Note: Descriptions are shown in the official language in which they were submitted.
2~879g3
Process for Removing Hydrogen Gas and Non-Metallic Inclusions
from Molten Aluminum or Aluminum Matrix Composites
Backqround of the Invention
This invention relates to a proc~ss for treating molten
unreinforced aluminum or aluminum matrix composites to remove
hydrogen gas and non-metallic inclusions, such as solid
particulates, oxides, alkali/alkaline earth metals, etc., from
the melt.
Molten aluminum prior to casting may contain many
impurities which, if not removed, can cause high scrap losses
in the casting, or otherwise poor metal quality in products
fabricated therefrom. In molten aluminum base alloys, the
principal objectionable impurities are dissolved hydrogen and
suspended non-metallic particles such as the oxides of
aluminum and magnesium, refractory particles, etc.
The solubility of hydrogen in aluminum alloys decreases
by about an order of magnitude when the metal solidifies.
Consequently, hydrogen gas is released from the metal during
casting if the hydrogen content of the molt~n metal is not
reduced below the solid solubility limit of hydrogen in the
metal~ Hydrogen causes pinhole porosity in rapidly solidified
metal such as direct-chill cast ingots, or fills shrinkage
cavities in slowly solidified metal. Even hydrogen remaining
dissolved in the metal after solidification may be harmful,
since it diffuses during heat treatment into voids and other
discontinuities in solid metal, thereby aggxegating the
harmful effects of these defect points in the properties of
the metal.
Solid, non-metallic particles suspended in the molten
metal cause serious difficulties during casting and
fabrication of aluminum alloys. These particles consist
mainly of oxides which are introduced into the melt with the
scrap during the melting operation, or ar~ produced by direct
oxidation with air, water vapour and other oxidizing gases
while the metal is processed in the molten state. Fine,
broken-up oxide films stirred into the molten metal are
, ......... . . , . . , ~ .
2087993
particularly harmful since in contrast to the more macroscopic
oxides in other solid particles, they cannot be skimmed off as
dross and remain suspended in the molten metal.
Also, in processes in which the aluminum matrix
composites are utilized, there i5 a proportion of waste or
scrap materials. The scrap from these metal matrix composites
represents a serious disposal problem since metal matrix
composites cannot be incorporated into standard alloys on
account of the danger of contamination by the reinforcing
particles present. For instance, a metal matrix composite
containing even a very low concentration of ceramic
particulates could have disastrous effects i~ mixed with metal
destined for thin gauge rolled products, such as can stock.
One technique for removing the ceramic particulates is
described in Provencher & Riverin, U.S. Patent No. 5,080,715.
Hydrogen gas, alkali~alkaline earth metals or compounds
and non-metallic inclusions are commonly removed from molten
aluminum by introducing an inert gas or chlorine gas into the
molten metal in the form of bubbles. Although chlorine gas
has been highly satisfactory in terms of its ability to remove
hydrogen and non-metallic inclusions from most aluminum
alloys, the use of chlorine presents serious problems due to
its corrosiveness and toxic nature. Thus, although the use of
chlorine for fluxing aluminum alloys has in the past been
considered a commercially acceptable practice, increasing
concern with air pollution and handling ha~ards in plants has
stressed the need for its elimination. As a result, numerous
attempts have been made to avoid the use of chlorine gas for
the treatment of molten aluminum.
One solution has been to utilize the chlorine in the form
of a powdered flux containing a chloride as the main
component. When ths flux is introduced into the molten metal
at high temperatures, it undergoes thermal decomposition to
generate corresponding gaseous chlorine or produce a liquid
salt, which carries out the refining action. In practice, a
powdered flux is fed through a blowing device which blows in
both inert gas and the flux simultaneously. Inert gas acts as
', ' ~ . ~ '
... . .
, . ,, ~ - .
2~87993
a carrier, transporting the flux. This can typically be done
by blowing the flux and inert gas into the molten metal
through a lance. Japanese published application 82-42118
describes such a method of blowing a flux-inert gas mixture
into molten aluminum.
It is also well known to feed refining gases into molten
aluminum by means of rotating impellers. These are described,
for instance, in U.S. Patents 3,839,019, 4,772,319, 4,992,241,
5,080,715, etc.
