Note: Descriptions are shown in the official language in which they were submitted.
. 163~PG/
18651
TITLE OF T~E INV$NTIOM
METHOD OF USING Ml-SELECTIVE ANTIMUSCARINIC
PYRIDOBENZODIAZEPINONES IN AXIAL M~O~PIA THERAPY
ACKGRO~ND ~F TEE INVENTION
Thi~ invention relates to control of ocular
development in general and, more~particularly, to the
: treatment of the eye to preve~t and/or arre~t the
: deve:lopment of myopia (nearsightedne~
~: Myopia a~flicts approximately 25% of the
: 25 world's population. In particular, myopia~af~lic~
15% to 75% of the youth of the world, depending ~pon
race, geographic distrib~tion and level:of edacation.
Approximately one-hal~ o~ all cases are classifiable
a~ axial myopia, where there is an e~ongation o~ the
~0 ~ye along the visual a~
2 ~
163FPGt - 2 - 18651
~ t birth, the human eye is about two-thirds
of the size o~ an adult eye. Thi~ i3 relatively
~hort in the directlon o the ~i~ual a~i~, or axial,
direction. As a result, children tend to be far-
sighted. Durin~ childhood, a~ the eye grows, therei8 a compensatory mechanism governing changes in
the structure of the cornea and lens in response
to increa~ing ocular lengt~ during childhood growth.
Often the proce~s is virtually perfect and no cor-
rection is needed ~or sharp vision at a distance.However, when regulatory mechanisms fail, the eye
tends to axially lengthen. As a result, di6tant
image~ focus in front of the plane of the retina
and the result for the patlent i~ axial myopia.
Myopia is ~ot a trivial maldevelopment
of the eye. In its pathologic form, the ~clera
cont;nues to grow as the retina stretches and
degenerates sesulting in permanent blindnes6.
The ~urgical therapies attempted for this condi-
tion are drastic and o~ten unsuccesE~ul.
Even in it8 milder forms, rnyopia has been
shown to ha~e an effect on the self-image of a child
during the critical s~ate o~ his development. Chil-
dren who wear gla66es, ~ustly or not, are con~idered
booki~h, i~troverted, and nonathletic (Curtin, 1985).
Because of the6e childhood memories, a parent that is
~ myopic i~ often particularly eager to inquire of the
doctor about a therapy to limit the development o~
the myopia when it has been dia~no~ed in hi6 child. - :
~ ~ $ ~
163FPG/ . - 3 - 186Sl
Furthermore, the optical correctionR whic~
exist for myopia are neither ideal nor risk-free.
There are roughly 24 million contact lens weareræ
in the United States, and ~he number i~ expected to
double in the next five years. No matter how well
fitted or cared ~or, complications of contact lens
wear range from allergic reaction~ to permanent loææ
of vi~ion due to corneal ulceration. The latter prob-
lem was ~o serious that the Food and Drug Adminiætra-
tion (FDA) reduced the recommended wearing time fore~tended wear contact lenses from 30 days to "one to
3even days". In 1988, over ~0,000 injuries related
~o contact lens wear were treated and reported by
hospital emergency roome.
While eyeglasses eliminate most ~f
the medical risks liæted above, they are not an
acceptable option as evidenced by the contact lens
wearer~ who tolerate the fruætration of contact lens
wear. As a result, large efforts have been devoted
to the correction o~ myopia by corneal surgery using
conventional knives or e~cimer laæer~. Neither of
these therapies are easily rever~ed or su~iciently
predictable in their re~ult~. De3pite the risk of
permanent visual impairment associated with the~e
~5 surgical the~apies, thousandæ of patient~ have
undergone radial keratotomy in the ~nited Stateæ.
While the optical and surgical correction of
~yopia corrects the refractivt ætate of the eye, theæe
therapies do not address the abnormal elongatlon of
the eye during development. Survey~ indicate that
2~751~
1~3FPG/ - 4 - 18651
even low degrees of myopia (-1.00 to -5.00 diopter~)
predispose patient~ to the development of glaucoma
(Perkin~, 1982). In myop;a, the retina is stretohed
and thinned, which predi~pose~ the eye to retinal
S detachment. Consequently, ~everal studies have docu~
mented myopia as a risk faetor ~or the development
of retinal detachment. All of the above highlight
the need for a pharmacologic therapy to address the
developmen~al abnormality of myopia.
lo Previously available therapies that relied
on administration of drugs began with the use of
cycloplegic agent~. Cycloplegics are topically
administered drugs that relax the ciliary muscle
of the eye, which is the muscle that focuses the
eye by controlling lens dimensions.
