Note: Descriptions are shown in the official language in which they were submitted.
- WO`91/20102 . 2 0 8 ~ 3 S ~ PCI/CA91/00192
II~ETAL AND I~ET~L OXIDE CAT~LYZED ELECTRODES
FOR l~:LECTROC~EI~ I CAL CELLS, ~ND UETEIODS
OF ~AK I NG S~E
FI15LD OY THE INVENTION:
This invention relates to porou~ electrodes for fuel
cells and similar cells such as air-metal cells, and
alkaline zinc/manganese dioxide cells (particularly
secondary cells), or other primary or secondary alkaline
cells, and to the methods of making the present
electrodes. In particular, the invention relates to
porous electrodes which may be used a~ fuel cell cathodes
or as auxiliary gas recombininy or transfer electrodes in
alkaline cells, wherein a catalytically active layer i~
formed on a porous conductive substrate, and the
catalytically active layer is derived from non-noble
~etals. In the event that the porous electrode is to be
used as a fuel cell anode, a further small amount of a
.. ,; ~.
noble metal is included in the catalytically active layer. ~.
In all events, electrodes according to the present
invention have a lower loading of catalytically active
.. ..,, ~, . . . . .
layer in terms of weight of catalyst per unit area of
geometric electrode surface than heretofore, but with
r r; ,~. , .
operating characteristics that compare favourably with
prior art electrodes having noble metal catalysts, but
being available at much reduced cost~.
., .. '' . ', ' ' ' . . ' " ~ ~ , ' . ', "' . , ~ , ,' ' ' ' ' ' ' .. , ' ' .. , ' ' ' ' ,.
.' ' ' ' ', . ' ', ~, . '. ' ' ' , . ' ,, ' , , , ", ~ .' ' ' , . . ' " ' , '
Wo91/20tO2 ` ' ~ ~8 ~ 3 ~ ~ PCT/CA91/OOt92 ~
. .,
The present invention teaches several alternative
methots for producing electrodes according to the present
invention; whereby the electrode, once produced, will in
all events have a catalytically active layer on a porous
conductive substrate. Electrodes of the present invention
may comprise a porous metal current collector, and a
further gas diffusion layer, with the current collector
being situated or embedded in the porous conductive
~ubstrate or within the gas diffusion layer.
J~CRGROIJND O~ T~l~ INVl~NTION:
The prior art has concerned itself, for many years,
with the provision of porous electrodes that are
particularly intended for use in fuel cells. However,
while many ~uite acceptable electrodes have bcen provided
in the prior art, it has been the general e~perience that
~uch electrodes are expensive to produce. This comes,
especially, due to the generally accepted reguirement ~or
the u~e of noble metals in fuel cell electrodes, including
. ~ , .
especially gas diffu~ion or poro~s electrodes. Moreover,
; whén the prior art has provided electrodes ~hich comprise
- , . - . - , . . .
a porous substrate having a porous catalytically active
-, . . : ~ . . ,
layer theroon, it has been common in the past for there to
be relatively high catalyst loading by weight per unit
,, . . :. ,. : ., , . .. . , . . i
ar~a of ~eometrical electrode sur~ace, thereby
contributing further to the cost of ~roducing such
' ' , ~ ` ', ~ ' ' ' . . ', ~ ' i ' ` .
WOsl/20102 ~ PCTtCA91/OOt92
20853~
electrodes.
Such prior art has included KORDESCH et al United
States Patent 3,405,010 dated October 8, 1968, and
XORDESCH United States Patent 3,310,434, dated March 21,
1967. The former patent relates to the catalyzing of
porous electrodes, using a heavy metal salt, an aluminum
salt, and a ruthenium salt. The latter patent is
particularly related to the use of noble metals as
catalysts on a porous electrode.
Yet another KORDESCH patent relating to the use of
wet proofed conductive substrates having an active
conductive layer with a surf?ce-deposited noble metal
catalyst is United States Pa~cnt 3,899,354 issued August
12, 1975.
BARER et al in United States Patent 3,935,029 issued
January 27, 1976 teach the use of fine graphite particles
enmeshed in a web of polytetrafluoroethylene (PTFE),
however, once again using noble metals.
