Note: Descriptions are shown in the official language in which they were submitted.
PEELABLE BARRIER r'! !gin
FOR VACUUM SKIN PACKAGES AND THE LIKE
FIELD OF THE INVENTION
This invention relates generally to thermof ormable barrier
films and to vacuum skin packages which can be made
therefrom. More specifically the invention relates to
multi-layer gas barrier films where the barrier layer or
layers are ethylene vinyl alcohol copolymer. Such films are
especially useful for vacuum skin packaging. Ethylene vinyl
alcohol copolymer is known to be of high gas impermeability;
it is a good barrier to oxygen. But its oxygen permeability
is known to increase in the presence of moisture. It has
been unexpectedly found that enclosing a layer of it with an
ionomer layer on each side will protect it from moisture.
Especially, particularly, the present invention relates to
such mufti-layer gas barrier films useful f or vacuum skin
packaging wherein the barrier layer or layers may be peeled
and separated from the gas permeable layer or layers.
~BACKGROUND OF THE INVENTION
Skin packaging can be classified as a vacuum forming process
for thermoformable polymeric films. The product on a
supporting member serves as the mold for the thermoformable
film which is formed about the product by means of
differential air pressure. However, the term "vacuum skin
packaging" or VSP as it is referred to hereinafter, refers
not to the f act that the thermoformable film is formed around
the product by vacuum or differential air pressure but to the
3/920727.4J/TXTJLS
1
<~uv~~~'~~
ac:. that the product is packaged under vacuum and the space
containing the product is evacuated from gases. Thus, there
is a need for the film formed around the product and for the
support member to be a barrier to o:cygen, air, and other
gaS2S.
Various skin packaging processes are disclosed in e.g. French
Patent No. 1,258,357, French Patent No. 1,286,01.8 (LaRoach
Freres), Australian Patent No. 245,774 ( Colbras Proprietary
Limited), U. S. Patent No. 3,491,504 (W. E. Young et al), U.
S. Patent No. RE.30,009 (Perdue et al), U. S. Patent DTo.
3,574,642 (Weinke), U. S. Patent No. 3,681,092 (Titchness et
al), U. S. Patent No. 3,713,849 (Grindrod et al), and U. S.
Patent No. 4,055,672 (H.irsch et a1). The Cryovac Division of
W.R. Grace & Co. has sold skin packaging thermoplastic
materials under the designations LDX°2986, V834HB, and
V836HB. LDX-2986 is of the structure: EVA(28oVA) +
antiblock/EVA+ionomer/et~hylene alpha olefin/EVOH / tie/
EVOH/tie/EVA + ionomer/HDPE. V834HB and V836HB are the same
structure except V834HB is 4 mil thick and V836HB is 6 mil
thick. The structure is: 5% antiblock + 95o ethylene butene
copolymer/ionomer/EVA/tie/EVOH/tie/EVA/ ionomer/HDPE. Tie is
jargon in the industry for an adhesive layer.
In order readily to open packages where plastic film layers
have been sealed together to close the package, various tear
tabs and easy open mechanisms have been devised. Exemplary
are U. S. Patent No. 4,889,731 (Williams, Jr.), U. S. Patent
No. 4,638,913 (Howe, Jr.), U.S. Pat. No. 4,886,690 (Davis et
al), and U.S. Pat. No. 4,956,212 (Bekele). It is a further
object of the present invention to provide an improved
peelable barrier film for vacuum skin packages having a
permeable layer with a high oxygen transmission rate and an
impermeable layer with a low oxygen transmission rate such
that fresh meat packaged with such film exhibits extended
shelf life and improved bloom.
3/920727.4J/TX~'JLS
2
('9
~~uu!x~~.~~'r~'
The foregoing and other objects are achieved by the present
invention which. is de~~cribed in the Summary of Invention
below, shown in the attached Drawings, and further described
in the Detailed Description.
SUMMARY OF THE INVENTION
In its broadest sense, the present invention is a mufti-layer
gas barrier film having an ethylene vinyl alcohol copolymer
(hereinafter EVOH) layer wherein said ethylene vinyl alcahol
copolymer layer is disposed between a first ionomer layer and
a second ionamer layer, each ionomer layer being directly
adhered to the EVOH layer without any adhesive therebetween.
Each ionomer may be the same ionomer or they may be different
ionomers. Optionally, this film may have another EVOH layer,
so it would be of the mufti-layer structure:
ionomer/EVOH/ionomer/EVOH. Each EVOH layer may be the same
or different EVOHs. With the other EVOH layer, this is
especially useful for vacuum skin packaging, particularly as
the gas impermeable portion of a peelable barrier film, as
further discussed below.
