Note: Descriptions are shown in the official language in which they were submitted.
2~7~89
S~C ~67gg
CHEMICAL COMPOSITIONS
This invention relates to inks, to a process for the
coloration of paper using such inks, to compounds and compositions which
may be used to prepare inks, and to a process for preparation of the
compounds.
During recent years developments in ink jet printing
technology have lead to desk-top printers capable of colouring paper
with yellow, magenta and cyan prints. Magenta inks currently used often
suffer from the problems of dull shade and poor light fastness leading
to prints which fade, and poor wet fastness leading to prints which
smudge. The present invention was conceived with these problems in
mind.
According to a first aspect of the present invention there is
provided an ink containing less than 5% inorganic compounds which
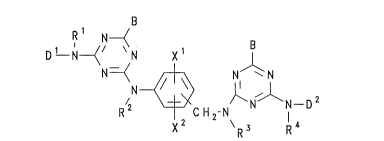
comprises a liquid medium and a dye of the Formula (1):
B
1. R ~CHz-N N I_D
( I )
wherein:
Dl and D2 are each independently a chromophore of the azo series;
Rl, R2, R3 and R4 are each independently H or optionally substituted
alkyl;
~20 B is a labile atom or group, hydroxy, amino or an ether,
thloether or amine group; and
xl and X2 are each independently ~, halo, alkyl, alkoxy, acylamino,
ureido, nitro, carboxy or sulpho.
2~87~9
2 SMC 367~g
, . .
It is preferred that the ink contains less than 2~ more
preferably less than 1~ inorganic compounds. The term 'inorganic
compounds' means any salt consisting of a metal cation and inorganic
anion. As examples of inorganic compounds there may be mentioned alkali
metal, alkali earth metal and transition metal halides, nitrates,
nitrites, sulphates, carbonates and bicarbonates, especially NaCl,
NaN03, NaN02, KCl, Na~lC03, CaC03 and CaC12.
The liquid medium is preferably an aqueous medium. It is
preferred that the dye of Formula (1) is completely dissolved in the
liquid medium to form a solution.
The ink preferably contains from 0.5% to 20Z, more preferably
from 0.5~ to 15Z, and especially from 1~ to 5~, by weight of the dye of
Formula (1), based on the to~al weight of the ink.
The liquid medium is preferably water or a mixture comprising
water and a water-soluble organic solvent, preferably in a weight ratio
from 99:1 to 1:99, more preferably from 95:1 to 50:50 and especially
from 90:10 to 60:40.
The water-soluble organic solvent is preferably a Cl_4-alkanol
such as methanol, ethanol, n-propanol, isopropanol, n-butanol,
sec-butanol, tert-butanol or isobutanol; an amide such as
dimethylformamide or dimethylacetamide; a ketone or ketone alcohol such
as acetone or diacetone alcohol; an ether such as tetrahydrofuran or
dioxane; a polyalkylene glycol such as polyethylene glycol or
polypropylene glycol; an alkylene glycol or thioglycol containing a
C2-C6 alkylene group such as ethylene glycol, propylene glycol, butylene
glycol or triethylene glycol; a thiodiglycol, hexylene glycol, or
diethylene glycol; a polyol such as glycerol or 1,2,6-hexanetriol; a
lower alkyl ether of a polyhydric alcohol such as Z-methoxyethanol,
2-(2-methoxyethoxy)ethanol, 2-(2-ethoxyethoxy)- ethanol,
2-[2-(2-methoxyethoxy)ethoxy]ethanol, 2-[2-(2-ethoxyethoxy)-
ethoxy]-ethanol; 2-pyrrolidone or N-methylpyrrolidone; or a mixture
containing two or more of the aforementioned water-soluble organic
solvents.
,
'
2~7~8~
3 S~C ~7gg
., ,
Especially preferred water-soluble organic solvents are
selected from Z-pyrrolidone, N-methylpyrrolidone, an alkylene glycol or
lower alkyl ether of a polyhydric alcohol such as ethylene glycol,
diethylene glycol, triethylene glycol or 2-methoxy-2-ethoxy-2-
ethoxyethanol; and a polyethylene glycol with a molecular weight of up
to 500. A preferred specific solvent mixture is a binary mixture
comprising water and either diethylene glycol, 2-pyrrolidone or
N-methylpyrrolidone in a weight ratio as mentioned above.
