Note: Descriptions are shown in the official language in which they were submitted.
WO 92/00092 _1- 2 0 8 031 2 5 PCT/US91/04682
LIGAND FOR CD28 RECEPTOR ON B CELLS AND METHODS
FIELD OF THE INVENTION
The present invention relates to the
identification of an interaction between the CD28
receptor and its ligand, the B7 antigen, and to a method
for regulating cellular interactions using the antigen,
fragments and derivatives thereof.
BACKGROUND OF THE INVENTION
The generation of a T lymphocyte ("T cell")
immune response is a complex process involving cell-cell
interactions (Springer et al., A. Rev. Immunol. 5:223-252
(1987)), particularly between T and B cells, and
production of soluble immune mediators (cytokines or
lymphokines) (Dinarello and Mier, New Engl..--Jour. Med.
317:940-945 (1987)). This response is regulated by
several T-cell surface receptors, including the T-cell
receptor complex (Weiss et al., Ann. Rev. Immunol. 4:593-
619 (1986)) and other "accessory" surface molecules
(Springer et al., (1987) supra). Many of these accessory
molecules are naturally occurring cell surface
differentiation (CD) antigens defined by the reactivity
of monoclonal antibodies on the surface of cells
(McMichael, Ed., Leukocyte TypiLicr III, Oxford Univ.
Press, Oxford, N.Y. (1987)).
One such accessory molecule is the CD28
antigen, a homodimeric glycoprotein of the immunoglobulin
superfamily (Aruffo and Seed, Proc. Natl. Acad. Sci.
84:8573-8577 (1987)) found on most mature human T cells
(Damle et al., J. Immunol. 131:2296-2300 (1983)).
Current evidence suggests that this molecule functions in
an alternative T cell activation pathway distinct from
that initiated by the T-cell receptor complex (June et
WO 92/00092 21 0 3 J 3 21 5 PCCT/US91/04682
2
al., Mol. Cell. $ia . 7:4472-4481 (1987)). Monoclonal
antibodies (mAbs) reactive with CD28 antigen can augment
T cell responses initiated by various polyclonal stimuli
(reviewed by June et al., supra). These stimulatory
effects may result from mAb-induced cytokine production
(Thompson et al., Proc. Natl. Acad. Sci 86:1333-1337
(1989); Lindsten et al., Science 244:339-343 (1989)) as a
consequence of increased mRNA stabilization (Lindsten et
al., (1989), supra). Anti-CD28 mAbs can also have
inhibitory effects, i.e., they can block autologous mixed
lymphocyte reactions (Damle at al., Proc. Natl. Acad.
Sci. 78:5096-6001 (1981)) and activation of antigen-
specific T cell clones (Lesslauer at al., Eur. J.
Immunol. 16:1289-1296 (1986)).,
The in vivo function of CD28 antigen is not
known, although its structure (Aruffo and Seed, (1987),
supra) suggests that like other members of the
immunoglobulin superfamily (Williams and Barclay, Ann.
Rev. Immunol. 6:381-405 (1988), it might function as a
receptor. CD28 antigen could conceivably function as a
cytokine receptor, although this seems unlikely since it
shares no homology with other lymphokine or cytokine
receptors (Aruffo and Seed, (1987) supra).
Alternatively, CD28 might be a receptor which
mediates cell-cell contact ("intercellular adhesion").
Antigen-independent intercellular interactions involving
lymphocyte accessory molecules are essential for an
immune response (Springer at al., (1987), supra). For
example, binding of the T cell-associated protein, CD2,
to its ligand LFA-3, a widely expressed glycoprotein
(reviewed in Shaw and Shimuzu, Current Opinion in
Immunology, Eds. Kindt and Long, 1:92-97 (1988)), is
important for optimizing antigen-specific T cell
activation (Moingeon at al., Nature 339:314 (1988)).
Another important adhesion system involves binding of the
LFA-1 glycoprotein found on lymphocytes, macrophages, and
granulocytes (Springer at al., (1987), supra; Shaw and
5
WO 92/00092 2u8 3w PCT/US91/04682
3
Shimuzu (1988), supra) to its ligands ICAM-1 (Makgoba et
al., Nature 331:86-88 (1988)) and ICAM-2 (Staunton et
al., d tune 339:61-64 (1989)). The T cell accessory
molecules CD8 and CD4 strengthen T cell adhesion by
interaction with MHC class I (Norment et al., Nature
336:79-81 (1988)) and class II (Doyle and Strominger,
Nature 330:256-259 (1987)) molecules, respectively.
"Homing receptors" are important for control of
lymphocyte migration (Stoolman, Cell 56:907-910 (1989)).
The VLA glycoproteins are integrins which appear to
mediate lymphocyte functions requiring adhesion to
extracellular matrix components (Hemler, Immunology Today
9:109-113 (1988)). The CD2/LFA-3, LFA-1/ICAM-1 and ICAM-
2, and VLA adhesion systems are distributed on a wide
variety of cell types (Springer et al., (1987), supra;
Shaw and Shimuzu, (1988,) supra and Hemler, (1988),
supra).
Intercellular adhesion interactions mediated by
integrins are strong interactions that may mask other
intercellular adhesion interactions. For example,
interactions mediated by integrins require divalent
cations (Kishimoto et al., Adv. Immunol. 46:149-182
(1989). These interactions may mask other intercellular
adhesion interactions that are divalent cation
independent. Therefore, it would be useful to develop
assays that permit identification of non-integrin
mediated ligand/receptor interactions.
T cell interactions with other cells such as B
cells are essential to the immune response. Levels of
many cohesive molecules found on T cells and B cells
increase during an immune response (Springer et al.,
(1987), supra; Shaw and Shimuzu, (1988), supra; Hemler
(1988), supra). Increased levels of these molecules may
help explain why activated B cells are more effective at
stimulating antigen-specific T cell proliferation than
are resting B cells (Kaiuchi et al., J. Immunol. 131:109-
114 (1983); Kreiger et al., J. Immunol 135:2937-2945
WO 92/00092 2 U 0 J PCr/US91/04682
4
(1985); McKenzie, J. Immunol. 141: 2907-2911 (1988); and
Hawrylowicz and Unanue, J. Immunol. 141:4083-4088
(1988)). The fact that anti-CD28 mAbs inhibit mixed
lymphocyte reactions (MLR) may suggest that the CD28
antigen is also an adhesion molecule.
Optimal activation of B lymphocytes and their
subsequent differentiation into immunoglobulin secreting
cells is dependent on the helper effects of major
histocompatibility complex (MHC) class II antigen (Ag)-
reactive CD4 positive T helper (CD4+ TO cells and is
mediated via both direct (cognate) Th-B cell intercellular
contact-mediated interactions and the elaboration of
antigen-nonspecific cytokines (non-cognate activation;
see, e.g. Noel and Snow, Immunol. Today 11:361 (1990)).
Although Th-derived cytokines can stimulate B cells
(Moller, Immunol. Rev. 99:1 (1987)), their synthesis and
directional exocytosis is initiated and sustained via
cognate interactions between antigen-primed Th cells and
antigen-presenting B cells (Moller, supra). The
successful outcome of Th-B interactions requires
participation of transmembrane receptor-ligand pairs of
co-stimulatory accessory/adhesion molecules on the
surface of Th and B cells which include CD2 (LFA-2); CD58
(LFA-3), CD4:MHC class II, CDlla/CD18 (LFA-1):CD54 (1CAM-
1).
During cognate Th:B interaction, although both
Th and B cells cross-stimulate each other, their
functional differentiation is critically dependent on the
provision by Th cells of growth and differentiation-
inducing cytokines such as IL-2, IL-4 and IL-6 (Noel,
supra, Kupfer et al., supra, Brian, supra and.Moller,
supra). Studies by Poo et al. (Nature 332:378 (1988)) on
cloned Th:B interaction indicate that interaction of the T
cell receptor complex (TcR) with nominal Ag-MHC class II
on B cells results in focused release of Th cell-derived
cytokines in the area of Th and B cell contact
(vectorially oriented exocytosis). This may ensure the
NO 92/00092 ~ 0 8 P 3( J PCT/US91/04682
activation of only B cells presenting antigen to Th cells,
and also avoids activation of bystander B cells.
It was proposed many years ago that B
5 lymphocyte activation requires two signals (Bretscher and
Cohn, Science 169:1042-1049 (1970)) and now it is
believed that all lymphocytes require two signals for
their optimal activation, an antigen specific or clonal
signal, as well as a second, antigen non-specific signal
(Janeway, supra).The signals required for a T helper cell
(Th) antigenic response are provided by antigen-presenting
cells (APC). The first signal is initiated by
interaction of the T cell receptor complex (Weiss, J.
Clin. Invest. 86:1015 (1990)) with antigen presented in
the context of class II major histocompatibility complex
(MHC) molecules on the APC (Allen, Immunol. Today 8:270
(1987)). This antigen-specific signal is not sufficient
to generate a full response, and in the absence of a
second signal may actually lead to clonal inactivation or
anergy (Schwartz, Science 248:1349 (1990)). The
requirement for a second "costimulatory" signal provided
by the MHC has been demonstrated in a number of
experimental systems (Schwartz, supra; Weaver and Unanue,
Immunol. Today 11:49 (1990)). The molecular nature of
these second signal(s) is not completely understood,
although it is clear in some cases that both soluble
molecules such as interleukin (IL)-1 (Weaver and Unanue,
supra) and membrane receptors involved in intercellular
adhesion (Springer, Nature 346:425 (1990)) can provide
costimulatory signals.
Freeman et al. (J. 1 unol. 143(8):2714-2722
(1989)) isolated and sequenced a cDNA clone encoding a B
cell activation antigen recognized by mAb B7 (Freeman et
al., J. immunol. 138:3260 (1987)). COS cells transffcted
with this cDNA have been shown to stain by both labeled
mAb B7 and mAb BB-1 (Clark et al., Human Immunol. 16:100-
113 (1986); Yokochi et al., J. Immunol. 128:823 (1981));
Freeman et al., (1989) supra; and Freedman et al.,
WO 92/00092 2, 0 3 J 1) ?, 5, PCT/US91/04682
6
(1987), supra)). Expression of the B cell activation
antigen has been detected on cells of other lineages.
For example, studies by Freeman et al. (1989) have shown
that monocytes express low levels of mRNA for B7.
Expression of soluble derivatives of cell-
surface glycoproteins in the immunoglobulin gene
superfamily has been achieved for CD4, the receptor for
HIV-1, using hybrid fusion molecules consisting of DNA
sequences encoding portions of the extracellular domain
of CD4 receptor fused to antibody domains (human
immunoglobulin C gamma 1), as described by Capon et al.,
Nature 337:525-531 (1989).
While the CD28 antigen has functional and
structural characteristics of a receptor, until now, a
natural ligand for this molecule has not been identified.
It would be useful to identify ligands that bind with the
CD28 antigen and other receptors and to use such
ligand(s) to regulate cellular responses, such as T cell
and B cell interactions, for use in treating pathological
conditions.
SUMMARY OF THE INVENTION
Accordingly, the present invention identifies
the B7 antigen as a ligand recognized by the CD28
receptor. The B7 antigen, or its fragments or
derivatives are reacted with CD28 positive T cells to
regulate T cell interactions with other cells.
Alternatively, the CD28 receptor, its fragments or
derivatives are reacted with B7 antigen to regulate
interactions of B7 positive cells with T cells. In
addition, antibodies or other molecules reactive with the
B7 antigen or CD28 receptor may be used to inhibit
interaction of cells associated with these molecules,
thereby regulating T cell responses.
WO 92/00092 2 " 6) " PCT/U591/04682
7
A preferred embodiment of the invention
provides a method for regulating CD28 specific T cell
interactions by reacting CD28 positive T cells with B7
antigen, or its fragments or derivatives, so as to block
the functional interaction of T cells with other cells.
The method for reacting a ligand for CD28 with T cells
may additionally include the use of anti-CD monoclonal
antibodies such as anti-CD2 and/or anti-CD3 monoclonal
antibody.
In an alternative embodiment, the invention
provides a method for regulating immune responses by
contacting CD28 positive T cells with fragments
containing at least a portion of the DNA sequence
encoding the amino acid sequence corresponding to the
extracellular domain of B7 antigen. In addition,
derivatives of B7 antigen may be used to regulate immune
responses, wherein the derivatives are fusion protein
constructs including at least a portion of the
extracellular domain of B7 antigen and another protein.,
such as human immunoglobulin C gamma 1, that alters tio:..
solubility, binding affinity and/or valency of B7
antigen. For example, in a preferred embodiment, DNA
encoding amino acid residues from about position 1 to
about position 215 of the sequence corresponding to the
extracellular domain of B7 antigen is joined to DNA
encoding amino acid residues of the sequences
corresponding to the hinge, CH2 and CH3 regions of human
Ig C71 to form a DNA fusion product which encodes B71g
fusion protein.
In another preferred embodiment, DNA encoding
amino acid residues from about position 1 to about
position 134 of the sequence corresponding to the
extracellular domain of the CD28 receptor is joined to
DNA encoding amino acid residues of the sequences
corresponding to the hinge, CH2 and CH3 regions of human
Ig Cy1 to form a CD28Ig fusion protein.
WO 92/00092 208 J ; j) ,-
WO
S
Alternatively, fragments or derivatives of the
CD28 receptor may be reacted with B cells to bind the B7
antigen and regulate T cell/B cell interactions. The
methods for regulating T cell interactions may be further
supplemented with the addition of a cytokine.
in another embodiment, the invention provides a
method for treating immune system diseases mediated by T
cell by administering B7 antigen, including B71g fusion
protein, to react with T cells by binding the CD28
receptor.
In yet another embodiment, a method for
inhibiting T cell proliferation in graft versus host
disease is provided wherein CD28 positive T cells are
reacted with B7 antigen, for example in the form of the
B71g fusion protein, to bind to the CD28 receptor, and an
immunosuppressant is administered.
The invention also provides a cell adhesion
assay to identify ligands that interact with target
receptors that mediate intercellular adhesion,
particularly adhesion that is divalent cation
independent.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 are bar graphs showing the results of cellular
adhesion experiments using CD28 positive (CD28'`) and CD28
negative (CD28') CHO cells as described in Example 1,
Infra.
Figure 2 are micrographs of the cellular adhesion studies
of Figure 1, as described in Example 1, infra.
Figure 3 are bar graphs of experiments testing the
ability of different human cell lines and normal and
activated murine spleen B cells to adhere to CD28+ CHO
cells, as described in Example 1, infra.
WO 92/00092 2 0 0 6 v 2" PCT/US91/04682
9
Figure 4 is a graph of the effects of blocking by mAbs on
CD28-mediated adhesion to human B cells, as described in
Example 1, infra.
Figure 5 is a bar graph of the results of adhesion
between COS cells transfected with B7 antigen and CD2' or
CD28" CHO cells, as described in Example 1, infra.
Figure 6 is a bar graph demonstrating the effect of anti-
CD28 and anti-B7 mAbs on T cell proliferation as
described in Example 2, infra.
Figure 7 is graphs showing the effects of DR7-primed
CD4'`CD45RO'' Th cells on differentiation of B cells into
immunoglobulin secreting cells, as'described in Example
2, infra (7a: IgM production by SKW B cells; 7b: IgG
production by CESS B cells).
Figure 8 is graphs showing the effect of anti-CD28 and
anti-B7 mAbs on the Th-induced production of
immunoglobulin by B cells as described in Example 2,
infra (8a: IgM production, 8b: IgG production).
Figure 9 is a diagrammatic representation of B71g (9a)
and CD28Ig (9b) protein fusion constructs as described in
Example 3, infra (dark shaded regions = oncostatin M;
unshaded regions = B7 and CD28, stippled regions = human
Ig Cyl).
Figure 10 is a photograph of a gel obtained from
purification of B71g and CD28 protein fusion constructs
as described in Example 3, infra.
Figure 11 depicts the results of FACSR analysis of binding
of the B71g and CD28Ig fusion proteins to transfected CHO
cells as described in Example 3, infra.
Figure 12 is a graph illustrating competition binding
analysis of Tut-labeled B71g fusion protein to
WO 92/00092 9 PCT/US91/04682
w V ) i!)I.r3
immobilized CD28Ig fusion protein as described in Example
3, infra.
Figure 13 is a graph showing the results of Scatchard
5 analysis of B71g fusion protein binding to immobilized
CD28Ig fusion protein as described in Example 3, infra.
Figure 14 is a graph of FACSR profiles of.B71g fusion
protein binding to PHA blasts as described in Example 3,
10 infra.
Figure 15 is an autoradiogram of 1251-labeled proteins
immunoprecipitated by B71g as described in Example 3,
infra.
Figure 16 is a graph showing the effect of B71g binding
to CD28 on CD28-mediated adhesion as described in Example
3, infra.
Figure 17 is a photograph of the results of RNA blot
analysis of the effects of B7 on accumulation of IL-2
mRNA as described in Example 3, infra.
DETAILED DESCRIPTION OF THE INVENTION
in order that the invention herein described
may be more fully understood, the following description
is set forth.
This invention is directed to the
identification of a ligand reactive with CD28 antigen
(hereafter referred to as "CD28 receptor"), and to
methods of using the ligand and its fragments and
derivatives, including fusion proteins. Also disclosed
is a cell adhesion assay method to detect ligands for
cell surface receptors.
Recently, Freeman et al., (3. Immunol.
143(8):2714-2722 (1989)) isolated and sequenced a cDNA
CA 02086325 2004-04-07
WO 92/00092 PCT/US91 /04682
11
clone encoding a B cell activation antigen recognized by
monoclonal antibody (mAb) B7 (Freedman et al., J.