In one aspect, the present invention is a peelable barrier
film suitable for vacuum skin packaging and the like, which
is separable into a gas permeable film and a gas impermeable
film which can be manually delaminated from each other,
wherein:
said gas permeable film comprises a plurality of layers
including a heat sealable, polymeric layer; a core layer
having an increased oxygen transmission rate; and an optical
layer comprising a linear ethylene/alpha-olefin copolymer
(EAO) having a density of between about 0.900 and 0.940
g/cc., and
said gas impermeable film comprises a plurality of
layers including a first EVOH layer immediately adjacent to
and in contact with said linear ethylene/alpha-olefin
3/920727.4J/TXTJLS
3
5'9 ~ .(1 rd ~ t~
~ilUil~~c
copolymer layer of said gas permeable film so that when
delaminat ion occurs the EAO layer will become a first surf ace
layer; a first intermediate layer comprising an ionomer, the
first intermediate layer being adjacent to the first EVOH
layer; a second EVOH layer adjacent to the first intermediate
layer; a second intermediate layer comprising an ionamer, the
second intermediate layer being adjacent to the second. EVOH
layer; an optional layer comprising a polymer selected from
an ionomer, ethylene vinyl acetate copolymer, or a mixture
thereof; and an outer ar second surface layer of polymeric
material; said heat sealable layer of the permeable film
being capable of sealing to a polymeri c surface with a bond
strength greater than the force required to delaminate said
impermeable film from said permeable film.
In still another aspect, the present invention includes a
novel vacuum skin package farmed from the above described
film.
DESCRIPTION OF THE DRAWINGS
In the drawings which are appended hereto and made a part of
this disclosure:
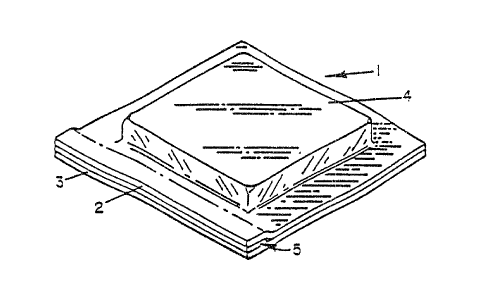
Figure 1 is a perspective view of one embodiment of a vacuum
skin package which can be made using the film and.process of
the present invention.
Figure 2 shows one embodiment of a tab arrangement for
delaminating or peeling apart the two films comprising the
composite peelable film of the present invention. Figure 2
shows the tab to be on film 6 but it could also be on film 7.
Figure 3 shows the peeling process shown in Fig. 2 underway
after rupture of a portion of the permeable film has taken
place; and
3/920727.4J/TXTJLS
4
F'? ,l~ ~ I'
~~uu
Figure ~ is a schematic cross section of the composite film
of the present invention.
DEFINITIONS
As used herein, the following abbreviations and terms have
the meanings defined below:
PVDC: PVDG stands for poly vinylidene chloride copolymers.
Typical ones are vinylidene chloride vinyl chloride
copolymers, vinylidene chloride methyl acrylate copolymers,
and vinylidene chloride acrylonitrile copolymers. These are
sold under the tradename saran by Dow, which name has become
generic in the United States but is a registered trademark in
other countries.
EVA: EVA designates ethylene/vinyl-acetate copolymers.
EMA: EMA designates ethylene/methyl acrylate copolymers.
EnBA: EnBA designates ethylene/n-butyl-acrylate copolymers.
LDPE: LDPE means law density polyethylene and designates
branched chain polyethylene made by the high pressure process
and will have a density below 0.940 g/cc and, most often a
density of 0.915 to 0.925 as the 0.926 to 0.939 range is
often referred to as the medium density range..
LLDPE: LLDPE;means linear low density polyethylene which
generally is understood to include that group of
ethylene/alpha-olefin copolymers having limited side chain
branching and which fall into a density range of 0.910 to
0.940 g/cc. Sometimes linear. polyethylene in the density
range from 0.926 to 0.940 is referred to as linear medium
density polyethylene (LMDPE). Typical brand names are Dowlex
from Dow Chemical Company, Ultzex and Neozex from Mitsui
Petro Chemical Company, and Eclair from duPont. The
3/920727.4J/TX'fJLS
~ ~1 C
~ iJ (s U ~ s
alpha-olefin copolymers are typically butene-1, pentene-1,
hexene-1, octene-1, etc.
VLDPE and ULDPE: Very low density polyethylene ('VLDPE) and
ultra-low density polyethylene (ULDPE) mean
ethylene/alpha-alefin copolymers which have a density of less
than about 0.915 arid, more specifically, usually 0.912 or
below . Typical VLDPE resins are those designated DFDA by
Union Carbide and are believed tcs principally or usually have
butene, or isobutene as a comonomer. ~fhe very low density
polyethylenes as compared to LLDPE, usually have
significantly higher copolymer: content and distinctly
different properties making them a distinct class of
polymers. Typically, resins designated "ULDPE" resins come
from Dow and are believed to have octene as 'the comonomer.
