Note: Descriptions are shown in the official language in which they were submitted.
2 ~
Cyclopentane Derivatives, Process for their Production
and their Pharmaceutical Use
Description:
The invention relates to cyclopentane derivatives, process
for their production as well as their use as auxiliary agents for
pharmacological studies and as pharmaceutical agents.
Cyclopentane derivatives have been dealt with intensively in
recent years, since prostaglandins derived from the cyclopentane
system, such as, e.g., PGA2, PGBz, PGE2, 6-oxo-PGE1, PGD2, PGF2a,
PGJz and their analogs, have the most varied biological actions,
e.g., on the cardiovascular system, the central nervous system or
immune system.
It has been found, surprisingly, that by the introduction of
a fluorine atom or a hydroxy group in 9 position (prostaglandin
numbering system) of the prostaglandin skeleton in combination
with the most varied structural features in the lower chain as
well as in 11-position, chemically and metabolically stable
prostaglandin analogs are obtained which are able to antagonize
the pharmacological properties of unstable thromboxane A2 (TXA2)
or PGH2 as well as its stable analogs, such as, e.g., U46619 or
U44069 on the receptor.
The compounds of this invention therefore constitute
valuable auxiliary agents for selective treatment of diseases,
which are attributable to an excess of TXA2 or PGH2.
.... . . .
,
g8~
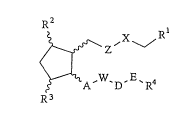
~he invention relates to cyclopentane derivatives of formula
I,
R2
,~~ ,X~" Rl (I),
A,W~D,E~R~
R3
in which ~ / ~ ~ ~ , N N
R1 can be o O ~ ~ ~ ' ~HN N' or Coo
and
R5 can mean hydrogen or C1-C10 alkyl, C3-C~o cycloalkyl, C7-C16
aralkyl, optionally substituted by halogen, phenyl, C~-C4 alkoxy
or di- (C~-C4) -alkylamino, phenacyl or C6-C12 aryl substituted by Y
or a 5- or 6-membered heterocyclic radical with at least one N, O
or S atom, or -CoNHR7 with R7 maaning hydrogen, Cl-c10 alkyl,
C6-C12 arylsulfonyl c1-C1o alkanoyl, or C1-C10 alkanesulfonyl,
X means ~(CH2)p-, -CH2-O-, or -CH2-S-,
Z,A, independent of one another, mean a direct bond,
(Z)-CH=CH-, (E)-CH=CH-, or -C-C ,
p means 0 to 5,
R2 means fluorine, OH,
n,r, independent of one another, mean o to 2,
R3 means oR6 or R6,
W means a direct bond, a ~[(CH2)n~V]q group, a -(CH2)n-V-
(CH2)q~V group, a free or functionally modified hydroxymethylene
': . '
~ ~ g '~
group, or a free or functionally modified 3 OH group, and
the hydroxy group can be respectively in ~- or ~ position,
q means l or 2,
D means a direct bond, a straight-chain saturated alkylene
group with 1-5 C atoms, a branched saturated alkylene group or a
straight-chain or branched unsaturated alkylene group with 2-5 C
atoms, which can be optionally substituted by fluorine atoms,
H H H
- ( CH2) n-NH -SO2-, 1 ' N ~ 1`N J ~T ~ N~
IJ
11 .Ll O (
-(CH2)n~NsN ~ N ~, ~(CH2)n N ~ N SO2-, n`N ~ N '
H V H H H H
/ ~`N J N ~ SO, ' ~(CH2)n~NH~NH~S02~' ~ /CH
V can be an O or S atom,
E can be a direct bond, -C_C- or -CH=CR8-, and R8 hydrogen,
C1-C5 alkyl, halogen or trifluoromethyl,
AWDE together can be a direct bond,
AW together can be a direct bond,
DE together can be a direct bond,
',, ~' .
" , ' `
R4 means ~ -(CH~
R4 - (CH~ ~ 2 ~ Y2 -(C~2)r N
~ ~ y _(CH~
-(CH2)-m ~ )r C3-C10 cycloalkyl, or C1-C10 alkyl
optionally substituted by Y,
m means 1 or 2,
y1 and y2 are the same or different and mean Y,
Y means hydrogen, halogen, N3, CF3, oR6~ NO2, NH2, CN, COOR6
or C1-C10 alkyl,
hydrogen, C1 C10 alkyl, C6-C12 aryl or C7-C16 aralkyl
optionally substituted by halogen and, if Rs means hydrogen,
their salts with physiologically compatible bases, as well as the
- or y-cyclodextrin clathrates, as well as the compounds of
formula I encapsulated with liposomes.
The definition of 5- or 6-memb~red heterocyclic radical
relates to heterocycles, which contain at least one heteroatom,
preferably nitrogen, oxygen or sulfur, and are monocyclic or
bicyclic. For example, there can be mentioned 2-furyl, 3-furyl,
2-thienyl, 3-thienyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, quinolyl,
isoquinolyl.
,~: : , .:
:, :, ' ' :
:
ik~g81~'1
As alkyl groups R4, Rs, R6, E and Y, straight-chain or
branched-chain alkyl groups ~ith 1-10 C atoms, such as, for
example, methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-
butyl, pentyl, isopentyl, neopentyl, heptyl, hexyl, decyl, are
suitable.