Thus, it is a principal purpose of the present
invention to provide catalyzed electrodes having excellent
performance charac~eristics, at low cost. The catalyzed
electrodes of the present-.invention are~-specifically
~dapted for use in fuel cells and metal-air cells; and
especially u~eful as auxiliary gas recombining electrodes
in alkaline zinc/manganese dioxide cells (particularly
secondary cells), or-other primary or secondary alkaline
-..
" . ~ '.' .~ ' ' ' ' ' ` ' . " ~ ,
WO91/20102 PCT/CA91/00192 -
2 08 ~:3`~
cells. The porous catalyzed electrodes of the present
invention have particular utility as oxygen reduction
electrodes in alkaline cells as noted above.
Thus, the present invention comprises the provision
of a porous electrode which comprises a porous conductive
~ubstrate and a porous catalytically active layer on the
porous conductive substrate; the porous conductive
substrate being chosen from the group consisting of
carbon, graphite, and metal: and the porous catalytically
active layer being chosen from the group consisting of a
catalytically active non-noble metal, an oxide of a
catalytically active non-noble metal, carbon, carbon
together with a catalytically active non-noble metal, and
carbon together with an oxide of a catalytically active
non-noble metal. (If used as an anode in a fuel cell, the
electrode of the present invention is as described above,
together with a further additional smaller amount of a
catalytically active noble metal or carbon together with a
catalytically active noble metal.)-
Catalytically active non-noble metals that are
particularly ¢ontemplated for use in the present invention
include iron, cobalt, nickel, manga~ese, chromium,-coppor,
and vanadium: and catalytically active noble~metals there
parki¢ularly.intended for~use in the present invention, in
anodes according to this invention, include platinum,
~allædium, rhodium, iridium, osmium, gold, silver, and
WO91/20102 2 0 8 5 ~ 5`: ~ PCT/CA91/00192
ruthenium.
In general, a porous electrode according to the .
present invention may include the porous active layer
cho~en from the group consisting of carbon together with a
catalytically active non-noble metal, and carbon together
with an oxide of a catalytically active non-noble metal;
and very often, the porous active layer may further ~.
comprise polytetrafluoroethylene (PTFE3, as a binder. The t~`
carbon may be graphite.
Generally speaking, fuel cells may be considered to :~
be galvanic cells, with the basic reaction being the
electrochemlcal oxidat.ion of a fuel and the
electrochemical reduction of an oxidant (e.g., oxygen).
It should be noted, however, that fuel cells differ from
ordinary primary cell9 ~uch a~ commercially available dry
cells, in that tho fuel and o~idant are generally
introducod continuously into the cell electrodes during
the production of electricity. Thus, theoretically, the
eloctrodes and electrolyte of fuel cells qhould maintain a
constant value, during the time when the fuel and the
o~idant are reacted electrochemically within the fuel
cell, and-electricity and the. product of~ reaction --
usually water -- ar- removed from the fuel cell.
~ ~ ~here ~has been, for the la t century or-so, a
.continuing search for ways to boost the electrical output
of fuel cell~, and/or to increa~e their ~ervice life, ~.
.:
, ~ .
Wo91/20102 2 0 8 ~ 3 ~ ~ ; PCT/CA91tO0192 .
and/or to lower the cost of producing fuel cells so as to
render them commercially feasible. Needless to say, one
major area for research has been the catalysis of
reactions which take place within the electrodes of fuel
cells, and thereby the requirement for discovering new
methods of depositing known catalysts either in a more
active form or more economically. However, the search
still continues for catalysts which will raise the current
density within electrodes, and/or the voltage of the cell,
to levels which approach those that are attainable in
theory.
On the other hand, .there has also developed a
prsssing need for gas recombining electrodes in closed
cells such as alkaline zinc/manganese dioxide cells --
especially secondary c-lls. In such cells, there may be a
periodic (or continuing) generation of gasses, and
particularly there may be evolution of gaseous oxygen on
charge, overcharge, or any reversal. of cell polarity.
Such cells typically operato over. a broad range of
temperatures (e.g. from -40 to ~65 degrees Celsius) and at
current densities where the auxiliary porous electrode may
itself be reguired to pass up..to 7~0 mA/sg. cm.