There is a slight difference in properties which is thought
to be attributable to the comonomer. As used herein the term
"linear ethylene/alpha-olefin copolymer having a density of
less than 0.915 g/cc" encompasses both VLDPE and ULDPE. (See
"Plastics Technology Magazine" f or September, 1984 at Page
113 where an article entitled, "INTRODUCING VERY LOW DENSITY
PE" appears.)
ETHYLENE COPOLYMER: Ethylene copolymers axe copolymers
of ethylene and vinyl acetate, alkyl acrylate or
alpha-olefin. Thus, all of the above deffined terms are
ethylene copolymers. Also within the scope of the present
definition are chemically modified derivatives of these
materials. ,
EVOH: EvOH means ethylene-vinyl alcohol copolymer or
hydrolyzed ethylene/vinyl-acetate copolymer and is sometimes
abbreviated "HEVA." EVOH resins are noted for their very
good gas barrier properties but tend to be quite moisture
sensitive. Typical suppliers of resins are Evalca in the
United States and Kuraray in Japan.
3/920727.4J/T~~TJLS
6
~" J1 l"7 I'a a w7 )
~a 1~ U ly ~'
,t i i.)
IONOi9ER: Ionomers are metal salts o:E acrylic acid
copolirmers, such as metal salts of ethylene/acrylic
copolymers or metal salts of ethylene/methacrylic acid
copolymers. A typical supplier is duPont, ~rrhich sells
ionomers under the trademark Surlyn.
OPTT_CAL LAYER: As used herein, an optical layer of a
multi-layer film designates a layer included to enhance the
appearance characteristics of a film so that a product
packaged in the film will have a better appearance.
HDPE: HDPE designates high density polyethylene resins.
Such resins are generally made by the low pressure process
and have a density of greater than 0.940 g/cc.
Antiblock: Antiblocks are very common additives that
alleviate the film sticking to itself when it is wound up or
the inner layer . of a tubular f ilm sticking to itself when the
tube is collapsed and laid flat. Antiblocks typically are
syloid in EVA or in LDPE. A common antiblock is sold under
the tradename 10075ACP Syloid by Teknor Color.
Tie: Tie is jargon in the plastics industry for an adhesive
layer in a film.
DETAILED DESCRIPTION
OF PREFERRED EMBODIMENTS
i
Turning now to the description of the invention reference is
first made to Figure 1 where vacuum skin package 1 is shown.
This package comprises a support member 3 which is a gas
impermeable member preferably formed of polyvinyl chloride
material (PVC) as a substrate material coated with a barrier
material and a heat sealing material; or, it can be a
material such as a polystyrene foam which also is coated with
a barrier material and a heat sealing material. Sometimes
cardboard coated with barrier material and heat sealing
3/920727.4J/TX'fJLS
7
~ ~ r~ r, ~ ~'l t?
~'iJa»:~ ~ iJ
n~acer ial is used as a support member . 'I'ypical heat sealing
materials are branched low density polyethylene (LDPE),
ionomers such as the Surlyn brand sold by duPant,
eth,rlene/methacrylic acid copolymers such as ~lucrel sold by
duPont, eth~~rlene/acrylic acid copolymers such as Primacor
sold by Dow, and EVA copolymers. The support member 3 may be
flat as shown or it may be formed in the shape of a tray.
The product 4 positioned on the support member 3 will, in
general, be a food product such as f rash, red meat.
Particularly, the prime cuts of beef, pork, and lamb would be
the preferred food products. When these products are vacuum
skin packaged or packaged in an atmosphere in the absence of
oxygen the fresh meat will tend to turn a purplish color and
remain that way as long as it is chilled and kept out of
contact with oxygen. The bright red "bloom" is restored when
the meat makes contact with oxygen again.
The thermoformable film 2 which covers the product 4 and is
sealed around the perimeter of the product in a manner to
assume the shape of the product and, thus, become a "skin" is
a composite film and is shown in greater detail in Figure 4.
In Figure 4, which is a schematic representation of the
layers in film 2, the two films 6 and 7 which make up the
composite 2 are shown bonded at interface 8. Film 2 is
preferably coextruded and as shown nine layers are coextruded
together. The two layers 15 and 16 which form the interface
8 are materials which do not readily adhere one to the other
and thus they, form a relatively weak bond. Preferred
materials are EVOH for layer 15 and E.~O far layer 16. More
preferably, layer 16 comprises I~PE.