A widely used cleaning/degassing agent is
hexachloroethane (C2Cl6) in tabletted form. However, this has
the disadvantages of forming a wet dross and contaminating
reclaimed particulate.
It is also known from Japanese patent publication JP
89210156A that NH~,Cl can be used together with a degassing
agent selected from a fluoride, chloride or sulphate as a
refining agent for molten aluminum. This procedure has the
disadvantage that the mixture of NH4Cl and the fluoride,
chloride or sulphate produces a liquid salt residue on the
melt surface, which is highly undesirable.
It is the object of the present invention to find an
improved method of removing from molten aluminum solid
inclusions, oxides, alkali/alkaline earth metals and hydrogen
while avoiding the use of chlorine gas.
Summary of the Invention
The invention relates to a process for treating
unrein~orced aluminum or aluminum alloy metal melts as well as
metal matrix composites thereof, to remove hydrogen gas and
non-metallic inclusions therefrom. In the procedure of the
present invention, a mixture of NH4Cl powder and inert gas is
injected into the molten metal beneath the surface thereof
through a rotating impeller or through a lance. With this
novel process, it is possible to remove significant quantities
of solid inclusions. For instance, the procedure is capable
of removing 20% by volume of alumina particles present in a
metal matrix composite. Hydrogen is also very significantly
reduced.
~, . , . , ,-. ~. , . . - . ~ .
- - ~ .
2~79~3
The fluxing and degassing process of this invention is
based on the co-injection of the inert gas and reactive
chemical agent in fine powder form, preferably followed by an
inert gas injection period. Non-metallic inclusions,
alkali/alkaline earth and hydrogen are removed by floatation.
Tha inert gas/NH4Cl mixture should be injected well
beneath the surface of the melt and in such manner that fine
gas bubbles are well distributed throughout the melt. Thus,
the mixture is preferably injected close to an impeller so
that there is a strong shearing action to break up the gas
into small, well distributed bubbles. For instance, the gas
may be injected through a hollow rotor with an outlet for the
gas at the bottom end of tha impeller rotor or the gas may be
injected by being discharged through an opening in the rotor
in the vicinity of the impeller bladesO Alternatively, a
separate injection lance may be used having an outlet close to
the impeller blades. For best results the melt is preferably
maintained at a temperature in the range of 690~C to 750C.
The inert gas may be any gas which is substantially
non-reactive toward liquid aluminum at reaction temperatures,
e.g. argon or nitrogen. However, argon is preferred.
The mixing device is preferably made from graphite,
silicon carbide or a ceramic material which is inert to the
molten metal. It comprises a crucible with an impeller
consisting of a central hub portion with radially projecting
vanes set to provide substantial shearing action. The vanes
may be vertically mounted or they may be inclined up to 45 to
the vertical. Typically, about 4 to 8 vanes are used and the
impeller is typically rotated at a rate of about 100-400 rpm.
The process of the invention functions to de-wet non-
metallic inclusions. Thus, the chemical agent wets the non-
metallic inclusions but is, itself, non-wetted by the liquid
aluminum phase. The reduction of surface tension between the
chemical agent wetted non-metallic and liquid aluminum is the
main mechanism of the non~metallic in~lusion removal by
floatation.
....
. :
.,,~ : -
20~7~93
The removal of alkali impurities is accomplished by areaction with the halide-containing treatment gas. When the
NH4Cl is injected into the molten aluminum, it decomposes into
volatile compounds N2, H2 and HCl. It also reacts with alkali
present at temperatures above 660~C to form NaCl, LiCl or
CaCl2. The product of the reaction is then entrained to the
surface by the gas bubbles and then trapped in the dross.
The hydrogen dissolved in the liquid aluminum is removed
by diffusion into the gas bubbles which are lifted to the melt
surface. The dross is regularly remov~d from the surface of
the melt, removing with it the contaminants and leaving a
purified aluminum.
The process of the invention can be used in either a
batch mode or in-line continuous mode for either foundry or
wrought alloys. The term "aluminum" as used herein is
intended to include the alloys thereof. It has been found to
be an effective means for cleaning molten aluminum which is
safe and environmentally sound without chlorine fluxing.