Alkyl groups R4, Rs, R6, E and Y can be substituted by
halogen atoms, hydroxy groups, C1-C4 alkoxy groups, C6-C12 aryl
groups, which can be substituted by halogen, di-(C1-C4~-
alkylamines and tri- (C~-C4) -alkylammonium. Those alkyl groups
which are singly substituted are preferred.
As substituents, for example, there can be mentioned
fluorinP, chlorine or bromine atoms, phenyl, dimethylamino,
diethylamino, methoxy, ethoxy.
As preferred alkyl groups R4, R5, R6, E and Y, those with 1-5
C atoms, such as, e.g., methyl, ethyl, propyl, isobutyl, butyl,
neopentyl, tert-butyl, chloroethyl, 1- or 2-chloropropyl,
hydroxyethyl and 1- or 2-hydroxypropyl can be mentioned.
As aryl groups Rs and R6, for example, phenyl, diphenyl, 1-
naphthyl and 2-naphthyl, which can be substituted by 1-3 halogen
atoms, a phenyl group, 1-3 alkyl groups each with 1-4 C atoms, a
chloromethyl group, fluoromethyl group, carboxyl group, C1-C4
alkoxy group or hydroxy group, are suitable. The substitution in
3- and 4-position on the phenyl ring i5 preferred, for example,
by fluorine, chlorine, C1-C4 alkoxy or trifluoromethyl or in 4-
position by hydro~y.
Cycloalkyl groups Rs can contain 3-10, preferably 3-6 carbon
atoms, in the ring. The rings can be substituted by alkyl groups
:
,
. ~ .
~g8~
with 1-4 carbon atoms. For example, there can be mentioned
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
methylcyclopentyl, methylcyclohexyl.
The C1-C10 alkyl groups mentioned under the definitions
should be straight-chain or branched alkyl groups, as they were
already mentioned for the alkyl groups above.
The hydroxy groups in R2, R3, Y and W can be functionally
modified, for example, by etherification or esterification, and
the free or modified hydroxy group in R2 and W can be in ~- or ~-
position, and free hydroxy groups are preferred.
As ether and acyl radicals, the radicals known to one
skilled in the art are suitable. Easily cleavable ether
radicals, such as, for example, the tetrahydropyranyl,
tetrahydrofuranyl, tert-butyldimethylsilyl, tert-
butyldiphenylsilyl, tribenzylsilyl radical, are preferred.
As acyl radicals, e.g., acetyl, propionyl, butyryl, benzoyl,
are suitable.
Halogen in the definitions for R5, R6, E and Y means
fluorine, chlorine, bromine and iodine.
Radicals "C1-C10 alkanoyl" or "C1-C10 alkanesulfonyl" for R7
correspond to the already m~ntioned alkyl groups of the same
length with the difference that they are bound on a carboxyl
group or sulfonyl group. C~-C4 alkanoyl or C~-C4 alkanesulfonyl
are preferred.
Inorganic and organic bases are suitable for salt formation
with the free acids (R5 = H), as they are known to one skilled in
the art for forming physiologically compatible salts. For
~ :. . : ,: :- ,: ' ~
.
': '
;
'~g81~ ,
example, there can be mentioned: alkali hydroxides, such as
sodium hydroxide or potassium hydroxide, alkaline-earth
hydroxides, such as calcium hydroxide, ammonia, amines, such as
ethanolamine, diethanolamine, triethanolamine, N~methylglucamine,
morpholine, tris-(hydroxymethyl)methylamine, etc.
Preferred are the compounds of formula I, in which
R1 means the group COORs or CoNHR7,
R3 means hydrogen, hydroxyl, C1-C4 alkyl, C6-C12 aryl or C7-C16
aralkyl optionally substituted by halogen,
Rs means hydrogen or C7~C16 aralkyl, C5-C6 cycloalkyl or C1-C10
alkyl optionally substituted by halogen,
R7 means C1-C7 alkanoyl, C6-C12 arylsulfonyl or C1-C7
alkanesulfonyl,
p means O to 4,
n,r, independent of one another, mean O or l.
Especially preferred are the compounds of formula I, in
which
R1 means the group CooR5,
R3 means hydrogen, hydroxyl, phenyl or phenylethyl,
~5 means hydrogen or methyl,
R7 means methanesulfonyl,
p means O to 4,
n,r, independent of one another, mean O or l.
The compounds of formula I according to the application can
~ . . . .
' .' ~
20~8~
be produced as described in more detail below:
A.
Z Hal-W-R4 ~ ~ Z~
R3 CH20H (I~ a) R3 A'W`D'E`
with R1, R2, R3, R4, X, Z in the above-indicated meanings,
Hal as bromine or chlorine,
A, DE as a direct bond,
Rl meaning a -COORs ester.
2 1 0 ~
,X~R (H3CO)~P`Jl`D~E`R4,
CHO (IV~ c)
R3
,X / ~ ~Z~X / ~,
= E R3 A' `D R
with R2, R3, R4 A, E, X, Z, in the above-indicated meanings,
. . ! ' " :
'
'
~g~
D as alkylene optionally substituted by alkyl or
CH
R1 meaning a -cooR5 ester
R9 as hydrogen or bromine,
W as -CH(OH)-.
