Note: Descriptions are shown in the official language in which they were submitted.
- 2 - 2 0 ~ ~43 ~3
Merck Patent Gesell~chaft
mit beschrankter Haftung
6100- D a r m ~ t a d t
Benzofuran~
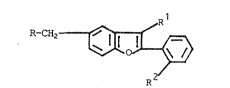
S The invention relates to novel benzofuran~ of
formula I:
R-CH2
wherein
R in the radical R3~
10 R1 is ~, ~al, COO~, COM~2, C~O, CN, NH2 or tetra-
zol-S-yl,
R2 is ~, CooR3, CN~ NO2~ NH2~ NHCOCF3~ NHSO2CF3
or tetrazol-5-yl,
R3 i ~ or alkyl, alkenyl or alkynyl eac~ having
up to 6 C atom~,
R~ , alkyl having 1-6 C atom~ or cyanoal~yl,
R300C-alkyl, tetrazol-5-ylalkyl orAr alkyl each
having 1-6 C atom~ in the Naikyl~ ty,
~A-B-C~D- i~ one of the groups -C~sC~-C~ , -C~=CH-
N-C~ C~sM-CH=CH-, -N~C~-C~=C~ C~=C~-CO-
NR~-, -C~QC~-NR~--CO-, -CO-NR~-C~=C~- or -~R4-
CO-C~-C~-, wher~in the H atom~ of the C~
group~ can be ~ub~tituted by alkyl having 1-6
C atoms 9 ~al, CooR3, CN and/or tetrazol-5-yl,
208~736
3 - 26474-263
~r i3 phenyl which i~ un~ubstituted or mono-
~ub~tituted or di~ub~tituted by ~al, R3, CF3,
- CooR3, CN~ oR3, N02, NH2, NHCOCF3, NHS02CF3
or tetrazol-5-yl, and
5 ~al i~ F, Cl, ~r or I,
and their ~alts.
SLmilar co~pounds are known from European patent
application A2-0 430 70g, but they contain a benzothio-
phene ring in place of the benzofuran ring and a sub-
stituted or unsubstituted Lmidazolyl group in place ofthe radical R.
The object of the invention was to find novel
compounds with valuable properties, e~pecially compounds
which can be used for ~he preparation of dru~.
It has been found that the compounds of formula
I and their salts possess very valuable pharmacological
properties coupled with a good tolerance. In particu-
lar, they exhibit antagoni~tic propertie~ towards
angiotensin II and can therefore be u~ed for the
treatment of angiotensin II-dep,endent hypertension,
aldo~teronism and cardiac in~uff:iciency, as well as
disorders of the central nervous s~y~tem. ~he~e effPcts
can be determined by conventional in vitro or in vivo
methods such as those de~crib~d for example in US patent
4 880 804 and in international patent application
91/14367, a8 w~ll a~ those de~cribed by A.T. Chiu et al.,
J~ Pharmacol. Exp~ Therap~ 250, 867-874 tl989), and by
POC. Wong et al~, ibid. 252t 719-725 (1990; in vivo, on
rat~).
The compound~ of formula ~ can b~ u~d a~ phar-
maceutical active ingredients in human and veterinary
med.ici~e, e~pecially for the prophylaxi~ and/or therapy
of cardiac, circulatory and vascular di~ea~es and in
particular of hypertonia, cardiac insufficiency and
208~73~
- 3a -
hyperaldosteronism, furthermore of hypertrophy and hyperplasy of the
blood vessels and the heart, angina p~ctoris, cardiac infarction,
haemorrhagjc stroke, restinosis after angioplasty or by-pass surgery,
arteriosclerosis, ocular hypertension, glaucoma, macular degeneration,
hyperuricaemia, disturbances of the renal functions such as renal
failure/diabetic complications such as nephropathia diabetica or
retinopathia diabetica, psoriasis, angiotensinII- induced disturDances
in female sexual organs, cognitive disorders, f.e. dementia~amnesia,
disturbances of the function of memory, states of fear, depr~ssions
and/or epilepsy.
The invention relates to the compounds o~ formula
I and their salt~ and to a process for the preparation
of the~e compounds and their salts, characteri~ed in that
736
-- 4 --
a compound of formula II:
E-CH~ II
wherein
E i~ Cl, Br, I9 a free OH group or an OH group which
has been functionally modified to acquire reacti-
vity, and
R~ and R2 are as defined in ClaLm 1,
is reacted with a compound of ~ormula III:
H-R III
wherein
R iQ as def ined in Claim 1,
or in that a compound o~ formula I is free~ from one of
its functional derivative~ by treatment with a solvo-
ly~ing or hydrogenoly~ing agent,
and/ox i~ that one or more radical~ R1, R2 and/or R in a
compound of for~ula I are converted to one or more other
radicals R1, R2 and/or R, and/or a ba~e or ~cid of ~ormula
I i~ coh~rted to o~e of it~ salts.
Abo~e and below, the radicals or parameters R, Rl
to R~ -A-B-C-D-, Ar, ~l and E are as defined in formulae
I and II unl~s exprss~ly indicated otherwi~e.
1~ the above formulae, ~alkyl~ has 1-6, pre-
ferably 1, 2, 3 or 4 C atoms. ~lkyln i~ preferably
methyl, nr el~a ethyl, propyl, i~opropyl, butyl~ iso-
b~tyl, sec-butyl or tert-butyl, or elsa pentyl, 1-, 2- or
3-methylbutyl~ 1, 20 or 292~dLmethylpropyl, 1-
ethylpropyl, hexyl, 1~, 2-, 3- or 4-methylpentyl, 1,l-o
1,2-, 1,3-, 2,2-, 2,3- or 3,3-dLmethylbutyl, 1- or 2-
ethylbutyl, 1-ethyl-1-methylpropyl, 1-ethyl-2-methyl-
propyl or 1,1,2- or 1,2,2-trlmethylpropyl. Alke~yl i~
7 3 ~
preferably vinyll prop-1-enyl, prop-2-enyl or but-l
enyl, or el~e pent-1-enyl or hex-l~enyl~ Alkynyl i
preferably ethynyl, prop-1-ynyl or prop-2-ynyl, or el~e
but-1-ynyl, pent-1-ynyl or hex-1-ynyl.
Hal is preferably F, Cl or Br, or else I.