In a preferred embodiment, in order to achieve excellent
barrier properties at both low and especially high relative
humidities, two layers of EVOH are employed. The: first layer
15 is the interface layer with layer 16; the second EVOH
layer 13 is disposed between two layers 12 and 14 of
ionomer. More preferably, layers 12 and 14 comprise a blend
3/920727.4J/TXTJLS
8
~,~(~il~~~
of ionomer and EVA and/or modified F>olymeric adhesives.
Preferred EVA has a vinyl acetate content of 12o to 2S% by
weight, most preferably 18%. Preferred polymeric adhesives
are EVA- and LLDPE- based adhesives.
The impermeable film further includes an abuse layer 10 which
is adhered to layer 12 either directly or by layer 11. Layer
11 typically is an implosion resistant layer such as an EVA
layer or is a moisture protection layer such as a layer of
ionomer, or layer 11 may be a blend of EVA and ionomer.
It is known that the oxygen barrier properties of EVOH
decrease in the presence of moisture. For instance, the EVOH
moisture problem is discussed by Dow Chemical Corporation in
its sales brochure entitled "Rigid Plastic Barrier Containers
far tJnrefrigerated Foods". The brochure describes the oxygen
transmission rate (OTR) for some typical thermoplastic
polymers as follows:
Oxygen Transmission
Polymer cc.mil/100 in2.atm.day
PVDC 0.15
Nylon 66 2.0
Nylon 6 2.6
Polypropylene 150
EVOH 0.01 at Oo relative humidity
EVOH , 1.15 at 100 o relative humidity
On page 14 of the brochure is a discussion of Dow's rigid
tubs of coextruded sheet containing a saran layer for retort
packaging. Dow°s laboratory staff tested the oxygen
permeability of several containers to illustrate the superior
oxygen barrier properties under moisture of saran tubs as
compared to EVOH tubs . The layers of the sheet were of the
structures: PP/tie layer/EVOH/tie layer/PP and PP/tie
3/920727. 4,7/TXT,7LS
9
s
~; ~ ~ a ~'~ "l r~
la7er/saran/tie layer/PP. (PP is an abbreviation far
polypropylene.) The tub containers were filled with hot
water, sealed, and retorted under water at 250 degrees F (121
degrees C) for 60 minutes at an air over pressure of 21 psig
(2.~kg/cm?). The retorted containers of both types were
then emptied and tested for oxygen transmission. The oxygen
transmission rate of the EVOH sheet was more than double that
of the saran sheet. Furthermore, although the EVOH sheet
dried out over time and its oxygen transmission rate
decreased, it was still double. that of the saran sheet.
Clearly due to the retort mois9=ure present, EVOH could not
provide the low oxygen transmission rate that saran did.
Many ways have been sought to protect an EVOH layer from
moisture. It has been unexpectedly found that enclosing EVOH
barrier layer Z3 with ionomer as described appears to improve
the oxygen barrier properties of the film. Since the EVOH
layer 13 is an anterior layer, it is protected from moisture
attack and will retain its low oxygen permeability. This
effect is enhanced by the presence of an ionomer layer on
each side of the EVOH layer 13. This is particularly so for
an EVOH with a lower mol% ethylene, such as 27 mol %
ethylene. It is not intended to be bound to any theory, but
it is believed that for an EVOH with a higher mol % ethylene,
such as 44 mol % ethylene, that such an EVOH has less of a
tendency to have a decrease of oxygen barrier properties in
the presence of moisture. Thus having an ionomer layer on
each of of the EVOH layer becomes more helpful for
alleviating this moisture problem as the mol % ethylene of
the EVOH decreases.
The excellent barrier properties achievable by the present
invention result in a VSP web having a wet (by wet is meant
1000 relative humidity) oxygen transmission rate (OTR) as low
as 70 cc. mil thickness/m?.atmosphere.day at room
temperature (which is equivalent to about 4.5 cc. mil
thickness/100 inZ.atmosphere.day at room temperature).
3/920727.4J/TXTJLS
~.'' .~9 C? ( ~ ~ v" !,()
~d ~ ~J ~ ~ ~ 't>
The test for oxygen transmission is conducted as per ASTM
D39~5.
The gas permeable film 7 preferably comprises an interface
lager 16 of EAO; a sealant layer 18; and a core layer 17
between the interface and sealant layers.
Sealant layer 18 preferably comprises EVA having 12a - 280
vinyl-acetate, and/or EMA having 15% to 25% methyl acrylate
by weight. A small amount of an anti-block agent can be
included. An example of EVA is Exxon XS74.16, a 12~ vinyl
acetate EvA. Examples of EMA are the resins sold under the
tradename EMAC by Chevron or other EMAs sold by Exxon.