It is the position of the present inventors that the
present i~vention provides a major step..in:the.required
direction, by providing.catalyzed porous condu~tors having
relatively low production costs, and with excellent
.. . .. .... .. . . ~ . . .. , . . .. . . , . ., .. . ,, .. .... .. . . ... ~ . .. .............. ... . ... . ... ..... .
. ... . .. .... . . .. . ...
wo gt/20l02 2 0 8 5 3 S ~ PcrtcAg1/00l92
. . . .
characteristics as to their efficiency, power density,
performance, and life. The characteristics of electrodes
according to the present invention compare most favourably
with the characteristics of certain higher efficiency
prior art fuel cell electrodes which, however, contained
noble metals in all instances and which therefore were
costly to produce.
When used as a catalyzed porous electrodes in fuel
cells or alkaline zinc/manganese dioxide cells, electrodes
of the present invention may comprise a porous gas
tiffusion layer adhered to the porous conductive substrate
spoken of above, at one side thereof. In fuel cells,
electrodes according to the present invention may also
generally include porous metal current collectors, as a
conveniont way of conducting electrical current produced
in the fuel cell out of the fuel cell.
In general, the catalyst provided by the present
invention is insoluble within the operating voltage and
the operating condition range of the electrode, thereby
contributing to the life of the electrode. This means
that-catalyzed porous electrodes according to the present
invention may be used with such electrolytes as alkaline
electrolytes,-for example potassium--hydroxide as used in
zinc/manganese dioxide cell~s. It also makes the use of
cataly~ed porous electrodes according to the present
i~vention in mstal-air batteries and cells more
. . . ~ . ,; :: , , ~ ~ .
. . . : . .,: . : . i . .
:-
WO91/20102 2 ;0 8 5 ~ ~ 5 PCT/CA9t/00192 .- .
8 ;~
attractive. -
. The inventors herein have discovered, guite
unexpectedly, that the addition of a metal or metal oxide
from the group consisting of iron, cobalt, nickel,
manganese, chromium, copper, and vanadium -- all non-noble .
metals -- to a porous electrode provides an electrode
having a service life and performance characteristics
comparable to those of platinum catalyzed electrodes.
Clearly, the cost of electrodes according to the present
invention, when compared to the cost of a platinum,
catalyzed electrode, may be considerably lower. Moreover,
platinum catalyzed electrodes may have significant carbon
corrosion o~ the carbon within the porous substrate
structure, whereas the use of metals and metal oxides
according to the present invention significantly decrea~es
the risk of carbon corrosion. That fact contributes,
therefore, to the attractiveness of electrodes accordinq
to the present invention for u~e in alkaline cell systems,
and metal-air batteries. In addition, dissolved trace
amounts of noble metals can cause problems in metal-air or
other-batteries containing a zinc, iron or aluminum anode
.. by.: enhancing the.hydrogen evolution (gassing;reaction).
.. This.enhanced. anode.corrosion cau~es high salf-discharge
and can result in cell leakage.
According to the present invention, the porous
conductive substrate of electrodes may be carbon,
.-
;
WO91/20102 2 0:8 3;3:S à PCT/CA91~00192
graphite, or metal; or, indeed, any other suitable
conductive material. The porous active layer may comprise
a catalytically active non-noble metal as discussed above,
an oxide of a catalytically active non-noble metal,
carbon, carbon toqether with a catalytically active non-
noble metal, and carbon together with a oxide of
catalytically active non-noble metal. Typically, the
porous active layer consists of carbon together with a
catalytically active non-noble metal or carbon together
with an oxide of catalytically active non-noble metal; and
in either case, it may further comprise a binder, usually
PTFE.
A typical porous electrode accordlng to the present
invontion would consist of an electrochemically active
layer which may be typically from 50 to 500 microns thick,
and it may further include a gas diffusion layer which may
also be 50 to 500 microns thick. If used in a fuel cell,
the clectrode would usually also have a metal scroen
current collector (a porous metallic current collector)
embedded in an electrode layer -- unless a bipolar
'con~truction method is applied. The electrochemically
active~'layer,-as noted,'-would contain the electrocatalyst
as described'àbove,'supported on ~ carbon, graphite or
'metal''porous~sub~trate,~"and''is located on the electrolyte
side of the electrode.