The elassic cycloplegic drug i8 the
belladonna alkaloid atropine, a~ailable for over
a century. Atropine i6 a long-acting non-specific
antimuscarinic agent that antagonizes the action
of the neurotransmitter acetylcholine (ACh) at auto-
nomic effector cells innervated by postganglionic
cholinergic nerves of the parasympathetic nervous
8y8tem .
In 1971 Bedros3ian completed a t~o-year
cros60ver study in 75 children with myopia. During
the first year, the right eye wa~ treated with l~O~o
atropine applled topically at bedtime. The left eye
was treated during the ~econd year. As shown by
Table 1, the progression of the myopia was halted in
the treated eye (See Table 1).
~ 3 ~ ~
,
~63FPG/ - 5 - 18651
.
Table 1. Effect of 1.0% Atropine Upon Myopia
(Bedro~æian, 1971)
Cmean change in refractlon in diopters~
~ for hyperopla, - for myopia]
~i~h~ ~ye Le~t ~ye
Year 1 +0.20 -0.85
~ear 1 -1.05 +0.17
The result~ were ~ignificant a~ le~s than
th@ 1% level.
A later study confirmed the findings of the
first ~udy and indicated that the greatest ef~ect of
atropine upon the ælowing of the rat~ of progre3sion
of myopia wa~ Reen in children under nine year o$ age
(See Table 2).
Table 2. Progression of Myopia
(Brodstein, 1984)
~progression in diopters per year~
[+ for hyperopia, - for myopia~
Age of Onset Therapy with
of ~hera~v 1.0% Ag~o~ine Control
< 9 year~ -0.23 -0.69
'9-12 year~ -0.24 -0.48
> 13 yearR -0.20 -0.23
',
Atropine ha8 not been widely u~ed for ~lowi~g
the progreasion of myopia due to drug effects includ~
ing glare from pupillary dilat~on, and inhibition of
ocular focu~ing that impair hear vi6ual work like
reading.In addition to the di comfort to the patient,
there i~ evidence that long-term u~e of atropine or
other long-term cycloplegies can harm the ret~a when
it i8 exposed to bri~,ht light.
2087 ~10
163EPG/ - 6 - 18651
Recently, ~elective-acting antimu~carinic
agent~ were discovered to be u~eful in myopia therapy.
This di~covery was made possible by a new body of
~ubstantial evidence to link the po~terior part of
the eye, ~pecifically the image quality region~ o~
the retina and hence an extension of the nervous
system, to the postnatal regulation of ocular growth.
There i8 ~i~nificant e~idence of myopia re~ulting ln
an eye that i6 subjected to retinal image degradation.
It has been shown that a~ial myopia can be experiment-
al.ly induced, in either birds or primates, in an eye
in which the retina i8 deprived of formed image6,
e.g., by suturing the eyelid~ or wearing an image
di~fusing goggle. The experimental myopia induced in
birds or primates 6uch aB monkeys mlmics the co~mon
axial myopia of humans.
Thus, the phenomenon of an animal's vision
procesæ apparently contributes to the feedback ~ech-
anism by which po3tnatal ocular growth is normally
regulated and re~ractive error is de~ermined. This
indicates that this mechanism i neural and likely
originates in the retina. R. A. Stone, et al. have
found a method of controlling the a~normal po6tnatal
growth of the eye of a maturing ani~al, which com-
25 pri8e8 controlling the presence of a neurochemical,it6 agonist or antagoni~t, which neurochemical i8
found to be changed under condition~ during matura-
tion leading to abnormal axial length. Therein it
i~ di~closed that in experimental animal~ such a6
chicks or monkeys ~ubjected to ocular image depriv-
ation ordinarily leading to the de~elopment of myopia,
the metabolism of certain retina~ neurochemicals ~
2 0 ~
163FPGt - - 7 - 18~51
altered leading to change6 in retinal concentrations
thereof. Specifically, retinal concentration~ of
dopamine were found to be reduced during such image
deprivation and the ocular administration of a
dopamine-related agent, e.g., apOmorphine, a dopamine
agonl~t, wa~ found to inhibit or actually prevent
the axial enlargement o~ the eye under conditions
ordinarily leading to such enlargement.