Examples of suit~ble ink media are given in US 4,963,189,
US 4,703,113, US 4,626,284 and EP 4,251,50A.
It is preferred that the inks of the present invention further
comprise one or more of a penetrant to assist permeation of the dye into
a paper substrate, a kogation-reducing agent to prevent or reduce the
build up of residue (koga) on the resistor surface in thermal ink jet
printers and a buffer such as sodium borate, to stabilise the pH of the
ink.
The kogation-reducing agent is preferably an oxo anion, such
as described in ~P 425150A. The oxo-anion may be C2O42-, S032-, S042-,
molybdate, As043- or more preferably a phosphate ester, a
diorganophosphate or more especially a phosphate salt which is
particularly effective in reducing kogation.
As examples of phosphate salts there may be mentioned dibasic
phosphate (~PO4Z-) monobasic phosphates (H2P04-) and polyphosphates
(P2074-). The selection of counter ion is not believed to be critical
and examples include alkali metals, ammonium and alkylammonium cations.
The kogation-reducing agent is preferably present in the ink
at a concentratlon from 0.001% to 1%, based on oxo-anion, and more
preferably from 0.01% to 0.5% (by weight).
The inks of the present invention are of particular ~alue in
ink jet printing of paper. We have found that paper printed with the
inks show acceptable water-fastness properties, a particularly bright
shade and good light fastness. The finding that certain dyes of the
invention have exceptional brightness on paper is particularly
surprising because super~icially similar dyes printed on paper have been
found to be less bright.
~87~g~
4 S~C 36799
It is preferred that Dl and D2 are each independently a
sulphonated monoazo radical, especially a phenylazonaphthalene radical,
more especially an azonaphthalene radical because of its attractive
shade.
A preferred sulphonated monoazo radical represented by Dl or
D2 is of the Formula (2) or (3):
H O
A--N=N,~ ' ~`W
V (2)
.
H O
A--N=N~ ~
U ( 3 )
wherein:
U is sulpho;
one of V ar.d W is sulpho and the other is H; and
A is a radical of the benzene of naphthalene series.
Particularly preferred dyes contain sulpho~ated monoazo
radicals of Formula (2) or (3) wherein A is a phenyl having 1 or 2
sulpho or carboxy groups or naphthyl having 1, 2 or 3 sulpho or carboxy
groups. It is especially preferred that A has a sulpho group in an
ortho position relative to the azo link.
2~87~8~
5 SMC 36799
As examples of phenyl radicals having 1 or 2 sulpho or carboxy
groups there may be mentioned phenyl; 2-, 3- or 4-sulphophenyl; 2,4-,
2,5- or 3,5-disulphophenyl; 2-methyl-4-sulphophenyl;
2-methyl-5-sulphophenyl; 4-methyl-2-sulphophenyl;
2-sulpho-5-methylphenyl; 2-methoxy-5-
sulphophenyl; 4-methoxy-2-sulphophenyl; 2-chloro-4-sulphophenyl;
2-chloro-5-sulphophenyl; 2,5-dichloro-4-sulphophenyl; 4-nitro 5-
sulphophenyl; 5-nitro-2-sulphophenyl; 2-carboxy-5-sulphophenyl; and 3,5-
and 3,4-dicarboxyphenyl.
As Examples of particularly preferred naphthyl radicals having
1, 2 or 3 sulpho or carboxy groups there may be mentioned 4-, 5-, 6- or
7-sulphonaphth-1-yl; 1- or 6-sulphonaphth-2-yl; 3,8- or
4,8-disulphonaphth-1-yl; 4,8- or 5,7-disulphonaphth-2-yl; 2,5,7- or
3,5,7-trisulphonaphth-2-yl; 1,5,7 or 3,6,8-trisulphonaphth-2-yl;
6-carboxy-8-sulphonaphth_2-yl and 6-carboxynaphth-2-yl.