C. II ~ ~ ,X~ ~R
R3 CON3 (VI~
R2 1 Hal-SO2-R (IX) R2
~ ,X " R CN R4(~ f - ` z
~ NH2 g) ~ A'W`D'E`R4
R3 R3
~V~I) (I)
with R2, R3, R4, X, Z, Hal in the above-indicated meanings,
AW, E as a direct bond,
D meaning ~-(C~I2)o~N ~ ~N ~ ,-(CH~o-NH-SO2-,
H H
R1 meaning a -CooR5 ester.
.
20~81~
D.
H2N-o-R4 (X) R2
H,2N-NH-So2-R4 (XIII)
oder ~r
H2N~ J~N~R ~"A,W`D E`R4
H H (~1) 0
h)
with R2, R3, R4, X, Z, in the above-indicated meanings,
AW, E as a direct bond,
R1 meaning a -COORs ester,
D meaning -~CH2)n\ ,O H H H
1~N~O~ N `, 1`N~N~N~,
v
H , H H
(CH2)n~N~N ~ ~, ~ ~N~ (CH2)D-NH-NH-SO2-.
H V
,
,, . ~ ~ ~ , , , , .:,
.
., ~ : .
: : :
, 11 2~88~
E.
R2 1 Hal-SO2-~4 (rX) R2
Il ) ~ Z ~ =C N R4~ ~ ,X~" R
R3 NH2 k) R3 A W`D E`R4
~XIV~ (~
with R2, R3, R4, X, Z, Hal in the above-indicated meanings,
AW, E as a direct bond,
O
D meaning 1`N ~ N ~ ,-(CH~1-NH-So2,
H H
R1 meaning a -CooR5 ester.
The compounds of formula I can be produced according to
claim 3 corresponding to the above-described process
alternatives. The initial compounds of formula II are produced
corresponding to the instructions given in examples lg-ln, 24a-
24g and 34a-34b.
The reaction conditions of the following proc~ss stages are:
a~ I (Process A)
In the presence of aqueous alkali or alkaline-earth
solutions and by using phase transfer catalysts (such as, e.g.,
tetrabutylammoniumhydrogen phosphate or
tetrabutylammoniumhydrogen sulfate) compounds of formula II are
reacted at 20 to 100C in 1 to 16 hours with reactand III as an
. ~, .
12
~8~
organic phase or a solution of III in an inert, water immiscible
organic solvent, optionally, as described in example l,
introducing a fluorine atom for R2 or a hydroxyl group, such as
described in examples 74 and 74a, is removed.
b) II ~ IV (Process B)
The oxidation of compounds of formula II takes place
according to known processes, such as, e.g., according to that of
Swern, Collins as well as with use of pyridinium dichromate or
pyridinium chlorochromate in solvents such as dichloromethane,
diethyl ether, tetrahydrofuran, benzene or toluene at -80C to
-50C (Swern) or up to +30C (in the other oxidations) within l0
minutes to 8 hours.
c) IV ~ VI (Process B), d) VI ~ I (Process B)
The reaction of compounds IV with phosphonates V as well as
the subsequent reduction or HBr elimination took place
analogously to the conditions mentionad in DE-OS 2845170.
e) II ~ VII (Process C)
The oxidation of the compounds of formula II is preferably
performed with Jones reagent or pyridiniumchlorochromate
according to reaction conditions known in the art. Then it is
reacted under usual conditions with thionyl chloride to acid
chloride, brought to reaction under phase transfer catalysis with
aqueous sodium azide solution and as descri~ed in example lc
rearranged to compounds of formula VIII, optionally a C-C
multiple bond is hydrogenated in Z or a fluorine atom, as
described in example l, is introduced.
., .
: : :.
.
.
: ' '; ' '
13
f) VII ~ VIII (Process C)
The reaction of the compounds of formula VII to compounds of
formula VIII takes place as described in example lc.
g) VIII ~ I (Process C)
The reaction of the compounds of formula VII with compounds
of formula IX or XII takes place as in examples lb and 78
mentioned in this respect.
h) IV ~ I ~Process D)
The reaction takes place analogously to the process
described in WO 90/02740 (process C, p. 16).
i) II ~ XIV (Process E)
The reaction of the compounds of formula II to compounds of
formula XIV takes place as described in examples 78a to 78c.
k) XIV ~ I (Process E)
The reaction of the compounds of formula XIV with the
compounds of formula IX takes place as described in examples lb
and 78.
The release of functionally modified hydroxy groups R2, R3,
R4 and W takes place according to the methods known to one
skilled in the ar~. For example, the cleavage of ether
protecting groups is performed in an aqueous solution of an
organic acid, such as, e.g., acetic acid, propionic acid, citric
acid, i.a, or in an aqueous solution of an inorganic acid, such
as, e.g., hydrochloric acid, or in the case of tetrahydropyranyl
ethers with use of pyridinium-p-toluenesulfonate, preferably in
alcohols as solvent or with use of anhydrous magnesium bromide,
preferably in diethyl ether as solvent.
., '
~88~
To improve the solubility, a water-miscible inert solvent is
suitably added with use of aqueous-acid reaction conditions.
Proven as suitable, there are, e.g., alcohols, such as methanol
and ethanol, ethers, such as dimethoxyethane, dioxane and
tetrahydrofuran, and tetrahydrofuran is preferably used.
The cleavage of silylether protecting groups takes place,
for example, with tetrabutylammonium fluoride according to the
methods known to one skilled in the art. As solvent, for
example, tetrahydrofuran, diethyl ether, dioxane, methylene
chloride, etc., are suitable. The cleavage is performed
preferably at temperatures between 20C and 80C.