A gas diffusion layer, when used, may typically
.. . .. .. . . . . . .. .. . . . . .. . . . .. ..... ..... . . .... .. . . ........ . .. . ..
WO91/20102 2 0 8`~ 3:~ ~ PCT/CA91/00192
consist of PTFE bonded carbon, and would normally have a
higher degree of hydrophobicity than the active layer.
During the manufacturing process of the gas diffusion
layer, discussed in greater detail hereafter, a pore
builder such as ammoniumbicarbonate, may be used. The
current collector, when used, may be embedded in either
the gas diffusion layer or the active layer; and when
electrodes according to the present invention are used in
bipolar cells, no current collector is reguired.
Typically, the total electrode thickness of an
electrode comprising an active layer, a gas diffusion
layer, and a current collector, may range from ioo to 750
microns.
Thus, porous olectrodes according to the present
invention may comprise a porous conductive substrate, and
a porous gas diffusion layer adhered to one side thereof.
As notcd, the porous active layer is at a first side of
the porous conductive substrate, and the porous gas
dif$usion layer is at the ~ide of the porous conductive
substrate opposite to that of the active layer. Of
course, the porous active layer may permeate the porous
conductive substrate, and in any event the porous gas
diffusion layer i adhered to one side thereof. ,-
~ - ~s noted above, electrodes according to the present
- invention may also ~e used as anodes in fuel cells, in
which ~ase a further additional smaller amount of a
.. .. . . . .. . . . ... . ... . . . ... .
WO91/20102 PCT/CA91/00192
~Q~3~
11
catalytically active noble metal or carbon together with a
catalytically active noble metal is added to the
catalytically active layer of the electrode. There is
little point in providing oxides of catalytically active
noble metals, since the oxide would be promptly reduced to
the noble metal per se when exposed to the hydrogen fuel
generally used ih a fuel cell.
The noble metals contemplated for use in this aspect
of the invention are discussed above.
The concentration of catalyst within the porous
catalytically active layer is generally in the range of
from O.l to lO mg per sguare centimeter of the gèometrical
electrode surface area. Typically, the concentration of
catalyst wit~in the porous catalytically active layer is
in the range of from l to 5 mg per square centimeter of
the geometrical electrode surfac0 area.
In general, cathodes for hydrogen/oxygen fuel cells,
according to the preqient invention, may have a service
life well in excess of two thousand hours at a current
density of lO0 mil1iamperes per siquare centimeter, at a
voltage above 0.85 volts--versus RHE (Reversible Hydrogen
Electrode). Similar electrodes, using non-noble metal
oxide catalysts, can be run at up to 300 mil?iamperes per
.. . .... ... .. . . . ..
s~uare centimeter on oxyg~n and air, with potentials of
about 0.8 volts and 0.79 volts versus RHE, respectively.
As auxiliary gas recombining electrodes used in alkaline
- . : .: , ~ , , ,- .,. : . . . . . .
, ; ~ , ~ ; . - .:
W O 9t/20102 2 0 8 5 3 5 ~ P~r/CA91/00192 `
electrochemical cells such as zinctmanganese dioxide cells
having potassium hydroxide electrolytes, the electrodes
may be successfully subjected to current densities up to
7~0 m~/sg. cm. at low temperatures.
The present invention provides three generally
related but tistinct processes for the production of
porous electrodes in keeping herewith. Those three
general methods may be characterized a~ follows:
I (a) impregnating a porous conductive substrate
structure with a compound containing the chosen
material for the porous catalytically active
layer; and
(b) forming the porous catalytically active layer:
II (a) mixing the chosen material for said porous
catalytically active layer with the chosen
material for the porous conductive substrate;
and
(b) fabricating the electrode:
III (a) depositing pyrolytic carbon from the gas phase
-onto a porous conductive substrate structure, at
an elevated temperature in a gas atmosphere.
The compound used in step (a) of Process I, above, is
- : . .
a metal salt solution of thè chosen catalytically active
.. . .
metal. The formation process of step (b) of the Process I
may be the chemical formation of the porous active layer,
or the thermal formation of the porous active layer.