Immunol. 139:3260 (1987)). COS cells transfected with
this cDNA were shown to stain by both mAb B7 and mAb BB-1
(Clark et al., Human Immunology 16:100-113 (1986), and
Yokochi et al., (1981), supra; Freeman et al., (1989)
supra; and Freedman et al., (1987), supra)). The ligand
for CD28 was identified by the experiments described
herein, as the-B7/BB-1 antigen isolated by Freeman et
al., (Freedman et al., and Freeman et al., supra.
For convenience, the ligand for CD28,
identified as the B7/BB-1 antigen, is referred to herein.
as the "B7 antigen".
The term "fragment" as used herein means, a
portion of the amino acid sequence corresponding to the
B7 antigen or CD28 receptor. For example, a fragment of
the B7 antigen useful in the method of the present
invention is a polypeptide containing a portion of the
amino acid sequence corresponding to the extracellular
portion of the B7 antigen, i.e. the DNA encoding amino
acid residues from position 1 to 215 of the sequence
corresponding to the B7 antigen described by Freeman et
al., supra 143(8):2714-2722 (1989). This reference dicloses
the amino acid sequence of the B7 polypeptide as shown in SEQ ID NO:8
Gly Leu Ser His Phe Cys Ser Gly Val Ile His Val Thr Lys Glu Val
1 5 10 15
Lys Glu Val Ala Thr Leu Ser Cys Gly His Asn Val Ser Val Glu Glu
20 25 30
Leu Ala Gln Thr Arg Ile Tyr Trp Gln Lys Glu Lys Lys Met Val Leu
40 45
Thr Met Met Ser Gly Asp Met Asn Ile Trp Pro Glu Tyr Lys Asn Arg
50 55 60
Thr Ile Phe Asp Ile Thr Asn Asn Leu Ser Ile Val Ile Leu Ala Leu
65 70 75 80
CA 02086325 2004-04-07
11-1
Arg Pro Ser Asp Glu Gly Thr Tyr Glu Cys Val Val Leu Lys Tyr Glu
85 90 95
Lys Asp Ala Phe Lys Arg Glu His Leu Ala Glu Val Thr Leu Ser Val
100 105 110
Lys Ala Asp Phe Pro Thr Pro Ser Ile Ser Asp Phe Glu Ile Pro Thr
115 120 125
Ser Asn Ile Arg Arg Ile Ile Cys Ser Thr Ser Gly Gly Phe Pro Glu
130 135 140
Pro His Leu Ser Trp Leu Glu Asn Gly Glu Glu Leu Asn Ala Ile Asn
145 150 155 160
Thr Thr Val Ser Gin Asp Pro Glu Thr Glu Leu Tyr Ala Val Ser Ser
165 170 175
Lys Leu Asp Phe Asn Met Thr Thr Asn His Ser Phe Met Cys Leu Ile
180 185 190
Lys Tyr Gly His Leu Arg Val Asn Gln Thr Phe Asn Trp Asn Thr Thr
195 200 205
Lys Gln Glu His Phe Pro Asp Asn
210 215
A fragment of the CD28 antigen that may be
used is a polypeptide containing amino acid residues from
about position 1 to about position 134 of the sequence
corresponding to the CD28 receptor as described by Aruffo
and Seed, Proc. Natl. Acad. Sci. (USA) 84:8573-8577
(1987). This reference discloses the amino acid sequence
of the CD28 receptor as shown in SEQ ID NO:9 :
Asn Lys Ile Leu Val Lys Gln Ser Pro Met Leu Val Ala Tyr Asp Asn
1 5 10 15
Ala Val Asn Leu Ser Cys Lys Tyr Ser Tyr Asn Leu Phe Ser Arg Glu
25 30
CA 02086325 2003-01-20
11-2
Phe Arg Ala Ser Leu His Lys Gly Leu Asp Ser Ala Val Glu Val Cys
35 40 45
Val Val Tyr Gly Asn Tyr Ser Gin Gln Leu Gin Val Tyr Ser Lys Thr
50 55 60
Gly Phe Asn Cys Asp Gly Lys Leu Gly Asn Glu Ser Val Thr Phe Tyr
65 70 75 80
Leu Gln Asn Leu Tyr Val Asn Gln Thr Asp Ile Tyr Phe Cys Lys Ile
85 90 95
Glu Val Met Tyr Pro Pro Pro Tyr Leu Asp Asn Glu Lys Ser Asn Gly
100 105 110
Thr Ile Ile His Val Lys Gly Lys His Leu Cys Pro Ser Pro Leu Phe
115 120 125
Pro Gly Pro Ser Lys Pro
130
The term "derivative" as used herein includes a
fusion protein consisting of a polypeptide including
portions of the amino acid sequence corresponding to the
B7 antigen or CD28 antigen. For example, a derivative of
the B7 antigen useful in the method of the present
invention is a B71g fusion protein that comprises a
polypeptide corresponding to the extracellular domain of
WO 92/00092 % 0 Is J 1 y PGT/US91/04682
12
the B7 antigen and an immunoglobulin constant region that
alters the solubility, affinity and/or valency (valency
is defined herein as the number of binding sites
available per molecule) of the B7 antigen.
The term "derivative" also includes monoclonal
'antibodies reactive with the B7 antigen or CD28 receptor,
or fragments thereof, and antibodies reactive with the
B71g and CD28Ig fusion proteins of the invention.
The B7 antigen and/or its fragments or
derivatives for use in the present invention may be
produced in recombinant form using known molecular
biology techniques based on the cDNA sequence published
by Freeman et al., supra. Specifically, cDNA sequences
encoding the amino acid sequence corresponding to the B7
antigen or fragments or derivatives thereof can be
synthesized by the polymerase chain reaction (see U.S.
Patent No. 4,683,202) using primers derived from the
published sequence of the antigen (Freeman et al.,supra).
These cDNA sequences can then be assembled into a
eukaryotic or prokaryotic expression vector and the
resulting vector can be used to direct the synthesis of
the ligand for CD28 by appropriate host cells, for
example COS or CHO cells. CD28 receptor and/or its
fragments or derivatives may also be produced using
recombinant methods.
In a preferred embodiment, DNA encoding the
amino acid sequence corresponding to the extracellular
domain of the B7 antigen, containing amino acids from
.about position 1 to about position 215, is joined to DNA
encoding the amino acid sequences corresponding to the
hinge, CH2 and CH3 regions of human Ig C71, using PCR, to
form a construct that is expressed as B71g fusion
protein. DNA encoding the amino acid sequence
corresponding to the B7Ig fusion protein has been
deposited with the American Type Culture Collection
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
13
(ATCC) in Rockville, Maryland, under the Budapest Treaty
on May 31, 1991 and accorded accession number 68627.
In another embodiment, DNA encoding the amino
acid sequence corresponding to the extracellular domain
of the CD28 receptor, containing amino acids from about
position 1 to about position 134, is joined-to DNA
encoding the amino acid sequences corresponding to the
hinge, CH2 and CH3 regions of human Ig C71 using PCR to
form a construct expressed as CD28Ig fusion protein. DNA
encoding the amino acid sequence corresponding to the
CD28Ig fusion protein has been deposited in the ATCC, in
Rockville, Maryland under the Budapest Treaty on May 31,
1991 and accorded accession number 68628.
The techniques for assembling and expressing
DNA encoding the amino acid sequences corresponding to B7
antigen and soluble B71g and CD28Ig fusion proteins, e.g
synthesis of oligonucleotides, PCR, transforming cells,
constructing vectors, expression systems, and the like
are well-established in the art, and most practitioners
are familiar with the standard resource materials for
specific conditions and procedures. However, the
following paragraphs are provided for convenience and
notation of modifications where necessary, and may serve
as a guideline.
Cloning and Expression of Coding Sequences for Receptors
and Fusion Proteins
cDNA clones containing DNA encoding CD28 and B7
proteins are obtained to provide DNA for assembling CD28
and B7 fusion proteins as described by Aruffo and Seed,
Proc. Natl. Acad. Sci. USA 84:8573-8579 (1987) (for
CD28); and Freeman et al., J. Immunol. 143:2714-2722
(1989) (for B7).
Alternatively, cDNA clones may be prepared from RNA
obtained from cells expressing B7 antigen and CD28
receptor based on knowledge of the published sequences
WO 92/00092 0 J PCT/US91/04682
14
for these proteins (Aruffo and Seed, and Freeman, su ra)
using standard procedures.
The cDNA is amplified using the polymerase
chain reaction ("PCR") technique (see U.S. Patent Nos.
4,683,195 and 4,683,202 to Mullis et al. and Mullis &
Faloona, Methods Enzymol. 154:.335-350 (1987)) using
synthetic oligonucleotides encoding the sequences
corresponding to the extracellular domain of the CD28 and
B7 proteins as primers. PCR is then used to adapt the
fragments for ligation to the DNA encoding amino acid
fragments corresponding to the human immunoglobulin
constant y 1 region, i.e. sequences encoding the hinge,
CH2 and CH3 regions of Ig C71 to form B7Ig and CD28Ig
fusion constructs and to expression plasmid DNA to form
cloning and expression plasmids containing sequences
corresponding to B7 or CD28 fusion proteins.
To produce large quantities of cloned DNA,
vectors containing DNA encoding the amino acid sequences
corresponding to the fusion constructs of the invention
are transformed into suitable host cells, such as the
bacterial cell line MC1061/p3 using standard procedures,
and colonies are screened for the appropriate plasmids.
The clones obtained as described above are then
transfected into suitable host cells for expression.
Depending on the host cell used, transfection is
performed using standard techniques appropriate to such
cells. For example, transfection into mammalian cells is
accomplished using DEAE-dextran mediated transfection,
CaPO4 co-precipitation, lipofection, electroporation, or
protoplast fusion, and other methods known in the art
including: lysozyme fusion or erythrocyte fusion,
scraping, direct uptake, osmotic or sucrose shock, direct
microinjection, indirect microinjection such as via
erythrocyte-mediated techniques, and/or by subjecting
host cells to electric currents. The above list of
transfection techniques is not considered to be
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
exhaustive, as other procedures for introducing genetic
information into cells will no doubt be developed.
Expression plasmids containing cDNAs encoding
5 sequences corresponding to CD28 and B7 for cloning and
expression of CD28Ig and B71g fusion proteins include the
OMCD28 and OMB7, vectors modified from vectors described
by Aruffo and seed, Proc. Natl.-Acad. Sci. USA (1987),
supra, (CD28);=and Freeman et al., (1989), supra, (B7).
Preferred host cells for expression of CD28Ig and B71g
proteins include COS and CHO cells.
Expression in eukaryotic host cell cultures
derived from multicellular organisms is preferred (see
Tissue Cultures, Academic Press, Cruz and Patterson, Eds.
(1973)). These systems have the additional advantage of
the ability to splice out introns and thus can be used
directly to express genomic fragments. Useful host cell
lines include Chinese hamster ovary (CHO), monkey kidney
(COS), VERO and HeLa cells. In the present invention,
cell lines stably expressing the fusion constructs are
preferred.
Expression vectors for such cells ordinarily
include promoters and control sequences compatible with
mammalian cei such as, for example, CMV promoter (CDM8
vector) and avian sarcoma virus (ASV) (ILN vector). Other
commonly used early and late promoters include those from
Simian Virus 40 (SV 40) (Fiers, et al., Nature 273:113
(1973)), or other viral promoters such as those derived
from polyoma, Adenovirus 2, and bovine papilloma virus.
The controllable promoter, hMTII (Karin, et al., Nature
299:797-802 (1982)) may also be used. General aspects of
mammalian cell host system transformations have been
described by Axel (U.S. Patent No. 4,399,216 issued Aug.
16, 1983). It now appears, that "enhancer" regions are
important in optimizing expression; these are, generally,
sequences found upstream or downstream of the promoter
WO 92/00092 2 0 S U '21 5 PCT/US91/04682
16
region in non-coding DNA regions. Origins of replication
may be obtained, if needed, from viral sources. However,
integration into the chromosome is a common mechanism for
DNA replication in eukaryotes.
Although preferred host cells for expression of
the DNA constructs include eukaryotic cells such as COS
or CHO cells, other eukaryotic microbes may be used as
hosts. Laboratory strains of Saccharomyces cerevisiae,
Baker's yeast, are most used although other strains such
as Schizosaccharomyces pombe may be used. Vectors
employing, for example, the 2 origin of replication of
Broach, Meth. Enz. 101:307 (1983), or other yeast
compatible origins of replications (see, for example,
Stinchcomb et al., Nature 282:39 (1979)); Tschempe et
al., Gene 10:157 (1980); and Clarke et al., Meth. Enz.
101:300 (1983)) may be used. Control sequences for yeast
vectors include promoters for the synthesis of glycolytic
enzymes (Hess et al., J. Adv. Enzyme Rea. 7:149 (1968);
Holland et al., Biochemistry 17:4900 (1978)). Additional
promoters known in the art include the CMV promoter
provided in the CDM8 vector (Toyama and Okayama, FEBS
268:217-221 (1990); the promoter for 3-phosphoglycerate
kinase (Hitzeman et al., J. Biol. Chem. 255:2073 (1980)),
and those for other glycolytic enzymes. Other promoters,
which have the additional advantage of transcription
controlled by growth conditions are the promoter regions
for alcohol dehydrogenase 2, isocytochrome C, acid
phosphatase, degradative enzymes associated with,nitrogen
metabolism, and enzymes responsible for maltose and
galactose utilization. It is also believed terminator
sequences are desirable at the 3' end of the coding
sequences. Such terminators are found in the 3'
untranslated region following the coding sequences in
yeast-derived genes.
Alternatively, prokaryotic cells may be used as
hosts for expression. Prokaryotes most frequently are
represented by various strains of E. coli; however, other
WO 92/00092 PCf/US91/04682
17
microbial strains may also be used. Commonly used
prokaryotic control sequences which are defined herein to
include promoters for transcription initiation,
optionally with an operator, along with ribosome binding
site sequences, include such commonly used promoters as
the beta-lactamase (penicillinase) and lactose (lac)
promoter systems (Chang et al., Nature 198: 1056 (1977)),
the tryptophan (trp) promoter system (Goeddel et al.,
Nucleic Acids Res. 8:4057 (1980)) and the lambda derived
PL promoter and N-gene ribosome binding site (Shimatake et
al., Nature 292:128 (1981)).
The nucleotide sequences encoding the amino
acid sequences corresponding to the CD28Ig and B71g
fusion proteins, may be expressed in a variety of systems
as set forth below. The cDNA may be excised by suitable
restriction enzymes and ligated into suitable prokaryotic
or eukaryotic expression vectors for such expression.
Because CD28 receptors occur in nature as dimers,'it is
believed that successful expression of these proteins
requires an expression system which permits these
proteins to form as dimers. Truncated versions of these
proteins (i.e. formed by introduction of a stop codon
into the sequence at a position upstream of the
transmembrane region of the protein) appear not to be
expressed. The expression of CD28 antigen in the form of
a fusion protein permits dimer formation of the protein.
Thus, expression of CD28 antigen as a fusion product is
preferred in the present invention.
Sequences of the resulting fusion protein
constructs are confirmed by DNA sequencing using known
procedures, for example as described by Sanger et al.,
Proc. Natl. Acad. Sc!. USA 74:5463 (1977) as further
described by Messing et al., Nucleic Acids Res. 9:309
(1981) or by the method of Maxam et al. Methods Enzyinol.
65:499 (1980)).
WO 92100092 , j ? 3 PCT/US91/04682
18
Recovery of Protein Products
As noted above, the CD28 receptor is not
readily expressed as a mature protein using direct
expression of DNA encoding the amino acid sequence
corresponding to the truncated protein. To enable
homodimer formation, it is preferred that DNA encoding
the amino acid sequence corresponding to the
extracellular domain of CD28 and including the codons for
a signal sequence such as oncostatin M in cells capable
of appropriate processing, is fused with DNA encoding
amino acids corresponding to the Fc domain of a naturally
dimeric protein. Purification of the fusion protein
products after secretion from the cells is thus
facilitated using antibodies reactive with the anti-
immunoglobulin portion of the fusion proteins. When
secreted into the medium, the fusion protein product is
recovered using standard protein purification techniques,
for example by application to protein A columns.
In addition to the fusion proteins of the
invention, monoclonal antibodies reactive with the B7
antigen and CD28 receptor, and reactive with B71g and
CD28Ig fusion proteins, may be produced by hybridomas
prepared using known procedures, such as those introduced
by Kohler and Milstein (see Kohler and Milstein, Nature,
256:495-97 (1975), and modifications thereof, to regulate
cellular interactions.
These techniques involve the use of an animal
which is primed to produce a particular antibody. The
animal can be primed by injection of an immunogen (e.g.
the B71g fusion protein) to elicit the desired immune
response, i.e. production of antibodies reactive with the
ligand for CD28, the B7 antigen, from the primed animal.
A primed animal is also one which is expressing a
disease. Lymphocytes derived from the lymph-nodes,
spleens or peripheral blood of primed, diseased animals
can be used to search for a particular antibody. The
WO 92/00092 2 0 8 6 3 3 PCT/US91/04682
19
lymphocyte chromosomes encoding desired immunoglobulins
are immortalized by fusing the lymphocytes with myeloma
cells, generally in the presence of a fusing agent such
as polyethylene glycol (PEG). Any of a number of myeloma
cell lines may be used as a fusion partner according to
standard techniques; for example, the P3-NS1/1-Ag4-1, P3-
x63-Ag8.653, Sp2/0-Ag14, or HL1-653 myeloma lines. These
myeloma lines are available from the ATCC, Rockville,
Maryland.