The saponification of the acyl groups and ester in COORs of
the cyclopentane derivatives is performed according to the
methods known to one skilled in the art, such as, for example,
with basic catalysts, such as, e.g., with alkali or alkaline-
earth carbonates or hydroxides in an alcohol or the aqueous
solution of an alcohol. As alcohols, aliphatic alcohols, such
as, e~g., methanol, e~hanol, butanol, etc., but preferably
methanol, are suitable. As alkali carbonates and hydroxides,
lithium, sodium and potassium salts can be mentioned. The
lithium and potassium salts are preferred. As alkaline-earth
carbonates and hydroxides, for example, calcium carbonate,
calcium hydroxide and barium carbonate are suitable. The
reaction generally takes place at -10C to ~70C, but preferably
at +25C.
The introduction of ester group Co2R5 for R1 or C02R6 ~or Y,
in which R5 or R6 represents an alkyl group with 1-10 C atoms,
`
.:
', ' '~ :
.
2~8~
takes place according to the methods Xnown to one skilled in the
art. The carboxy compounds (Rs = H or R6 = H) are reacted, for
example, with diazohydrocarbons in a way known in the art. The
esterification with diazohydrocarbons takes place, e.g., in that
a solution of the diazohydrocarbon in an inert solvent,
preferably in diethyl ether, is mixed with the carboxy compound,
dissolved in the same or in another likewise inert solvent, such
as, e.g., methylane chloride. After completion of the reaction
within 1 to 60 minutes, the solvent is removed and the ester is
purified in the usual way. Diazoalkanes are either known or can
be produced according to known methods [Org. Reactions, Vol. 8,
pages 389-394 (1954)].
The introduction of ester group CO2Rs for R1 or CO2R6 for Y,
in which R5 or R6 represents a substituted or unsu~stituted aryl
group, takes place according to the methods known to one skilled
in the art. For example, the 1-carboxy compounds are reacted
with the corresponding arylhydroxy compounds with
dicyclohexylcarbodiimide in the presence of a suitable base, such
as, e.g., pyridine, DMAP, triethylamine, in an inert solvent,
su~h as, e.g., methylene chloride, ethylene chloride, chloroform,
ethyl acetate, tetrahydrofuran, but preferably with chloroform.
The reaction is performed at temperatures between -30C and
~50C, preferably at +10C.
The cyclopentane derivatives of formula I with Rs or R6
meaning a hydrogan atom can be convarted to salts with suitable
amounts of the corresponding inorganic bases with neutralization.
For example, by dissolving the corresponding acids in water,
. ...
~'
,: ~
2~8~ 1
which contains stoichiometric amounts of the base, the solid
inorganic salt is obtained after evaporation of the water or
after addition of a water-miscible solvent, e.g., alcohol or
acetone.
The production of the amine salts takes place in the usual
way. For this purpose, the acid is dissolved in a suitable
solvent, such as, e.g., ethanol, acetone, diethyl ether or
benzene and 1 to 5 equivalents of the respective amine is added
to this solution. In this case, the salt usually accumulates in
solid form or is isolated in the usual way after evaporation of
the solvent.
The functional modification of the free hydroxy groups takes
place according to the methods known to one skilled in the art.
For the introduction of the ether protecting groups, the reaction
is performed, for example, with dihydropyran or methyl vinyl
ether in methylene chloride or chloroform with use of catalytic
amounts of an acid condensing agent, such as, e.g., p-
toluenesulfonic acid. The respecti~e enol ether is added in
excess, preferably in 1.2 to 10 times the amount of the
theoretical requirement. The reaction normally takes place at -
10C to +30C and is completed after 2 to 45 minutes.
For the introduction of silylether protecting groups, the
reaction is performed, for example, with t-butyl-
diphenylchlorosilane or t-butyl-dimethylchlorosilane in
dimethylformamide with use of a base, such as, e.g., imidazole.
The respective silyl chloride is added in excess, preferably in
1.05 to 4 times the amount of the theoretical requirement. The
-
reaction normally takes place at 0C to 30C and is completed
after 1 to 24 hours.
The introduction of the acyl protecting groups takes place
by a compound of formula I being reacted in a way known in the
art with a carboxylic acid derivative, such as, e.g., acid
chloride, acid anhydride, etc.
Cyclodextrin clathrates are obtained analogously to the
instructions in WO 87/05294.
Liposomes are produced according to t~e production process
described in "Pharmazie in unserer Zeit [Pharmaceutics in Our
Time] 11, 98 (1982)."
All stereoisomeric forms are also part of the object of the
invention.
-, ~
.
., ~ ` ~ :
Biolo~ical Ac_ion and Ran~e of APplication of the New TXA2
Antagonists:
The compounds of this invention are suitable for treatment
of diseases of the cardiovascular system, the stomach, the
pancreas, the liver and the kidneys. They work in an
antihypertensive and bronchodilatory manner. They are
excellently suited for inhibition of the activation of platelets.
Consequently, the new TXA2 antagonists of formula I represent
valuable pharmaceutical active ingredients. Moreover, the
compounds are distinguished by higher selectivity, a
substantially longer effectiveness and a greater stability than
similar TXA2 antagonists.
