which
works with alkaline and/or alkaline-earth compounds would be used
for this purpose. If only alkaline-earth compounds are available,
then these would be added directly to the hot combustion gases,
or immediately prior to the splitting off of CO2. Thereby, the
system, especially the waste-heat boiler which might be present,
.
- 26 -
.
`
,
2~83~1
1 is protected against corrosion. The halogens that were possibly
split off during the first extruder phase are suctioned off and
are also added to the smoke gas purification to be neutralized.
If the waste waters containing the alkalis and earth-alkalis can
be added to water without a problem, for example to brackish water
or to the ocean, there is no need to further process the wash
water.
Fig. 1 and Fig. 4 each show a method flow-chart from which the
above described method becomes clear. The method flow-chart is
self-explanatory and needs no further description.
Fig. 3 shows an alternative construction of the extruder station
with reference to the system shown in Fig. 2. Fig. 3 is also
self-explanatory, so that here too there is no need for further
explanation. The temperatures indicated on the extruders are
temperature limits. The preferred temperatures can be seen in
Fig. 4.
- 27 -
,
' "