The composition of the core layer 17 allows for maximum gas
permeability. Layer 17 is typically of ethylene copolymers
such as EVA, EnBA, ULDPE, or blends thereof , which have high
OTRs allowing for a more rapid bloom and longer bloomed color
retention of meats which have aged in the VSP barrier
package. Also, blends of EVA/ionomer or of EnBA/ionomer
could be used. These blends of EVA/ionomer or of EnBA
ionomer for layer 17 are known from U.S. Patent 4,956,212,
mentioned above. Most ionomers are stiffer than most EVAs,
so ionomer toughens the gas permeable film 7. But ionomer is
also a better oxygen barrier than EVA, so ionomer is
decreasing the gas permeability whereas it is desired to have
film 7 be of high gas permeability. So it is desired to have
plasticizer such as white mineral oil in the ionomer to
increase the oxygen transmission rate of the ionamer.
Generally, ionomers (such as Surlyn, which are metal
neutralized ethylene methacrylic acid copolymers) do not hold
plasticizers. The plasticizer blooms out of such materials.
However, when blended with an EVA or EnBA at 40/60, 30/70,
20/80 ratios, ionomers can be plasticized. Another group of
Surlyn ionomers (1856) which are terpolymers of ethylene
methacrylic acid ester can be plasticized by themselves. But
the plasticizer can cause other problems when it migrates, so
3/920727.4J/TXTJLS
11
C1 ~'~, l1 F~ ~7 ~"~ f
l., U a U .H ( ~,~
it is a trade of.f with how much ionomer for stiffening is
used as use of ionomer is desirably with plasticizer to
increase oxygen transmission rate. This can readily be
determined by the person of ordinary skill in the art without
undue experimentation.
It has also been found that, when EnBA and EVA have the same
molo comonomer of nBA and VA respectively, then the use of
EnBA alone as a core component produces a film of higher gas
permeability as compared to an analogous film having an EVA
core component. Other materials which can be used with or
without plasticizer modification include copolymers of
ethylene and ester type acrylic derivatives such as ethylene
methyl acrylate, ethylene ethyl acrylate, etc. However, a
preferred core composition for the present permeable film is
a EnBA (30oBA) or a blend of EnBA(30oBA) + EnBA(lBoBA). A
preferred EnBA for use in the present invention has about 300
butyl acrylate although copolymers having from about 5o to
35% of butyl acrylate, such as EnBA with 18o butyl acrylate
are also within the scope of the present invention. It has
further been found that inclusion of a small amount of a
plasticizer into a core of EVA produces a permeable film with
an acceptably high oxygen transmission rate.
Layer 16 can be a ULDPE of density of between about 0.900 and
0.915 grams/ce. For obtaining good package optics, a density
of about 0.912 is preferred. An example of such a material
is Attane 4002 from Dow. Materials of 0.906 grams/cc can
also be used. Also preferred are L1'~PE materials, e.g. 0.935
grams/cc. A commercial example of LI~PE is Dowlex 2037. Use
of LI~mPE in this construction improves the peelability of the
impermeable film 6 from the permeable film 7.
Layers 15 and 13 are both EVOH layers. Preferred resins
contain between about 27 and 48 male percent of ethylene. An
especially preferred commercial resin is EVAL from Evalca.
3/920727.4J/TXTJLS
12
63 .f'1 ft /> !1 N~1
lw J' 1~ il >.~ 1 i~
Layers 15 and/or 13 can also include amorphous nylon.
Commercial examples of semiaromatic amorphous nylons of
hexamethylene diamine, isophthalic and terephthalic acids
containing bis(p-amino cyclohexy) methane for inclusion in
the present composite axe Selar PA 3426, from Du Pant; and
Grivory 21, from Emser Industries. Others include Selar PA
3508; Gelon A 100 from General Electric; Durethane T 40 from
Mobay; Allied XA 1722 from Allied Signal; Novamid X 21 from
Mitsubishi Chemical Tndustries Limited; and MXD6 from
Mits~,;bishi Gas Chemical Co., Tnc.
Layers 12 and 14 are implosion resistant. layers which also
serve to enhance the oxygen barrier properties of 'the film by
shielding the EVOH of layer 13 from moisture. Preferably,
layers 12 and 14 comprise a blend of ionomer and EVA and/or
modified polymeric adhesives. Preferred EVA has a VA content
of 12o to 25o by weight, most preferably 180. Preferred
polymeric adhesives are EVA- and LLDPE- based adhesives. Most
preferred compositions for layers 12 and 14 are:
35o ionomer + 35%EVA + 30% EVA based adhesive; or
70o ionomer + 30o EVA based adhesive; or
70o ionomer + 3f% LLDPE based adhesive.
Layer 10 is an outer layer, normally an outermost layer which
provides high gloss and good moisture barrier properties. A
preferred material for layer 10 is HDPE such as Soltex
J60-800C-147 from Solway.
i
Optional layer 11 functions as an implosion resistant layer
when it is of a polymer such as EVA or as a moisture
protection layer when' it is or an ionomer, and is preferably
an ionomer or a blend such as that disclosed for layers 12
and 14.