The resulting cells, which include the desired
hybridomas, are then grown in a selective medium such as
HAT medium, in which unfused parental myeloma or
lymphocyte cells eventually die. Only the hybridoma
cells survive and can be grown under limiting dilution
conditions to obtain isolated clones. The supernatants
of the hybridomas are screened for the presence of the
desired specificity, e.g. by immunoassay techniques using
the B71g fusion protein that has been used for
immunization. Positive clones can then be subcloned
under limiting dilution conditions, and the monoclonal
antibody produced can be isolated.
Various conventional methods can be used for
isolation and purification of the monoclonal antibodies
so as to obtain them free from other proteins and
contaminants. Commonly used methods for purifying
monoclonal antibodies include ammonium sulfate
precipitation, ion exchange chromatography, and affinity
chromatography (see Zola et al., in Monocl2nal Hybridama
Antibodies: Techniques and Applications, Hurell (ed.) pp.
51-52 (CRC Press, 1982)). Hybridomas produced according
.to these methods can be propagated in vitro or in vivo
(in ascites fluid) using techniques known in the art (see
generally Fink et al., rog. Clin. Pathol., 9:121-33
(1984), Fig. 6-1 at p. 123).
Generally, the individual cell line may be
propagated in vitro, for example, in laboratory culture
WO 92/00092 2 0 8 6 32'^')' PCT/US91/04682
~j 20
vessels, and the culture medium containing high
concentrations of a single specific monoclonal antibody
can be harvested by decantation, filtration, or
centrifugation.
In addition, fragments of these antibodies
containing the active binding region of the extracellular
domain of B7 or CD28 antigen, such as Fab, F(ab')2 and Fv
fragments, may be produced. Such fragments can be
produced using techniques well established in the art
(see e.g. Rousseaux at al., in Methods Enzymol., 121:663-
69, Academic Press (1986)).
USES
General
The experiments described below in the
Examples, suggest that the CD28 receptor and its ligand,
the B7 antigen, may function in vivo by mediating T cell
interactions with other cells such as B cells. The
functional consequences of these interactions may be
induced or inhibited using ligands that bind to the
native CD28 receptor or the B7 antigen.
It is expected that administration of the B7
antigen will result in effects similar to the use of
anti-CD28 monoclonal antibodies (mAbs) reactive with the
CD28 receptor in vivo. Thus, because anti-CD28 mAbs may
exert either stimulatory or inhibitory effects on T
cells, depending, in part, on the degree of crosslinking
or "aggregation" of the CD28 receptor (Damle, J. Immunol.
140:1753-1761 (1988); Ledbetter at al., Blood 75(7):1531-
1539 (1990)) it is expected that the B7 antigen, its
fragments and derivatives, will act to stimulate or
inhibit T cells in a manner similar to the effects
observed for an anti-CD28 monoclonal antibody, under
similar conditions vivo. For example, administration
of B7 antigen, e.g. as a soluble B71g fusion protein to
react with CD28 positive T cells, will bind the CD28
receptor on the T cells and result in inhibition of the
WO 92/00092 s , q ~, PCT/US91/04682
-iliUt)od~
21
functional responses of T cells. Under conditions where
T cell interactions are occurring as a result of contact
between T cells and B cells, binding of introduced B7
antigen in the form of a fusion protein that binds to
CD28 receptor on CD28 positive T cells should interfere,
i.e. inhibit, the T cell interactions with B cells.
Likewise, administration of the CD28 antigen, or its
fragments and derivatives in vivo, for example in the
form of a soluble CD28Ig fusion protein, will result in
binding of the soluble CD28Ig to B7 antigen, preventing
the endogenous stimulation of CD28 receptor by B7
positive cells such as activated B cells, and interfering
with the interaction of B7 positive cells with T cells.
Alternatively, based on the known effects of
aggregating the CD28 receptor, either by reacting T cells
with immobilized ligand, or by crosslinking as described
by Ledbetter et al., Blood 75(7):1531-1539 (1990)), the
B7 antigen, and/or its fragments or derivatives, may be
used to stimulate T cells, for example by immobilizing B7
antigen or B71g fusion protein, for reacting with the T
cells. The activated T cells stimulated in this manner
in vitro may be used in vivo in adoptive therapy.
Therefore, the B7 antigen and/or fragments or
derivatives of the antigen may be used to react with T
cells to regulate immune responses mediated by functional
T cell responses to stimulation of the CD28 receptor.
The B7 antigen may be presented for reaction with CD28
positive T cells in various forms. Thus, in addition to
employing activated B cells expressing the B7 antigen,
the B7 antigen may be encapsulated, for example in
liposomes, or using cells that have been genetically
engineered, for example using gene transfer, to express
the antigen for stimulation of the CD28 receptor on T
cells.
The CD28 receptor, and/or its fragments or
derivatives, may also be used to react with cells
WO 92/00092 PCF/US91/04682
208634'
22
expressing the B7 antigen, such as B cells. This
reaction will result in inhibition of T cell activation,
and inhibition of T cell dependent B cell responses, for
example as a result of inhibition of T cell cytokine
production.
In an additional embodiment of the invention,
other reagents, such as molecules reactive with B7
antigen or the CD28 receptor are used to regulate T
and/or B cell responses. For example, antibodies
reactive with the CD28Ig fusion proteins, and Fab
fragments of CD28Ig, may be prepared using the CD28Ig
fusion protein as immunogen, as described above. These
anti-CD28 antibodies may be screened to identify those
capable of inhibiting the binding of the B7 antigen to
CD28 antigen. The antibodies or antibody fragments such
as Fab fragments may then be used to react with the T
cells, for example, to inhibit CD28 positive T cell
proliferation. The use of Fab fragments of the 9.3
monoclonal antibody, or Fab fragments of the anti-CD28Ig
monoclonal antibodies as described herein, is expected to
prevent binding of CD28 receptor on T cells to B7
antigen, for example on B cells. This will result in
inhibition of the functional response of the T cells.
Similarly, anti-B7 monoclonal antibodies such
as. BB-1 mAb, or anti-B71g monoclonal antibodies prepared
as described above using B71g fusion protein as
immunogen, may be used to react with B7 antigen positive
cells such as B cells to inhibit B cell interaction via
the B7 antigen with CD28 positive T cells.
In another embodiment the B7 antigen may be
used to identify additional compounds capable of
regulating the interaction between the B7 antigen and the
CD28 antigen. Such compounds may include soluble
fragments of the B7 antigen or CD28 antigen or small
naturally occurring molecules that can be used to react
with B cells and/or T cells. For example, soluble
CA 02086325 2000-08-17
WO 92/00092 PCr/US91 /04682
23
fragments of the ligand for CD28 containing the
extracellular domain (e.g. amino acids 1-215) of the B7
antigen may be tested for their effects on T cell
proliferation.
Uses in vitro and In Vivo
In a. method of the invention, the ligand for',
CD28, B7 antigen, is used for regulation of CD28 positive
(CD28+) T cells. For example, the B7 antigen is reacted
with T cells in vitro to crosslink or aggregate the CD28
receptor, for example using CHO cells expressing B7
antigen, or immobilizing B7 on a solid substrate, to
produce activated T cells for administration in vivo for
use in adoptive therapy. In adoptive therapy T
lymphocytes are taken from a patient and activated in
vitro with an agent. The activated cells are then.
reinfused into the autologous donor to kill tumor cells
(see Rosenberg et al., Science 223:1318-1321 (1986)).
The method can also be used to produce cytotoxic T cells
useful in adoptive therapy.
Alternatively, the ligand for CD28, its
fragments or derivatives, may be introduced in a suitable
pharmaceutical carrier in vivo, i.e. administered into a
human subject for treatment of pathological conditions
such as immune system diseases or cancer. Introduction
of the ligand in vivo is expected to result in
interference with T cell/B cell interactions as a result
of binding of the ligand to T cells. The prevention of
normal T cell/B cell contact may result in decreased T
cell activity, for example, decreased T cell
proliferation.
In addition, administration of the B7 antigen
in vivo is expected to result in regulation of in vivo
levels of cytokines, including, but not limited to,
WO 92/00092 ''~ PCT/U591/046H32
24
interleukins, e.g. interleukin ("IL"')-2, IL-3, IL-4, IL-
6, IL-8, growth factors including tumor growth factor
("TGF""), colony stimulating factor (""CSF""), interferons
("IFNs"), and tumor necrosis factor ("TNF"") to promote
desired effects in a subject. It is anticipated that
ligands for CD28 such as B71g fusion proteins and Fab
fragments may thus be used in place of cytokines such as
IL-2 for the treatment of cancers in vivo. For example,
when the ligand for CD28 is introduced in vivo it is
available to react with CD28 antigen positive T cells to
mimic B cell contact resulting in increased production of
cytokines which in turn-will interact with B cells.
Under some circumstances,=as noted above, the
effect of administration of the B7 antigen, its fragments
or derivatives in vivo is stimulatory as a result of
aggregation of the CD28 receptor. The T cells are
stimulated resulting in an increase in the level of T
cell cytokines, mimicking the effects of T cell/B cell
contact on triggering of the CD28 antigen on T cells. In
other circumstances, inhibitory effects may result from
blocking by the B7 antigen of the CD28 triggering
resulting from T cell/B cell contact. For example, the
B7 antigen may block T cell proliferation. Introduction
of the B7 antigen in vivo will thus produce effects on
both T and B cell mediated immune responses. The ligand
may also be administered to a subject in combination with
the introduction of cytokines or other therapeutic
reagents. Alternatively, for cancers associated with the
expression of B7 antigen, such as B7 lymphomas,
carcinomas, and T cell leukemias, ligands reactive with
the B7 antigen, such as anti-B71g monoclonal antibodies,
may be used to inhibit the function of malignant B cells.
Because CD28 is involved in regulation of the
production of several cytokines, including TNF and gamma
interferon (Lindsten et al., supra, (1989)), the ligand
for CD28 of the invention may be useful for in v
regulation of cytokine levels in response to the presence
WO 92/00092 PCT/US91/04682
of infectious agents. For example, the ligand for CD28
may be used to increase antibacterial and antiviral
resistance by stimulating tumor necrosis factor (TNF) and
IFN production. TNF production seems to play a role in
5 antibacterial resistance at early stages of infection
(Havell, J. Immunol. 143:2894-2899 (1990)). In addition,
because herpes virus infected cells are more susceptible
to TNF-mediated lysis than uninfected cells (Koff and
Fann, Lymphokine Res. 5:215 (1986)), TNF may play a role
10 in antiviral immunity.
Gamma interferon is also regulated by CD28
(Lindsten et al., supra). Because mRNAs for alpha and
beta IFNs share potential regulatory sequences in their
15 3' untranslated regions with cytokines regulated by CD28,
levels of these cytokines may also be regulated by the
ligand for CD28. Thus, the ligand for CD28 may be useful
to treat viral diseases responsive to interferons (De
Maeyer and De Maeyer-Guignard, in Interferons and other
20 Regulatory Cytokines, Wiley Publishers, New York (1988)).
Following the same reasoning, the ligand for CD28 may
also be used to substitute for alpha-IFN for the
treatment of cancers, such as hairy cell leukemia,
melanoma and renal cell carcinoma (Goldstein and Laszio,
25 CA: a Cancer Journal for clinicians 38:258-277 (1988)),
genital warts and Kaposi's sarcoma.
In addition, B71g fusion proteins as described
above may be used to regulate T cell proliferation. For
example, the soluble CD28Ig and B71g fusion proteins may
be used to block T cell proliferation in graft versus
host (GVH) disease which accompanies allogeneic bone
marrow transplantation. The CD28-mediated T cell
proliferation pathway is cyclosporine-resistant, in
contrast to proliferation driven by the CD3/Ti cell
receptor complex (June et al., 1987, supra).
Cyclosporine is relatively ineffective as a treatment for
GVH disease (Storb, Blood 68:119-125 (1986)). GVH
disease is thought to be mediated by T lymphocytes which
WO 92/00092 PCT/US91/04682
26
express CD28 antigen (Storb and Thomas, Immunol. Rev.
88:215-238 (1985)). Thus, the B7 antigen in the form of
B71g fusion protein, or in combination with
immunosuppressants such as cyclosporine, for blocking T
cell proliferation in GVH disease. in addition, B71g
fusion protein may be used to crosslink the CD28
receptor, for example by contacting T cells with
immobilized B71g fusion protein, to-assist in recovery of
immune function after bone marrow transplantation by
stimulating T cell proliferation.
The fusion proteins of the invention may be
useful to regulate granulocyte macrophage colony
stimulating factor (GM-CSF) levels for treatment of
cancers (Brandt et al., N. Ena. J. Med. 318:869-876
(1988)), AIDS (Groopman et al., N. Eng. J. Med. 317:593-
626 (1987)) and myelodysplasia (Vadan-Raj et al., N. Ena.
J. Med. 317:1545-1551 (1987)).
Regulation of T cell interactions by the
methods of the invention may thus be used to treat
pathological conditions such as autoimmunity,
transplantation, infectious diseases and neoplasia.
In a preferred embodiment, the role of CD28-
mediated adhesion in T cell and B cell function was
investigated using procedures used to demonstrate
intercellular adhesion mediated by MHC class I (Norment
et al., (1988) supra) and class II (Doyle and Strominger,
(1987) supra) molecules with the CD8 and CD4 accessory
molecules, respectively. The CD28 antigen was expressed
to high levels in Chinese hamster ovary (CHO) cells and
the transfected cells were used to develop a CD28-
mediated cell adhesion assay, described infra. With this
assay, an interaction between the CD28 antigen and its
ligand expressed on activated B lymphocytes, the B7
antigen, was demonstrated. The CD28 antigen, expressed
in CHO cells, was shown to mediate specific intracellular
adhesion with human lymphoblastoid and leukemic B cell
WO 92/00092 Vin{ U ~ 0' ^) PCT/US91/04682
2~V ~iv
27
lines, and with activated murine B cells. CD28-mediated
adhesion was not dependent upon divalent cations. A mAb,
BB-1, reactive with B7 antigen was shown to inhibit CD28-
mediated adhesion. Transfected COS cells expressing the
B7 antigen were also shown to adhere to CD284 CHO cells;
this adhesion was blocked by mAbs to CD28 receptor and B7
antigen. The specific recognition by CD28 receptor of B7
antigen, indicated that B7 antigen is the ligand for the
CD28 antigen.
The results presented herein also demonstrate
that antibodies reactive with CD28 and B7 antigen
specifically block helper Th-mediated immunoglobulin
production by allogeneic B cells, providing evidence of
the role of CD28/B7 interactions in the collaboration
between T and B cells.
In additional preferred embodiments, B71g and
CD28Ig fusion proteins were constructed by fusing DNA
encoding the extracellular domains of B7 antigen or the
CD28 receptor to DNA encoding portions of human
immunoglobulin C gamma 1. These fusion proteins were
used to further demonstrate the interaction of the CD28
receptor and its ligand, the B7 antigen.
The cell adhesion assay method of the invention
permits identification and isolation of ligands for
target cell surface receptors mediating intercellular
adhesion, particularly divalent cation independent
adhesion. The target receptor may be an antigen or other
receptor on lymphocytes such as T or B cells, on
monocytes, on microorganisms such as viruses, or on
parasites. The method is applicable for detection of
ligand involved in ligand/receptor interactions where the
affinity of the receptor for the ligand is low, such that
interaction between soluble forms of the ligand and
target receptor is difficult to detect. In such systems,
adhesion interactions between other ligands and receptors
that are. divalent cation dependent may "mask" other
~,~' r) r'
WO 92/00092 2 0 i3 u ,~ 2: PCF/US91/04682
28
interactions between ligands for target receptors, such
that these interactions are only observed when divalent
cations are removed from the system.
The cell adhesion assay utilizes cells
expressing target cell surface receptor and cells to be
tested for the presence of ligand mediating adhesion with
the receptor. The cells expressing target receptor may be
cells that are transfected with the receptor of interest,
such as Chinese hamster ovary (CHO) or COS cells. The
cells to be tested for the presence of ligand are
labeled, for example with 51Cr, using standard methods and
are incubated in suitable medium containing a divalent
cation chelating reagent such as ethylenediamine
tetraacetic acid (EDTA) or ethyleneglycol tetraacetic
acid (EGTA). Alternatively, the assay may be performed
in medium that is free of divalent cations, or is
rendered free of divalent cations, using methods known in
the art, for example using ion chromatography. Use of a
divalent cation chelating reagent or cation-free medium
removes cation-dependent adhesion interactions permitting
detection of divalent cation-independent adhesion
interactions. The labeled test cells are then contacted
with the cells expressing target receptor and the number
of labeled cells bound to the cells expressing receptor
is determined by measuring the label, for example using a
gamma counter. A suitable control for specificity of
adhesion can be used, such as a blocking antibody, which
competes with the ligand for binding to the target
receptor.
The following examples are presented to
illustrate the present invention and to assist one of
ordinary skill in making and using the same. The
examples are not intended in any way to otherwise limit
the scope of the disclosure or the protection granted by
Letters Patent hereon.
WO 92/00092 V 3U e- 25 PCr/US91/04682
29
EXAMPLE 1
Identification of the Ligand For CD28 Receptor
If CD28 receptor antigen binds to a cell
surface ligand, then cells expressing the ligand should
adhere more readily to cells expressing CD28 receptor
than to cells which do not. To test this, a cDNA clone
encoding CD28 under control of a highly active promoter
(Aruffo and Seed, (1987) supra) together with a
selectable marker (pSV2dhfr) (Mulligan and Berg, Science
209:1414-1422 (1980)) was transfected into dihydrofolate
reductase (dhfr)-deficient CHO cells.