Especially preferred radicals represented by A are of ~ormula
(4) or (5):
U U
~' ~
zl ( 4 ~ z 2 ( 5 )
wherein:
zl is H or sulpho;
U is sulpho, and
z2 is ~; sulpho; Cl_4-alkyl, especially methyl; or Cl_4-alkoxy,
especially methoxy.
It is preferred that Dl and D2 are the same as each other.
Optionally substituted alkyl radicals which may be represented
by Rl, R2, R3 and R4 include Cl_4-alkyl and Cl_4-alkyl substituted by a
halogen, hydroxy, cyano, carboxy or alkoxy group.
Lt is preferred that Rl is the same as R4.
~087~8~
6 SMC 36799
By labile atom or group it is meant any atom or group attached
directly to the triazine ring which is displaceable by a nucleophile
under alkaline conditions. The preferred labile atom is a halogen,
especially F or Cl. The preferred labile group is sulpho, more
preferably a quaternary am~onium group. As examples of quaternary
ammonium groups there may be mentioned trialkyl ammonium groups and
optionally substituted pyridinium groups, especially 3-carboxypyridinium
and 4-carboxypyridinium groups.
xl and x2 are prèferably each independently H; Cl; Cl_4-alkyl,
especially methyl; Cl_4-alkoxy, especially methoxy; carboxy or sulpho.
It is particularly preferred that xl and x2 are H.
When B is an ether group it is preferably -O-(Cl_4-alkyl),
especially methoxy.
When B is a thioether group it is preferably -S-(Cl_4-alkyl),
especially -S-CH3.
When B i5 an amine group it preferably contains less than 13
carbon atoms. Preferred amine groups represented by B are of the
formula:
- ~ -R5
R6
wherein:
R5 and R6 are each independently H; optionally substituted phenyl;
Cl_4-alkyl; hydroxy-Cl_4-alkyl, especially hydroxyethyl;
-(CH2CH2NH)XCH2CH2NH~ wherein x is 2, 3 or 4; or R5 and R6
together with the nitrogen to which they are attached form a 5
or 6 membered ring, especially a morpholine or piperidine
ring.
When R5 or R6 is optionally substituted phenyl the optional
substituent or substituents are preferably selected from Cl_4-alkyl,
especially methyl; Cl_4-alkoxy, especially methoxy; halo, especially
chloro; sulpho and carboxy.
. .
~87~8~
7 SMC 36739
As examples of amine groups there may be mentioned
methylamino, ethylamino, butylamino, dimethylamino, diethylamino,
morpholino, piperidino, hydroxyethylamino, diethanolamino, phenylamino,
3- or 4-carboxyphenylamino, 3,4- or 3,5-dicarboxyphenylamino, 2-, 3- or
4-sulphophenylamino and 2-methyl-4-sulphophenylamino.
According to a second aspect oE the present invention there is
provided a dye of Formula (1) as shown above, wherein Dl, D2, Rl, R2,
P~3, R4, xl and x2 are as hereinbefore defined and B is an amino, ether,
thioether or amine group. Preferred ether and amine groups are as
hereinbefore described.
The dyes of the invention may be prepared by a process
comprising the condensation of one mole of an o-, m- or
p-aminobenzylamine of the Formula (8~:
Xl
~CH2N R3H
H R2N~
( 8
with a triazine compound of the Formula (9a) followed by condensation
with a triazine c~mpound of the Formula (9b):
R4 R
D2_N~'N~B 1 D 1 N~"N~B 1
B ( 9 a ~ ( 9 b )
- ~0~7~8~
8 SMC 36799
., .
wherein:
Dl, D2, Rl, R2, R3, R4, xl and x2 are as defined above and sl is a
labile atom or group as described above. When the groups D2-NR4- and
Dl-NRl- are identical one mole of the aminobenzylamine of Formula (8)
may simply be condensed with about two moles, preferably from 1.7 to 2.3
moles, of the triazine compound of Formula (9a).
Dyes of Formula (1) in which B is a hydroxy, amino or an
ether, thioether or amine group may be prepared by condensing a reactive
dye of Formula tl) in which B is a labile atom or group with
respectively water or ammonium hydroxide or an ethoxide, thioethoxide or
an amine, optionally in the presence of an alkali.