There have ~een recent advances made in
the understanding of the cholinergic nervous system.
Cholinergic receptors are proteins embedded in the
wall of a cell that respond to ~he chemical acetyl-
choline. The~e receptoræ are broadly broken down
into nicotinic and muscarinic receptors. In this
respect, it i~ now known that the ~uscarinic recept-
ors are not all of one type. Recent ~indings show
that there are at least ~ive, if not more, types ~f
cholinergic ~uscarinic receptors (types Ml throug~
M5). Type Ml receptors are those pre~ent in abun-
dance and thought to be enriched irl the brain neuraltiRæue and neural ganglla. Other receptors are con-
. centrated in other tis~ues, such a~ in heart1 smooth
:; muscle ti~ue, or glands. While many pharmacological
agents interactin~ with muscarinic reCeptorB influe~ce
2~ several typeæ, ~ome agents are known to have a majoreffect on a single type of receptor with rela~ive
selectivity and other agents ca~ have a relatively
~elective e~ect on a different 6ingle receptor.
Still other agents may have a ~ignificant e~fect on
more than o~e or even all ~ypes o~ receptors. It is
known, ~or example, that pirenzepine, (~astrozepin,
LS ~19) 5,11-Dihydro~ 4-methyl-1-piperazinyl)
~7~
163FP&/ - ~ - 18651
acetyl3-6~-pyrido~2,3-b~1,4Jbenzodiazepin-6-
one, and it~ dihydrochloride, are anticholinergic,
antimuscarinic, and relatively selective ~or ~1
receptor~. It i~ al~o known that 4-DAMP S4-
s diphenylacetoxy-N-methylpiperadine methiodide) is
a relatively ~elective antagoni~t for mooth musc1e
(ordinarily called M3 type but variouæly called type
M2 or M3, as the current cla~sification of reCeptorB
i~ ~n flux). In contrast, it is believed that
lo atropine, discussed above a~ a cycloplegic agent,
i~ a non-~pecific antagoni~t for all types of
cholinergic ~usearinic receptors.
- Pirenzepine, being primarily an Ml
antagoni6t, inhibit~ a~ial elongation, but i8 ~ar
le~s effective at pupil ~ilation than atropine or
another cycloplegic agent. This ma~es it possible to
suppress the development of myopia without dilating
the pupil and paralyzing the accommodation activity
of the ciliary body.
. Pirenzepine, however, has a disadvantage.
The admini~tration of a drug ~opically into the eye
oP a developing child for a long period of ti~e make~
it desireable to have a minimal li~elihood o~ sensi-
tization of the eye. Pirenzepine te~ts positive in
æen~itization a66ay8. There i8 therefore a need in
the art for a ~elective anti-muscarinic agent that
can be ù~ed to treat myopia, which will not cau~e
effects on pupil ~ize or accommodatlon of the ciliary
body, and which will be les8 likely to cause
~ensitization than pirenzepine.
2 0 ~ 7 3~ ~
163FPG/ - 9 - 18651
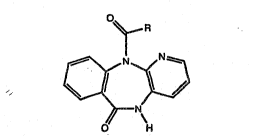
SUMMARY OE T~E INvENTIQ~
The invention i~ a method of using a
compound of the ~ormula:
~_R
10 ~;j
H
and the pharmaceutically acceptable salt~ thereof,
where R is piperidine substituted by Cl_5 alkyl,
in the therapy of myopia by ocular administration
of cUch a compound, which will prevent abnormal
increases in the axial length of the eye.
Specifically, the compounds rispenzepine (ulenzepine)
,~N ~ CH9
,~N~
O tl
a~d nuvenzepine
2087~10
163FP~/ - 10 ~651
O~\N--~H3
S ~)
N~
' 10
can be used .in the method of the pre~en~ invention,
by their ocular administration to ~revent abnormal
increa~es in the axial length of the eye and thus
prevent progression of axial myopia.