Cell Cul:.,. =e. T51, 1A2, 5E1, Daudi, Raji, Jijoye,
CEM, Jurkat, HSB2, THP-1 and HL60 cells (Bristol-Myers
Squibb Pharmaceutical Research Institute, Seattle, WA)
were cultured in complete RPMI medium (RPMI containing
10% fetal bovine serum (FBS), 100 U/ml penicillin and 100
pg/ml streptomycin. Dhfr-deficient Chinese hamster ovary
(CHO) cells (Urlaub and Chasin, Proc. Natl. Acad Sci.,
77:4216-4220 (1980)) were cultured in maintenance medium
(Ham's F12 medium (GIBCO, Grand Island, NY) supplemented
with 10% FBS, 0.15 mM L-proline, 100 U/ml penicillin and
100 ,ug/ml streptomycin). Dhfr-positive transfectants
were selected and cultured in Selective Medium (DMEM,
supplemented with 10% FBS, 0.15 mM L-proline, 100 U/ml
penicillin and 100 lAg/ml streptomycin).
Spleen B cells were purified from Balb/c mice
by treatment of total spleen cells with an anti-Thy 1.2
mAb (30H12) (Ledbetter and Herzenberg, Immunol. Rev.
47:361-389 (1979)) and baby rabbit complement. The
resulting preparations contained approximately 85% B
cells, as judged by FACSR analysis following staining with
fluorescein isothiothiocyanate (FITC)-conjugated goat
anti-mouse immunoglobulin (TAGO). These cells were
activated by treatment for 72 hrs with E. coli
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
lipopolysaccharide (LPS, List Biological Laboratories,
Campbell, CA) at 10 g/ml in complete RPMI.
Monoclonal Antibodies. Monoclonal antibody (mAb) 9.3
5 (anti-CD28) (ATCC No. HB 10271, Hansen et al.,
Immunogenetics 10:247-260 (1980)) was purified from
ascites before use. mAb 9.3 F(ab')2 fragments were
prepared as described by Parham, in J. Immunol. 131:2895-
2902 (1983). Briefly, purified mAb 9.3 was digested with
10 pepsin at pH 4.1 fpr 75 min. followed by passage over
protein A Sepharose to remove undigested mAb. A number
of mAbs to B..cell-associated antigens were screened for
their abilities to inhibit CD28-mediated adhesion. mAbs
60.3 (CD18); 1F5 (CD20); G29-5 (CD21); G28-7, HD39, and
15 HD6 (CD22); HD50 (CD23); KB61 (CD32); G28-1. (CD37); G28-
10 (CD39); G28-5 (CD40); HERMES1 (CD44); 9.4 (CD45); LB-2
(CD54) and 72F3 (CD71) have been previously described and
characterized in International Conferences on Human
Leukocyte Differentiation Antigens I-III (Bernard et al.,
20 Eds., Leukocyte Typing, Springer-Verlag, New York (1984);
Reinherz et al., Eds., Leukocyte Typing II Vol. 2 New
York (1986); and McMichael et al., Eds., Leukocyte Typing
III Oxford Univ. Press, New York, (1987)). These mAbs
were purified before use by protein A Sepharose.
25 chromatography or by salt precipitation and in exchange
chromatography. 8TA401 (Kuritani and Cooper, J. Exp.
Med. 155:839-848 (1982)) (Anti-IgD); 2C3 (Clark et al.,
(1986), supra) (anti-IgM); Nambi, H1DE, P10.1, W6/32
(Clark et al., (1986) supra; and Gilliland et al., Human
30 Immunology 25:269-289 (1989), anti-human class I); and
HB10A (Clark et al., (1986), supra, anti-MHC class II)
were also purified before use. mAbs B43 (CD19); BL-40
(CD72); AD2, 1E9.28.1, and 7G2.2.11 (CD73); EBU-141, LN1
(CDw75); CRIS-1 (CD-76); 424/4A11, 424/3D9 (CD77) Leu 21,
Ba, 1588, LO-panB-1, FN1, and FN4 (CDw78); and M9, G28-
10, HuLym10, 2-7, F2B2.6, 121, L26, HD77, NU-B1, BLAST-1,
BB-1, anti-BL7, anti-HC2, and L23 were used as coded
samples provided to participants in the Fourth
International Conference on Human Leukocyte
2086325'
WO 92/00092 PCT/US91/04682
31
Differentiation Antigens (Knapp, Ed., Leukocyte Typing
IV, Oxford Univ. Press, New York (1990). These were used
in ascites form. mAbs BB-1 and LB-1 (Yokochi et al.,
(1981), supra) were also purified from ascites before
use. Anti-integrin receptor mAbs P3E3, P4C2, P4G9
(Wayner et al., J. Cell. Biol. 109:1321-1330 (1989)) were
used as hybridoma culture supernatants.
Immunostaining Techniques. For indirect
immunofluorescence, cells were incubated with mAbs at 10
jg/ml in complete RPMI for 1 hr at 4 C. mAb binding was
detected with a FITC-conjugated goat anti-mouse
immunoglobulin second step reagent. For direct binding
experiments, mAbs 9.3 and BB-1 were directly conjugated
with FITC as described by Goding in Monoclonal
Antibodies: Principles and Practices Academic Press,
Orlando, FL (1983), and were added at saturating
concentrations in complete RPMI for 1 hr at 4 C. Non-
specific binding of FITC-conjugated mAbs was measured by
adding the FITC conjugate following antigen pre-blocking
(20-30 min at 4 C) with unlabeled mAb 9.3 or BB-1.
Immunohistological detection of adherent lymphoblastoid
cells was achieved using the horseradish peroxidase (HRP)
method described by Hellstrom et al., J. Immunol.
127:157-160 (1981).
Plasmids and Transfections. cDNA clones encoding
the amino acid sequences corresponding to T cell antigens
CD4, CD5 and CD28 in the expression vector p7rH3M (Aruffo
and Seed (1987), supra)), were provided by Drs. S. Aruffo
and B. Seed, Massachusetts General Hospital, Boston, M.A.
An expressible cDNA clone in the vector CDM8 encoding the
amino acid sequence corresponding to B7 antigen (Freeman
et al., J. Immunol. 143:2714-2722 (1989)) was provided by
Dr. Gordon Freeman (Dana Farber Cancer Institute, Boston,
MA).
Dhfr-deficient CHO cells were co-transfected
with a mixture of 9 Mg of plasmid irH3M-CD28 (Aruffo and
2u31)3r~
WO 92/00092 PCT/US91/04682
32
Seed, (1987) supra) and 3 ig of plasmid pSV2dhfr
(Mulligan and Berg, (1980), supra) using the calcium
phosphate technique (Graham and Van Der Eb, Virology
52:456-467 (1973)). Dhfr-positive colonies were isolated
and grown in Selective Medium containing increasing
amounts of methotrexate (Sigma Chemical Co., St. Louis,
MO). Cells resistant to 10 nM methotrexate were
collected by incubation in PBS containing 10 mM EDTA,
stained for presence of the CD28 receptor by indirect
immunofluorescence, and separated by FACSR into CD28-
positive (CD28+) and CD28-negative (CD28') populations.
Both populations were again cultured in Selective Medium
containing increasing concentrations of methotrexate to 1
AM, stained for the CD28 antigen and again sorted into
CD28+ and CD28' populations.
COS cells were transfected with B7, CD4 or CD5
cDNAs as described by Malik et al., Molecular and
Cellular Biology 9:2847-2853 (1989). Forty-eight to
seventy-two hours after transfection, cells were
collected by incubation in PBS containing 10 mM EDTA, and
used for flow cytometry analysis or in CD28-mediated
adhesion assays as described, infra.
Cell lines expressing high (CD28+) and low
(CD28') levels of the CD28 receptor were isolated from
amplified populations by FACSR sorting following indirect
immunostaining with mAb 9.3. After two rounds of FACSR
selection, the CD28+ population stained uniformly positive
with FITC-conjugated mAb 9.3 (mean channel, 116 in linear
fluorescence units), while the CD28' population stained no
brighter (mean channel, 3.9) than unstained cells (mean
channel, 3.7). Staining by CD28+ CHO cells was
approximately ten-fold brighter than phytohemagglutin-
stimulated T cells (mean channel, 11.3). The CD28+ and
CD28' populations stably maintained their phenotypes after
more than 6 months of continuous culture in Selective
Medium containing 1 M of methotrexate.
20863 25
WO 92/00092 PCT/US91/04682
33
Cell Adhesion Assay for a Ligand For CD28
An adhesion assay to detect differential
binding to CD28+ and CD28' CHO cells by cells 3xpressing a
ligand for CD28 was developed. Since mAb 9.3 has been
shown to inhibit mixed lymphocyte reactions using B
lymphoblastoid cells lines as a source of alloantigen
(Damle et al., (1981) supra; and Lesslauer et al., Eur.
J. Immunol. 16:1289-1296 (1986)) B lymphoblastoid cell
lines were initially tested for CD28-mediated adhesion.
CD28-Mediated Adhesion Assay. Cells to be tested
for adhesion were labeled with 51Cr (0.2-1 mCi) to
specific activities of 0.2-2 cpm/cell. A mouse mAb
having irrelevant specificity, mAb W1, directed against
human breast carcinoma-associated mucin, (Linsley at al.,
Cancer Res. 46:5444-5450 (1986)), was added to the
labeling reaction to a final concentration of 100 Mg/ml
to saturate Fe receptors. Labeled and washed cells were
preincubated in complete RPMI containing 10 leg/ml of mAb
W1, and unless otherwise indicated, 10 mM EDTA. mAb 9.3
or mAb 9.3 F(ab')2 was added to some samples at 10 pg/ml,
for approximately 1 hr at 23 C.
Labeled cells (1-10 x 106/well in a volume of
0.2 ml complete RPMI, containing EDTA and mAbs, where
indicated) were then added to the CHO monolayers.
Adhesion was initiated by centrifugation in a plate
carrier (1,000 rpm, in a Sorvall HB1000 rotor,
approximately 210 X g) for 3 min at 4 C. Plates were
then incubated at 37 C for 1 hr. Reactions were
terminated by aspirating unbound cells and washing five
times with cold, complete RPMI. Monolayers were
solubilized by addition of 0.5 N NaOH, and radioactivity
was measured in a gamma counter. For most experiments,
numbers of bound cells were calculated by dividing total
bound radioactivity (cpm) by the specific activity
(cpm/cell) of labeled cells. When COS cells were used,
their viability at the end of the experiment was
2083225
WO 92/00092 PCT/US91/04682
34
generally less than 50%, so specific activity
calculations were less accurate. Therefore, for COS
cells results are expressed as cpm bound.
In pilot experiments, T51 lymphoblastoid cells
were found to adhere more to CD28+ CHO cells, than to
CD28' CHO cells. Furthermore, adhesion of T51 cells to
CD28;' CHO cells was partially blocked by mAb 9.3, while
adhesion to CD28' CHO cells was not consistently affected.
Adhesion was not affected by control mAb L6 (ATCC No. HB
8677, Hellstrom et al., Cancer Res. 46:3917-3923 (1986)),
which is of the same isotype as mAb 9.3 (IgG2a). These
experiments suggested that T51 cells adhered specifically
to CD28'' CHO cells. Since blocking of adhesion by mAb 9.3
was incomplete, ways to increase the specificity of the
CD28 adhesion assay were explored.
The effects of divalent cation depletion on T51
cell adhesion to CD28"* and CD28' CHO cells were examined.
Preliminary experiments showed that EDTA treatment caused
loss of CHO cells during washing, so the CHO cell
monolayers were fixed with paraformaldehyde prior to EDTA
treatment. Fixation did not significantly affect CD28-
mediated adhesion by T51 cells either in the presence or
absence of mAb 9:3. Monolayers of CD28+ and CD28" CHO
cells (1 to 1.2 X 105/cm2 in 48 well plastic dishes) were
fixed in 0.5% paraformaldehyde for 20 min at 23 C, washed
and blocked in Complete RPMI for 1 hr, then pre-incubated
with or without mAb 9.3 or mAb 9.3 F(ab')2 at 10 Ag/ml in
Complete RPMI for 1 hr at 37 C. T51 cells were labeled
with "Cr, preincubated with or without 10 mM EDTA, added
to CHO cells and cellular adhesion was measured. The
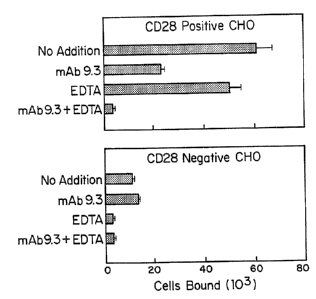
results are presented in Figure 1. Mean and standard
deviation (error bars) are shown for three replicate
determinations.
The specificity of CD28-mediated adhesion was
greatly increased in the presence of EDTA (Figure 1).
Adhesion to CD28+ cells in the presence of EDTA was 17-
WO 92/00092 2 0 8 u J r~ PCr/US91/04682
fold greater than to CD28' cells in the presence of EDTA,
compared with 5.5-fold greater in its absence. Adhesion
to CD28* cells in the presence of mAb 9.3 plus EDTA was
reduced by 93%, compared with 62% in the presence of mAb
5 alone. CD28-mediated adhesion of T51 cells in the
presence of EDTA could also be seen quite clearly by
microscopic examination following immunohistological
staining of T51 cells. Cellular adhesion between
unlabeled T51 cells and CD28+ or CD28' CHO cells was
10 determined in the presence of 10 mM EDTA as described
above. Adherent T51 cells were stained with biotinylated
anti-human Class II Ab, HB10a, fixed with 0.2%
glutaraldehyde and visualized by sequential incubation
with avidin-conjugated HRP (Vector Laboratories, Inc.,
15 Burlingame, CA) and diaminobenzidine solution (Hellstrom
and Hellstrom, J. Immunol. 127:157-160 (1981)). The
results of staining are shown in Figure 2. A similar,
but slightly less significant increase in adhesion
specificity, was also observed in the presence of the
20 calcium-specific chelator, EGTA.
The Ligand For CD28 is a B Cell Activation Marker
The increased specificity of CD28-mediated
25 adhesion in EDTA made it possible to more readily detect
adhesion by cells other than T51. A number of additional
cell lines were tested, including three lymphoblastoid
lines (T51, 1A2, and 5E1); four Burkett's Lymphoma lines
(Daudi, Raji, Jijoye, and Namaiwa); one acute
30 lymphoblastic (B cell) leukemia (REH); three T cell
leukemias (CEM, Jurkat and HSB2); and two monocytic
leukemias (THP-1 and HL60). As a source of primary B
cells, murine splenic B cells, before and after
activation with LPS, were tested. All cells were tested
35 for adhesion to both CD28* and CD28' CHO cells, in the
absence and presence of MAb 9.3. The cells were labeled
with 51Cr and CD28-mediated adhesion was measured as
described above.` Three representative experiments
showing adhesion to CD28+ CHO cells are shown in Figure 3.
WO 92/00092 2 0 U U a r) PCT/US91 /04682
36
Inhibition by mAb 9.3 is shown as an indicator of
specificity; in most cases, adhesion measured in the
presence of mAb 9.3 was approximately equal to adhesion
to CD28' cells.
CD28-specific adhesion (i.e., adhesion being
greater than 70% inhibitable by mAb 9.3), was observed
with T51, 5E1, Raji, and Jijoye cells. Daudi cells also
showed-specific adhesion, although to a lesser extent.
Other cell lines did not show specific CD28-mediated
adhesion, although some (e.g., Namalwa) showed relatively
high non-specific adhesion. Primary mouse splenic B
cells did not show CD28-mediated adhesion, but acquired
the.ability to adhere following activation with LPS. In
other experiments, six additional lymphoblastoid lines
showed CD28-mediated adhesion, while the U937 cell line,
unstimulated human tonsil B cells, and phytohemagglutinin
stimulated T cells did not show adhesion. These
experiments indicate that a ligand for CD28 is found on
the cell surface of activated B cells of human or mouse
origin.
CD28-Mediated Adhesion is Specifically Blocked by a mAb
(BB-1) to B7 Antigen
In initial attempts to define B cell molecules
involved in CD28-mediated adhesion, adhesion by
lymphoblastoid cell lines having mutations in other known
cellular adhesion molecules was measured using the
adhesion assay described above. The 616 lymphoblastoid
line (MHC class II-deficient) (Gladstone and Pious,
Nature 271:459-461 (1978)) bound to CD28 CHO cells
equally well or better than parental T51 cells.
Likewise, a CD18-deficient cell line derived from a
patient with leukocyte adhesion deficiency (Gambaro
cells) (Beatty et al., Lancet 1:535-537 (1984)) also
adhered specifically to CD28. Thus, MHC class II and
CD18 molecules do not mediate adhesion to CD28.
WO 92/00092 208632PCT/US9]/04682
37
A panel of mAbs to B cell surface antigens were
then tested for their ability to inhibit CD28-mediated
adhesion of T51 cells. For these experiments, a total of
57 mAbs reactive with T51 cells were tested, including
mAbs to the B cell-associated antigens CD19, CD20, CD21,
CD22, CD23, CD37, CD39, CD40, CD71, CD72, CD73, CDw75,
CD76, CD77, CDw78, IgM, and IgD; other non-lineage-
restricted antigens CD18, CD32, CD45, CD54, and CD71;
CD44 and another integrin; MHC class I and class II
antigens; and 30 unclustered B cell associated antigens.