The aminobenzylamine and triazine compounds are preferably
condensed in an aqueous medium, optionally in the presence of a
water-soluble organic solvent, at a temperature of from 20 to 60C, and
preferably maintaining the pH at from 5 to 9 by adding an acid-binding
agent to neutralise any hydrogen halide formed during the condensation.
Suitable acid-binding agents are- alkali metal hydroxides, carbonates and
bicarbonates.
As examples of diamines of Formula (8) there may be mentioned
the three isomeric aminobenzylamines and derivatives in which one or
both nitrogen atoms have a Cl_4-alkyl substituent.
Compounds of Formula (9a) and (9b) may be obtained, for
example, by reacting cyanuric chloride at 0-20C in aqueous medium, with
a water-soluble compound of the formulae D2NR4H or DlNRlH wherein Dl,
D2, Rl and P~4 are as defined above. Such compounds have been fully
described in the prior art and especially include monoazo dyes obtained
from diazotising an amine, for example an aniline or naphthylamine, and
coupling with a coupling component having a substituent defined by -NR4H
or -NRlH, for example a hydroxynaphthylamine.
The above-mentioned diazotisation may be achieved using
methGds known per se in the dyestuff art, for example by reacting the
amine with an acidic solution of NaN02 at below 5C.
2087~8~
g S~C 367g9
Examples of the aforementioned coupling components include
l-hydroxy-8-aminonaphthalene-3,6-disulphonic acid,
l-hydroxy-8-aminonaphthalene-3,5-disulphonic acid,
l-hydroxy-6-aminonaphthalene-3-sulphonic acid,
1-hydroxy-6-methylaminonaphthalene-3-sulphonic acid,
l-hydroxy-7-aminonaphthalene-3-sulphonic acid,
l-hydroxy-7-methylaminonaphthalene-3-sulphonic acid, and
l-hydroxy-6-aminonaphthalene-3,5-disulphonic acid.
It is convenient to isolate and use the dyes in the form of
their water-soluble salts, particularly their alkali metal (especially
sodium~ salt and especially their ammonium or substituted ammonium
salts. It is to be understood that the invention relates to both the
free acids and their salts.
Preferred substituted ammonium salts are of formula +NT4 or
+NHT3 wherein each T independently is Cl_4-alkyl or two or three Ts
taken together with the nitrogen atom to which they are attached from a
5- or 6-membered heterocyclic ring and any remaining T is Cl_4-alkyl.
Preferably each T independently is selected from Cl_4-alkyl. Examples
of substituted ammonium salts of formula +NT4 or +NHT3 include
(CH3)3N+H~ (CH3)2N+~2. H2N+(CH3)(CH2CH3), CH3N+H3, CH2CH2N+H3,
H2N+(CH2CH3)2. CH3CH2CH2N~H3~ CH3CH2CH2N+H3, (CH3)2CNH+H3, pyridinium,
piperidinium, morpholinium, N+(CH3)4, N+tCH2CH3)4, N-methyl pyridinium,
N,N-dimethyl piperidinium and N,N-dimethyl morpholinium.
Reactions for preparing dyes of the invention may be performed
using analogous conditions to those known in the art. Similarly, the
dyes may be isolated by kno~n methods, for example spray drying or
precipitation and filtration.
Inorganic compounds may be substantially removed from the dyes
by processes known ~Q~ ss, for sxample revsrse osmosis, ultrafiltration,
dialysis or a combination thereof. Removal o~ the inorganic compounds
enables inks to be prepared which are particularly suited for ink jet
printing.
'' ' '' - ' '
SMC 36799
According to a further aspect of the present invention there
is provided a composition comprising a dye of the Formula (1) as defined
in the first aspect of the present invention and a dye of Formula (10~:
Yl y2
3 ~3 N H ; ~N H
( 1 0 ) ( X ) m
whsrein:
X3 is a sulpho or carboxy group;
each X4 independently is a substituent;
m has a value of from 0 to 2;
yl ~ y2 are each independently alkyl or halo; and
Z4 is a carboxy group.