R i3 a radical derived from lH- or 3~-imidazo-
[4,5-b]pyridine or a radical derived from 1~- or 3H
imidazo[4,5-c]pyridine or, more precisely:
(a) 2-R3-3~-imidazo[4,5-b]pyridin-3-yl (if -A-B-C-D- =
-C~-C~-C~=N-),
(b) 2-R3-3~-imidazo[4~5-c]pyridin-3-yl (if -A-B-C-D- =
-C~-C~ N-CH ),
(c) 2-R3-l~-imidazo[4,5 c]pyridin-1-yl (if -A-B-C-D- =
-C~=N-CH=C~-),
lS (d) 2-R3~ imidazo[4,5-b]pyridin-l-yl (if -A-B-C-D- =
-~=CH-CH=C~-),
(e) 2-R3-4-~4-4,5-dihydro-5-oxo-~-imidazo[4,5-b~pyri-
din-3-yl (if -A-B~C-D- = -C~=CH-Co-NR4-),
(f) 2-R3-5-R4-4~5odihydro-4-oxo-3~-imidazo[4,5-c]pyri-
din-3-yl (if -A~B-C-D- = -C~-:CH-NR4-Co~
(g) 2 -R3- 5 -R4-4,5-dihydro-4-oxo-].~-imidazo[4,5-c]pyri-
din-3-yl (if -A-B-C-D- - -CO-NR~-C~=C~
(h) ~-R3-4-R4-4,5-dihydro-S-oxo-l~-Lmidazo[4,5-b]pyri-
din-3-yl (if -A-B-C-D- - -NR4-co-~
In the radical~ A-~-C-~- here, the ~ atoms
located on the C atomh can be ~ub~tituted by alkyl
(preferably met~yl), ~al (preferably F or Cl), CoOR3
(preferably COO~, COOc~3 or COOC2~5), CN and/or t~tra-
zol-S-yl. Preferred sub~titue~ts are C~3 and CO~.
Preferably only o~ or two of the~e ~ atoms are sub-
~tituted by one of the ~ubstitu~nt~ indicated~
Accordingly, the co~pounds of formula I i~clude
tho~e of formulae Ia to Ih~ wherein R i~ a~ defined in
each case under (a) to ~h). The compound~ of f ormulae Ia
and If are preferred.
The radical Rl i8 prefera~ly ~ or Br.
The radical R2 i~ preferably CN, or el~e pre-
ferably tetrazol-5-yl, COO~, COOCH3, COOC2~5 or N~SO2CF3.
20~73~
-- 6
The radical R3 (if it i~ not H) i8 preferably
linear and i8 preferably alkyl or alkenyl each having
2-6 C atoms, especially butyl, or el~e ethyl or propyl,
or else pentyl, hexyl, allyl or pxop-l-enyl, or else
but-1-enyl, pent-l-enyl, hex-1-enyl, ethynyl, prop-l-
ynyl, but-l-ynyl, pent-1-ynyl or hex-1-ynyl.
The radical R4 i8 preferably ~, or else preferably
alkyl (especially CH3), cyanoalkyl (e~pecially cyano-
methyl, 2~cyanoethyl, 3-cyanopropyl), AOOC-alkyl
~especially methoxycarbonylmethyl, ethoxycarbonyl methyl,
2-methoxycarbonylethyl, 2-ethoxycarbo~ylethyl), carboxy-
alkyl (especially carboxymethyl, 2-carboxyethyl,
3-carboxypropyl) or tetrazol-5-ylalkyl [especially
tetrazol-5-ylmethyl, 2-(tetrazol-5-yl)ethyl, 3-(tetra-
zol-5-yl)propyl], it being possible for all these
radicals preferably to contain a total of up t3 6 C atoms
in each case. Also, the radical R4 iS preferably Ar-alkyl
having 7-11 C atom~, especially benzyl, 1- or 2-phenyl~
ethyl, 1-, 2- or 3-phenylpropyl, 1-, 2-, 3- or 4-phenyl-
butyl, o-, m- or p-fluorobenzyl, (preferably) o-, m- or
p-chlorobenæyl, o-, m- or p-bromobenzyl, o-, m- or
p-methylbenzyl, o-, m- or p-tri~luoromethyl benzyl, o-,
m- or p-methoxycarbonylbenzyl, o-, m- or p-ethoxy-
carbonylbenzyl~ (preferably) o-~ m-- or p-cyanobenzyl, o-,
m or p-carboxybenzyl, o-, m- or p-nitrobenzyl, (prefer-
ably) o-, m- or p~aminobenzyl or (pr~ferably) o-, m- or
p-(tetrazol-5-yl)benzyl.
The compounds of formula I can pnsse~ one or
more chiral centres and ca~ therefore exist in different
form~ (optically active or optica~ly inactive)~ Formula
I include~ all the~e fo~ms.
Accordingly, the inventio~ relate~ especlally to
those compounds of forml?la I in which at lea~t one of
said radicals ha3 one of the preferred meanings indicated
above. Some preferred groups of compounds can be
expres~ed by the following partial formulae Ii, Ij, Iai
to Ihi and Iaj to Ihj, which correspond to formulae I and
Ia to Ih and wherein the radicals not described more
_ 7 _ 2-6~74~2~6
precisely are ac defined in formulae I and Ia to Ih~
compound~ of formulae Ii and Iai, Ibi, Ici, Idi, Iei,
Ifi, Igi and Ihi, which correspond to formula~ I and Ia
to Ih except that in addition R1 is ~ therein; and
S compound~ of formulae Ij and Iaj, Ibj, Icj, Idj, Iej,
If3, Igj, Ihj, Iij, Iaij, Ibij, Icij, Idij, Ieij, Ifij,
Igij and Ihij, which correspond to formulae I, Ia to Ii
and Iai to Ihi except that in addition R2 is CN or
(preferably) tetrazol-5-yl therein.
Another sel~cted group of preferred compounds has
formula I wherein Rl is ~, R2 is tetrazol-5-yl, R3 is
alkyl having 2-4 C atom~ and -A-B-C-D- is -C(C~3) e
C~-C~=N-,-C(CH3)=C~-C( CH3)=N-,-C~=C~-N(o-~OOC-C6H4-CH2)-
-CO-, ~CH=CH-NH-CO- or -CH=CH-N(CH2COOH)-CO-.
The compounds of fo~mula I and also the starting
mat~rial~ for th~ir preparation are moreover prepared by
methods known per qe, such a~ those de~cribed in the
litexature (for example in the ctandard works like
Houben-Weyl, Methoden der organischen Chemie (Methods of
~0 Organic Chemistry), Georg-Thieme-V~rlag, Stuttgart, but
e~pecially in European patent application A2-0 430 709
and US patent 4 880 804)~ under reaction condition~ which
are known and uitable for ~aid reactions~ it al~o being
pos~ible to make use of variant~ ~lown per ~e, which are
not mentioned in greater detail here.
If desired, the st~rting l~aterials can also be
formed i~ ~itu, so that they are not i~olated from the
reaction mixture but Lmm~diately reacted further to give
the compounds of f ormula I.
~ The compounds of for~ula I can preferably be
obtained by rea~ting compound~ of formula TI with com-
pound~ of formula IIIo
In the compound~ of formula II, ~ is preferably
Cl, Br, I or an 0~ group which ha~ bee~ functionally
modified to acguire reactivity, ~uch as alkyl~ulfonyloxy
having 1-6 C atoms ~preferably methyl~ulfonyloxy) or
arylaulfonyloxy having 6-10 C atoms ~preferably phenyl-
or p-tolyl-sulfonyloxy).