.. ~ ... .... , ,.: .. ....... .. . . .... ..
, . . . .. . .. .
W O 91/20102 . P~r/CA91/00192
2~8~3~5 ` "
~he chosen active material that is used in step (a)
of the Process II, may further be mixed with carbon and ~ ,7'!'
PTFE. ,
The Process III is carried out at least 600 degrees '
C, and the gas atmosphere may be steam, carbon dioxide, ' "
carbon monoxide, ammonia, nitrogen, argon, or hydrogen. ' ".
~RIEF DESCRIPTION OF THE DRAWINGS:
A typical embodiment of the present invention when
used as an auxiliary electrode as an oxygen gas
recombining electrode in an alkaline zinc/manganese
dioxide cell is shown in the singlo Figure of drawing
which accompanies the following di~cusslon.
DETAILE~ DESCRIP~ION OF THE ~REFERRED EMBODIMENTS: j'.
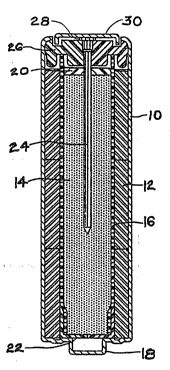
The Figure shows a typical cmbodiment of an alkaline
zinc/manganese dioxide cell having an auxiliary porous
o~ygen ga~ recombining electrode in koeplng with the '
present invention. The cell comprises a steel can 10
having a :conventional.metal oxide cat~ode 12 which is
typically fonmed cylindrically around an anode 14. The
cathode`l'2'.may.comprise ~inely.divide&.manganese dio~ide '-'
and 'gr~phite,-:and the anode. ~ay~compri~e zinc powder.
Between the cathode and the anode is an .electrolyte ' .
permeable separator 16; When the cathode is manganese~ ~'
dio~ide and the anode is zinc, then the electrolyte may be
an aqueous 301ution of potassium hydroxide. A boss or a
.
.. . . . .... .. . . . .. .. . . . . ..... . .... .. . . . . . .. . ... . .
W O 91/20102 PC~r/CA91/OOt92
2`~`g~)35~ ` , .
.
14
pip 18 is ~ormed at the base of the can 10, to give the
cathode or positive contact for the cell.
The electrolyte permeable separator 16 is typically
formed of rayon/polyvinyl alcohol. The anode may have at
its upper end an auxiliary porous gas recombining
electrode 20, which is arranged so as to be wetted by the
electrolyte. At its lower end, the anode may be provided
with an insulated disc or basket 22, to preclude anode
contact with the cathode pip 18.
A current collector nail 24 projects into the anode
14 through a casing cover 26, with the head 28 of the
collector nail 24 being on the outside of tho cover 26 to
form the anode contact ~or the cell. The cover 26 seals
the can 10 by crimping formed around its edge. An
additional anode cover 30 i~ provided to give the anode or
negative contact for the cell.
The cathode may be provided with auxiliary cathode
material to catalyze the reabsorption of hydrogen as set
forth in KORDE5CH and TOMANTSCHGER United States Patent
4,925,747 issued May 15, 1990. The ausiliary eloctrode 20
may have the characteristics set forth in TOMANTSCHGER and
--KORDESCH United States Patent 4,900,642 issued February
13,-1990. ~
- There follo~ several examples of various electrode
-structures according to the present invention, their
~anufacture, and their operating characteristics,
... ... ~. ~.................. .
,~: . . , . - , ,, , .
.. - . , -
WO 91/20102 PCltCA9ltOOt92
2'~ 3 5 a' `' ''"
~XAMPLE I:
Under the general steps of Process I noted above, a
graphite felt (for example, a graphite product available
commercially as PANEX~ CFP 30-10) was impregnated with
manganose nitrate solution and pressed into a layer which
comprised a mixture of carbon available commercially as
SHAWINIGAN BLACRT~ and PTFE. The carbon/PTFE layer
comprised 62.5% carbon, 37.5% PTFE.