The new TXA2 antagonists have the properties typical for
this family of compounds, such as, e.g., reduction of the
peripheral arterial, the coronary and the pulmonary vascular ~
resistance, reduction of the pulmonary blood pressure, reduction
of the systemic blood pressure without at the same time reducing
the cardiac output and coronary blood circulation, promotion of
the kidney blood circulation and the blood circulation of other
peripheral organs, increase of the cerebral blood circulation,
inhibition of the platelet activation and dissolution of blood
clots, inhibiti~n of bronchoconstriction, inhibition of gastric
acid secretion, cytoprotection of the heart, the stomach and
intestinal mucous membrane, the liver, cytoprotection in the
pancreas and in the kidneys as well as antiallergic properties.
Therefore, the new TXA2 antagonists are suitable on principle for
treatment of stroke, prophylaxis and treatment of coronary heart
', ' ' ' . ' ' ` : ~ ~
:~ :
', . :.. , ~
19
`~88~ ~
diseases, for example, coronary thrombosis, for treatment of
myocardial infarction, peripheral arteriopathies, for prophylaxis
and treatment of other thromboembolic diseases and in
arteriosclerosis, in ischemic attacks of the central nervous
system and other disturbances of the blood circulation of the
brain, such as, e.g., migraine, for treatment of hypertonia and
for treatment of diseases which accompany an increase of the
pulmonary vascular resistance, such as, e.g., the pulmonary
hypertonia and for treatment of shock, asthma and allergic
rhinitis. They can further be used to inhibit labor pains and
for treatment of toxicoses in pregnancies.
Further, the new TXA2 antagonists can be used to improve the
organ function after transplantation, for example, in kidney
transplantation, to prevent rejection reactions, instead of
heparin or as adjuvant in the case of dialysis or hemofiltration
and in the case of storing dried blood plasma, for example, dried
blood platelets.
The new TXA2 antagonists have an antimetastatic action and
antiproliferative properties. They are suitable on principle for
treatment o~ neoplasias. The new TXA2 antagonists can be used in
combination with, for example, carbacyclins, prostacyclin and its
analogs, 7-oxoprostacyclins, prostaglandins and their derivatives
and 6-oxo-PGE1- and 6-oxo-9-fluoroprostaglandin derivatives, with
TXA2-synthetase inhibitors, with phosphodiesterase inhibitors,
with antagonists and receptor antagonists of various platelet
stimulators (e.g., ADP, thrombin, collagen, PAF, adrenaline,
serotonin, fibrinogen), with calcium antagonists, with
. .
:
2 ~
fibrinolytic agents and thrombolytic agents, e.g., t-PA,
streptokinase, with heparin and other anticoagulants, with
cyclooxygenase inhibitors, e.g., acetylsalicylic acid, with
inhibitors of lipoxygenases as well as antagonists of
lipoxygenase products, with vasodilators, such as, e.g., nitro
compounds, with antihypertensive agents, such as, e.g., ~-
blockers or with diuretics.
The dose of the compounds is 0.1-1000 mg/day, preferably
0.1-500 mg/day, also in several partial doses, if they are
administered to human patients. The unit dose for the
pharmaceutically acceptable vehicle is 0.1-100 mg. For
parenteral administration, sterile, injectable aqueous or oily
solutions are used. For oral administration, for example,
tablets, coated t~blets, or capsules are suitable.
Thus, the invention also relates to pharmaceutical agents
based on the compounds of general formula I and usual auxiliary
agents and vehicles.
The active ingredients according to the invention are to be
used in connection with the auxiliary agents known and usual in
galenicals, e.g., for the production of antihypertensive agents.
The unit dose range for the ampule is 0.1-100 mg, for the
tablet 0.1-100 mg.
;~
. ~ ~
:,
~ ~ 8 '~
Example 1:
7-[lR,2S,5R)-2-(4-Toluenesulfonylamino)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
55 mg (139 ~mol) of the alcohol produced according to
example la is dissolved in 2.5 ml of anhydrous toluene, mixed
with 56 ~1 of anhydrous pyridine, cooled under an atmosphere of
dry argon to -60C and mixed with 42 ~1 of diethylaminosulfur
trifluoride. It is allowed to heat within 3.5 hours to 0C,
quenchsd by adding 1 ml of a saturated sodium bicarbonate
solution, diluted with water and extracted several times with
dichloromethane. The combined organic extracts are dried on
magnesium sulfate and the residue obtained after removal of the
solvent is purified by chromatography on 4 analytic thin-layer
slabs. A mixture of n-hexane and acetone is used as mobile
solvent, diethyl ether is used as eluant. 20 mg (53 ~mol, 38%)
of 7-[5(S)-(4-toluenesulfonylamino)-cyclopent-lenyl]-5(Z)-
heptenoic acid methyl ester as well as 16 mg (40 ~mol, 29~) of
the title compound are each isolated as colorless oil.
IR (KBr): 3430, 3310, 3050, 3020, 2940, 2860, 1735, 1600,
1450, 1320, 1240, 1160, 1095, 920, 815 and 670, 580 and 550 cm~1.
Exa~æle la.
7-[(lR,2S,5S)-2-(4-Toluenesulfonylamino)-5 hydroxy-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
The solution of 140 mg (280 ~mol) of the compound, produced
according to example lb, in 1.5 ml of methanol is mixed with 20
mg of finely pulverized potassium carbonate and heated for 1 hour
.. . `, ~
~' .~ ,, ' ~ ' .
?
~, , .