20~4~3~
-- 8 --
The reaction of II with III i8 conveniently
carried out by fir~t converting III to a salt by
treatment with a base, ~or ex~mple with an alkali metal
alcoholate such as CH30Na or pota~ium t~rt-~utylate in an
alcohol such as C~30~, or with an alkali metal hydride
gUCh a8 ~aH or an alkali metal alcoholate in dimethyl-
formamide (DMF), and then reacting said salt with II in
an inert solvent, for example an amide such as DMF or
dimethylacet~mide, or a 3ulfoxide such a~ dimethyl
sulfoxide (DMSO), conveniently at temperatures of between
-20 and 100, pref~rably of between 10 and 30. Other
suitable ba~es are alkali metal carbonate~ such as ~a2CO3
or ~2CO3, or alkali metal hydrogen carbonates such as
NaHCO3 or ~03.
Some of the starting materials, especially those
of formula III, are known. If they are not known, they
can be prepared by known method~ analogously to known
substances.
Compounds of formula II are novel. Compourlds of
formula II lE = Br~ can be obtained for example by
reaction of 5-methylsalicylaldehyde with an ~-R1-2-R2-
benzyl bromide of formula IV to glve an ~-Rl-2-R2-benzyl
ether of formula V:
R~ H3C CHO
Br~C~ ~ ~ CHR
R2 R2
IV V
2S cycli~ation with the eliml~ation of water ~for example
with ~/DMF) ~o give the corresponding 3-Rl-2-(2-R2-
phenyl)-5-methylbenzofuran ~formula II with ~ in place of
E) and bromination (for ~xample with N-bromo~uccinLmide~.
A compound of formula I can also be freed from
one of it~ functional derivative~ by treatment with a
solvolysing (for example hydrolysing) or hydrogenolysing
2~3~73~
agent.
Thu~ it i~ po~ible, usins one of the methods
indi~ated, to prepare a compound which has formula I but
in which a tetrazol-S-yl group is replaced with a
S tetrazol~S-yl group functionally modified in the 1-
position (protected by a protecting group). Example~ of
suitable protecting groups are: triphenylmethyl, which
can be cleaved with ~Cl or formic acid in an inert
solvent or solvent mixture, for example ether/methylene
chloride/methanol; 2-cyanoethyl, which can be cleaved
with NaO~ in water/~F; and p-nitrobenzyl, which can be
cleaved with H2/Raney nickel in ethanol (compare European
patent application A2-0 291 969~
It is also possible to convert one compound of
formula I to another compound of formula I by converting
one or more of the radicals R~, R2 and/or R to other
radicals Rl, R2 ~nd/or R, for example by reducing nitro
groups to amlno groups (for example by hydrogenation on
Raney nickel in an inert solvent such as methanol or
ethanol), and/or functionally modifying free amino and/or
hydroxyl groups, and/or freeing functionally modified
amlno and/or hydroxyl groups by solvolysis or hydro-
genoly~is, and/or replacing halogen atom~ with C~ groups
(for example by reaction with copper(I3 cyanide), and/or
2g hydrolysing nitrile group8 to COO~ groups, or co~verting
nitrile group~ to tetrazolyl group~ with hydrazoic acid
derivatives, for e~ample ~odium azide in N-methyl-
pyrrolidone ~r trimethyltin a~ide in toluene.
Thus, for e~ample, free amino group~ can be
acylated in conventional ~an~er wi~h an acid chloride or
anhydride, or free hydro~yl and/or N~ groups ca~ be
alkylated with an u~ub~titu~ed or ~ub~tituted alkyl or
Ar alkyl halide, conveniently in an inert ~olv~nt ~uch a~
methylene chloride or T~E', and/or in the pre~ence of a
35 base such a~ triethylamine or pyridine, at temperature~
of between -60 and +30. The conver~ion of a radical R
wherein R~ = ~ to another radical R wherein R4 i~ other
than H i8 Lmportant. Thi~ reaction i~ preferably carried
2~7~
-- 10 --
out in an acid amide ~uch a~ D~F, N methylpyrrolido~e,
1,3-dimethyl~2-oxohexahydropyrimi dine or h~xametbyl-
phosphorotriamide, an alcohol such a~ m thanol or
tert-butanol, an ether such a~ THF, or a halogenated
S hydrocarbon such a~ methylene chloride, or mixtures
thereof, a the solvent, and/or in the pressnce of an
alkali metal alcoholate such as sodium methylate or
potassium tert-butylate, an alkali metal hydride such as
odium or potassium hydride, an alkali metal carbonate
such a~ sodium or potassium carbonat0, an alkali metal
bicar~onate such as ~odium or pota~sium bicar~onate, or
a tertiary amine such a~ triethylamine or ethyldii~o
propylamine, at temperature~ of between about -30 and
200, preferably of between 20 and 60.
1~ If desired, a functionally modified amino and/or
hydroxyl group in a compound of formula I can be freed by
solvolysi~ or hydrogenolysi~ using conventional method~.
Thu~, for example, a compound of for~ula I containing an
N~COCF3 or CoOR3 group (wherein R3 i3 other than ~) can be
conv~rted to the corre~ponding compound of formula I
containing an N~2 or ~OOC group instead. Ester groups can
be saponified for example with NaO~ or KO~ in water,
watèr~T~F or water/dioxane, at temperatures of between 0
and 100.
The reaction of nitrile~ of formula T (R1 and/or
R2 - C~) with hydrazoic acid derivatives leads to
t~trazoles o~ formula I (R1 and/or ~2 = tetrazol-5-yl).
It iB preferable to U8~ trialkyltin azide~ such as
trLm~thyltin azide, in an inert ~olvent, for example a~
aro~atic hydr~carbo~ su~h a~ toluene, at temperatures of
between 20 and 150, preferably of b~tween 80 and 140,
or 30dium azide in ~-methylpyrrolidone at temperatures of
between abol1t 100 and 200.
A ba~e of formula I can be converted with an acid
to the corre~ponding acid addition ~alt. Po~ible acids
~or this reaction are especially tho~e which yield
physiologically acceptable salts. Thus it i~ possible to
use inor~anic acids, for example sulfuric acid, nitric
2~8~ 736
acid, hydrohalic acid3 such a~ hydrochloric acid or
hydrobromic acid, phosphoru~ acids such as ortho-
phosphoric acid, and sulfamic acid, a~ well as organic
acids, especially aliphatic, alicyclic, araliphatic,
aromatic or heterocyclic monoba6ic or polyba~ic
carboxylic, Rulfonic or sulfuric acids, for example
formi c acid, acetic acid, propionic acid, pivalic acid,
diethylacetic acid, malonic acid, succinic acid, pimelic
acid, fumaric acid, maleic acid, lactic asid, tartaric
acid, malic acid, citric acid, gluconic acid, ascorbic
acid~ nicot~nic acid, i~onicotinic aeid, methane- or
ethane-sulfonic acid, ethanedisulfonic acid,
2-hydroxyethanesulfonic acid, benzenesulfonic acid,
p-toluenesulfonic acid, naphthalene~mono~ulfonic and
-disulfonic acids and laurylsulfuric acid. Salts with
physiologically unacceptable acids, for example picrates,
can be nsed for isolating and/or purifying the compounds
of formula I.