The catalyst was thermally formed at 300 degrees C,
in air, a~d resulted in an electrode having catalyst
loading of 1.5 mg. of manganese per sguare centimeter of - :
geometrical electrode surace area. The following table
demonstrates the electrode p¢rf ormance, as a cathode at
the oxygen side of an o~ygen/hydrogen fuel cell system
having 9 N ~OH electrolyte, at 65 degrees C.
Current Density IR free potential
~mA/s~.cm.~ [mV vq. RHE]
0 993
882
838
828
100 822
150 814
-- 200 - - 806
2S0 802
- 300 800
400 796
500 - 788-
(~he IR free potential is determined using laboratory
proc~dures a~d standards, and is measured in millivolts as
... , . . . , ., . . .. , ..... , ........ .. ...... ,, .. ... .. , .. ,, .,~. .... ,., .. ~, .. .. . . . ... . . ... .. .. ....
. . ... . .. . .
W O 91t20102 2 a 8 5 3 ~ ~ PC~r/CA91/00192
:: " . ` I
16
against a Reversible Hydrogen Electrode reference).
~X~hE II:
Again, using the general Process I, an active layer
of nickel FIBREX~ felt was impregnated with manganese
nitrate solution, and again pressed into a carbon/PTFE
layer as in Example I. The catalyst was thermally formed
at 300 degrees C in air, with a catalyst loading of 7.6
mg. of manganese per square centimeter of geometrical
electrode surface area.
The electrode performance in an oxygen/hydrogen fuel
cell system, having 6 N ~OH electrode, at 20 degrees C,
was as foll GWg: :
Current Density IR free potential
~mA/sq.cm.~ tmV vs. RHE]
0 942
828
809
801
100 793
-125 789
150 764 ;
~AMP~
In thi~ case, activated carbon plus an active
catalyst waq used, in a multi-layer PTFE bonded carbon
electrode. .The gas diffusion layer was a carbon~PTFE
layer as described above in Examples I and II.
The active layer comprised carbon black available
commercially as VULCANIM XC 72R which was acti~ated to 30%
weight loss in the ~resence of cobalt-aluminum spinel (5
SUBST~TUT~ SHI~
.. . . . . ..
,.' 1 . '. ' ' . ' . , , . ~,.. .. ~ : ' ' ' ' ' . .. ' . '
... . . ~ . . ..... , . . .. ~ .. :
~ . .... ... - .... . : - :-
. ~ . . ' . , - ~, . .
W O 91/20102 2 b 8 5 3 s ~ PC~r/CA91/00192
mmol cobalt, 10 mmol aluminum per 100 grams of carbon), in
the amount of 67~ together with graphite in the amount of
22~, and PTFE in the amount of 11%.
The catalyst loading was 5 mg. of manganese dioxide
per square centimeter of geometrical electrode surface
area, which may also be expressed as 3.2 mg. of manganese
per s~uare ~entimeter of geometrical electrode surface
area.
The electrode was operated for 220 hours at 100
milliamperes per square centimeter at 65 degrees C in 12 N
KOH. The oxygen and air operation, both, of thé electrode
are set forth in the ~ollowing table:
Current Density IR free potential IR free potential
~mA/sg.cm.~ ~mV vs. RHE~ ~mV vs. RHE~
Oxygen Air
0 1025 1021
937 915
900 880
895 864
100 888 857
150 881 845
200 870 827
250 864 810
300 857 792
.....
The electrode wa~ also operated at 150 milliamperes
per square centimeter, over a varying range of
tomperatures, with air operation being observed as set
.... . .. , . . . . . . ...................... . , . ,. , . ~ . . . . . .
-: .
- . : . - : ,; . ... . . .
. ., ,. ,. , ., . . ::
WO9lt20102 2 0 8 5 3 ~ j PCT/CA91/00192
18
forth in the following table. . -
Temperature IR free potential at 150 mA/cm .,
tc~ Air operation ~mV vs. RHE]
845
841 -
6~ 834
836
831
822
812
810
788
749
737 ~.
Finally, oxygen long term performance in 12 N KOH at 65
degrees C, operating at 100 milliamperes per square
centimoter, was determined as follows:
Oxygen long term performanco in 12 N K~H at 65 C and 100
mA/cm.l~1 .
time IR free potential at 100
ma/cm
~hrs] ~mV vs. RHE~
820
100 890
500 893.