When X3 ls a carboxy group it is preferred that one or both of
the groups represented by Z4 are at the 3-, 4~ or 5-position, more
preferably at the 3- or 5-position because we have found that such
compounds usually have better solubility in aqueous media than analogous
compounds wherein the groups represented by Z4 are at the 4- or
6-position.
It is preferred that X3 is a carboxy group.
The nature and position of the substituent or substituents
defined by -(X4)m is generally selected on the basis of synthetic
convenience. It is preferred that each X4 independently contains less
than 7 carbon atoms. ~s examples of substituents represented by X4
there may be mentioned halo, especially chloro; sulpho; alkyl,
especially Cl_4-alkyl; alkoxy, especially Cl_4-alkoxy; and carboxy. It
is preferred that m has a value of 1, more preferably 0.
~087~g
11 SMC 367g9
As will be appreciated the compound of Formula (10) can exist
in other tautomerlc forms than that shown and these are intended to be
included in the definition provided by Formula (lO).
When yl or y2 is an alkyl group it preferably has less than
seven carbon atoms, and more preferably is Cl_4-alkyl, especially
methyl. When yl or y2 is halo it is preferably Chloro. It is preferred
that yl and y2 are identical to each other.
It is preferred that one or both of the groups represented by
Z4 are at the 5-position indicated in Formula (10).
The positive charge shown in Formula (10) may be balanced by a
negatively charged carboxy or sulpho group in the same molecule or by an
anion of a different molecule or atom. The anion is preferably
colourless. Examples of colourless anions include carbonate,
bicarbonate, sulphate, sulphite, phosphate, halide, acetate and
benzoate.
The compound of Formula (10) preferably has at least as many
carboxy groups as sulpho groups.-
The compounds of Formula (10) may be prepared by condensingtogether compounds of Formula (11), (lZ) and tl3) wherein m, X3, yl, y2
and Z4 are as hereinbefore defined and X5 is C=0 or S02:
~NH2 1
X 2 )
( ) ( ~ ( l 3 )
Condensation of compounds (11), (12) and (13) is preferably
performed at a high temperature, more preferably above 150C, especially
in the range 170-190C. The condensation may be performed in a high
~5 boiling solvent, for example sulpholane.
~8~
12 S~.C 36799
.,
The relative amounts of the dyes of Formula (1) and (10~ may
be varied between wide limits, preferably so as to ensure that the
composition has acceptable brightness and light fastness for practical
use in ink jet printing. It is preferred that the relative amounts (by
weight) or the dye of Formula (10) and the dye of Formula (1) are in the
range 95:5 to 5:95, more preferably 90:10 to 10:90.
The present invention also provides inks comprising a
composition as hereinbefore described and a liquid medium. The liquid
medium is preferably as described in the first aspect of the present
invention.
~ further aspect of the present invention provides a process
for printing a substrate with an ink using an ink jet printer,
characterised in that the ink is as defined in the first aspect of the
present invention or contains a dye or composition according to the
present invention.
A suitable process for the application of an ink as herein-
before defined comprises forming-the ink into small droplets by ejection
from a reservoir through a small orifice so that the droplets of ink are
directed at a substrate. This process is commonly referred to as ink
jet printing, and the ink jet printing processes for the present inks
are preferably piezoelectric ink jet printing, and mors especially
thermal ink jet printing. In thermal ink jet printing, programmed
pulses of heat are applied to the ink by msans of a resistor, adjacent
to the orifice during relative movement between the substrate and the
reservoir.
A preferred substrate is an overhead projector slide or a
paper, especially plain paper, which may have an acid, alkaline or
neutral character.
According to a still further aspect of the present invention
there is provided a paper or an overhead projector slide printed with an
ink according to the first aspect of the invention or a dye according to
the second aspect of the invention.
~7~
13 SMC 36799
The inks of the present invention may be used in admixture
with other colorants and inks or separately with other colorants and
inks in an ink jet printer to give a printer capable of printing a
variety of colours and shades. Suitable other colorants and inks for
use with the invention are described in GB Patent application 9204903.0,
EP 468647 Al, EP 468648 Al and EP 468649 Al.