On the other hand, compounds of formula I con-
taining COOH or tetrazolyl groups can be converted withbase~ t~or example ~odium or potas~ium hydroxide or
carbonate) to the corresponding ~tal salts, e~pecially
alkali metal or alkaline earth ~3tal salts, or to the
corre~ponding ammonium salt~. ~he potas~ium ~alt4 of the
2S tetrazolyl derivative~ are particularly preferred.
The novel co~pound~ of formula I and their
phy~iologically acceptable salt~ can be used for the
manufaoture of pharmaceuti~al preparation~ by incor-
poratio~ into a suitable dosage form together with at
lea~t one excipient or ad~unct and, if desired, togPther
with one or ~ore other active ingredi~t~O The resulting
fo~mulations can be u~ed a~ drug~ in hu~an or veterinary
medicine. Pos3ible excipient~ are organic or inorganic
sub~tance~ which are ~litable for e~teral (for example
oral or rectal) or parenteral administration or for
administration in the form of a~ inhalation ~pray, and
which do not react with ~he novel compoundY, examples
being water, vegetable oil~, benzyl alcohol~,
208~736
- 12 -
polyethylene glycols, glycerol triacetate and other fatty
acid glyceride~, gelatin, soya lecithin, carbohydrat~s
such as lactose or ~tarchf magne~ium stearate, talc and
cellulos0. Tablets, coat~d tablet~, cap~ule~, ~yrups,
S juices or drops~ in particular, are used for oral
administration; lacquered tablets and capsules with
coatings or shells resistant to gastric juices are of
special interest. Suppositories are used for rectal
administration and solutions, prefer~bly oily or aqueous
solutions, as well as suspensions, emulsions or implants,
are used for parenteral administration. For admini~-
tration as inha~ation sprays, it i~ possible to use
sprays containing the active ingredient either dissolved
or suspended in a propellant mixture (for example fluoro-
chlorohydrocarbon~j. It i~ convenient here to use theactive ingredient in micronised form, it being possible
for one or more additional physiologically compatible
solvents, for example ethanol, to be present. Inhalation
solutions can be administered with the aid of conven-
~0 tional inhalers. The novel compounds can also be lyo-
philised and the resulting lyophili~ates used for example
for the manufacture of injectable preparations. The
indicated formulation~ can be st:erilised and/or can
contain adjun~t~ such as preservative~, stabilisers
and/or wetting agent3, emulsifier~, ~alts for influencing
the o~motic pre~sure, buffer 3ub~tance~ and colours
and/or flavouring~ de~ired~ they can al~o contain
one or more other active ingredien~ or ex~ple one or
more vitsmin3, diuretic~ or antiphlogistic~.
. 30 The ~ub~tances according to the invention are
normally admini~tered analogously to other known, com-
mercially available preparations, but in particular
analogou~ly to the compounds described in US patent
4 880 804, preferably in dose3 of between about 1 m~ and
35 1 g, especially of between 50 and 500 mg per dosage unit.
The daily do~e i8 preferably between abollt 0.1 and
50 mg/kg, e~pecially between 1 and 100 mg/~g of body
weight. However, the particular dose for each individual
2~73~
- 13 -
patient depends on a ve~y wide variety of factor~, for
example on the efficacy of the particular compound u8ed,
age, body weight, general ~tate of health, sex, diet~
time and mode of administration, rate of excretion, d~ug
combination and ~everity of the particular disease to
which the therapy i~ applied. Oral administration is
preferred.
Above and below, all temperatures are given in
C. In the following Examples, ~conventional working-
upl~ means: Water is added if necessary, the p~ isadjusted to between 2 and 10 if nece~sary, depending on
the constitution of the end product, extraction is
carried out with ethyl acetate or methylene chloride and
the organic phase i~ ~eparated off, dried over sodi~m
sulfate/ evaporated and purified by chromatography on
silica gel and/or by cry tallisation. MS (FAB) = mass
spectrum (obtained by the fast atom bo~bardment method).
A solution of 0.23 g of Na in 20 ml of methanol
i~ added dropwise over 15 min to a solution of 3.2 g of
2-butyl-1(or 3)~-Lmidazo[4,5-b]pyr}.dine in 75 ml of
methanol. The mixture i8 ~tirred for a further 30 min at
20 and evaporated, the re~idue $~ dis~olved in 20 ml of
~MF, a~d ~ ~olution o 3.45 g of 5-bromomethyl-2-
(2~methoxycarbonylphenyl)ben~ofuran in 10 ml of DMF i8
added dropwise at 0, with ~irring. The mixture is
~tirred for 16 hour~ at 20, evaporated, worked up ~n
conventional mann~r a~d chromatographed on silica ~el to
give 2-~utyl-3-(2-(2-methoxqcarbonylphenyl)benzo-
furan 5-ylm~thyl) 3H-imidazo[4,5-b3pyridine.
xamDl~ 2
~a) l-e9 g f 2-butyl-7-methyl-3H-Lmidazot4,5-b~-
pyridine (m.p. 75-76) are di~olved in 60 ml o~
DMF, 1.44 g of potas3ium carbonate are added at ~10
to -14, the mixture i~ ~tirred for 40 min at -14
and a ~olution of 5.97 g of 5-hromomethyl-2-(2~
2~8~73~ `
- 14 -
triphenylmethyl- lH-tetrazol-5-yl)phenyl~benæofuran
("A") in 120 ml of DMF i~ added dropwise. The
mixture is then stirred for a further 2 hour~ at
-14 and 16 hours at 20, evaporated and worked up
with water/ethyl acetate. After chromatography
first with methylene chloride/methanol ~8:2 and then
with toluene/ethyl acetate 8:2, 2-butyl-7-methyl-3-
(2-(2-(1-triphenylmethyl-1~-tetrazol-5-yl)phenyl)-
benzofuran-5-ylmethyl)-3~-Lmidazo[4,5 b]pyridine is
obtained: Rf 0.61 (methylene chloride/methanol
97:3); MS ~FAB) 70~.