DETAILED DESCRIPTION OF T~E INVENTXON
Compounds of formula I are prepared e~arting
from compound3 of formula II, de~cribed in J~ Med
Chem. 6, 255, 1963; Bull. Soc. Chim. Fran. 7, 2316,
lg66; and German Pat. No. 1,179,943, by acylation
with the acyl chlorides of formula III, according to
the follo~ing ~cheme: -
O~
H ~ R
CICOR ~
O H H
Il
.
20~7~1 ~
163FPG/ ~ 18651
where R is defined as before, that is piperidille
substituted by Cl_5 alkyl. The reaction iB carried
out in polar solv~nts such as dimethylformamide and
d-methylsulfo~ide in the presence of bases sueh a~
triethylamine, alkaline hydroxides and alkaline
earbonate~ at temperatures ranging from room
temperature to 150C, with reaction times ranging
from 2 to 24 hours.
Representative pharmaceuticaily acceptable
lo ~alts include, for e~ample, hydrochloride and maleate.
Salts are generally prepared as acid addition salts
by combining the core compound with one-to-three equi~
valents of an appropriate acid in an inert solYent.
The ~alt i3 then recovered by solvent evaporation or
by fYltra~ion if the ealt precipitates ~pontaneously,
or by precipitation using a co-solvent or a non-~olar
co-solvent followed by filtration.
The term "pharmacologically effective amount"
shall mean that amount of a drug or pharmaceutical
agent that will elicit the ~iological or medical
response of a tiæsue, sy tem or organ that iæ being
sought by a veterinarian or phy6ician.
The term ~'alkyl~ ~hall mean linear ~traight
.or branched chain alkane or alkene.
The term 't~ubætitutedi' shall be deemed to
include multiple degrees o~ substitution by a named
subætituent. : .
The compounds u~ed in the method of the
invention can be prepared readily according to the
~ollowing detailed example~ using readily available
stasting mater~als, reagents and conventional syn-
thesiR procedure~. In the~e reactions, it i~ also
2 ~
163FPG/ ~ 18651
po~sible to ma~e use of variantæ whioh are themselves
known to those o~ ordinary skill in this art, ~ut
which are not mentioned in greater detail. Addi-
tional background ;nformation is taught in U.S.
Patent No. 4,556,653, the entire disclosure of which
is incorporated herein by reference.
~XAMPLE 1
ll-(l-Methylpiperidin-3-carbonyl~-5,11-dihydro-
lo 6H-pYrido r 2.3-bl r 1~4lbenzodiaz~pin-6-one
The chloride o~ l-methylpiperidine-3-
carboxylic acid hydrochloride (4.7g) is added to
a solution o~ 5,11-dihydro-6~-pyr;do[2,3-b]~1,4]
benzodiazepin-6-one (5g) and triethylamine ~7.5ml)
in dimethylformamide (200ml). The reaction mixture
is heated to 90C for 24 hour~.
EXAMP~$_~
ll-(l-Me~hylpiperidin-4-carbonyl)-5, ll-dihydro-
6H-pvridor2.3-blrl.4l~nzodiaz~in-6-one
The chloride of l-methylpiperidine-4-
carboxylic acid hydrochloride ~4.7g) was added to
a ~olutio~ of 5,11-dihydro-6~-pyrldo~2,3-b~[1,4~
benzodiazepin-6-o~e (5g) and triethylamine (7.5ml)
in dimethylformamide (200ml). The reaction mixture
wa6 heated to 90C for 24 hour~.
The ~olvent was evaporated under ~aeuum and
30 the re~idue was di~olved in lOZ acetic acid (150ml).
The æolution waæ repeatedly washed with methylene
chloride, decolored wi~h charcoal, alkalinized to
2~7~
163FPG/ . - 13 - 18651
pH 8 with a ~aturated solution of æodium bicarbonate
and extracted with methylene chloride (lOOml ~ 5).
The collected organic extracts were dried on ~odium
sulphate and evaporated. The residue, cry~tallized
from ethanol-ethylether, yielded 2.4g (30%) o 11-
~l-methylpiperidin-4~carbonyl)-5,11-dihydro-6H-pyrido
C2,3-b]~1,43benzodiazepin-6-one melting at 265-267C
dec.