In addition to these, many other mAbs which did not react
with T51 by FACSR analysis were tested. Initial screening
experiments were carried out in the absence of EDTA, and
any mAbs which blocked adhesion were subsequently
retested in the presence and absence of EDTA. Of these
mAbs, only those directed against MHC class I molecules
(Nambi, HIDE, P10.1, W6/32), and one to an unclustered B
cell ar.- .gen (BB-1), originally described as a B cell
activat:.,:)n marker (Yokochi at al., (1981) supra) were
consistently able to block CD28-mediated adhesion by
greater than 30%.
The dose-dependence of adhesion inhibition by
the anti-Class I mAb, HIDE, by BB-1 and by 9.3 were '
compared in the presence of EDTA in the experiment shown
in Figure 4. Jijoye cells were labeled with S1Cr and
allowed to adhere to CD28' CHO cells in the presence of 10
mM EDTA as described above. Adhesion measured in the
presence of the indicated amounts of mAbs 9.3, HIDE
(anti-human class I MHC, Gaur at al., Immunogenetics
27:356-361 (1988)), or mAb BB-1 is expressed as a
percentage of maximal adhesion measured in the absence of
mAb (45,000 cells bound). mAb 9.3 was most effective at
blocking, but mAb BB-1 was able to block approximately
60% of adhesion at concentrations less than 1 Mg/ml. mAb
H1DE.also partially blocked adhesion at all
concentrations tested. When EDTA was omitted from the
adhesion assay, blocking by class I mAbs was consistently
WO 92/00092 J PC'T/US91/04682
38
less, and required higher mAb concentrations, than mAbs
9.3 or BB-l.
Binding of mAb 5B-1 by Different Cells Correlates With
CD28-Speci c Adhesion
To investigate the roles of molecules
recognized by anti-class I and BB-1 mAbs in CD28-mediated
adhesion, levels of these antigens on certain of the cell
lines tested for CD28-specific adhesion in Figure 3 were
compared. Cells were analyzed by FACSR following indirect
immunofluorescence staining with mAbs H1DE and BB-1.
Cell lines 1A2, Namalwa, REH and HL60 (which did not
adhere specifically to CD28) all bound high levels of mAb
HIDE, whereas Daudi cells (which did adhere) did not show
detectable binding. Therefore, a direct correlation
between CD28-mediated adhesion and expression of class I
antigens was not observed. On the other hand, these
experiments suggested a correlation between adhesion to
CD28 and staining by mAb BB-1.
To confirm this correlation, cell lines
examined for CD28-mediated adhesion in Figure 3 were
tested for staining by direct immunofluorescence using
FITC-conjugated mAb BB-1 (Table 1). Cell lines were
incubated with no mAb or with FITC-conjugated mAb BB-1
with or without preincubation with cold (unlabeled) BB-1
mAb. Values shown in Table 1 represent mean fluorescence
in linear units. All of the cell lines which adhered
specifically to CD28 receptor (Figure 3) bound higher
levels of the FITC-conjugate than those which did not
adhere specifically. Antigen specificity was
demonstrated in all cases by the ability of unlabeled mAb
BB-i to compete for binding of the FITC-conjugate.
WO 92/00092 208602, PCT/US91/04682
39
Table 1
CELLS WHICH ADHERE TO CD28 RECEPTOR ALSO BIND mAb BS-1
FITC-BB-1
SPECIFIC
LINE CELL TYPE' 110 mAb -COLD +COLD BINDING
Positive for CD28 Adhesion2
T51 B-LCL 2.3 16.4 3.0 13.4
5E1 B-LCL 2.1 13.0 2.4 10.6
Jijoye BL 2.3 17.8 2.8 15.0
Raji BL 2.1 7.1 2.8 4.3
Daudi BL 2.1 6.4 2.8 3.6
Negative for CD28 Adhesion3
1A2 B-LCL 2.1 4.5 4.3 <1
Namalwa BL 2.2 3.8 2.2 1.6
REH B-ALL 2.0 2.1 2.0 <1
CEM T-ALL 2.3 2.0 1.9 <1
Jurkat T-ALL 2.2 2.2 2.0 <1
HSB2 T-ALL 2.1 2.3 2.3 <1
HL60 AML 2.3 3.1 3.1 <1
THP-1 AML 2.3 3.1 3.0 <1
' B-LCL B-lymphoblastoid cell line
BL - Burkett's lymphoma
B-ALL - B cell-derived acute lymphoblastoid leukemia
T-ALL - T cell-derived acute lymphoblastoid leukemia
AML - Acute Monocytic leukemia
2 Positive for CD28 adhesion - >70% inhibition of adhesion by
mAb 9.3
3 Negative for CD28 Adhesion - < 70% inhibition of adhesion by
mAb 9.3.
Specific binding - (FITC-BB-1 + cold) subtracted from (FITC
BB-1 - cold).
COS Cells Expressing the B7 Antigen Adhere Specifically
to CD28
The role of the B7 antigen recognized by mAb
BB-1 in CD28-mediated adhesion was investigated using a
cDNA clone isolated and sequenced by Freeman et al. as
described in J. Immunol. 143:2714-2722 (1989). COS cells
were transfected with an expression vector containing the
cDNA clone encoding the B7 antigen, as described by
Freeman et al., (1989), supra as described above. Forty-
eight hours later, transfected COS cells were removed
from their dishes by incubation in PBS containing 10 mm
EDTA, and were labeled with 51Cr. Cells were shown to
express B7 antigen by FACSR analysis following indirect
staining with mAb BB-1 as reported by Freeman et al,
supra. Adhesion between B7 transfected COS cells and
CD28+ or CD28' CHO cells was then measured in the presence
of 10 mM EDTA as described above. Where indicated,
WO 92/00092 w U U U d a c) PCT/US91/04682
adhesion was measured in the presence of mAbs 9.3 or BB-1
(10 pg/ml). As shown in Figure 5, B7/BB-1-transfected
COS cells adhered readily to CD284 CHO cells; adhesion was
completely blocked by both mAbs 9.3 and BB-1. No
5 adhesion to CD28' CHO cells was detected. This experiment
was repeated five times with identical results.
In other experiments, adhesion was not blocked
by non-reactive, isotype matched controls, mAb W5 (IgM)
10 (Linsley, (1986) sutra) and .b L6 (IgG2A) (Helistrom et
al., (1986) su r ), or by mAb H1DE, which reacts with
class I antigens on COS cells. CD28-mediated adhesion by
B7 transfected cells could also be clearly seen by
microscopic examination of the CHO cell monolayers after
15 the assay. When COS cells were transfected with
expressible CD4 or CD5 cDNA clones, no CD28-mediated
adhesion was detected. Expression of CD4 and CD5 was
confirmed by FACSR analysis following immunofluorescent
staining. When EDTA was omitted from the assay, adhesion
20 measured with CD5-transfected COS cells was greatly
increased but not inhibited by mAb 9.3. In contrast,
adhesion by B7 transfected COS cells under these
conditions was still partially blocked (approximately
40%) by mAb 9.3. Thus, transfection of B7 into COS cells
25 confers the ability on the cells to adhere specifically
to CD28 receptor.
The above assay for intracellular adhesion
mediated by the CD28 receptor, described above,
30 demonstrated CD28-mediated adhesion by several
lymphoblastoid and leukemic B cell lines, and by primary
murine spleen cells following activation with LPS. These
results indicate the presence of a natural ligand for the
CD28 receptor on the cell surface of some activated B
35 lymphocytes.
Several lines of evidence show that the B cell
molecule which interacted with the CD28 receptor is the
B7 antigen. mAb BB-1 was identified from a panel of mAbs
WO 92/00092 2 0 8 6 5~ 5 PCT/U591/04682
41
as the mAb which most significantly inhibited CD28-
mediated adhesion. Furthermore, a correlation was
observed between the presence of B7 antigen and CD28-
mediated adhesion (Table 1). Finally, COS cells
transfected with B7 cDNA demonstrated CD28-mediated
adhesion. Taken together, these observations provide
strong evidence that B7 antigen is a ligand for CD28
receptor. Because both CD28 (Aruffo and Seed, (1987)
supra) and B7 (Freeman et al., (1989) sum) are members
of the immunoglobulin superfamily, their interaction
represents another example of heterophilic recognition
between members of this gene family (Williams and Barclay
(1988), supra).
CD28-mediated adhesion differs in several
respects from other cell adhesion systems as shown in the
above results. CD28-mediated adhesion was not blocked by
mAbs to other adhesion molecules, including mAbs to ICAM-
1 (LB-2), MHC class II (HB10a) CD18 (60.3), CD44 (HERMES-
1 homing receptor), and an integrin (P3E3, P4C2, P4G9).
CD28-mediated adhesion was also resistant to EDTA and
EGTA, indicating that this system does not require
divalent cations, in contrast to integrins (Kishimoto et
al., Adv. Immunol. 46:149-182 (1989)) and some homing
receptors (Stoolman, gall 56:907-910 (1989)) which
require divalent cations. In the system described
herein, in which CD28 receptor was expressed to high
levels relative to those on activated T cells, it was
sometimes difficult to measure CD28-mediated adhesion
because of cation-dependent "background" adhesion (i.e.,
that not blocked by MAb 9.3, see Figure 1). Preliminary
experiments suggest that background adhesion in the
absence of EDTA was also blocked by MAb 60.3, which
inhibits adhesion mediated by LFA-1 (Pohlman et al., J.
35. Immunol. 136:4548-4553 (1986)). Even under optimal
conditions, some cells (such as Namalwa, see Figure 3)
showed significant non-CD28 dependent adhesion to CHO
cells. Non-CD28 mediated. adhesion systems may also be
responsible for the incomplete blockage by mAb BB-1 of B
WO 92/00092 'v 0 3 4 D PCT/US91/04682
42
cell adhesion (Figure 4). That this mAb is more
effective at blocking adhesion by transfected COS cells
(Figure 5) may indicate that non-CD28 mediated systems
are less effective in COS cells.
Finally, CD28-mediated adhesion appears more
restricted in its cellular distribution to T and B cells
as compared to other adhesion molecules. CD28 receptor
is.primarily expressed by cells of the T lymphocyte
lineage. The B7 antigen is primarily expressed by cells
of the B lymphocyte lineage. Consistent with this
distribution, the ligand for CD28 was only detected on
cells of B lymphocyte lineage. Thus, available data
suggest that CD28 mediates adhesion mainly between T
cells and B cells. However, since CD28 expression has
been detected on plasma cells (Kozbor et al., J. Immunol
138:4128-4132 (1987)) and B7 on cells of other lineages,
such as monocytes (Freeman et al., (1989) supra), it is
possible that other cell types may also employ this
system. Many adhesion molecules are known to mediate T
cell-B cell interactions during an immune response and
the levels of several of these, including CD28 and B7
antigen, have been reported to increase following
activation. Increased levels of these molecules may help
'25 explain why activated B cells are more effective at
stimulating antigen-specific T cell proliferation than
are resting B cells. Because the B7 antigen is not
expressed on resting B cells, CD28-mediated adhesion may
play a role in maintaining or amplifying the immune
response, rather than initiating it. Such a role is also
consistent with the function of CD28 in regulating
lymphokine and cytokine levels (Thompson et al., (1989),
supra.; and Lindsten et al., (1989), supra).
WO 92/00092 ' 3 PCT/US91/04682
43
EXAMPLE 2
Characterization of Interaction Between CD28 Receptor
and B7 Anticten
This example used alloantigen-driven maturation
of B cells as a model system to demonstrate the
involvement of the CD28 receptor on the surface of major
histocompatibility complex (MHC) class II antigen-
reactive CD4 positive T helper (Th) cells and antigen
presenting B cells during the Th-B cell cognate
interaction leading to B cell differentiation into
immunoglobulin-secreting cells (IgSC).
Cognate interaction between CD4` Th and antigen-
presenting B cells results in the activation and
differentiation of both cell types consequently leading
to the development of immunoglobulin-secreting cells
(Moller (Ed) Immunol Rev. 99:1 (1987), sup-ra). Allogenic
MLR offers an ideal system to analyze cognate Th-B cell
interaction because alloantigen-specific CD4'` Th induce
both the activation and differentiation of alloantigen-
bearing B cells into immunoglobulin secreting cells
(Chiorazzi et al., Immunol Rev. 45:219 (1979); Kotzin et
al., J. Immunol, 127:931 (1981); Friedman et al., J.
Immunol. 129:2541 (1982); Goldberg et al., J. Immunol.
135:1012 (1985); and Crow et al., J. Exp. Med. 164:1760
(1986)). The involvement of the CD28 receptor on Th cells
and its ligand B7 during the activation of T. and B cells
in the allogeneic MLR was first examined using murine mAb
directed at these molecules.
Culture medium. Complete culture medium (CM)
consisted of RPMI 1640 (Irvine Scientific, Santa Ana, CA)
supplemented with 100 U/ml of penicillin G, 100 Ag/ml of
streptomycin, 2 mM L-glutamine, 5 X 10"5 M 2-ME, and 10%
FBS (Irvine Scientific).
Cells and mAbs. EBV-transformed B cell lines CESS
(HLA-AS1, A3; B5, B17; DR7), JIJOYE, and SKW6.4 (HLA-Ala;
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
44
B27, B51; DR7), were obtained from the ATCC. EBV-
transformed B cell lines ARENT (HLA-A2; B38, B39, DRw6)
and MSAB (HLA-Al, A2; B57; DR7) were provided by Dr. E.
G. Engleman, Stanford University School of Medicine,
Stanford, CA. Hybridomas OKT4 (IgG anti-CD4), OKT8 (IgG
anti-CD8) and HNK1 (IgM anti-CD57) were obtained from the
ATCC and ascitic fluids from these hybridomas were
generated in pristane-primed BALB/c mice. Production and
characterization of anti-CD28 mAb 9.3 (IgG2a) has been
described by Ledbetter et al., J. Immunol. 135:2331
(1985); Hara et al., J. Exp. Med. 161:1513 (1985) and
Martin et al,, J. Immunol. 136:3282 (1986).
mAb 4H9 (IgG2a anti-CD7)--as
described by Damle and Doyle, J. Immunol 143:1761 (1989)
was provided by Dr.
Engleman and mAb anti-B7 antibody (BB1; IgM)' as described
by Tokochi et al., J. Immunol. 128:823 (1981)
was provided by Dr. E.
Clark, University of Washington, Seattle, WA.
Peripheral blood mononuclear cells (PBMC) from
healthy donors were separated into T and non-T cells
using a sheep erythrocyte rosetting technique, and T
cells were separated by panning into CD4+ subset and
further into CD4+CD45RA - CD45R0+ memory subpopulation as
described by Damle et al., J. Immunol. 139:1501 (1987).
Proliferative responses of T cells. To examine the
effect of anti-CD28 and anti-B7 mAbs on the proliferative
responses of T cells, fifty-thousand CD4+CD45R0+ T cells
were stimulated by culturing with 1 x 104 irradiated (8000
rad from a 137Cs source) EBV-transformed allogenic B cells
(or 2.5 X 104 non-T cells) in 0.2 ml of CM in round-bottom
microtiter wells in a humidified 5% CO. and 95% air
atmosphere in the presence of 10 Ag/ml of mAb reactive
with CD7, CD28, CD57 or B7 antigen. CD4+CD45R0+ T cells
also were also independently stimulated with 100 gg/ml of
soluble purified protein derivative of tuberculin (PPD,
WO 92/00092 0 u ~~ D PCT/US91/04682
Connough Laboratories, Willowdale, Ontario, Canada) in
the presence of 1 X 104 irradiated (3000 rad) autologous
non-T cells in the presence of the above mAbs.
Triplicate cultures were pulsed with 1 ;CCi/well = 37
5 kBq/well of (3H]dThd (6.7 Ci/mmol, NEN, Boston, MA) for 16
h before harvesting of cells for measurement of
radiolabel incorporation into newly synthesized DNA. The
results are expressed as cpm SEM. Proliferative
responses were examined on day 7 of culture. EBV-
10 transformed B cell lines were used as stimulator cells in
these experiments because these B cells exhibit various
features of activated B cells such as the expression of
high levels of MHC class II and B7 molecules (Freeman et
al., J. Immunool 139:3260 (1987); and Yokochi et al., J.
15 Immunol. 128:823 (1981)).
Figure 6 shows the results of these
experiments. The presence of anti-CD28 mAb (9.3 IgG2a)
but not that of isotype-matched anti-CD7 mAb (4H9, IgG2a)
20 consistently inhibited the MLR proliferative response of
CD4+ T cells to allogeneic B cells. Similarly, the
addition of anti-B7 mAb (BB1; IgM) but not that of
isotype-matched anti-CD57 HNK1; IgM) to the allogeneic
MLR resulted in the inhibition of T cell proliferation.
25 The inhibitory effects of anti-CD28 mAb 9.3 on the MLR
responses of T cells are consistent with previous
observations reported by Damle et al., J. Immunol.
120:1753 (1988) and Damle et al., Proc. Natl. Acad. Sci.
TSA 78:5096 (1981). Similar to the allogeneic MLR,
30 proliferative response of CD4'' T cells to soluble Ag PPD
presented by autologous non-T cells was also inhibited by
anti-CD28 and anti-B7 mAb. Although both anti-CD28 mAb
9.3 (IgG2a) and anti-B7 mAb, BB1 (IgM) inhibited the
allogeneic MLR and the soluble antigen-induced
35 proliferative responses, anti-CD28-mediated inhibition
was always stronger than that by anti-B7 for all the
responder-stimulator combinations examined. These
observations are also consistent with the weaker ability
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
46
of anti-B7 mAb to block the CD28-mediated adhesion to B7+
B cells as described above.