The invention is further illustrated by the following Examples
in which all parts and percentages are by weight unless otherwise
indicated.
Exam~le 1
Preparation of
C I
N~
SO3H OH NH~/ \N
N--N~N~ --H N~
SO3H -HNCH2
( ~ 1 )
Sta~e 1
A solution of 3-aminobenzylamine (0.59g) in acetone (lOml) was
added to a stirred solution of 1-hydroxy-2-~1',5'-disulphonaphth-2'-yl-
azo)-8-(4n,6n-dichloro-s-triazin-2n-ylamino)-n~phthalene-3,6-disulphonic
acid t21.89g, M,I. 2188) in water (200ml). The reaction mixture was
stirred at 20C and pH 6 to 7 for 2 hours, then at 30C to 35C and pH
8.0 to 8.5 for 3 hours. After filtering to remove any traces of
insoluble product, ethanol was added and the precipitated bluish-red dye
which resulted was collected and dried.
Yield 14.~g, M.I. 2737.
2~L~
14 SM~ 367gg
Sta~e 2 - removal of inor~anic compounds
58.88g of the title dye obtained by a method analogous to
Stage 1 was suspended water (400ml) and dialysed to remove inorganic
compounds. The water was evaporated to give the title dye in the form
of its sodium salt. When made into an ink and printed onto plain paper
using a thermal ink jet printing machine, the printed image was found to
have moderate water fastness (about 60%) and an attractive bright shade.
The title dye was found to have an EmaX f 70,000 at 543nm and
a brightness of 69, as measured on a Minolta Chroma Meter.
Examples of specific inks containing the title dye are:
Ink ~y~ Liquid medium and other components
(parts) (Parts
1 2.5 Water (60)
Ethylene glycol (40)
2 4.0 Water (85)
Diethylene glycol (15)
3 5.0 Water (90)
N-methylpyrrolidone tlO)
Ammonium phosphate (O.2)
4 3.0 Water (65)
Glycerol (25)
Triethanolamine (10)
2.0 Water (80)
Ethylene glycol (15)
Polyethylene glycol (~W 200) (5)
6 2.5 Water (80)
Diethylene glycol (20)
~ Sodium borate (O.2)
7 3.0 Water (92.5)
Dlethylene glycol (7.5)
Dimethyl phosphate (0.3)
~onomethyl phosphate (0.2)
Purther inks containing the product from stage 2 may be
prepared according to the following formulations shown in Table I and
Table II wherein figures denote parts by weight for each stated
coMponent:
2~8~
15S~C 367~g
The following Abbreviations are used:
PG = propylene glycol,
DEG = diethylene glycol,
NMP = N-methyl pyrollidone,
DMK = dimethylketone,
IPA = isopropanol,
MEOH = methanol,
2P = 2-pyrollidone,
MIBK = methylisobutyl ketonè,
P12 = Propane-1,2-diol,
BLL = Butane-2,3-diol,
CET = Cetyl ammonium bromide ta surfactant),
BAS = 1:1 mixture by weight of ammonia and methylamine,
PHO = Na2HP04, and
TBT = Tertiary butanol.
Table I~
Ink Dye _ Na _ _
No. Content Water PG DEG NMP DMK NaOH Stearate IPA MEO~ 2P MIBK BAS
_ _ _ _ _ _
l 2.~ 80 5 6 4 5 3
2 3.0 90 5 2 0.2
3 1.0 85 5 2 2 0.1 5 1
4 2.1 91 8 1
3.1 86 5 0.2 4 5
6 1.1 91 9 0.5 0.5 9
7 2.5 60 4 153 3 6 10 5 4
8 1.9 70 20 10
9 2.4 75 5 4 6 5 5
4.1 80 3 52 10 0.3
11 3.~ 65 54 6 5 4 6 5
12 4.6 96 4
13 0.8 90 5 5
14 1.2 80 2 61 5 1 4 1
1.8 80 5 15
16 2.6 84 11 5
17 3.3 80 2 10 2 6
18 1.7 90 7 0.3 3
19 1.5 69 2 202 1 3 3
1.6 91 _ 4 _ _ 4
,
`:
8 ~
16 SMC 36799
Table II
I Ink Dye ~ _ ~ - Na I _ ~ _
No. Content W~ter PG DEG NMP CET TBT St ~ BDL ~ 2P
21 3.0 80 15 0.2 5
22 2.0 90 5 1 2 5
23 1.5 85 5 5 0.15 5.0 0.2 .