Preferably, the present composite film is crosslinked. The
preferred method for crosslinking is irradiation, although
chemical crosslinking such as by peroxides is also within the
3/920727.4J/TXTJLS
13
~,tJ~i~~.~r~l~
sccpe of the present invention. Irradiation of the film rnay
be accomplished by the use of high energy electrons, ultra
violet radiation, X-rays, gamma rays, beta particles, etc.
Preferably, electrons are employed from about 0.5 megarads up
to about 18 megarads (MR) dosage level. The irradiation
source can be any electron beeun generator operating in a
range of about 150 kilovolts to about 6 megavolts with. a
power output capable of supplying the desired dosage. The
voltage can be adjusted to appa:opriate levels which may be
for example 1,000,000 or 2,000,000 or 3,000,000 or 6,000,000
or higher or lower.
Many apparatus for irradiating films are known to those of
skill in the art. For purposes of the present invention, the
irradiation is preferably carried out at a dosage within the
range of from about 9 MR to about 18 MR and most preferably
within 'the range of from about 12 MR to abo~zt 14 MR.
The specific best mode of the forming web for vacuum skin
packaging and the like Which is separable into permeable and
impermeable films has as the permeable skin film or layer 7,
a construction as follows:
Sealant layer (18) / Core layer (17) / Optical layer (16)
0.20 - 0.60 1.00 - 2.50 0.10 - 0.20 mils
Wherein:
Sealant = EVA or EMA
EVA copolymer having 4o to 28% VR from Du
Pont or Exxon, or EMA copolymer having 40
to 24o MA from Exxon or Chevron with an
anti-block agent;
3/920727.4J/TXTJLS
14
~G~~~~r~~
Core - EnBA with nBA content of 15%~30~ such as
Lotryl 30 BA 2 from :Elf Atochem which has
30o nBA, with or without a plasticizer,
or an EnBA/EVA blend with or without a
plasticizer, or an EnBA/EMA blend with or
without a plasticizer, or an EnBA( 18 oBA) /
EnBA(30oBA) blend with or without a
plasticizer;
Optical = LNmPE from Dow, DOWLEX 2047.
The impermeable or peelable barrier film has the following
construction:
EVO~i/ IONOMER /EVOH/ IONOMER / OPTIONAL /I~7PE ( Outside )
+ +
EVA EVA
+ +
EVA based tie EVA based tie
wherein the ionomer layer is a blend of ionomer with tie and
with EVA or modified LLDPE or modified EVA. One of the EVA
resins that is preferred is Elvax 3165 from DuPont. The
preferred EVA based tie is Bynel CXA 3062 from DuPont.
Further, although in its preferred embodiment, the present
invention is directed to a composite film having a permeable
web and an impermeable web, it is also within the scope of
the present invention to provide the present improved barrier
web with a prior art permeable web and further, to provide
the present improved permeable web with a prior art barrier
web depending on the requirements of any given application.
To make the composite film or web, a coextrusion process
similar to that desoribed in U.S.Pat. No. 4,287,151 to Esakov
et a1 may be employed. Suitable annular or flat sheet
mufti-layer dies must, of course, be used and these are well
known in the art.
3/920727.4J/TXTJLS
~~i~~~~l
Looking now at Figures 2 and 3, tab 5 has been laid across
one edge of the vacuum skin package as can be seen by the
dotted line in Figure 1 so that in forming of upper web 2
(the composite film) around product 4, it does not adhere to
the support 3. This allows for a tab to form as can be seen
in Figure 2 that can be gripped and moved upwardly by the
fingers to a position shown in Figure 3. In this position it
can be seen that the permeable film 7 has delaminated at
interfacing rather than its bond with the support member 3.
This leaves the film portion 7' :firmly adhered to the support
member 3. This type of strong bond is formed between the EMA
or EVA surf ace of the film 7 with the coated PVC of the
support member. Surfaces such as EVA and EnBA adhere
strongly to each other and their bond strength is greater
than the internal cohesive strength of film 7.
Also seen in Figure 3 is the beginning of the delamination of
the permeable film 6 from the impermeable film 7. The
already peeled apart portion 6' has separated from the bonded
portion 7' so that the entire impermeable film 6 may be
peeled from the permeable film 7 leaving the inner "skin"
package comprising support member 3, peeled, permeable film
7', and product 4. SVhen product 4 is a fresh red meat
product, it will, within half an hour to an hour, regain its
bright red bloom and is then ready for display in a showcase
or retail case.
i
Upon reading and becoming familiar with the disclosure
herein, equivalent layer combinations and packages will
likely become evident or obvious to those skilled in the
art. For example, instead of or in addition to blending EVA
or polymeric adhesives into the intermediate layers of the
impermeable film, separate tie layers caa be included in the
film structure to bond, e.g. the intermediate ionomer layers
to the EVOH layers. Although the film of the invention is
3/920727.4J/TX°.C'JLS
16
~~~~~r~B
especially useful for VSP applications, it can also be 'used
in other packaging applications.