T cell-induced Immunoalobulin (Ia) production by B cells
To further examine the roles of CD28 and B7
during cognate Th-B interactions, two EBV-transformed B
cells lines, IgG-secreting DR7+ CESS and IgM-secreting
DR7+ SKW were used. When appropriately stimulated, both
these B cells lines significantly increase their
production of the respective Ig isotype. First, the
effects of DR7-specific CD4+ CD45RO+ Th line on the ig
production of both CESS and SKW B cells was examined.
DR7-primed CD4+ Th cells were derived from the allogeneic
MLC consisting of responder CD4+CD45RO+ T cells (HLA-A26,
A29; B7, B55; DR9, DR10) and irradiated MSAB,(DR7+) B
cells as stimulator cells as described by Damle et al.,
J. Immunol, 133:1235 (1984).
The isolation of resting CD4+CD45RO+ T cells and
that of DR7-primed CD4+ CD45RO+ T lymphoblasts using
discontinuous Percoll density gradient centrifugation was
also as described by Damle, supra (1984). These DR7-
primed CD4+ Th cells were continuously propagated in the
presence of irradiated MSAB B cells and 50 U/ml of IL-2.
Prior to their functional analysis, viable DR7-primed Th
cells were isolated by Ficoll-Hypaque gradient
centrifugation and maintained overnight in CM without DR7+
feeder cells or IL-2, after which immunoglobulin secreted
in the cell-free supernatant (SN) was quantitated using a
solid-phase ELISA.
To examine the effect of Th cells on Ig
production, by both CESS and SKW B cells 2 x 104-2.5 x 104
cells from HLA-DR7+ EBV-transformed B cell lines, IgM-
producing SKW or IgG-producing CESS were cultured with
varying numbers of DR7-primed CD4+CD45R0+ Th cells for 96
h after which cell-free SN from these cultures were
collected and assayed for the quantitation of IgM (SKW
cultures) or IgG (CESS cultures) using solid-phase ELISA.
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
47
Exogenous IL-6 (1-100 U/ml) induced Ig production by
these B cells was also used as a positive control to
monitor the non-cognate Ig production by these B cell
lines. Ig production by freshly isolated resting
CD4+CD45RO+ Th cells (autologous to the DRt-primed CD4+ Th
cells) was also simultaneously examined as a control for
DR7-primed CD4+ Th cells. - - .
Ia cruantitation. IgG or IgM in culture SN were
measured using solid-phase ELISA as described by Volkman
et al., Proc. Natl. Acad. Sci. USA 78:2528 (1981).
Briefly, 96-well flat-
bottom microtiter ELISA plates (Corning, Corning NY) were
coated with 200 p1/well of sodium carbonate buffer (pH
9.6) containing 10 pg/ml of affinity-purified goat anti-
human Ig or IgM Ab (Tago, Burlingame, CA) incubated
overnight at 40 C, and then washed with PBS and wells
were further blocked with 2% BSA in PBS (BSA-PBS).
Samples to be assayed were added at appropriate dilution
to these wells and incubated with 200 gl/well of 1:1000
dilution of horseradish peroxidase (HRP)-conjugated
F(ab')2 fraction of affinity-purified goat anti-human IgG
or IgM Ab (Tago). The plates were then washed, and 100
p1/well of o-phenylenediamine (Sigma, St. Louis, MO)
solution (0.6 mg/ml in citrate-phosphate buffer with pH
5.5 and 0.045% hydrogen peroxide). Color development was
stopped with 2N sulfuric acid. Absorbance at 490 rim was
measured with an automated ELISA plate reader. Test and
control samples were run in triplicate and the values of
absorbance were compared to those obtained with known IgG
or IgM standards run simultaneously with the SN samples
to generate the standard curve using which the
concentrations of Ig in culture SN were quantitated.
Data are expressed as ng/ml of Ig SEM of either
triplicate or quadruplicate cultures.
Figure 7 shows the Ig production by either B
cell line as a function of the concentration of DR7-
primed Th with optimal Ig production induced at either 1:1
WO 92/00092 2 ~} !a-3 , 3 PCT/US91/04682
48
or 1:2 Th:B ratios. At Th:B ratios higher than 1:1
inhibition of Ig production was observed. Hence, all
further experiments were carried out using a Th:B ratio of
1:2. As shown in Figure 7, these unprimed resting CD4+Th
cells slightly induced IgM production by SKW B cells but
has no effect on the IgG production by CESS B cells in 4-
day cultures. This slight helper effect observed with
unprimed CD4+CD45RO+ population during the Ig induction
cultures. The production of Ig by CESS (IgG) or SKW
(IgM) B cells induced by DR7-primed CD4+ Th was specific
for HLA-DR7 because similarly activated DRw6-primed CD4+
Th (stimulated with DRw6+ ARENT B cells and autologous to
the DR7-primed TO were unable to induce Ig production by
either CESS or SKW B cells.
The roles of CD28 and B7 during cognate Th:B-
induced Ig production were further examined using anti-
CD28 and anti-B7 mAbs. Both CESS and SKW B cells
constitutively express B7 antigen on their surface and
thus, represent a source of uniformly activated B cell
populations for use in Th-B cognate interactions or in
cytokine-driven non-cognate maturation. Thus, DR7+ B
cells (CESS or SKW) were cultured for 4 days with DR7-
specific CD4+ Th line at Th:B ratio of 1:2 and mAb to CD28
and B7, (and CD7 and CD57 as controls) were added to
these cultures at different concentrations. Ig
production (IgM, Figure 8a and IgG, Figure 8b) at the end
of 3-day cultures was quantitated in cell-free SN.
Figure 8 shows that both anti-CD28 and anti-B7 mAbs but
not their isotype-matched mAb controls (anti-CD7 and
anti-CD57, respectively) inhibited Th induced Ig
production by B cells in a does-dependent manner. Once
again, anti-CD28 mAb-mediated inhibition of Ig production
was stronger than that by anti-B7 mAb. In contrast, Ig
production by either B cells induced by exogenous IL-6
(non-cognate differentiation) was not affected by any of
the above mAb.
WO 92/00092 2 0 S u .3 3 PCT/US91/04682
49
These results strongly suggest that the
interaction between CD28 and B7, during cognate Th-B
collaboration, in addition to activation of Th cells, is
pivotal to the differentiation of activated B cells into
Ig secreting cells.
The above results demonstrate the relationship
of CD28 receptor and its ligand, the B7 antigen, as a co-
stimulatory transmembrane receptor-ligand pair
influencing Th:B interactions. Involvement of both CD28
and B7 during Th:B collaboration was demonstrated by
inhibition by anti-CD28 and anti-B7 of not only Th cell
activation but also Th-induced differentiation of B cells
into IgSC. It appears as if the observed inhibitory
effects of anti-CD28 and anti-B7 mAbs are due to the
inhibition of CD28:B7 interaction underlying these
responses.
Interaction between CD28 receptor and B7
antigen may influence the production of cytokines and
thus B cell differentiation. Ligation of CD28 by B7
during Th:B collaboration may facilitate sustained
synthesis and delivery of cytokines for their utilization
during the differentiation of B cells into immunoglobulin
secreting cells. The lack of inhibition by anti-CD28 and
anti-B7 mAbs of cell dependent differentiation of CESS or
SKW B cells induced with exogenous IL-4 or IL-6 suggests
that CD28:B7 interaction controls either production of
these cytokines, or their targeted delivery to B cells,
or both of these events.
The interaction of CD28 and B7 is most likely
not restricted to Th:B cell interactions, and applies more
generally to other antigen-presenting cells such as
monocyte/Mo, dendritic cells, and epidermal Langerhans
cells. Ligation of a nominal antigen presented in
conjunction with MHC class II molecules on the surface of
antigen-presenting cells by the TcR/CD3 complex on the
surface of Th cells may lead to elevated expression of B7
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
antigen by these cells, which, via the interaction with
CD28, then facilitates the production of various
cytokines by Th. This in turn drives both growth and
differentiation of both Th and B cells.
5
EXAMPLE 3
Characterization of the Interaction between CD28 Receptor
and B7 Antigen
I. Preparation=of Fusion Proteins
To further characterize the biochemical and
functional aspects of the interactions between the CD28
receptor and B7 antigen, fusion proteins of BT and CD28
with human immunoglobulin C gamma 1 (human Ig Cyl) chains
were constructed and expressed and used to-measure the
specificity and apparent affinity of interaction between
these molecules. Purified B71g fusion protein, and CHO
cells transfected with B7 antigen were used to
investigate the functional effects of this interaction on
T cell activation and cytokine production.
Preparation of B7Ig and CD28Ig Fusion Proteins
B71g and CD28Ig fusion proteins were prepared
as follows. DNA encoding the amino acid sequence
corresponding to the extracellular domain of the
respective protein (B7 and CD28) was joined to DNA
encoding the amino acid sequences corresponding to the
hinge, CH2 and CH3 regions of human immunoglobulin Cyl.
This was accomplished as follows.
Piasmid Construction. Expression plasmids were used
containing cDNA encoding the amino acid sequence
corresponding to CD28 (pCD28) as described by Aruffo and
Seed, Proc. Natl. Acad. Sci. USA 84:8573 (1987).
and provided by Drs. Aruffo
and Seed, Mass General Hospital, Boston, MA. Expression
plasmids containing cDNA encoding the amino acid sequence
corresponding to CD5 (pCD5) as described by Aruffo, Cell
WO 92/00092 V CS 0 PCT/US91 /04682
51
61:1303 (1990), and also provided by Dr. Aruffo, and cDNA
encoding the amino acid sequence corresponding to B7
(pB7) as described by Freeman et al., J. Immunoi.
143:2714 (1989)) and provided by Dr. Freeman, Dana Farber
Cancer Institute, Boston, MA, were also used.
For initial attempts at expression of soluble
forms of CD28 and B7, constructs were made (OMCD28 and
OMB7) in which stop codons were introduced upstream of
the transmembrane domains and the native signal peptides
were replaced with the signal peptide from oncostatin M
(Malik et al., Mol. Cell Biol. 9:2847 (1989)). These
were made using synthetic oligonucleotides for
reconstruction (OMCD28) or as primers (OMB7) for PCR.
OMCD28, is a CD28 cDNA modified'for more efficient
expression by replacing the signal peptide with the
analogous region from oncostatin M. CD28Ig and B71g
fusion constructs were made in two parts. The 5'
portions were made using OMCD28 and OMB7 as templates and
the oligonucleotide,
CTAGCCACTGAAGCTTCACCATGGGTGTACTGCTCACAC (SEQ ID NO:1)
(corresponding to the oncostatin M signal peptide) as a
forward primer, and either
TGGCATGGGCTCCTGATCAGGCTTAGAAGGTCCGGGAAA (SEQ ID NO:2),
or, TTTGGGCTCCTGATCAGGAAAATGCTCTTGCTTGGTTGT (SEQ ID NO:3)
as reverse primers, respectively. Products of the PCR
reactions were cleaved with restriction endonucleases
(Hind III and Boll) as sites introduced in the PCR
primers and gel purified.
The 3' portion of the fusion constructs
corresponding to human Ig C71 sequences was made by a
coupled reverse transcriptase (from Avian myeloblastosis
virus; Life Sciences Associates, Bayport, NY)-PCR
reaction using RNA from a myeloma cell line producing
human-mouse chimeric mAb L6 (provided by Dr. P. Fell and
M. Gayle, Bristol-Myers Squibb Pharmaceutical Research
Institute, Seattle, WA) as template. The
oligonucleotide, AAGCAAGAGCATTTTCCTGATCA
WO 92/00092 i3J L F'CT/US9l/04682
52
GGAGCCCAAATCTTCTGACAAAACTCACACATCCCCACCGTCCCCAGCACCTGAACT
CCTG (SEQ ID NO:4), was used as forward primer, and
CTTCGACCAGTCTAGAAGCATCCTCGTGCGACCGCGAGAGC (SEQ ID NO:5)
as reverse primer. Reaction products were cleaved with
Bc1I and XbaI and gel purified. Final constructs were
assembled by ligating HindIII/BclI cleaved fragments
containing CD28 or B7 sequences together with BclI/XbaI
cleaved fragment containing Ig Cyl sequences into'
HindIII/XbaI cleaved CDM8. Ligation products were
transformed into MC1061/p3 E. coli cells and colonies
were screened for the appropriate plasmids. Sequences of
the resulting constructs were confirmed by DNA
sequencing. The DNA used in the B7 construct encodes
amino acids from about position I to about position 215
of the sequence corresponding to the extracellular domain
of the B7 antigen, and for CD28, the DNA encoding amino
acids from about position 1 to about position 134 of the
sequence corresponding to the extracellular domain of the
CD28 receptor.
CDSIg was constructed in identical fashion,
using CATTGCACAGTCAAGCTTCCATGCCCATGGGTTCTCTGGCCACCTTG
(SEQ ID NO:6), as forward primer and
ATCCACAGTGCAGTGATCATTTGGATCCTGGCATGTGAC (SEQ ID NO:7) as
reverse primer. The PCR product was restriction
endonuclease digested and ligated with the Ig Cyl
fragment as described above. The resulting construct
(CD5Ig) encodes an amino acid sequence containing
residues from about position 1 to about position 347 of
CD5, two amino acids introduced by the construction
procedure (amino acids DQ), followed by the ig Cy1 hinge
region.
In initial attempts to make soluble derivatives
of B7 and CD28, cDNA constructs were made encoding
molecules truncated at the NH2-terminal side of their
transmembrane domains. In both cases, the native signal
peptides were replaced with the signal peptide from
oncostatin M (Malik, su r , 1989), which mediates
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
53
efficient release of secreted proteins in transient
expression assays. The cDNAs were cloned into an
expression vector, transfected into COS cells, and spent
culture medium was tested for secreted forms of B7 and
CD28. In this fashion, several soluble forms of B7 were
produced, but in repeated attempts, soluble CD28
molecules were not detected.
The next step was to construct receptor Ig C71
fusion proteins. The DNAs encoding amino acid sequences
corresponding to B7 and CD28 extracellular regions,
preceded by the signal'peptide to oncostatin M, were -
fused in frame to an Ig C'yl cDNA, as shown in Figure 9.
During construction, the Ig hinge disulfides were mutated
to serine residues to abolish intrachain disulfide
bonding. The resulting fusion proteins were produced in
COS cells and purified by affinity chromatography on
immobilized protein A as described below. Yields of
purified protein were typically 1.5-4.5 mg/liter of spent
culture medium.
Polymerase Chain Reaction (PCR). For PCR, DNA
fragments were amplified using primer pairs as described
below for each fusion protein. PCR reactions (0.1 ml
final volume) were run in Taaq polymerase buffer
(Stratagene, La Jolla, CA), containing 20 gmoles each of
dNTP; 50-100 pmoles of the indicated primers; template (1
ng plasmid or cDNA synthesized from < 1 g total RNA
using random hexamer primer, as described by Kawasaki in
PCR Protocols, Academic Press, pp. 21-27 (1990));
and q polymerase
(Stratagene). Reactions were run on a thermocycler
(Perkin Elmer Corp., Norwalk, CT) for 16-30 cycles (a
typical cycle consisted of steps of 1 min at 94 C, 1-2
min at 50 C and 1-3 min at 72 C).
Cell Culture and Transfections. COS (monkey kidney
cells) were transfected with expression plasmids using a
modification of the protocol of Seed and Aruffo ( oc.
CA 02086325 2000-08-17
WO 92/00092 PCr/US91 /04682
54
Natl. Acad. Sci. 84:3365 (1987)).
Cells were seeded at 106 per 10 cm
diameter culture dish 18-24 h before transfection.
Plasmid DNA was added (approximately 15 gg/dish) in a
volume of 5 ml of serum-free DMEM containing 0.1 mM
cloroquine and 600 g/ml DEAE Dextran, and cells were
incubated for 3-3.5 h at 37 C. Transfected cells were
then briefly treated -(approximately 2 min) with 10%
dimethyl sulfoxide in PBS and incubated at 37 C for 16-24
h in DMEM containing 10% FCS. At 24 h after
transfection, culture medium was removed and replaced
with serum-free DMEM (6 ml/dish). Incubation was
continued for 3 days at 37 C, at which time the spent
medium was collected and fresh serum-free medium was
added. After an additional 3 days'at 37 C; the spent
medium was again collected and cells were discarded.
CHO cells expressing CD28, CD5 or B7 were
isolated as described by Linsley et al., (1991) supra, as
follows: Briefly, stable transfectants expressing CD28,
CD5, or B7, were isolated following cotransfection of
dihydrofolate reductase-deficient Chinese hamster ovary
(dhfr- CHO) cells with a mixture of the appropriate
expression plasmid and the selectable marker, pSV2dhfr,
as described above in Example 1. Transfectants were then
grown in increasing concentrations of methotrexate to a
final level of 1 pM and were maintained in DMEM
supplemented with 10% fetal bovine serum (FBS), 0.2 mM
proline and 1 M methotrexate. CHO lines expressing high
levels of CD28 (CD28` CHO) or. B7 (B7+ CHO) were isolated
by multiple rounds of fluorescence-activated cell sorting
(FACSR) following indirect immunostaining with mAbs 9.3 or
BB-1. Amplified CHO cells negative for surface
expression of CD28 or B7 (dhfr+ CHO) were also isolated by
FACSR from CD28-transfected populations.