The mu~carinic agents for u e in this
invention are those relatively 3elective in blocking
the Ml type receptors and which are relatively les~
selective for the type M3 smooth muscle receptor~.
Relative affinitie~ for Ml-M5 receptor~ were deter-
mined using the following as~ay.
XAMPIJ~ ~
Three radioligand binding assays were
establi~hed in order to determine the affinitie~ of
mu~carinic antagoni6ts for M~, M2 and M3 muscarinic
receptor subtypes. Ml receptors in rat cerebral
cortex were labeled with 3~-pirenzepine, 3~-AEDX-384
labeled M2 receptor~ in rat heart and M3 receptor~ in
rabbit iris ~ ciliary body (minus ciliary processes
which contain Ml receptor~ were labeled with 3~-QNB.
Scatchard analy~is revealed that one population o~
binding ~ites wa6 labeled in each ti~sue and t~e
following Kd and Bmax values were obtained: cortex
(2.36 nM, 1781 fmol/mg protein), heart ~5.77 nM~ 164
fmoltmg protein) and iris + ciliary body ~24.5 pM and
210 fmol/mg protein). Ki values in the three bind-
ing a~say~ were obtained ~or atropine, rispenzepi~e,
2 0 ~
~63FPG/ - 14 - 18651
pirenzepine and nuvenzepine. The most potent of the
agents at Ml binding æites was atropine with a Ki
value o~ 0.13 nM. Pirenzepine, nuvenzepine and
ri~penzepine posses~ed Ki value~ of 4.87 nM, 1.50 nM
and 2.61 ~M, respectively. Atropine was the mo~t
potent of the antagonists at M2 receptors pos~eesing
a Ki of 0.82 nM while pirenæepine (532 nM~, nuvenze-
pine (248 nM) and ri~penzepine (226 nM) were much
le~ effective. Atropine wa3 al~o the mo~t potent
(0.37 nM)-at M3 binding sites be~ng followed by
nuvenzepine (29.7 nM>, pirenzepine (37.2 nM) and
rispenzepine (38.9 nM). In term~ of their ability to
distingui6h between Ml and M3 receptors, atropine waæ
eæsentially devoid of selectivity while pirenzepine,
nuvenzepine and ri~penzepine were re8pecti~ely 8-,.
20- and 15-~old more selective for Ml than for M3
receptors.
~7~
1ii3FPG/ . -- 15 -- 1~651
~ ~ o
r I ~ OD 0~
~ o o
.
rt O
~ ~ ~ O Q~
~ 1 oc~ o
r~
s > ~~ ~ c~ a~
0 M a
~ ~ ~ r~ .
~ L ~1 ~ ~ ~
d ~ ,~ o ,~ a
~ ~ ~ -
~: ~ ~ .o ~ `_
~ ~ t` J
.~ ., æ . ~ ~ ~ ~ a
.r r r H u H ~ 3
t~S t~
~q u ~ 0~
~ ~ Ou~
-I
~ X U~ o o~ d v
P 1:
~` ~ ~q ~ .,
0 ~ . ~~.D td
C~
rl j~¦ S '~¦ ~ v , A ,U
25 .~ ~ ~ 1 o ~, ~
a ~ ^
o ~ 3
aJ ~ ~ oo
r~ W ~ I
3 0 r ~ ~rl G~ r 1 ~4 cY
-- 1:~ O N
~ . ~ N ~ ~ r l Itri
~::g ~
G~ P
rl rl ~ r~
~C ~ P; Zi S~ ~ X ~ X
2~7~0
163FPG/ - 16 - 18651
Al~erna~ively, ~h~ affini~y and relative
affinity of ~uscarinic antagoni~ts for Ml-M5
receptor~ can be determined by other means ~nown in
the art. ~or example, ~ee Buckley, et al., Molecular
Pharmacology, 35: 469-476 (1989) or Dorje, et al., J.
Pharmacol. Exp. Ther. 256:727-733 (1991) for detailed
description~ of techniques known in the art for
determining the a~tagoni~t binding properties o~ five
cloned human muscarinic receptor~. Similarly, there
are other ways in which to accomplish functional
6tudie~ to measure Ml ~en~itivity. For instance,
one popular method at present is to u~e vas deferens
of the guinea pi~ which has an Ml ~ensitivi~y. First
it i8 ~et up so that its tension i~ measured and a
known 6timulat~r such a~ the Ml agonist McNeil A343
i8 given to change ~Pnsion by a predictable amount.