EXAMPLE i
Percentages in the examples are percentages by weight, unless
otherwise indicated as mol%.
Example I
Film (1) and film (2) were made in which a layer on each
side of the EVOH (EVOH layer 13 in the drawings) was a blend
of EVA based tie, ionomer, and EVA as described below. The
EVOH was 27 mol % ethylene. The films were fully coextruded
and after coextrusion, irradiated with an electron beam at 13
MR. These films (1) and (2) were of the multi-layer
structure, from sealing layer to outside abuse layer as
follows
~____pe~e~le film 7--____~~_______impermeable film 6-______~
90%EVA /90%EnBA /90%ULDPE/EVOH/blend/EVOH/blend/ionomer/FmPE
+ (18% +
,BA)
10% LDPE+ 10% EVA
based 10% based
anti mineral anti
block oil block
The films were tested at 40 degrees F and 100 % relative
humidity (RH) for oxygen transmission rate (OTR), and the
results were a.s follows
3/920727.4J/TXTJLS
17
r rJ r~
~a V (J t3 '~ 1 ~
F i lm OTR 0TR
blend layer 40 degrees F 40 degrees F
of :ionomer 100° RH/ 100 o RH/cc/
+ EVA -+- EvA cc.mil/day/ day/m2.atm
based tie mz.atm at X
thickness-1
(1) 35 o ionomer 356 49
35 % EVA
30 o EVA based tie
(2) 20 % ianomer
0 o EVA
30 °s EVA based tie 477 70
1 where fox the OTR of the right-hand calumn, the X mil
actual thickness far the first film with the 49 OTR was 7.2
mils and far the second film with the 70.OTR was 6.8 mils.
Example II
Several additional films were made as in Example I, except
these were of the mufti-layer structures of film (I) or of
film (II) as follows, with the mil thickness of each layer
indicated below that layer for a total film thickness of 6.35
mils:
Film (I)
~____permeable film-_><---________impermeable film 6_--_____~
W / U / X / Y / Z / Y / Z / ionomer/ HDPE
0.40 2.0 0>2 0.3 1.1 0.25 1.20 0.5 0.4
3/920727.4J/TXTJLS
18
WIi~U~.~r3~f)
Film (I:L)
<---permeacle film--><-----------impermeable film 6--------_>
w / U l x / v / z / Y / v / HDPE
0.40 2.0 0.2 0.3 1.1 0.25 1.7 0.4
where:
U = EnBA ( 3 0 %BA ) or
50%EnBA( 18%BA) + 50%EnBA( 30%BA)
W = EVA (18 to 25% VA) + LD:PE based antiblock or
= EIYIA ( 18 to 24 % MA) + LDPE based antiblock
X = LLDPE (density 0.912 g/cc) + EVA based antiblock or
ULDPE (density 0.905 g/cc) + EVA based antiblock or
LNIDPE (density 0.935 g/cc) + EVA based antiblock
Y = EVOH (27 or 44 mol % ethylene) or
85% EVOH + 15 % amorphous nylon
Z = a blend of
35% ionomer + 35% EVA (18% VA) + 30% EVA based tie or
70% ionomer + 30% EVA based tie or
70% ionomer,+ 30% LLDPE based tie
V = a blend of:
35% ionomer + 35% EVA (18% VA) + 30% EVA based tie or
ionomer + EVA based tie or
ionomer + LLDPE based tie
Of these the preferred was Film (I) where:
3/920727.4,7/TXTJLS
19
r ~-
t~ (~ U 3 S
w = EvA +~ LDPE based antiblock
X = L'~PE (density 0.935 g/cc) + EVA based antiblock
Y = EVOH (27 or 44 molo ethylene)
Z = 35 o ionomer + 35 % EVA ( :L8 % VA) + 30 o EVA based 'tie
Exar~ole IIT
The peel strength of some of th.e films of Examples I and II
were tested. This was done by ;pulling apart the film at the
interface, i.e. at the junction of the ethylene alpha olefin
internal layer of permeable film 7 and the EVOH layer of
impermeable film 6, and measuring the pounds of force per
linear inch. of film to pull apart the permeable film from the
impermeable film. This was done before electron beam
irradiation. Then it was done after electron beam
irradiation to cross link the film.
The cross linking is desirable to toughen the film, but it
can also increase peel strength at the interface. Increase
in peel strength at the interf ace is undesirable as it is
wanted to pull apart the film at this interf ace after
packaging the meat so the meat will bloom.