Immunostainina and FACSR Analysis. Transfected CHO
cells or activated T cells were analyzed by indirect
immunostaining. Before staining, CHO cells were removed
WO 92/00092 P(T/US91/04682
from their culture vessels by incubation in PBS
containing 10 mM EDTA. Cells were first incubated with
murine mAbs 9.3 (Hansen et al., Immunogenetics 10:247
(1980)) or BB-1 (Yokochi et al., supra) at 10 g/ml, or
5 with Ig fusion proteins (CD28Ig, B71g, CD5Ig or chimeric
mAb L6 containing Ig Cy1, all at 10 gg/ml in DMEM
containing 10% FCS) for 1-2 h at 4 C. Cells were then
washed, and incubated for an additional 0.5-2h at 4 C with
a FITC-conjugated second step reagent (goat anti-mouse Ig
10 serum for murine mAbs, or goat anti-human Ig Cy serum for
fusion proteins (Tago, Inc., Burlingame, CA).
Fluorescence was analyzed on 10,000 stained. cells using a
FACS IVR cell sorter (Becton Dickinson and Co., Mountain
View, CA) equipped with a four decade logarithmic
15 amplifier.
Purification of Ia Fusion Proteins. The first,
second and third collections of spent serum-free culture
media from transfected COS cells were used as sources for
20 the purification of Ig fusion proteins. After removal of
cellular debris by low speed centrifugation, medium was
applied to a column (approximately 200-400 ml medium/ml
packed bed volume) of immobilized protein A (Repligen
Corp., Cambridge, MA) equilibrated with 0.05 M sodium
25 citrate, pH 8Ø After application of the medium, the
column was washed with 1 M potassium phosphate, pH 8, and
bound protein was eluted with 0.05 M sodium citrate, pH
3. Fractions were collected and immediately neutralized
by addition of 1/10 volume of 2 M Tris, pH 8. Fractions
30 containing the peak of A280 absorbing material were pooled
and dialyzed against PBS before use. Extinction
coefficients of 2.4 and 2.8 ml/mg for CD28Ig and B71g,
respectively, by amino acid analysis of solutions of
known absorbance. The recovery of purified CD28Ig and
35 B71g binding activities were nearly quantitative as
judged by FACSR analysis after indirect fluorescent
staining of B7` and CD28'`
CHO cells.
WO 92/00092 208632' 5) rcriuS91i04682
56
SDS Page. SDS-PAGE was performed on linear
acrylamide gradients gels with stacking gels of
acrylamide. Aliquots (1 g) of B71g (lanes 1 and 3 of
Figure 10) or CD28Ig (lanes 2 and 4) were subjected to
SDS-PAGE (4-12% acrylamide gradient) under nonreducing (-
(3ME, lanes 1 and 2) or reducing (+0ME, lanes 3 and 4)
conditions. Lane 5 of Figure 10 shows molecular weight
(Md markers. Gels were stained with Coomassie Brilliant
Blue, destained, and photographed or dried and exposed to
X-ray film (Kodak XAR-5; Eastman Kodak Co., Rochester,
NY) for autoradiography to visualize proteins.
As shown in Figure 10, the B71g fusion protein
migrated during SDS-PAGE under nonreducing conditions
predominantly as a single species of Mr 70,000, with a
small amount of material migrating as a Mr approximately
150,000 species. After reduction, a single Mr
approximately 75,000 species was observed. CD28Ig
migrated as a Mr approximately 140,000 species under non-
reducing conditions and a Mr approximately 70,000 species
after reduction, indicating that it was expressed as a
homodimer. Since the Ig C71 hinge cysteines had been
mutated, disulfide linkage probably involved cysteine
residues which naturally form interchain bonds in the
CD28 homodimer (Hansen et al., Immunogenetics 10:247
(1980)).
DNAs encoding the amino acid sequences
corresponding to the B71g fusion protein and CD28Ig
fusion protein have been deposited with the ATCC in
Rockville, Maryland, under the terms of the Budapest
Treaty on May 31, 1991 and there have been accorded
accession numbers: 68627 (B71g) and 68628 (CD28Ig).
II. Characterization of B7Ig and CD28Ig Cyl Fusion
Proteins
To investigate the functional activities of
B71g and CD28Ig, binding of CHO cell lines expressing
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
57
CD28 or B7 was tested as follows. In early experiments,
spent culture media from transfected COS cells was used
as a source of fusion protein, while in later
experiments, purified proteins were used (see Figure 11).
Binding of B7Ig and CD28Ig to CHO cells. Binding of
CD28Ig and B71g fusion proteins was detected by addition
of FITC-conjugated goat anti-human Ig second step reagent
as described above. B71g was bound by CD28; CHO, while
CD28Ig was bound by B7` CHO. B71g also bound weakly to
B7' CHO (Figure 11), suggesting that this molecule has a
tendency to.form homophilic interactions. No binding.was
detected of-chimeric mAb L6 containing human Ig Cyl, or
another fusion protein, CD5Ig. Thus B71g and CD28Ig
retain binding activity for their respective counter-
receptors.
The apparent affinity of interaction between B7
and CD28 was next determined. B7Ig was either iodinated
or metabolically labeled with [35S] methionine, and
radiolabeled derivatives were tested for binding to
immobilized CD28Ig or to CD28` CHO cells.
Radiolabeling of B7Ig. Purified B71g (25 g) in a
volume of 0.25 ml of 0.12 M sodium phosphate, pH 6.8 was
iodinated using 2 mCi 1 I and 10 g of chloramin T.
After 5 min at 23 C, the reaction was stopped by the
addition of 20 g sodium metabisulfite, followed by 3 mg
of KI and 1 mg of BSA. Iodinated protein was separated
from untreated 125I by chromatography on a 5-ml column of
Sephadex G-10 equilibrated with PBS containing 10% FCS.
Peak fractions were collected and pooled. The specific
activity of 125I-B7Ig labeled in this fashion was 1.5 x 106
cpm/pmol.
B7Ig was also metabolically labeled with
[35S]methionine. COS cells were transfected with a
plasmid encoding B71g as described above. At 24 h after
transfection, [35S]methionine (<800 Ci/mmol; Amersham
WO 92/00092 it 3 i I N 5 PCT/US91/04682
t~ 58
Corp., Arlington Heights, IL) was added to concentrations
of 115 pCi/ml) in DMEM containing 10% FCS and 10% normal
levels of methionine. After incubation at 37 C for 3 d,
medium was collected and used for purification of B71g as
described above. Concentrations of (35S)methionine-
labeled B71g were estimated by comparison of staining
intensity after SDS-PAGE with intensities of known
amounts of unlabeled B7Ig. The specific activity of
[35S]methionine-labeled B71g was approximately 2 x 106
cpm/ g.
Binding Assays. For assays using immobilized
CD28Ig, 96-well plastic dishes were coated for 16-24 h
with a solution containing CD28Ig (0.5 pg in a volume of
0.05 ml of 10 mM Tris, pH 8). Wells were then blocked
with binding buffer (DMEM containing 50 mM BES, pH 6.8,
0.1% BSA, and 10% FCS) (Sigma Chemical Co., St. Louis,
MO) before addition of a solution (0.09 ml) containing
125I-B7Ig (approximately 3 x 106 cpm, 2 X 106 cpm/pmol) or
[35S]--B7Ig (1.5 x 105 cpm) in the presence or absence of
competitor to a concentration of 24 nM in the presence of
the concentrations of unlabeled chimeric L6 mAb, mAb 9.3,
mAb BB-1 or B71g, as indicated in Figure 12. After
incubation for 2-3 h at 23 C, wells were washed once with
binding buffer, and four times with PBS. Plate-bound
radioactivity was then solubilized by addition of 0.5 N
NaOH, and quantified by liquid scintillation or gamma
counting. In Figure 12, radioactivity is expressed as a
percentage of radioactivity bound to wells treated
without competitor (7,800 cpm). Each point represents
the mean of duplicate determinations; replicates
generally varied from the mean by a 20%. Concentrations
were calculated based on a Mr of 75,000 per binding site
for mAbs and 51,000 per binding site for B71g. When
binding of 125I-B7 to CD28* CHO cells was measured, cells
were seeded (2.5 x 104/well) in 96-well plates 16-24 h
before the start of the experiment. Binding was
otherwise measured as described above.
WO 92/00092 2 0 8 6 3 5 PCT/US91/04682
59
The results of a competition binding experiment
using 1251-B71g and immobilized CD28Ig are shown in Figure
12. Binding of 125I-B7Ig was competed in dose-dependent
fashion by unlabeled B71g, and by mAbs 9.3 and BB-1. mAb
9.3 was the most effective competitor (half-maximal
inhibition at 4.3 nM), followed by mAb BB-1 (half-maximal
inhibition at 140 nM) and B7 Ig (half-maximal inhibition
at 280 nM). Thus, mAb 9.3 was approximately 65-fold more
effective as a competitor than B71g, indicating that the
mAb has greater apparent affinity for CD28. The same
relative difference in avidities was seen when
(35S]methionine-labeled B71g was used. Chimeric mAb L6
did not significantly inhibit binding. The inhibition at
high concentrations in Figure 12 was not seen in other
experiments.
When the competition data shown in Figure 12
were replotted in the Scatchard representation (Figure
13), a single class of binding sites was observed
(binding constant (Kd) estimated from the slope of the
line best fitting the experimental data (r = -0.985), Kd
of approximately 200 riM. An identical Kd was detected for
binding of 1 I-B7Ig to CD28* CHO cells. Thus, both
membrane bound CD28 and immobilized CD28Ig showed similar
apparent affinities for '251-B7.
Binding of B7Ig to CD28 a ,pressed on T cells
Although B71g bound to immobilized CD28Ig, and
to CD28* CHO cells, it was not known whether B71g could
bind to CD28 naturally expressed on T cells. This is an
important consideration since the level of CD28 on
transfected cells was approximately 10-fold higher than
that found on PHA-activated T cells as shown above in
Example 1. PHA-activated T cells were prepared as
follows.
Cell separation and Stimulation. PBL were isolated
by centrifugation through Lymphocyte Separation Medium
(Litton Bionetics, Kensington, MD) and cultured in 96-
CA 02086325 2000-08-17
WO 92/00092 PCT/US91/04682
well, flat-bottomed plates (4 x 104 cells/well, in a
volume of 0.2 ml) in RPMI containing 10% FCS. Cellular
proliferation of quadruplicate cultures was measured by
uptake of [3H]thymidine during the last 5 h of a 3 day (d)
5 culture. PHA-activated T cells were prepared by
culturing PBL with 1 pg/ml PHA (Wellcome) for 5 d, and 1
d in medium lacking PHA. Viable cells were collected by
sedimentation through Lymphocyte Separation Medium before
use.
PHA-activated T cells were then tested for
binding of B.7Ig (10 pg/ml) by FACSR analysis after
indirect immunofluorescence as described above: Where"
indicated (Figure 14), mAbs 9.3 or BB-1 were also added
at 10 pg/ml to cells simultaneously with B7Ig. Bound mAb
was detected with a FITC-conjugated goat anti-human Ig
Cy1 reagent.
As shown in Figure 14, these cells bound
significant levels of B71g, and binding was inhibited by
mAbs 9.3 and
BB-1.
The identity of B71g-binding proteins was also
determined by immunoprecipitation analysis of 125I-surface
labeled cells as follows.
Cell Surface Iodination and Immunonrecigitation
PHA-activated T cells were cell-surface labeled with 1251
using lactoperoxidase and H20 2 as described by Vitetta et
al., J. Exp. Med. 134:242 (1971).
Aliquots.of a nonionic detergent
extract of labeled cells (approximately 3 X 108 cpm in a
volume of 0.12 ml) were prepared as described by Linsley
et al., J. Biol. Chem. 263: 8390 (1988)
and subjected to immunoprecipitation
analysis and SDS-PAGE, as described above using a 5-15%
acrylamide gradient, under reducing (Figure 15, +$ME,
lanes 1-7) or non-reducing conditions (-$ME, lanes 8 and
WO 92/00092 Z 0 8 6 J ('5 PCT/US91/04682
61
9), with no addition (lane 1), addition of mAb 9.3 (5 g,
lane 2), addition of B71g (10 g, lane 3), or addition of
chimeric L6 mAb (10 g, lane 7).
As shown in Figure 15, Both mAb 9.3 and B71g
immunoprecipitated a protein having a Mr of approximately
45,000 under reducing conditions, and proteins having a Mr
of approximately 45,000 and approximately 90,000 under
nonreducing conditions, with the latter form being more
prominent. The protein having a Mr of approximately
45,000 found in the sample precipitated with chimeric mAb
L6 was due to spillover and was not observed in other
experiments. mAb 9.3 was more effective at
immunoprecipitation than B71g, in agreement with the
greater affinity of the mAb (Figures 12 and 13).
Identical results were obtained when CD28' CHO cells. were
used for immunoprecipitation analysis. Preclearing of
CD28 by immunoprecipitation with mAb 9.3 also removed
B71g-precipitable material, indicating that both mAb 9.3
and B71g bound the same 125I-labeled protein.
Taken together, the results in these
experiments indicate that CD28 is the major receptor for
B71g on PHA-activated T cells.
Effects of B7 Binding to CD28 on CD28-mediated Adhesion
mAbs to CD28 have potent biological activities
on T cells, suggesting that interaction of CD28 with its
natural ligand(s) may also have important functional
consequences. As a first step in determining functional
consequences of interaction between B7 and CD28, it was
determined whether B71g could blo:,'c the CD28-mediated
adhesion assay described above. The adhesion of 51Cr-
labeled PM lymphoblastoid cells to monolayers of CD28+ CHO
cells was measured as described above, in the presence of
the indicated amounts of mAb 9.3 or B71g. Data are
expressed in Figure 16 as a percentage of cells bound in
the absence of competitor (40,000 cpm or approximately
WO 92/00092 j O J J PCT/US91/04682
62
1.1 X 105 cells). Each point represents the mean of
triplicate determinations; coefficients of variation were
< 25%.
As shown in Figure 16, B71g blocked CD28-
mediated adhesion somewhat less effectively than mAb 9.3
(half-maximal inhibition at 200 nM as compared with 10 nM
for mAb 9.3). The relative effectiveness of these
molecules at inhibiting CD28-mediated adhesion was
similar to their relative binding affinities in
competition binding experiments (Figure 12). CD281g
failed to inhibit CD28-mediated adhesion at
concentrations of up to 950 nM, suggesting that much
higher levels of CD28Ig were required to compete with the
high local concentrations of CD28 present on transfected
cells.
The Effects of B7 On T Cell Proliferation
It was further investigated whether triggering
of CD28 by B7 was costimulatory for T cell proliferation.
The ability of B71g to costimulate proliferation of PBL
together with anti-CD3 was first explored. PBL were
isolated and cultured in the presence of the
costimulators of T cell proliferation indicated, in Table
2. Anti-CD3 stimulation was with mAb G19-4 at 1 pg/ml in
solution. For CD28 stimulation, mAb 9.3 or B7Ig were
added in solution at 1 pg/ml, or after immobilization on
the culture wells by pre-incubation of proteins at 10
pg/ml in PBS for 3 h at 23 C and then washing the culture
wells. B7+ CHO and control dhfr* CHO cells were
irradiated with 1,000 rad before mixing with PBL at a 4:1
ratio of PBL/CHO cells. After culture for 3 d,
proliferation was measured by uptake of [3H) thymidine for
5 h. Values shown are means of determinations from
quadruplicate cultures (SEM < 15%).
In several experiments, B7Ig in solution at
concentrations of 1-10 pg/m1 showed only a modest
!3'U 92/00092
208632, 0 PCT/US91/04682
63
enhancement of proliferation even though the anti-CD28
mAb 9.3 was effective. Because CD28 crosslinking has
been identified as an important determinant of CD28
signal transduction (Ledbetter at al., o0 75:1531
(1990)), B71g was also compared to 9.3 when immobilized
on plastic wells (Table 2, Exp. 1).
Table 2
[3H]-T incorporation
EXR 1 CD28 Stimulation -Anti-CD3 +Anti-CD3
cpm X 1.0'3
1 None 0.1 26.0
mAb 9.3 (soln.) 0.3 156.1
mAb 9.3 (immob.) 0.1 137.4
B71g (immob.) 0.1 174.5
2 None 0.2 19.3
mAb 9.3 (soln.) 0.4 75.8
B7 + CHO cells 9.4 113.9
dhfr + CHO cells 23.8 22.1
Under these conditions, B71g was able to
enhance proliferation and compared favorably with mAb
9.3. B7+ CHO cells also were tested and compared with
control dhfr+ CHO cells for costimulatory activity on
resting lymphocytes (Table 2, Exp. 2'). In this
experiment, proliferation was seen with dhfr+ CHO cells in
the absence of anti-CD3 mAb because of residual
incorporation of [3H]thymidine after irradiation of these
cells. The stimulation by dhfr+ cells was not enhanced by
anti-CD3 mAb and was not observed in other experiments
(Tables 3 and 4) where transfected CHO cells were added
at lower ratios.
For the experiments shown in Table 3, PHA
blasts were cultured at 50,000 cells/well with varying
amounts of irradiated CH) cell transfectants. After 2 d
of culture, proliferation was measured by a 5 h pulse of
[3H]thymidine. Shown are means of quadruplicate
determinations (SEM < 15%). Background proliferation of
WO 92/00092 U U J J?., PCT/US91/04682
64
PHA blasts without added CHO cells was 11,200 cpm.
[3H]thymidine incorporation by irradiated B7'' CHO and CD5+
CHO cells alone was > 1,800 cpm at each cell
concentration and was subtracted from the values shown.
For the experiments summarized in Table 4, PHA blasts
were stimulated as described in Table 3, with irradiated
CHO cells at a ratio of 40:1 T cells/CHO cells. mAbs
were added at 10 ug/ml at the beginning of culture. mAb
LB-1 (Yokochi et al., supra) is an isotype-matched
control for mAb BB-1. Proliferation was measured by
uptake of (3H]thymidine during a 5 h pulse after 2 d of
culture. Values represent means of quadruplicate
cultures (SEM <15%).