The starting material "A" i~ obtained as follows:
The bromlnation of o-tol~onitrile in carbon
tetrachloride ( W irradiation~ gives o-bromomethyl-
benzonitrile (m.p. 69). This is rea~ted with 5-
methylsalicylaldehyde in acetone, in the presence of
potassium carbonatel to give 2-(o-cyanobenzyloxy)-5-
methylbenzaldehyde (m.p. 99). Cyclisation with Na~ in
DMF under argon at 20 give~ 2-o-cyanophenyl-5-methyl-
benzofuran (mOp. 112-113), which i9 converted to
2-o-(1-trimethyl~tannyl-1~-tetrazol-S-yl)phenyl-5-
methylbenzofuran ~m.p. 289 (decomposition)] with
trimethyltin azide in boiling toluene. Cleavage of the
protecting group with ~Cl in methanol/ether gives
2-~2 (1~-tetrazol-5-yl)phenyl~-5-methylbenzofuran, which
i~ converted in ~he crude ~tate to 2-~2~ triphenyl-
methyl~lH-tetrazol-S-yl)phenyl]-5-methylbenzofuran (m.p.
167~ with triphenylchloromethane in methylene chloride,
in the pre3e~ce of triethylAmine. Bromination with
M-bromo3uccini~;de gives ~A~ ~m.p. 169)~
(b) The product obtained according to (a) (Rf 0.61;
1 g) i~ dissolved in 60 ml of 4 n ~Cl i~ dioxane and
th~ solution i~ ~tirred for 16 hours at 20. After
evaporatio~ and conventional working-up, 2-butyl-7
. 35 methyl-3-(2-t2-(lH-tetrazol-5-yl)phenyl)-
benzof uran-S-ylmethyl ) - 3~-imidazo t 4, 5 -b ] pyridine is
obtained: m.p. 250 (decompo~ition); MS (FAB) 464.
The following are obtained analogously:
2~736
- 15 -
from 2-ethyl-5,7-dLmethyl~3~ imidazo[4,5-b]pyridine and
"A": 2~ethyl-5,7~dimethyl-3-(2-(2 (l-triphenylmethyl-
1~-tetrazol-5 yl~phenyl~benzofuran-5-ylmethyl)-3~-
imidazo[4,5-b3pyridine, and from the latter with
~Cl/dioxane 2-ethyl-5,7-dim~thyl-3-(2-(2-(lH-tetrazol-
5-yl)phenyl)benzofuran-5-ylmethyl)-3H-imidazo[4,5-b]
pyridine, m.p. 256;
from 2-butyl~5-(2-carboxybenzyl)-4,5-dihydro-4-oxo-3~-
imidazo[4,5-c]pyridine t"C"; obtainable by the hydro-
genolysi~ of 3-benzyl-5-(2-benzyloxycarbonylbenzyl)-2-
~utyl-4,5-dihydro-4-oxo-3~-imidazo[4,5-c]pyridine) and
"A": 2-butyl-5-(2-carboxybenzyl)-4,5-dihydro-4-oxo-3-
(2-(2-(1-triphenylmethyl-lH-tetrazol-5-yl)phenyl)-
benzofuran-5-ylmethyl)-3~-Lmldazo[4,5-c~pyridine, and
from the latter with ~Cl/dioxane: 2-butyl-5-(2-carboxy-
benzyl)-4,5-dihydro-4-oxo-3-(2-(2-(lH-tetrazol-5-yl)-
phenyl)benzofuran-5-ylmethyl)-3B-imidazo[4,5-c]pyridine;
from 2-butyl-4,5-dihydro-4-oxo-3~-imidazo r 4,5-c]pyridine
and "A"- 2-butyl-4,5-dihydro-4-oxoo3-(2-(2-(1-
triphenylmethyl-1~-tetra~ol-5-yl)phenyl)benzofuran-5-
ylmethyl)-3~-imidazo[4,5-c]pyridine, and from the latter
with ~Cl/dioxane: 2-butyl-4,5-dihydro-4-oxo-3-
t2-(2-(1~-tetrazol-5-yl)phenyl) benzo f uran-5 -ylmethyl)~
3~-imidazo~4,5-c~pyridine;
f rom 2-bu~yl-5-carboxymethyl-4,5 dihydro-4-oxo-3~-
Lmidazo[4,5-c]pyridine tobtainable by the hydrogenoly~is
of 3-benzyl-5-benzyloxycarbonylmethyl-2-butyl-
4,5-dihydro-4-oxo-3~imidazot4,5-c3pyridine) and "A":
2-butyl-5-carboxymethyl-4~5-dihydro 4-oxo-3~(2-(2~
triphenylmethyl-1~-tetrazol-5-yl)phenyl)benzofuran-5-
ylmethyl3-3H-Lmidazo~4,5-c]pyridine, and from the latter
with HCl/dioxane: 2-butyl-5-carboxymethyl-4,5-
dihydro-4-oxo-3-(2-t2-(1~-tetrazol-5-yl)phenyl)benzo
furan-5-ylmethyl ) -3~-imidazo[4,5-c]pyridine.
35 ~3xample 3
The following are obtained analogously to Example
2a) with 5-bromomethyl-2-(2-cyanophenyl)benzofuran :
2~8~73~
- 16 -
2-butyl-3-(2-(2-cyanophenyl)benzofuran-5-ylmethyl)-7-
methyl-3~-imidazo[4~5-b]pyridine;
3-(2-(2-cyanophenyl)benzofuran-5-ylmethyl)-2-ethyl-5,7-
dimethyl-3~-imidaza[4,5-b]pyridine;
2-butyl-5-(2-carboxyb~nzyl)-3-(2-(2-cyanophenyl)benzo-
furan 5-ylmethyl)-4,5-dihydro-4-oxo-3H-imidazo~4,5-
c]pyridine;
2-butyl-3-(2-(2-cyanophenyl)benzofuran-5-ylmethyl)-4,5-
dihydro-4-oxo-3~-imidazo~4,5-c]pyridine;
2-butyl-5-carboxymethyl-3-(2-(2-cyanophenyl)benzofuran-
5-ylmethyl)-4,5-dihydro-4-oxo-3H-imidazo[4,5-c]pyridine.