Under this condition, the predicted effect of the
agonist is first carefully plotted and then the
degree to which one or another antalgoni~t block~
this agonist effect i~ measured.
. An in vivo animal model of myopia i~ avail-
able for ~creening, which i~ de6cri~ed a~ follows.
Form-deprivation myopia i~ induced in
day-old White Leghorn chick under aseptic condi-
tion~ and other anesthesia by eyelid ~uture toone eye. The chicks are maintained on a 12 hour
~ight:dark cycle. The ~utured eyes are treated .
with nuvenzepine, rispenzepine and pirenzepine and
saline solution as a eontrol. Drug i8 injected daily
subconjunctivally during the light cyc~e. At two
week~ of agc the animal~ ~re ~acrificed and axia~
and equatorial dimension~ o~ unfixed eyes are
measured with vernier calipers independently by two
~7~
163FPG/ - 17 - 18651
observers. Ri~penzepine and nuvenzepine, because
of their ~omewhat greater potency at the Ml receptor
relative to pirenzepine, would be expected to be at
least as effective as pirenzepine in myopia ther~py.
EXAMPLE 4
The guinea pig magimlzation test is re~uired
by the U.S. Food and Drug Administration of all drug~
lQ intended for ophthalmic admini~tration. The test
population for nuvenzepine and ri~penzepine versus
pirenzepine consisted of at least nine female Dunkin
Hartley al~ino guinea pig~ between 300 and 500g, w.ith
10 control animal~. In Pha~e I, which i~ the induc-
tion phase, o~ day 1 two intradermal injection~ atthe highe~t generally tvlerated coneentration of the
test material were administered in the shaved ~ter-
capsular area (0.lml of the test agent in a 50:50
mixture of Freund'æ complete ad juYant and distilled
~0 water). On day 7, approximately 400mg of a mixture
of 10% ~odium laurel sul~ate in petrolatum wa6 applied
to the ~haYed intercapsular area. On day 8, 400mg of
a mixture of the te~t compound in petrolatum was
-applied, on a 2 ~ 4 cm piece of Whatman paper to the
2s chaved intercapsular area, along with Blenderm tape.
I~ Phase II, which i8 the challenge phase,
on ~ay 22, 200mg o~ petrolatum was applied ~o the
sha~ed right flank, wh:ile 200mg of test compound at
the highest non-irritant concentration (micronized
in petrolatum~ wae applied to the ~haved le~t flank,
with each application being occluded ~or 24 hours.
2 ~
163FPG/ - 18 - 18651
Readings were taken on days 24 and 25, with grades
assigned as follows:
O = no reaction
1 = scattere~ mild redne~
. 5 2 = moderate and diffused redne6s
3 = intense redneæs and æwelling
Nuvenzepine and ri~penzepine bo~h ~howed O, no
reaction in any test animal. Pirenzepine, however,
showed a grade of"l"in ~ix of the animals and a
lo grade o~ "2" in two of the animals, out o~ a ~otal
population of nine animals.
Diagnosis and treatment, using the method
of the invention, is well known to those of ordinary
skill in tne art~ .
1~ The refractive state o~ the eye i~ ea~ily
determined even in newborn in~ant~ by the use of a
retinoscope, a handheld instrument. This objective
mea~urement i~ performed after cycloplegia o~ the
eye has been obtalned by topical administration of
cyclopentolate or a~ropine. Furthermore, the axial
length of the eye can easily be ~easured using
ultrasonography.
Myopia become~ manifest as children reach
the age of 7 to 12 year~. Once identified, the
2S myopia usually progre~se~ until the eye completes
development after the age o~ 14.
Once identified, the progression of myopia
can be documented by examination every 6 to 12
months. Risk ~actors for the development of high
degrees of myopia include race and ~amily h~story.
Once the myopia has been documen~ed a~
progressing, pharmacolog~c therapy could be initiated
~7~
163FPG/ - 19 - 18651
to reduce the rate of progre~sisn. Therapy would be
continued until the ocular development is completed.