Moreover, with the process of this invention, it is
possible to remove very significant amounts of solid
inclusions. For instance, it has been demonstrated that a
complete and efficient removal of 20% by volume of alumina
particulate having particle sizes of 5-75 ~m from the aluminum
matrix composite material, DUR~LCAN~, can be achiev~d. Also,
hydrogen is typically reduced from about 0.27 ml/lOOg to about
0.10 ml/lOOg.
Brief Description of the Drawinqs
The invention is illustrated by way of example with
reference to the drawings in which:
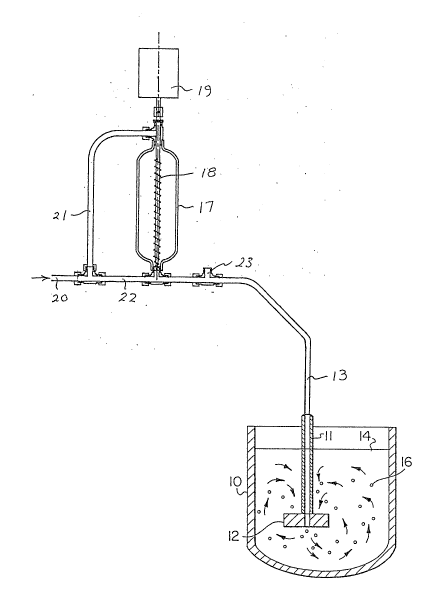
Figure 1 is a schematic illustration of a treatment
system for carrying out the invention; and
Figure 2 is a plot showing calcium and hydrogen removal
as a ~unction of time.
Description of the Preferred Embodiments
A suitable system for carrying out the invention is
depicted in Figure 1 where a vessel 10 is provided which may
conveniently be a furnace in the form of a 300 kg crucible
:. ~ .. .-: .
2087~3
electrically heated from the sides. It is filled with a
molten metal or metal matrix composite to level 14.
Extending down into the molten material is a rotatable
mixer consisting of a hollow rotatable drive shaft 11 with an
impeller 12 with radially projecting vanes at the bottom
thereof. One embodiment of the impeller has six blades set at
a ~5 angle, with a diameter of 25 centimetres and a thickness
of 8 centimetres. The bottom of the impeller 12 is also
preferably positioned about 8 centimetres from the bottom of
the vessel. The impeller may be located at the centre of the
crucible or it may be off-set from the centre.
The mixture of NH4Cl and inert gas is fed through the
hollow shaft 11 by a feed line 13. This feed line 13 connects
to a reservoir 17 for holding NH4Cl powder. A rotating feeder
18 is connected to an electric motor 19. The inert gas is fed
in through inlet line 20 and is divided into lines 21 and 22
feeding to the top and bottom of the reservoir 17 to maintain
a constant pressure. The NH4Cl powder feeds out of the bottom
of vessel 17 into line 22 and is carried along by the inert
gas into line 13 through safety valve 23. A mixing action
takes place in the vessel 10 as shown with gas bubbles 16
being formed.
Dross forms on the surface of the melt and this is
regularly removed from the surface, removing with it the
contami~ants and particulate and leaving a purified aluminum.
Preferred embodiments of the invention are further
illustrated by the following non-limiting examples.
Example 1
A test was carried out using the equipment described in
Figure 1 to remove ceramic particulate from scrap DURALCAN
metal matrix composite material. The impeller had six blades
set at a 45 angle, with an impeller diameter of 25
centimetres and a thickness of 8 centimetres. The bottom of
the impeller was about 8 centimetres from the bottom of the
vessel and ~as positioned off-centre at one-quarter of the
vessel diameter. The composite was an AA 6061 aluminum alloy
containing 20% by volume of alumina particles havin~ particle
... . .
, .
; -:
208 7 993
sizes in the range of 5-75 ~m. An amount of 214 kg of the
scrap composite was added to the furnace and was heated to a
temperature of 720C. Argon was fed in at a rate of 6 l/min.
and two additions of NH4Cl were made, each addition being in
the amount of 15g mixed with 200g of pure alumina powder. The
alumina powder was used only for security reasons to prevent
any excessive solid/gas transformation during the addition
into the molten metal, which can cause metal splashing.
The total time of the test was 23 minutes and all 20% by
volume of alumina particulate was removed.