Example 4
The following are obtained analogously to Example
2 from the starting materials of formula III given
therein with 3-bromo-5-bromomethyl-2-(2-(1-tri-
- phenylmethyl~ tetrazol-5-ylphenyl)benzofuran:
3-(3-bromo~2-~2-(1-triphenylmethyl-lH-tetrazol-5-yl)
phenyl)benzofuran-S-ylmethyl)-2-butyl-7-methyl-3H-
imidazot4,5 b]pyridine, and from the latter: 3-~3-
bromo-2-(2-(1~-tetrazol-5-yl)phenyl)benzofuran-5-yl
methyl)-2-butyl-7-methyl-3~-imidazo r 4,5-b]pyridine;
3-(3-bromo-2-(2~ triphenylmeth~yl-1~-tetrazol-5-yl)
phenyl~be~zofura~-5-ylmethyl)-2-ethyl-5,7-dimethyl-3~-
LmidazoL4,5-b~pyridine, and from the latter: 3-(3-
bromo-2-(2~ tetrazol-5-yl~phenyl)benzofuran-5~yl-
methyl)-2-ethyl-5,7-dimethyl-3~-Lmidazo[4,5-b~pyridine;
3-(3-bromo 2~ triphenylmethyl~ tetrazol-5-yl)-
phenyl)benzofuran-5-ylmethyl)-2-butyl-5-(2-~arboxy-
benzylj-4,5-dih~dro-4-oxo-3~-imidazo[4,5-~]pyridine, and
from the latter: 3-(3-bromo-2-(2-tlH-tetrazol-5-
yl)phenyl)benzofuran-5-ylmethyl)-2-butyl-5-(2-carboxy-
benzyl)-4,~ dihydro 4-oxo-3~-imidazo[4,5-c]pyridine;
3-(3-bromo-2-(2-(1-triphenylmethyl~ tetrazol-S-yl)-
phenyl ) benzofurarl-5-ylmethyl ) ~2-butyl-4, 5-dihydro-4-
oxo-3E~-imidazot4,5-c]pyridine, and from the latter: 3-
(3-bromo-2-(2-(1~-tetrazol-5-yl)ph~nyl)benzofuran-5-
ylmethyl)-2-butyl-4,5-dihydro-4-oxo-3~-imidazo~4,5-c]-
2~73~
- 17 -
pyridine;
3-(3-bromo-2-(2-(1-triphenylmethyl-1~-tetrazol-5-yl)-
phenyl)benzofuran-5-ylmethyl)-2-butyl-5-carboxymethyl-
4,5-dihydro-4-oxo-3H-Lmidazo[4,5-c~pyridine, andfromthe
latter: 3 (3-bromo-2-(2~ tetrazol-5-yl~phenyl)-
benzofuran-5 ylmethyl3-2-methyl-5-carboxymethyl-4,5-
dihydxo-4-oxo-3H-imidazo[4,5-c]pyridine.
Example 5
The following are obtained analogously to Example
2a) from 2-butyl-l(or 3)~-imidazo[4,5-b]pyridine:
with 5-bromomethyl-2-(2-~itrophenyl)benzofuran: 2-
butyl-3-(2-(2-nitrophenyl)benzofuran-5-ylmethyl)-3H-
imidazo[4,5-b]pyridine;
with S~bromomethyl-2-(2-trifluoroacetamidophenyl)-
benzofuran: 2-butyl-3-(2-(2-trifluoroacetamldophenyl)-
benzofuran-5-ylmethyl) 3H imidazo[4,5-b]pyridine;
with 5-bromomethyl-2-(2-cyanophenyl)-3-fluorobenzofuran:
2-butyl-3-(2-(2-cyanophenyl~-3-fluorobenzofuran-
5-ylmethyl)-3H-imldazot4,5-b~pyridine;
with 5-bromomethyl-3-chloro-2-(2-ethoxycarbonylphenyl)-
benzofuran: 2 butyl-3-(3-chloro-2-(2-ethoxycarbonyl-
phenyl)benzofuran-5-ylmethyl)-3~-Lmidazo[4,5-b3pyridine~
:~x~.
A mixture of 1 g of 2-butyl-3-(2-(2-methoxy-
carbo~ylphenyl)benzofura~-5-ylmethyl)-3H-imidazo[4,5-
b]pyridîne, 12 ml of 2 n aqueou~ NaO~ solution and 48 ml
of etha~ol i~ boiled for 2 hour~ and then evaporated.
Acidification to p~ 3 with HCl give~ 2-butyl-3-
(2-(2-carboxyphenyl)benzofuran~5~ylmethyl)~3~-imidazo
~0 t4,5-b]pyridine, which i8 filtered off, washed with water
and dri~d.
Exa~ple 7
A mixture of 420 mg of 2-butyl-3-(2-(2-cyano-
phenyl)benzofuran-5-ylmethyl)-7-methyl-3~-imidazo-
[4,5b]pyridine, 206 mg of trimethyltin azide and 15 ml of
- 18 - 2~ 7~ ~
xylene i~ boiled for 96 hour~. After 48 hours, a further
O.2 g of trLmethyltin azide is added. The mixture i8
cooled, treated with et~ereal hydrochloric ac.id and
evaporated. Conventional working-up give~ 2-
butyl-7-methyl-3 (2-(2~ tetrazol-5-yl)phenyl)benzo-
furan-5 ylmethyl)-3~Lmidazo[4,5-b]pyridine, mOp. 250
(decomposition).
The corresponding K salt is prepared therefrom in
conventional manner.
~xam~le 8
A solution of 1 g of 2-butyl-3-(2-(2-nitro-
phenyl)benzofuran-5-ylmethyl)-3~-im~dazot4,5-b]pyridine
in 30 ml of ethanol i~ hydrogenated on 1 g of Raney Ni at
20 and normal pressure until the uptake of H2 ha~ ceased~
The m1xture is filtered and evaporated to give 3-(2-(2-
aminophenyl)benzofuran-5-ylmethyl)-2-butyl-3~-
imidazo[4,5-b]pyridine.
~xample 9
A solution of 2.82 g of trifluoromethanesulfonic
anhydride in 10 ml of methylene chloride is added
dropwi~e at -50 to -60 to a ~olution of 3.96 g of
3-(2-(2 aminophenyl)benzofuran-5-ylmethyl) 2-butyl-3~-
Lmidazot4,5-b]pyridine and 1.01 g of triethylamine in
30 ml of methylene chloride. The mixture is left to warm
up to 20 and poured into dilu~e acetic acid to give
2-butyl 3-(2-(2-trifluoromethan~sulfonamidophenyl)-
benzofuran-5-ylmethyl)~3~-Lmidazol4,5-b~pyridine after
conventional working-up.
xample 10
A qolution of 0.79 g of chloroacetonitrile in
5 ml of DMF i~ added dropwise at 20 to a ~olution of
7.07 g of 2 butyl-3-(2-(2-tl-triphenylmethyl 1~-tetra-
zol-5-yl3phenyl)be~zofuran-s-ylmethyl)-4~5-dihydro-4-
oxo-3~-Lmidazot4,s-c]pyridine and 1.17 g of potassi~m
tert-butylate in 25 ml of DMF, with stirring. The
2~73~
-- 19 --
mixture iB stirred for a further 30 min at 20 and poured
on to ice, hydrochloric acid i5 added to pH 6 and the
mixture ic worked up in conventional manner to give
2-butyl-5-cyanomethyl-3~ (2~ triphenylmethyl-lH-
S tetrazol-5-yl)phenyl)benzofuran-5-ylmethyl)-4,5-di-
hydro-4-oxo-3~-imidazo[4,5-c]pyridine.