8~
to 70C. After the cooling, it is acidified with saturated
citric acid, diluted with water and extracted several times with
dichloromethane. The combined organic extracts are dried on
magnesium sulfate and the residue obtained after removal of the
solvent is purified by chromatography on 2 analytic thin-layer
slabs. A mixture of n-hexane and ethyl acetate is used as mobile
solvent, diethyl ether is used as eluant. 96 mg t243 ~mol, 87%)
of the title compound is isolated as colorless oil.
IR (Film): 3600-3200, 3270, 2940, 2870, 1735, 1600, 1450,
1330, 1270, 1160, 1110, 1090, 1025, 815 and 665 cm~1.
Example lb:
7-[(lR,2S,5S)-2-(4-Toluenesulfonylamino)-5-benzoyloxy-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
The solution of 340 mg (98~ ~mol) of the amine, produced
according to example lc, in 30 ml of anhydrous dichloromethane is
mixed with 1.52 ml of triethylamine, 483 mg of p-toluenesulfonic
acid chloride and stirred for 1.5 hours at 23C under an
atmosphere of dry argon. It i5 poured on a semiconcentrated
sodium chloride solution and extracted several times with
dichloromethane. The combined organic extracts are dried on
magnesium sulfate and the residue obtained after removal of the
solvent is purified by chromatography on about 70 ml of fine
silica gel with a gradient system of n-hexane and ethyl acetate.
330 mg (660 ~mol, 67%) of the title compound is isolated as
colorless oil.
~ ; , , , , . ~
.,
,
2 ~
IR (Film): 3270, 2940, 2870, 1730, 1710, 1600, 1450, 1330,
1270, 1160, 1110, 1090, 1025, 905, 815, 710 and 665 cm~~.
Example lc:
7-[(lR,2S,5S)-2-Amino-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester (A) and 7-[(lR,2S,5S)-2-
trifluoroacetamido-5-benzoyloxy-cyclopentyl]-5(Z)-heptenoic acid
methyl ester (B):
The solution obtained according to example ld is mixed with
1.47 ml of trifluoroacetic acid and refluxed for 6 hours. It is
allowed to stand for 14 hours at 23C, concentrated by
evaporation and the residue is purified by chromatography on
about 250 ml of fine silica gel with a gradient system of
dichloromethane and methanol. 2.0 g (5.79 mmol, 43% relative to
the feedstock in example le) of title compound A as well as
1.53 g (3.47 mmol, 26% relative to the feedstock in example le)
of title compound B are isolated.
IR (CHCl3) of A: 3500-2600, 2950, 2870, 1710, 1670, 1450,
1275, 1190, 1140 and 835 cm-1.
IR (~ilm) of B: 3320, 2950, 2870, 1740-1690, 1600, 1550,
1450, 1435, 1270, 1210, 1180, 1110, 1070, 1025 and 710
cm .
.- .: . -
-
,
.
. ' ~
24
~81~
Example ld:
7-[(lR,2S,5S)-2-Azidocarbonyl-5-benzoyloxy-cyclopentyl]-
5(Z)-heptenoic acid methyl ester:
The residue obtained according to example le is dissolved in
20 ml of dichloromethane, cooled to 3C, mixed with 10 mg of
tetrabutylammonium hydrogen sulfate and the solution of 1.04 g of
sodium azide in 3.5 ml of water. It is stirred for 2.5 hours,
diluted with dichloromethane, the organic phase is separated and
dried on freshly annealed magnesium sulfate. The solution
obtained after filtration is immediately further reacted.
Example le:
7-[(lR,2S,5S)-2~Chlorocarbonyl-5-benzoyloxy-cyclopentyl]-
5(Z)-heptenoic acid methyl ester:
The solution of 5.0 g (13.4 mmol) of the compound, produced
according to example lf, in 133 ml of anhydrous dichloromethane
is mixed under ice cooling with 2.13 ml of freshly distilled
thionyl chloride and allowed to stir for 20 hours at 23~C under
an atmosphere of dry argon. It is concentrated by evaporation
and the resulting residue is further reacted without
purification.
Example lf:
7-[(lR,2S,5S)-2~Hydroxycarbonyl-5-benzoyloxy-cyclopentyl]-
5(Z)-heptenoic acid methyl ester:
The solution of 30.2 g (83.9 mmol~ of the alcohol, produced
according to example lg, in 725 ml of acetone is cooled to -15C,
- ~`
-
,: , ,
,, ' ,
~ ,
2~81~1
mixed with 44 ml of a standardized chromosulfuric acid solution
(Jones reagent), stirred for 3 hours at -10C and excess
oxidizing agent is d~composed by adding 13 ml of isopropanol. It
is diluted with water, extracted several times with diethyl
ether, the combined organic extracts are washed with water and
saturated sodium chloride solution and dried on magnesium
sulfate. The residue obtained after filtration and removal of
the solvent is purified by chromatography on about 1 l of coarse
silica gel with a gradient system of n-hexane and ethyl acetate.
29.3 g (78.3 mmol, 93%) of the title compound is isolated as
colorless oil~
IR (Film): 3600-2500, 3060, 2950, 2860, 1740-1690, 1600,
1580, 1450, 1435, 1360, 1310, 1270, 1170, 1110, 1070, 1025 and
710 cm~1.