The results obtained are shown in Table 1 below:
TABLE 1
Chemical Composition of the Aluminum Metal Matrix
ELEMENT BEFORE AFTER
TREATMENT TREATMENT ¦
_ _ - - I
~Cu 0.~9 _ 0.28
Fe 0.16 0.13
I _ _ I
¦Mg 0.71 0.55 ¦
¦Mn 0.002 0.002
Ni 0.019 0.002
l _ I
ISi 0.58 0.57
¦Ti O. 034 0.024
¦ Cd < 0.001 < 0.001
Co < O.001 < O.001
I - _
I V 0.008 0.008
25 Alkali/Alkaline Earth Removal
Sr _ 0.16 0.078
Na 0.0057 0.0017
Sr removal: 51%
Na removal: 70%
~r,~ . . ' . ~'. '
. ~.,~ ~ . ' ' `' ' :
" . ' ` . ' ~ : ~ '
::;
203799~
Typical hydrogen removal was 50-80% depending upon the
initial level of hydrogen. ~ typical hydrogen concentration
after treatment was found to be 0.10 ml/lOOg.
Example 2
Another test was carried out for cleaning and degassing
of AA3004 aluminum alloy. For this test, a different impeller
was used, this one having eight blades set at right angles
with an impeller diameter of 15 centimetres and thickness of 4
centimetres. The impeller was located at the centre of the
vessel about 8 centimetres from the bottom.
An amount of 204 kg of the AA3004 aluminum alloy was
added to the furnace and the temperature of the melt was
maintained at 720C. Argon gas was injected at a rate of 8
l/min. and the NH4Cl powder was injected at a rate of 1 g/min.
The injection time was 15 minutes.
The results obtained are shown in Table 2 below:
TABLE 2
_ I
ELEMENT BEFORE AFTER
TREATMENT TREATMENT
. . _ _
Cu .16 .16
I
Fe .33 .33
I
Mg .99 .96
I_ . _
Mn 1.07 1.08
I _ _ _
Ni .004 .004
Si .14 .14 ~
. I
Ti .007 .007
l _
¦ Cd 0002 0001
~ .008 .007
I _ _ .
C~ .0008 O
Na .0001 O
.. ~. . . .:
"=,
r:'' ` ~ l - -'' .:
r~
;.-, . :. ~ -
20~9~
Example 3
Using the same basic procedure as in Example 2, a 200 kg
batch test was conducted with an injection of 0.075 kg/ton of
NH4Cl during a period of 15 minutes. This resulted in complete
removal of calcium from an initial concentration of 8 ppm. No
significant loss of magnesium or other alloying elements was
observed. The dross generated was dry, indicating a good
reactivity in the melt during the fluxing.
Example 4
The procedure and equipment of Example 2 were again used
with 204 kg of AA3004 aluminum alloy being added to the
furnace. This was heated to 720C and argon was injected at
rates of 8 and 10 l/min. The NH4Cl was added at amounts
between 0 and 0.075 kg/ton. The results obtained are shown in
Tables 3 and 4 below:
TABLE 3
.
Argon NH4Cl _ Ca (ppm) Removal
flow rate Injection efficiency
(L~min) (kg~ton) Before 10 min 20 min(~)
_ _ ~
8 0 210520.5_ 19 12
8 0.037 34.523.5_ 16 54
8 0.075 _7.5 1 0 100
8 0.075 24 8.5 1.5 94
_ 11
~0 0.075 26 12 8 69
11
0.075 26.5 7 74
TABLE 4
Argon NH~Cl Inclusion (PoDFA)RemOva
flow rate ~njection efficiency I
(L/min) (kg/ton)BeforeAfter (%) ¦¦
_0.075 1.44 0.37 74
0.075 0.94 0.3~ 64
,: :
:~ - - . -:
,..... ..
2887993
Exam~le 5
Again using the same procedure and equipment as in
Example 2, the removal of calcium and hydrogen was determined
as a function of time. The furnace was charged with 204 kg of
AA3004 aluminum alloy and heated to 7~0C. Argon was injected
at a rate of 10 l/min and NH4Cl was injected in an amount of
0.075 kg/ton. The results obtained were measured over a
treatment time of 45 minutes and the results obtained are
shown in Figure 2.
:,.,,~.,.