B7+ CHO cells were very effective at
costimulation with anti-CD3 mAb, indicating that cell
surface B7 had similar activity in this assay as the
anti-CD28 mAbs.
B7+ CHO cells were also tested as to whether
they could directly stimulate proliferation of resting
PHA blasts which respond directly to CD28 crosslinking by
mAb 9.3. Again, the B7+ CHO cells were very potent in
stimulating proliferation (Table 3) and were able to do
so at very low cell numbers (PHA blast:B7'' CHO ratios of
>800:1). The control CD5+ CHO cells did not possess a
similar activity. (In a number of different experiments
neither dhfr CHO, CD5+ CHO, nor CD7+ CHO cells stimulated
T cell proliferation. These were therefore used
interchangeably as negative controls for effects induced
by B7+ CHO cells. The stimulatory activity of B7+ CHO was
further shown to result from CD28/B7 interaction, since
mAb BB1 inhibited stimulation by the B7+ CHO cells without
affecting background proliferation in the presence of CD7`
CHO cells (Table 4). mAb LB-1 (Yokochi et al., supra),
an IgM mAb to a different B cell antigen, did not inhibit
proliferation. mAb 9.3 (Fab fragments) inhibited
proliferation induced by B7+ CHO and as well as background
proliferation seen with CD7+ CHO cells.
WO 92/00092 C n
S J 3 PCf/US91/04682
Table 3
[3H] incorporation
5 T cells/CHO cells + B7+ CHO + CD5+ CI-bO
cpm X 10'3
25:1 92.7 15.5
10 50:1 135.4 19.4
100:1 104.8 16.8
200:1 90.3 17.7
400:1 57.0 13.7
800:1 42.3 17.6
Table 4
Stimulation mAb j3H1
incorporation
cpm X 10"3
None None 10.8
B7+ CHO None 180
B7+ CHO 9.3 Fab 132
B7+ CHO BB-1 98.3
B7+ CHO LB-1 196
CD7+ CHO None 11.5
CD7+ CHO 9.3 Fab 10.0
CD7+ CHO BB-1 10.0
CD7` CHO LB-1 11.3
These experiments show that B7 is able to
stimulate signal transduction and augment T cell activity
by binding to CD28, but that crosslinking is required and
B7 expressed on the cell surface is most effective.
The Effects of B7 on IL-2 mRNA Accumulation
Effects of CD28/B7 interactions on IL-2
production were investigated by analyzing transcript
levels in PHA-blasts stimulated with B7+ CHO cells or CD7+
CHO cells. RNA was prepared from stimulated cells and
CA 02086325 2000-08-17
WO 92/00092 PCT/US91 /04682
66
tested by RNA blot analysis for the presence of IL-2
transcripts as follows.
PHA blasts (5 X 107) were mixed with transfected
CHO cells at a ratio of 40:1 T cells/CHO cells, and/or
mAbs as indicated in Figure 17. mAb 9.3 was used at 10
g/ml. mAb BB-1 was added at 20 g/ml 1 h before
addition of B7` CHO cells. When mAb 9.3 was crosslinked,
goat anti-mouse Ig (40 gg/ml) was added 10 min after
addition of mAb 9.3. Cells were incubated for 6 h at 37 C
and RNA was isolated and subjected to RNA blot analysis
using 32P-labeled IL-2 or GAPDH probes as described below.
RNA was prepared from stimulated PHA blasts by
the procedure described by Chomczynki and Sacchi, Anal.
Biochem 162:156 (1987).
Aliquots of RNA (20 g) were fractionated on formaldehyde
agarose gels and then transferred to nitrocellulose by
capillary action. RNA was crosslinked to the membrane by
UV light in a Stratalinker (Stratagene, San Diego, CA),
and the blot was prehybridized and hybridized with a 32P_
labeled probe for human IL-2 (prepared from an
approximately 600-bp cDNA fragment provided by Dr. S.
Gillis; Immunex Corp., Seattle, WA). Equal loading of
RNA samples was verified both by rRNA staining and by
hybridization with a rat glyceraldehyde-6-phosphate
dehydrogenase probe (GAPDH, an approximately 1.2-kb cDNA
fragment provided by Dr. A Purchio, Bristol-Myers Squibb
Pharmaceutical Research Institute, Seattle, WA).
As shown in Figure 17, B7` CHO cells, but not
CD7` CHO cells, induced accumulation of IL-2 mRNA
transcripts. Induction by B7' CHO cells was partially
blocked by mAb BB-1. Induction by B7' CHO cells was
slightly better than achieved by mAb 9.3 in solution, but
less effective than mAb 9.3 after crosslinking with goat
anti-mouse Ig. Thus, triggering of CD28 by cell surface
B7 on apposing cells stimulated IL-2 mRNA accumulation.
WO 92/00092 Z 0 8 J 3 , 1 PCT/US91/04682
67
The apparent Kd value for the interaction of
soluble Ig Cy fusions of CD28 and B7 (approximately 200
nM), obtained from the above experiments, is within the
range of affinities observed for mAbs (2-10,000 nM;
Alzari at al., Annu. Ref. Immunol. 6:555 (1988)) and
compares favorably with the affinities estimated for
other lymphoid adhesion molecules. Schneck at al., (Cell
56:47 (1989)) estimated the affinity (Kd approximately 100
nM) between a murine T cell hybridoma TCR and soluble
alloantigen (class I MHC molecules). A Kd of 400 nM was
measured between CD2 and LFA3 (Recny at al., a. Biol.
C e . 265:8542 (1990)). The affinity of CD4 for class II
MHC, while not measured directly, was estimated (Clayton
at al., Nature (Loud.) 339:548 (1989)) to be > 10,000
times lower than the affinity'of gp120-CD4 interactions
(Kd = 4 nM; Lasky at al., Cell 50:975 (1987)). Thus, the
affinity of B7 for CD28 appears greater than affinities
reported for some other lymphoid adhesion systems.
The degree to which the apparent Kd of CD28/B7
interaction reflects their true affinity, as opposed to
their avidity, depends on the valency and/or aggregation
of the fusion protein preparations. The degree of
aggregation of these preparations was examined by size
fractionation (TSK G3000SW column eluted with PBS).
Under these conditions, B71g eluted at M. approximately
350,000, and.CD28Ig at M. approximately 300,000. Both
proteins thus behaved in solution as larger molecules
than they appeared by SDS-PAGE (Figure 10), suggesting
that they may form higher aggregates. Alternatively,
these results may indicate that both fusion proteins
assume extended conformations in solution, resulting in
large stokes radii. Regardless, the interaction that was
measured using soluble proteins probably underestimates
the true avidity between CD28 and B7 in their native
membrane-associated state.
The relative contribution of different adhesion
systems to the overall strength of T cell-B cell
WO 92/00092 PCT'/US91/04682
68
interactions is not easily gauged, but is likely a
function of both affinity/avidity and the densities on
apposing cell surfaces of the different receptors and
counter-receptors involved. Since both CD28 and B7 are
found at relatively low levels on resting lymphoid cells
(Lesslauer et al., Eur. J. Immuno. 16:1289 (1986);
Freeman et al., supra 1989), they may be less involved
than other adhesion systems (Springer Nature Mond).
346:425 (1990)) in initiating intercellular interactions.
The primary role of CD28/B7 interactions may be to
maintain or amplify a response subsequent to induction of
these counter-receptors on their respective cell types.
Binding of B7 to CD28 on T cells was
costimulatory for T cell proliferation (Tables 2-4)
suggesting that some of the biological effects of anti-
CD28 mAbs result from their ability to mimic T cell
activation resulting from natural interaction between
CD28 and its counter-receptor, B7. mAb 9.3 has greater
affinity for CD28 than does B71g (Figures 15 and 16),
which may account for the extremely potent biological
effects of this mAb (June et al., supra 1989) in
costimulating polyclonal T cell responses. Surprisingly,
however, anti-CD28 mAbs are inhibitory for antigen-
specific T cell responses (Damle et al., Proc. Natl.
Acad. Sci. USA 78:5096 (1981); Lesslauer et al., supra
1986). This may indicate that antigen-specific T cell
responses are dependent upon costimulation via CD28/B7
interactions, and that inhibition therefore results from
blocking of CD28 stimulation. Despite the inhibition,
CD28 must be bound by mAb under these conditions,
implying that triggering by mAb is not always equivalent
to triggering by B7. Although mAb 9.3 has higher
apparent affinity for CD28 than B7 (Figure 12), it may be
unable under these circumstances to induce the optimal
degree of CD28 clustering (Ledbetter et al., supra 1990)
for simulation.
WO 92/00092 2 0 8 6 3 2 5 PCT/US91/04682
69
CD28/B7 interactions may also be important for
B cell activation and/or differentiation. As described
above in Example 2, mAbs 9.3 and BB-1 block Th cell-
induced Ig production by B cells. This blocking effect
may be due in part to inhibition by these mAbs of
production of Th-derived B cell-directed cytokines, but
may also involve inhibition of B cell activation by
interfering with direct signal transduction via B7.
These results suggest that cognate activation of B
lymphocytes, as well as T. lymphocytes, is dependent upon
interaction between CD28 and B7.
The above results demonstrate that the ligand
for CD28 receptor, the B7 antigen, is expressed on
activated B cells and cells of other lineages. These
results also show that CD28 receptor and its ligand, B7,
play a pivotal role during both the ctivation of CD4* Th
cell and Th-induced differentiation of B cells. The
inhibition of anti-CD28 and anti-B7 mAbs on the cognate
Th:B interaction also provide the basis for employing the
CD28Ig and B7Ig fusion proteins, and monoclonal
antibodies reactive with these proteins, to treat various
autoimmune orders associated with exaggerated B cell
activation such as insulin-dependent diabetes mellitus,
myasthenia gravis, rheumatoid arthritis and systemic
lupus erythematosus (SLE).
As will be apparent to those skilled in the art
to which the invention pertains, the present invention
may be embodied in forms other than those specifically
disclosed above without departing from the spirit or
essential characteristics of the invention. The
particular embodiments of the invention described above,
are, therefore, to be considered as illustrative and not
restrictive. The scope of the present invention is as
set forth in the appended claims rather than being
limited to the examples contained in the foregoing
description.
WO 92/00092 2 0 i3 a 5 PCT/US91/04682 "
SEQUENCE LISTING
(1) GENERAL INFORMATION:
5 (i) APPLICANT: Linsley, Peter S
Ledbetter, Jeffrey A
Damle, Nitin K
Brady, William
10 (ii) TITLE OF INVENTION: LIGAND FOR CD28 RECEPTOR ON
B CELLS AND METHODS
(iii) NUMBER OF SEQUENCES: 7
15 (iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Sheldon & Mak
(B) STREET: 201 South Lake Avenue, Suite 800
(C) CITY: Pasadena
(D) STATE: California
20 (E) COUNTRY: United States
(F) ZIP: 91101
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
25 (B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: Patentln Release #1.0, Version
#1.25
30 (vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
35 (viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Mandel, SaraLynn
(B) REGISTRATION NUMBER: 31,853
(C) REFERENCE/DOCKET NUMBER: 7794
WO 92/00092 2 0 8 6 3 2 5 PCT/US91/04682
71
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (818) 796-4000
(B) TELEFAX: (818) 795-6321
(2) INFORMATION FOR SEQ ID NO:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(iv) ANTI-SENSE: NO
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Homo sapiens
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:1:
CTAGCCACTG AAGCTTCACC ATGGGTGTAC TGCTCACAC 39
(2) INFORMATION FOR SEQ ID NO:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(iv) ANTI-SENSE: NO
(vi) ORIGINAL SOURCE:
u80
WO 92/00092 2 PCf/US91l04582
72
(A) ORGANISM: Homo sapiens
(Xi) SEQUENCE DESCRIPTION: SEQ ID NO:2:
TGGCATGGGC TCCTGATCAG GCTTAGAAGG TCCGGGAAA 39
(2) INFORMATION FOR SEQ ID NO:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 base pairs
(B) TYPE: nucleic acid
,(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(iv) ANTI-SENSE: NO
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Homo sapiens
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:3:
TTTGGGCTCC TGATCAGGAA AATGCTCTTG CTTGGTTGT 39
(2) INFORMATION FOR SEQ ID NO:4:
(1) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 84 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
WO 92/00092 0 8 6 3 29. 5 PCT/US91/04682
73
(iv) ANTI-SENSE: NO
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Homo sapiens
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:4:
AAGCAAGAGC ATTTTCCTGA TCAGGAGCCC AAATCTTCTG ACAAAACTCA
CACATCCCCA 60
CCGTCCCCAG CACCTGAACT CCTG
84
(2) INFORMATION FOR SEQ ID NO:5:
(1) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 41 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(iv) ANTI-SENSE: NO
(vi) ORIGINAL SOURCE:.
(A) ORGANISM: Homo sapiens
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:5:
CTTCGACCAG TCTAGAAGCA TCCTCGTGCG ACCGCGAGAG C 41
(2) INFORMATION FOR SEQ ID NO:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 47 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
WO 92100092 2 0 PPCT/US91/04682
74
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(iv) ANTI-SENSE: NO
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Homo sapiens
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:6:
CATTGCACAG TCAAGCTTCC ATGCCCATGG GTTCTCTGGC CACCTTG 47
(2) INFORMATION FOR SEQ ID NO:7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(iv) ANTI-SENSE: NO
(vi) ORIGINAL SOURCE:
(A) ORGANISM: Homo sapiens
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:7:
ATCCACAGTG CAGTGATCAT TTGGATCCTG GCATGTGAC 39
CA 02086325 2003-01-20
74-1
(2) INFORMATION FOR SEQ ID NO:8:
<210> 8
<211> 216
<212> PRT
<213> HOMO SAPIENS
<300>
<301> Gordon J. Freeman, et al.
<302> B7, A NEW MEMBER OF THE Ig SUPERFAMILY WITH UNIQUE EXPRESSION ON
ACTIVATED AND NEOPLASTIC B CELLS
<303> Journal of Immunology
<304> 143
<305> 8
<306> 2714-2722
<30'7> 1989-10-15
<309>
<313> (1)..(216)
<400> 1
Gly Leu Ser His Phe Cys Ser Gly Val Ile His Val Thr Lys Glu Val
1 5 10 15
Lys Glu Val Ala Thr Leu Ser Cys Gly His Asn Val Ser Val Glu Glu
25 30
20 Leu Ala Gln Thr Arg Ile Tyr Trp Gln Lys Glu Lys Lys Met Val Leu
35 40 45
Thr Met Met Ser Gly Asp Met Asn Ile Trp Pro Glu Tyr Lys Asn Arg
50 55 60
Thr Ile Phe Asp Ile Thr Asn Asn Leu Ser Ile Val Ile Leu Ala Leu
65 '70 75 80
Arg Pro Ser Asp Glu Gly Thr Tyr Glu Cys Val Val Leu Lys Tyr Glu
85 90 95
Lys Asp Ala Phe Lys Arg Glu His Leu Ala Glu Val Thr Leu Ser Val
100 105 110
Lys Ala Asp Phe Pro Thr Pro Ser Ile Ser Asp Phe Glu Ile Pro Thr
115 120 125
Ser Asn Ile Arg Arg Ile Ile Cys Ser Thr Ser Gly Gly Phe Pro Glu
130 135 140
CA 02086325 2003-01-20
74-2
Pro His Leu Ser Trp Leu Glu Asn Gly Glu Glu Leu Asn Ala Ile Asn
145 150 155 160
Thr Thr Val Ser Gin Asp Pro Glu Thr Glu Leu Tyr Ala Val Ser Ser
165 170 175
Lys Leu Asp Phe Asn Met Thr Thy. Asn His Ser Phe Met Cys Leu Ile
180 185 190
Lys Tyr Gly His Leu Arg Val Asn Gln Thr Phe Asn Trp Asn Thr Thr
195 200 205
Lys Gln Glu His Phe Pro Asp Asn.
210 215
(2) INFORMATION FOR SEQ ID NO.:9:
<210> 9
<211> 134
<212> PRT
<213> HOMO SAPIENS
<300>
<301> Alejandro Aruffo and Brian Seed
<302> MOLECULAR CLONING OF A CD28 cDNA BY A HIGH-EFFICIENCY COS CELL
EXPRESSION
SYSTEM
<303> The Proceedings of the National Academy of Sciences USA
<304> 84
<305> 23
<306> 8573-8577
<307> 1987-12
<309>
<313> (1)..(134)
<400> 2
3 0 Asn Lys Ile Leu Val Lys Gin Ser Pro Met Leu Val Ala Tyr Asp Asn
1 5 10 15
Ala Val Asn Leu Ser Cys Lys Tyr Ser Tyr Asn Leu Phe Ser Arg Glu
20 25 30
Phe Arg Ala Ser Leu His Lys Gly Leu Asp Sex Ala Val Glu Val Cys
35 40 45
Val Val Tyr Gly Asn Tyr Ser Gan Gln Leu Gin Val Tyr Ser Lys Thr
50 55 60
CA 02086325 2003-01-20
74-3
Gly Phe Asn Cys Asp Gly Lys Leu Gly Asn Glu Ser Val Thr Phe Tyr
65 70 '75 80
Leu Gin Asn Leu Tyr Val Asn Gin Thr Asp Ile Tyr Phe Cys Lys Ile
85 90 95
Glu Val Met Tyr Pro Pro Pro Tyr Leu Asp Asn Glu Lys Ser Asn Gly
100 105 110
Thr Ile Ile His Val Lys Gly Lys His Leu Cys Pro Ser Pro Leu Phe
3.0 115 120 125
Pro Gly Pro Ser Lys Pro
130