Example la:
7-[(lR,2S,5S)-2-Hydroxymethyl-5-benzoyloxy-cyclopentyl]-
5(Z)-heptenoic acid methyl ester:
The solution of 57.9 g (96.7 mmol) of the com~ound, produced
according to example lh, in 124 ml of anhydrous tetrahydrofuran
is mixed with 155 ml of a 1 M solution of tetrabutylammonium
fluoride in tetrahydrofuran and stirred for 17 hours at 23C
under an atmosphere of dr~ argon. It is mixed with water,
extracted several times with diethyl ether, the combined organic
extracts are washed with water and saturated sodium chloride
solution and dried on magnesium sulfate. The residue obtained
after filtration and removal of the solvent is purified by
:, . i , ,
.
.. ' , : ~
~, . , , :.
. i ,,
26
,- ~Z ~
chromatography on about 2 l of coarse silica gel with a gradient
system of n-hexane and ethyl acetate. 30.2 g (83.8 mmol, 87%) of
the title compound is isolated as colorless oil.
IR (Film): 3600-3200, 3060, 2940, 2860, 1735, 1710, 1600,
1580, 1360, 1310, 1275, 1170, 1110, 1070, 1025 and 715 cm~1.
Example lh:
7-[(lR,2S,5S)-2-(tert-Butyldiphenylsilyloxymethyl)-5-
benzoyloxy-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
The solution of 47.9 g (96.7 mmol) of the compound, produced
according to example li, in 212 ml of anhydrous pyridine is
cooled under an atmosphere of dry argon to 5C, mixed T~ithin 30
minutes with 29 ml of benzoyl chloride and stirred for 1.5 hours
at 23C. It is poured on 600 ml of ice water, extracted several
times with diethyl ether, the combined organic extracts are
washed with 2 n hydrochloric acid, watar and saturated sodium
chloride solution and dried on magnesium sulfate. The residue
obtained after filtration and removal of the solvent is purified
by chromatography on about 2 l of fine silica gel with a gradient
system of n-hexane and ethyl acetate. 57.9 g (96.7 mmol, 100%)
of the title compound is isolated as colorless oil.
IR (Film~: 3070, 3040, 3000, 2950, 2930, 2850, 1735, 1710,
1600, 1450, 1425, 1360, 1310, 1270, 1110, 820, 740 and 705 cm~l.
, . . - .
-, -
' ' , , .
Example li: 2~83~1
7-[(lR,2S,5S)-2-(tert-Butyldiphenylsilyloxymethyl)-5-
hydroxy-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
The solution of 154 g of the crude product, produced
according to example lj, in 150 ml of acetone is mixed with
33.4 g of potassium carbonate, 41.2 g of methyl iodide and heated
for 6 hours to 80C. It is concentrated by evaporation, taken up
in 400 ml of dichloromethane, washed with water and saturated
sodium chloride solution and dried on magnesium sulfate. The
residue obtained after filtration and removal of the solvent is
purified by chromatography on about 2 1 of coarse silica gel with
a gradient system of n-hexane and ethyl acetate. 47.9 g (96.8
mmol, 88% relative to the feedstock in example lj) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3070, 3040, 3000, 2940, 2920, 2850,
1735, 1585, 1460, 1425, 1240, 1165, 1110, 1005, 820, 740 and 700
cm .
Example li:
7-[(lR,2S,5S)-2-(tert-Butyldiphenylsilyloxymethyl)-5-
hydroxy-cyclopentyl]-5(Z)-heptenoic acid:
A total of 50 g of finely pulverized potassium-tert-
butanolate is added within one hour in portions to the emulsion
of 99.7 g of carboxybutyltriphenylphosphonium bromide in 256 ml
of anhydrous tetrahydrofuran and 600 ml of anhydrous dimethyl
sulfoxide. It is stirred until a clear red solution results, the
solution of 43.8 g (110 mmol) of the compound, produced according
,
, : .
- : :
,, , ,: , ,
28
, :
to example lk, is instilled continuously in 130 ml of anhydrous
tetrahydrofuran and allowed to react for 2 hours at 23C under an
atmosphere of dry argon. It is poured in ice water, adjusted to
a pH of 4-5 by adding a saturated citric acid solution and
extracted several times with dichloromethane. It is washed with
water and saturated sodium chloride solution, dried on magnesium
sulfate, filtered and concentrated by evaporation. The resulting
residue is further reacted without purification.
Example lk:
(lS,3RS,5R,6S)-3-Hydroxy-6-(tert-
butyldiphenylsilyloxymethyl)-2-oxabicycio[3.3.0]octane:
The solution of 45.8 g (116 mmol) of the compound, produced
according to example 11, in 1.4 l of anhydrous toluene is cooled
under an atmosphere of dry argon to -70C, 202 ml of a 1.2 M
diisobutylaluminum hydride solution in toluene is instilled
within one hour and stirred for 1 hour. Excess reducing agent is
decomposed ~y adding 13 ml of isopropanol. It is allowed to heat
to 0C, 100 ml of water is instilled and stirred at 23C until a
fine granular precipitate has formed. It is suctioned off,
rewashed with dichloromethane and, after removal of the solvent,
43.8 g (110 mmol, 95%) of the title compound is isolated as
colorless oil.
IR (Film): 3600-3200, 3070, 3050, 2940, 2850, 1590, 1465,
1425, 1390, 1360, 1260, 1110, 1010, 940, 820, 740 and 700 cm-1.
:
29
~.