24 2.5 90 6 4 0 lZ
3.1 82 4 8 0.3 . 6
26 O.g 85 10 5 0 2
27 1.5 90 5 5 0.3 .
229 22 2 775o 4 10 4 21 46 11
2.6 91 6 3
31 3.2 769 7 3.0 0 95 5
32 4.0 785 11 . 6
33 3.3 86 7 7
34 1.1 70 5 ~ ~ ~ ~ 0.1 50 1 5 5
Example 2
The method of Example 1, stage 1, was repeated except that in
place of 3-aminobenzylamine there was used 3-amino-N-methylbenzylamine
(0.68g). The resultant product was collected as described in Example 1
to give 14.lg of a bluish-red dye. The dye may be dialysed to remove
chloride ions, inorganic compounds and made into an ink as described in
Example 1, stage 2.
Examvle 3
Preparation of
- H2NCH2CH2(NHCH2CH2)"jNH
N~--N H
SO3H OH NH~/ \ N`I I ~1
~N=I\J ~ N-- \y
~J Ho3s~so3H _NHCH2
SO3H
112) 2
~n87~
17 SMC 367g5
13.79g of the product from Example 1, stage 1, was added to water
(SOOml) followed by pentaethylene hexamine (9.Og). The mixture was
stirred until a complete solution was obtained at about pH 10. The
solution was maintained at 70-75C for about 2 hours until reaction was
essentially complete. After cooling to 20C, the solution was
neutralised to pH 7 using concentrated hydrochloric acid. The
precipitated product was filtered o~f and washed with (lOOml) of water.
The product was dissolved in water (200ml) and dialysed to remove
chloride ions, inorganic compounds and the solvent e~aporated to give
the title dye having a water fastness of approximately 86%.
~xample 4
A solution of l-hydroxy-2-(2'-sulphophenylazo)-8-(4 n ~ 6 n _
dichloro-s-triazine-2n-ylamino)-naphthalene-3,6-disulphonic acid (27.6g,
M.I. 1380, 0.02 mole) and 3-amino-N-methylbenzylamine (1.36g, 0.1 mole)
was stirred at 20 to 25C and pH 6 for 0.5 hours followed by 3 hours at
30 to 35C and pH 8.5. On cooling, the pH was adjusted to 7.0 with 2N
hydrochloric acid, a small amount of insoluble matter was filtered off
and salt solution (22% w/v) was added with stirring. The precipitated
product was collected, washed with brine (20%) and dried.
Yield 18.0g, M.I. 2000.
The product may be diaIysed and made into an ink as described in
~xample 1, stage 2.
Example 5
A solution of l-hydroxy-2-(1',5'-disulphonaphth-2'-ylazo)-
6-(4n,6~-dichloro-s-triazinylamino)-naphthalene-3,5-disulphonic acid
(22.6g, M.I. 1355, 0.0167 mole) and 2-aminobenzylamine (1.016g,
0.0083 mole) was stirred at pH 8 and 35C for 2 hours. Further
2-aminobenzylamine (0.2g) was added and hea~ing continued for a further
2 hours. On cooling to 20C, salt (25% w/v) was added and the mixture
was stirred for 16 hours. Precipitated solid was collected and dried.
Yield 31.0g, M.I. 3728.
The product may be dialysed and made into an ink as described in
Example 1, stage 2.
2~87~
18 S~C 367g9
Example 6
A solution of 3-amino-N-methylbenzylamine (1.36g) in acetone
(20ml) was added to a stirred solution of 1-hydroxy-2-(1'-sulphonaphth-
2'-ylazo)-8_(4",6n_dichloro_s_triazin_2n-ylamino)naphthalene-3,6-
disulphonic acid (21.28g, M.I. 1064). After stirring at pH 6 and 20C
for 1 hour, the temperature was maintained at 35C for 28 hours. Any
insoluble material was filtered off, the filtrate concentrated to lOOml
and the bluish-red dye precipitated by adding ethanol ~rith stirring.