The following 2-butyl-3-l2-(2-(1-triphenyl-
methyl-l~-tetrazol-5-yl)phenyl)benzofuran-5-ylmethyl)-
4,5-dihydro-4-oxo-5-R4-3~-imidazo[4,5-c]pyridines are
10 obtained analogously:
with methyl iodide: 5-methyl-
with ethyl iodide: 5-ethyl~
with isopropyl iodide~ S-isopropyl-
with butyl bromide: 5-butyl-
15 with tert-butyl bromide: 5-tert-butyl-
with 3-bromopropionitrile: 5-(2-cyanoethyl)-
with 4-bromobutyronitrile: 5-(3-cyanopropyl)-
with methyl bromoaeetate: 5-methoxycarbonylmethyl
with ethyl 3-bromopropionate: 5-(2-ethoxycarbonylethyl)-
with benzyl bromide: 5-benzyl-
with o-fluorobenzyl bromide: 5-to-fluorobenzyl)-
with m-fluorobenzyl bromlde: 5-(m-fluorobenzyl)-
with p-fluorobenzyl bromlde: 5-(p-fluorobenzyl)-
with o-chlorobenzyl bromide~ 5-(o-chloro~enzyl)-
~5 with m-chlorobenzyl bromide: 5-(m-chlorobenzyl)-
with p-chlorobenæyl bromide: 5~(p-chlorobenzyl)-
with o-bromobenxyl bromide: 5-(o-bromobenzyl)-
with m-bro~ob zyl bromide: 5-(m-bromobenzyl)-
with p-bromobe~zyl brom1de: 5~-(p-b~omobenzyl)-
with p-~ethylbenzyl bromide: 5-(p-m~thylbenzyl~-
with o-trifluoromethylbenzyl
bromide: 5-(o-trifluoroMethyl-
ber~zyl ) -
with m-trifluoromethylbenzyl
bromide: 5 (m-trifluoromethyl-
benzyl)-
with p-trifluoromethylbenzyl
bromide: 5-(p-trifluoromethyl-
7 3 6
20 -
benzyl)-
with o-methoxycarbonylbenzyl
brom1de: 5-(o-methoxycarbonyl-
benzyl)-
with m-methoxycar~onylbenzyl
bromide: 5-(m-methoxycarbonyl-
benzyl)-
with p-methoxycarbonylbe~yl
bromide: 5-(p-m~thoxycarbonyl-
benzyl)-
with o-cyanobenzyl brcmide: 5-~o-cyanobenzyl)-
with m-cyanobenzyl bromide: 5-(m-cya~ob nzyl)-
with p-cyanobenzyl bromlde: 5-(p-cyanobenzyl)-
with o-nitrobenzyl chloride: S-(o-nitrobenzyl)-
with m-nitrobenzyl chloride: 5-(m-nitro~enzyl)-
with p-nitrobenzyl chloride: 5-(p-nitrobenzyl)-
with o-trifluoroacetamido-
benzyl bromideo 5-(o-trifluoroacetamido-
benzyl)-
with m-trifluoroacetamido-
benzyl bromlde: S-(m~trifluoroacetamldo-
benzyl)-
with p-trifluoroacetamido
benzyl bromide: 5-(p-trifluoroacetamido~
benzyl)~
with o-trifluoromethyl~ul-
fo~amidobenzyl bromide: 5-~o-trifluoromethylsul-
fon~midobenzyl)-
with m-trifluoro~ethylsul-
fonamidobenzyl bromide: 5-(m-trifluoromethylsul-
fonamldobenzyl)-
with p-trifluoromethyl~ul~
fonamidobenzyl bromide: 5-(p~trifluoromethyl~ul-
fonamidobenzyl)-.
2~8~73~
- 21 -
~am~le 11
The following 2-butyl-4,5-dihydro-4-oxo-3-(2-
~2-(1~-tetrazol-5-yl)phenyl)benzofuran-5~ylmethyl)-5-
R4-3H-imidazo[4,5-c]pyridines are obtained analogously to
Example 2b) ~rom the compounds described in Example 10:
5-cyanomethyl-
5-methyl-
5-ethyl-
5-isopropyl-
5-butyl-
5-tert-butyl-
5-(2-cyanoethyl)-
5-(3-cyanopropyl)-
5-methoxycarbonylmethyl- (alRo 5-carboxymethyl-)
5-(2-ethoxycarbonylethyl)- [also 5-52-carboxyethyl)-]
5-benzyl-
5-(o-fluorobenzyl)-
5-(m-fluorobenzyl)-
S-(p-fluoroben~yll-
5-(o-chlorobenzyl)-
5-(m-chlorobenzyl)-
5-(p-chlorobenzyl)-
5-(o~bromobenzyl)-
5-(m-bromobenzyl)-
5-(p-bromobenzyl)-
5-(p-methylbenzyl)-
5-(o-trifluoromethylbenzyl)-
5-(m-trifluoromethylbenzyl)-
5-(p-trifluoromethylbenzyl)-
5-(o-methoxycarbonylbenzyl)- [al~o 5-(o-~arboxy
benzyl)]
s-~m-methoxycarbonylbenzyl)- tal~o 5-tm-carboxy
benzyl)]
5-(p-methoxycarbonylbenzyl)- [ al80 5-(p-carbo~y
benzyl)]
5-(o-cyanobenzyl)-
5-(m-cyanobenzyl)-
5-(p-cyanobenzyl)-
2~8~73~
- 22 - 26474-263
5-(o-nitrobenzyl)-
5-(m-nitrobenzyl)
5-(p-nitrobenzyl)-
S-(o-trifluoroacetamidobenzyl)-
S-(m-trifluoroacetamidobenzyl)-
5-(p-trifluoroacetamidobenzyl)-
S-(o-trifluoro~ethylsulfonamidobenzyl)-
5-(m-trifluoromethylsulfonamidohenzyl)-
5-(p-trifluoromethylsulfonamidobenzyl)-.
xample 12
Tha following 3-(3-bromo-2-(2-(1-triphenyl-
methyl~ tetrazol-5-yl)phenyl)benæofuran-5-ylmethyl)-
2-butyl-4,5-dihydro-4-oxo-5-R4-3~-imidazo[4,5-c]pyridines
are obtained analogously to Example 10 by reaction of
3-(3-bro~o-2-(2-(1-triphenylmethyl-1~-tetrazol-
S-yl)phenyl)benzofuran-5-ylmethyl)-2-butyl-4,5-dihydro-
4-oxo-3~-imidazo[4,S-c]pyridine with chloroacetonitrile
or with the other alkylating agents indicated in said
~xample-
5-cyanomethyl-
S-methyl-
S-ethyl-
5-i~opropyl-
5-tert-butyl-
5~ cyanoethyl)-
5-(3 cyanopropyl)- .