For most childre~, therapy would be initiated between
the ~ges of 7 and 10 years and discontinued around
the age of 14 years.
Therapy to inhibit axial-elongation myopia
during maturation can be admin~ætered by the use of
the agent in eye drops. Indeed, in the va~t majority
of ca~es, treatment agents are adminiRtered to human
eyes by the application of eye droRs. Eye drops are
typically made up at a concentration of active agent
between about O.S and 2 percent in the ophthalmic
medium. A 1% ~olution o riæpenzepine or nuvenzepine
in water would be a likely concentration for clinical
lS use. Some constraint6 in formulation may e~i~t having
to do with p~ and preæervative. A p~ of about 6.5 i8
expected to be acceptable a~ an ophthalmic drop and
practical in terms o~ known solubility and stability
of pyridobenzodiazepinones. Since pirenzepine ie
known to form very acidic solutions in physiological
saline, treatment with known compatible bases to bring
the p~ up to about 4.5 to 7.5 ~preferably 6 or 6.5)
is recommended. Phosphate buf~ering i8 al~o common
for eye drop3 and i~ compatible with ri~penzepine
and nuvenzepine. A common regimen for ~pplication of
e~e dropæ i8 two to three time~ a day spaced eve~ly
throughout waki~g hours. More e~fective agent6 may
require fewer applications or enable the u6e of more
dilute ~olution~.' Alternatively, ointment~ and ~olid
in~erts are now coming into increased use in clinical
practiee. They avoid problemæ o~ drug decompo~ition
while deliverlng a def ined amount of drug. It i~,
2~7~
163FPG/ - 20 - 18651
of cour~e, also possible to administer the above-
described active agent~ in therapeutically effective
amounts and dosages in pill~, capsults, or other
preparatio~s of sy~temic admini~tration.
In experiments in animals, such a~ tho~e
mentioned hereinabo~e in which axial myopia has
been experimentally induced by depriving the retina
o~ formed images, it has been noted by other~ in
primates that amblyopia waB al60 e~perimentally and
coincidentally induced. Amblyopia i8 evidenced by
poor vi~ual acuity in the eye re~ultlng in poor
vi3ual performance. Nor~ally, visual acuity improves
during maturation. It i8 known that amblyopia may
occur in humans from unknown causes or a~ part of
ætrabismus. It iæ po~ible that adminiEtration of
therapeutically effective amounts and doæages o.~ the
muscarinic antagonist6 relatively selective in block-
ing the Ml cholinergic receptors, but les~ ~elective
in blocking cholinergic receptors in smooth muscle
cells, e.g. rispenzepine and nuvenzepine, might
prevent or inhibit the development of permanent
or persistent amblyopia in maturing human~ with
decreased likelihood of ~ensitizat~on of the eye.
It is al~o possible t~at humans who have already
developed amblyopia from other or even unk.nown
causes might be aided by similar therapeutic
treatmen`t with the aforementioned agent~.
. In addition to rispenzepine and nuvenzepine,
o~her selective antimu~carinic pyridobenzodiaze-
pinone6 within the scope of the invention includethe following.
~$ 7 ~ ~ ~
163FPG/ - 21 - lB651
~ N,~-Dime:thyl-4-t[~,ll-dihydro-6-o~o-~-
pyrido~2,3-b~[lm4~benzodiazepin~ yl~carbonyl~
piperldinium iodide
~ N~Jf
~
O H
ll-C(l-ethylpiperidirl-4-yI)carbonyl~-6,11-
dihydro-5-oxo-~-pyridotl,2-b~[1,5]benzodia~epine
lS .
~ N
: O H
3-Methyl-4-Z(l-methylpiperidin-4-yl)
carbony~]-4,9-dihydro-10-oxo-10~-thicno[3,4-b~
tl,~]benzodiazepine
0~
~N~
.~ N
O H
~0~7~ 1 ~
163FPG/ 22 - lB651
4- ~ ( 1 methylpiper 1 d in-4-yl 3 carbonyl ~ -3-
methyl-4, 9-dihydro-10 oxo-lOH-pyrido~3, 2-b]~thieno
[3 9 4-e~ ~1, 5~diazepiJIe
~ .
~
S~N~
O H