For the ethylene alpha olefin, it was found that use of LN1DPE
at the interface and also use of higher 44 mol% ethylene EVOH
at the interface, resulted in a peel strength at the
interface which was not deleteriously aff acted (i.e. not
increased in a statistically significant manner) by electron
beam cross linking. Also, when amorphous nylon was added to
the EVOH layer at the interface, the peel strength was not
deleteriously affected by cross linking. The results are
summarized below, where the ethylene alpha olefin (EAO) of
the permeable film at the interface and the EVOH of the
impermeable film at the interf ace are noted:
3/920727.4J/TXTJLS
~~ p f,1 f~ ~! r C
r:,u~~ ~ I '~
Peel Peel
Strength Strength
before after
Peel interface Cross Cross
Film EAO EVOH Linking Linking
(1) LMDPE 27 mol% 0.035 0.046
(0.935)
(2) IlLDPE 44 mol% 0.034 0.038
(0.905)
(3) ULDPE 27 mol% 0.367 0.326
(0.905) (85% EVOH +
15% amorphous
nylon)
(4) ULDPE
(0.905) 27 mol% 0.027 0.422
Example IV
Two films were made as in Example II, and one film had an
ionomeric polymer layer on each side of the second EVOH layer
of the impermeable film and the other film had a
non-ionomeric polymer layer on each side of the second EVOH
layer of the impermeable film. The EVOH was EVOH with 44 mol
% ethylene. ,The OTR of these films was measured. Two
packages of fresh read meat were packaged with each film and.
stored for 14 days in a high humidity chamber of 100%
relative humidity at 40 degrees F. Then the top of the meat
was observed for discoloration. It was found from the OTR
that the film with the non-ionomeric palymer layer on each
side of the EVOH layer had a lower, and thus more desirable,
OTR, but in the actual packaging tests of fresh red meat,
this film resulted in actual discoloration of 5% of the top
of the meat i:or one package, whereas the film that had an
3/920727.4J/TXTJLS
21
~~~~!~"1
ionomeric polymer layer on each side of the second EVOH layer
did nat result in any discoloration at all of the fresh red
meat. It is believed this discoloration was due to use of
EVOH that had 44 molo ethylene, i.e. the presence of i.onomer
on each side of the EVOH was less helpful for protection of
the EVOH from moisture with 44 molo ethylene EVOH than with
27 mol% ethylene EVOH. The resu:Lts are summarized below.
Normalized Normalized 40 degrees
F
OTR OTR 100 oRH after
40 degrees 73 degrees F/ 14 days
F/
cc.mil/m2/ cc.mil/m~/ o of Top
day/ atm day/ atm Surf ace
100 o RH 100 o RH of Meat
Discolored
Pack Pack
1 2
Film (1) 128 812 Oo Oo
with ionomer
Film (2) 109 610 Oo 50
without ionomer
Example V
Film samples are made, as described above, but where the
films are of tl~e 3-layer structure: A1/B/A2, where A1 and A2
are ionomeric layers, and may be the same ionomeric
composition or each may be a different ionomeric composition,
and each of layers A1 and A2 may be the same thickness or
each may be a different thickness, and where B is a barrier
layer of EVOH. Thus, the 3-layer film is:
ionomer/EVOHfionorner. Preferred is:
B = EVOH (27 or 44 mol o ethylene) or
85% EVOH + 15 % amorphous nylon
3/920727.43/T~TJZS
22
t~
~~ U (j t3~ (,k ; l
and
A1 and A2 are the same or different and = a blend of:
35o ionomer -~- 35o EVA (18a VA) + 30o EVA based tie or
70o ionomer + 30o EVA based t:ie or
70o ionomer + 30o LLDPE based tie or
20o ionomer + 50a EVA + 30o E~VA based tie
Most preferred is A1 and A2 each is 35o ionomer + 35o EVA
(18% VA) + 30°s EVA based tie, and B is EVOH (27 molo
ethylene).
Also, 4-layer films are made where the above-described
3-layer film has another EVOH layer so that the 4-layer film
is of the structure:
ionomer/EVOH/ionomer/EVOH
The other EvOH layer may be EVOH (27 or 44 mal o ethylene) or
may be 85o EVOH + 15 o amorphous nylon, and preferably is
EVOH (27 mol% ethylene).
For the 3-layer films or the 4-layer films, preferred
thicknesses are 0.20 to 0.30 mil for each of the EVOH layers,
and 1.0 to 1.8 mil for each of the ionomer layers.
All of these films should have excellent, low OTRs at high
humidity of 100oRH.
While certain representative embodiments and details
have been shown for the purpose of illustration, numerous
modifications to the formulations described above can be made
without departing from the invention disclosed.
3/920727.4J/TXTJLS
23