S-metho~ycarbo~ylmethyl-
5-(2-ethoxycarbonylethyl)-
S-benzyl-
5-(o-fluorobenzyl)-
S-(m-fluorobenzyl)-
S-(p-fluorobenzyl~-
S~(o-chlorobenzyl)-
5-(m-chlorob~nzyl)-
5-(p-chlorobenzyl)-
5-(o-bromobenzyl)-
5-(m-bromobenzyl)-
7 3 6
- 23 -
26474-263
S-(p~bromobenzyl)-
5~(p-methylbenzyl)-
5-(o-trifluoromethylbenzyl)-
5-(m-trifluoromethylb~nzyl)-
5-(p-trifluoromethylbenzyl)-
5~(o-methoxycarbonylbenzyl)-
5-(m-methoxycarbonylbenzyl)-
5-(p-methoxycarbonylbenzyl)-
5-(o-cya~obenzyl)-
5-(m-cyanobenzyl)-
5-(p-cyanobenzyl)-
5-(o-nitrobenzyl)-
5-~m-nitrobenzyl)-
5-(p-nitrobeazyl)-
5-(o-trifluoroacetammdobenzyl)-
5-(m-trifluoroacetamidobenzyl)-
5-(p-trifluoroacetamidob~nzyl)-
5-(o-trifluoromethylsulfonamidobenzyl)-
5-(m-trifluoromethylsulfonamidobenzyl)-
5-(p-trifluoromethylsulfonamidobenæyl)-.
The followi~g 3-(3-bromo-2-(2-(1~-tetrazol-5-
yl)phenyl)benzofuran-S-ylmethyl)-2-butyl-4,5-dihydro-4-
oxo-5-Rs-3~-imidazot4,5 c~pyridine~ are obtained analo-
~ously to Exa~ple 2b~ fr3m the compounds de~c~ibed in~xample 12:
5-cyallomethyl-
5-me~hyl-
3 0 S -ethyl -
5 -i80propyl -
5-tert-butyl
5-(2~cyanoethyl~
5O(3-cya~opropyl)-
5-methoxycarhonylmethyl- (al~o 5-carboxymethyl-)
5-(2-ethoxycarbonylethyl~- talso 5~(2-carboxyethyl.)-]
5-benzyl-
2~8~73~
- 24 -
5-(o fluorobenzyl~-
5-(m-fluorobenzyl)-
5~(p-fluorobenzyl)-
5 (o-chlorobenzyl)-
5-(m-chlorobenzyl)-
5-(p-chlorobenzyl)-
5-(o-bromobenzyl)-
5-(m-bromobenzyl)-
5-(p-bromobenzyl)-
5-(p-methylbenzyl)-
5-(o-trifluoromethylbenzyl)-
5-(m~trifluoromethylbenzyl)-
5-(p-trifluoromethylbenzyl)-
5-(o-methoxycarbonylbenzyl)- [al~o 5-(o-carboxy
benzyl~]
5-(m-methoxycarbonylben~yl)- [also 5-(m-carboxy
benzyl)]
5-(p-methoxycarbonylbenzyl)- ~also 5 (p carboxy
benzyl)]
5-(o-cyanobenzyl)-
S-(m-cyanobenzyl)- ~ -
5-(p-cyanobenzyl)
5-~o nitrobenzyl)-
5-(m-nitrobenzyl)- ~-
5-(p-nitrobenzyl)-
5- ~ o-trif luoroacetamidobenzyl)-
5~ trifluoroacetamldobenzyl)-
5-(p trifluoroacetamldobenzyl)-
5-(o-trifluoro~ethyl~ulfona~idobenzyl)-
5-(m-trifluoro~ethyl~ulfonamidoben2yl)-
5-(p-trifluoromethylsulfonamidobenzyl)-.
E~ample 14
A ~olution of 1 g of 2-butyl-4,5-dihydro-5-(o-
nitrobenzyl)-4-oxo-3-(2-(2~ -tetrazol-5-yl)phenyl)-
benzofuran-5-ylmethyl)-3~-Lmidazol4,5-c]pyridine in 20 ml
of methanol is hydrogenated on 0.3 g of 5% Pd-on-
charcoal at 20 and normal pres~ure until the calculated
2~8~7~6
- 25 -
a~ount f ~2 ha~ been taken up. The catalyst is filtered
off and the filtrate i8 evaporated to give 5-~o amino-
benzyl) 2-butyl 4,5-dihydro-4-oxo-3-(2-(2~
tetrazol-5 yl)phenyl)benzofura~-5-ylmethyl)-3B-imidazo-
[4~5-c]pyridine~
The following 2-butyl-4,5-dihydro-4-oxo~3-(2-
~2-(1~-tetrazol-5-yl)phenyl)benzofuran-5-ylmethyl)-3~-
imidazo[4,5-c]pyridines:
.5-(m-aminobenzyl)
5-(p-aminobenzyl)-,
or the followiny 3-t3-bromo-2-(2~ -tetrazol-5-yl)-
phenyl)benzofuran-5-ylmethyl)-2-butyl-4,5-dihydro-4-
oxo-4~ imidazo[4,5-c]pyridine~:
5-(o-aminobenzyl)-
5-(m-aminobenzyl)-
5-(p-aminobenzyl)-,
are obtained analogously by hydxoqenation of the cor-
responding nitro compounds mentioned in Example 11 or 13
re~pectively.
The following Examples relate to pharmaceutical
formulations containing active ingredients of formula I
or their salts.
~xa~le As Tablet~ and coated tablets
Tablet3 of the following composition are produced
by compression in conve~tional manner and, where
required, are provided with a co~ventional sucrose based
co~ting:
Active i~gredient of formula ~100 mg
~icrocrystalline cellulose 278.8 mg
30 Lacto~e 110 mg
M~ize ~tarch 11 mg
Magne~ium stearate 5 mg
Fi~ely divided silicon dioxide0.2 mg
~x~Epl~ B: ~ard gelati~ cap~ules
Co~ventional two-part hard gelatin cap~ules are
each filled with
2~8~ 7~6
- 26 -
Active ingrsdient of formula I100 mg
Lactose 150 mg
Cellulose 50 mg
Magne~ium stearate 6 mg
~xam~le C: 5Oft gelatin sap~ule~
Conventional ~oft gelatin capsule~ are filled
with a mixture of 50 mg of active ingredient and 250 mg
of olive oil in each case~
~ample D: ampoules
A solution of 200 g of active ingredient in 2 kg
of propane-1,2-diol i~ made up to 10 l with water and
filled into ampoules so that each ampoule contains 20 mg
of active ingredient.
~xa~ple ~: Aqueou~ ~uspen~ion for oral adm;ni~tration
An aqueou~ suspension is prepared in conventional
manner. The unit dose (5 ml) contains 100 mg of active
ingredient, 100 mg of ~odium carboxymethyl cellulose,
S ml of sodium benzo~te and 100 mg of sorbitol.