Yield 20.4g, M.I. 2065.
The product may be dialysed and made into an ink as described in
Example l, stage 2.
Examples 7 t~o 10
Further dyes prepared by a similar procedure are listed in Table
III, the chlorotriazine group being attached to the coupling component.
The dyes may be made into inks as described in Example 1.
Table III
Example Diazo Coupling Linking ¦ Shade
Component Component Diamine
_
7 2-amino- l-hydroxy- o-amino- Bluish~red
naphthalene- 8-amino- benzylamine
1,5-disulpho naphthalene-
3,6-disulpho
8 2-amino- ll m-amino- n
naphtbalene- benzylamine
l-sulpho
9 2-amino- n N-beta- n
naphthalene- hydroxyethyl-
1,5-disulpho m-amino-
benzylamine
2-amino- 1-hydroxy-8- 3-amino- n
naphthalene- amino- benzylamine
l-sulpho naphthaLene
3,5-disulpho
.. .. ~ .. ",~ _ .
2 0 ~
19 S~C 3679g
Example ll - Composition and Inks
Preparation of the compound of Formula (13) where X is carboxy and ~1
and Q2 are both 2-methyl-5-carboxyphenyl
a' Hll~"NH+_Q2
~,X
( l 3 ) ~
A mixture of 3,6-dichlorofluoran (7.4g), 3-amino-4-
methylbenzoic acid (9.lg), zinc chloride (4.2g) and sulpholane (20g) was
stirred at 180C for 3 hours. After cooling, the mixture was added to
ice/water (200g) and concentrated hydrochloric acid (10 ml) was added.
The resultant precipitate was filtered off and washed with water.
The precipitate was added to water (600ml) and the pH adjusted
to 9.0-9.5 by addition of sodium hydroxide solution. The solution was
screened and the filtrate acidified to pH 3 with concentrated
hydrochloric acid. The title product in the free acid form was filtered
off and washed with a little water to give a solid.
The solid was added to water (600ml) and concentrated ammonium
hydroxide added to pH 9-9.5. The solution was dialysed until no further
chloride ion was detected, the solution was screened, and the water
evaporated to give the title compound as the am~onium salt.
Preparation of Ink
A composition may be prepared by mixing 3.2 parts of the
compound of Formula (13) and 0.8 parts of the compound of Formula (11).
The composition may be dissolved in a mixture of water (92.5 parts) and
diethylene glycol (7.25 parts) to give an ink. When printed onto plain
paper using a thermal ink jet printer the printed image has good light
fastness and a bright magenta shade.
-- 2~8~
20 SMC 35799
Further inks may be prepared according to the formulations
described in Example 1, Tables I and II except that in place of the dye
of Example l there is used the above composition.
Example 12
Preparation of the compound of Formula (14) wherein B is hydroxy
N ~
,~ H N C H2
S03H 2
(1 4)
The product from Example 1, Stage I, (27.37 g) and sodium
hydroxide (40 g) were added to water (500 ml) and the mixture stirred at
75-80C for about 4 hours. After cooling to 20C the solution was
neutralised using concentrated hydrochloric acid. The precipitated
product was filtered off, washed with 20% brine solution, dissolved in
water (200 ml), dialysed to remove chloride ions, and the water
evaporated to give the title dye.
Ex ~
The method of Example 12 may be repeated except that in place of
sodium hydroxide there is used the basic compound listed in Table IV
below to give a compound of Formula (14) wherein B is as indicated in
the final column.
Table IV
Example ¦ Basic Compound
_ _ .. .
13 Ammonium hydroxide (12 ml) -NH2
14 Ethanolamine (8 g) -NHCH2CH20H
Butylamine (8.52 g) -NH(CH2)3CH3
16 Diethanolamine (12.6 g) -N(cH2cH2oH)2
_ _