1000 . 890
lS00 ` 851
2000 822
.. ~
-. . . . . - ., -
~: ~ :
Under the Process III, VULCANTN XC 72R carbon was
activat~d in the presence of 5 mmol of cobalt per 100
',.
-~ WO91/20102 2 0 8 ~ 3 5 S PCT/CA91/00192
`' .:
19
grams of carbon in the gas phase, in a carbon dioxide ~as
atmosphere, at lO00 degrees C. This resulted in an active
layer consisting of carbon activated at lOOO-C in carbon
dioxide atmosphere, in the amount of 95.2% containing
cobalt oxide; with PTFE in the amount of 4.8%
The catalyst loading was determined to be l.3 mg. of
cobalt per square centimeter of geometrical electrode
sur$ace area.
The electrode performance was determiner in 9 N XOH,
at 20 degrees C with oxygen, as follows:
Current Density IR free potential
~mA/sq.cm.] tmV vs. R~E~
0 gO5
l 832
796
765
lO0 755
lS0 725
250 689
500 631
750 607
E:Xl~MPLE V:
Along the general lines of preparation of an
electrode in keeping with Example II, an anode was
fabricated, as follows:
The gas dlffusion layer comprised carbon (SHAWINIGAN
BLACK) in the-amount of 50%, with 50% PTFE.
The actiYe layer comprised superconductive carbon
bla~ available commercially as BLACK PEAR~S~ activated
, . . , , . .~; . : ,
WO 91/20102 ~ 2 0 8 ~ 3 5 ~ PCT/CA91/00192
" .
to 30% weight loss in the presence of cobalt-aluminum
spinel (5 mmol cobalt, 10 mmol aluminum per 100 grams of
carbon), together with ruthenium (0.2 grams Ru per 100
grams of carbon) all in the amount of 80~; with 20% PTFE.
The following table demonstrates the performance of
this electrode, as an anode, after 165 hours at 100
milliamperes per square centimeter at 65 degrees C in 12 N :-
XOH, with hydrogen:
Current Density IR free potential
tmA/sq.cm.] tmV vs. RHE]
O
100 11
150 15 `.
200 23
250 28
Opcration at 150 milliamperes per sguare centimeter
over a range of temperatures, resulted in the following: :
:
Temperature IR free potential at 150 :
mA/cm
~C] tmV vs. RXE]
23
23 .
23
. 23
23
' ~ 565 . - . 26
- 50 - - 33
~5 36 i:~
; - 40 ` 43
46
63
92
.
;
.- . ....... , - - - . . . . :
W091/20102 2 0 8 ~3S 5 PCT/CA91/OOt92
21
. ~
Finally, hydrogen long term performance in 12 N KOH ";-
at 65 degrees C and lOO milliamperes per square centimeter
was determined, with the following results:
timeIR free potential at lOO mA/cm
~hrs] ~mV vs. RHE3
10 .j
lOO 39
500 ~S
lOOO 49
lSOO 83
2000 lO9
2500 85
3000 68
There have been described 0lectrodes that are
~ntended ~or use as cathodes in fuel cells (or metal-air
cells), or as auxiliary gas recombining electrodes in
sealed alkaline cells, which electrodes have excellent
operatiny characteristics and which are produced without
the requirement for use of noble metals. When a small
additional amount of noble metal has been used, however,
the electrodes are suitable for uqe as anodes in fuel
c~lls, with egually good operating characteristics and
comparatively low costs o~ ~roduction.
It should be noted that electrodes according to the
present inYention may be used as gas recombining
electrodes in rechargeable alkaline zinc/manganese dioxide
~ells, as woll as in other primary or secondary alkaline
cells. In particular, electrodes such as those described
aboYe in Examples I to IV have utility as gas recombining
.: - ,, - . . . ,-, .,
WO91/20102 ~85~ PCT/CA91/00192 ~:
electrodes such as for oxyqen recombination in alkaline
cells. The ability of such auxiliary porous electrodes to
function at low temperatures and high current densities
has been demonstrated.
The scope of this invention is defined in the
accompanying claims. `:.
,,,, , - :
.. ........ . .... . .... .. . .. . .. .