~88161
Exam~le 11:
(lS,5R,6S)-3-Oxo-6-(tert-butyldiphenylsilyloxy~methyl)-2-
oxabicyclo[3.3.0]octane:
The solution of 67 g (119 mmol) of the tosylate, produced
according to example lm, in 1.3 1 of dimethoxyethane is mixed
with 136 g of sodium iodide, 118 g of zinc dust, 79 ml of water
and refluxed for 16 hours. After the cooling, it is filtered off
from undissolved res~dues, the filtrate is concentrated by
evaporation to about 200 ml, mixed with water and extracted
several times with diethyl ether. The combined organic extracts
are washed with 10% sodium hydrogensulfate solution, water,
saturated sodium chloride solution and dried on magnesium
sulfate. The residue obtained after filtration and removal of
the solvent is purified by chromatography on about 2 l of coarse
silica gel with a gradient system of n-hexane and ethyl acetate.
45.8 g (116 mmol, 98~) of the title compound is isolated as
colorless oil.
IR (Film): 3070, 3050, 2950, 2930, 2850, 1775, 1590, 1470,
1425, 1165, 1110, 1005, 820, 740 and 705 cm~1.
Example lm:
(lS,5R,6S,7R)-3-Oxo-6-(tert-butyldiphenylsilyloxymethyl)-7-
toluenesulfonyloxy-2-oxabicyclo[3.3.0]octane:
The solution of 67.3 g (164 mmol) of the alcohol, produced
according to example ln, in 260 ml of anhydrous pyridine is mixed
with 62.8 g of p-toluenesulfonic acid chloride and stirred for 27
hours under an atmosphere of dry argon at 50C. It is
:
,
?
~o
~a~
concentrated by evaporation, mixed with water and extracted
several times with dichloromethane. It is washed with water and
saturated sodium chloride solution and dried on magnesium
sulfate. The residue obtained after filtration and removal of
the solvent is purifie~d by chromatography on about 2 l of coarse
silica gel with a gradient system of n-hexane and ethyl acetate.
67 g (119 mmol, 72%) of the title compound is isolated as
colorless oil.
IR (CHCl3): 3070, 3030, 2960, 2930, 2860, 1770, 1600, 1470,
1425, 1365, 1190, 1175, 1110, 1040, 980, 960, 895, 875, 820 and
700 cm-1.
Example ln:
(lS,5R,6S,7R)-3-Oxo-6-(tert-butyldiphenylsilyloxymethyl)-7-
hydroxy-2-oxabicyclo[3.3.03octane:
The solution of 129 g (251 mmol) of (lS,5R,6S,7R)-3-oxo-6-
(tert-butyldiphen~lsilyloxymethyl)-7-benzoyloxy-2-
oxabicyclo[3.3.0]octane in 1 l of methanol is mixed with 14.9 g
of potassium carbonate and stirred for 3 hours at 23C under an
atmosphere of dry argon. ~t is mixed with water, neutralized by
carefully adding 2 n hydrochloric acid, concentrated by
evaporation and extracted several times with dichloromethane. It
is washed with water, saturated sodium chloride solution and
dried on magnesium sulfate. The residue obtained after
filtration and removal of the solvent is purified by
chromatography on about 2 l of fine silica gel with a gradient
~, ; - , , ,~ . . .
. ' , . .
system of n-hexane and ethyl acetate. ~99 ~ 8 ~36 mmol, 94%) of
the title compound is isolated as colorless oil.
IR (Film): 3600~3200, 3070, 3050, 2940, 2960, 1770, 1590,
1430, 1240, 1170, 1115, 1075, 825, 745, 705, 615 and 510 cm~1.
Example 2:
7-[(lR,2S,5R)-2-(4-Toluenesulfonylamino)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
The solution of 16 mg (44 ~mol) of the compound, produced
according to example 1, in 1 ml of methanol is mixed with 0.5 ml
of a 5% lithium hydroxide solution and stirred for 1.5 hours at
23C. It is acidified by adding saturated citric acid, diluted
with water and extracted several times with dichloromethane. It
is washed with water, saturated sodium chloride solution and
dried on magnesium sulfate. The residue obtained after
filtration and removal of the solvent is purified by
chromatography on an analytic thin-layer slab. A mixture of
dichloromethane and methanol is used as mobile solvent, a mixture
of chloroform and isopropanol is used as eluant. 15.7 mg (41
~mol, 93%) of the title compound is isolated as colorless solid.
IR (KBr): 3600-2400, 3430, 3310, 3050, 3020, 2950, 2930,
2860, 1715, 1600, 1450, 1320, 1240, 1160, 1095, 920, 815, 670,
580 and 550 cm~1.
,
' ~,
~. ~ . , .
~81~
Example 3:
7-[(lR,2S,5R)-2-(Benzenesulfonylamino)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
80 mg (210 ~mol) of the compound produced according to
example 3a is reacted analogously to example 1 and, after working
up and purification, 23 mg (63 ~mol, 30%) of 7-[5(S)-
(benzenesulfonylamino)-cyclopent-1-enyl]-5(Z)-heptenoic acid
methyl ester as well as 26 mg (68 ~mol, 32%) of the title
compound are isolated as colorless oil.
IR (Film): 3430, 3310, 3040, 3020, 2950, 2860, 1730, 1600,
1450, 1320, 1240, 1160, 1095, 930, 755, 720 and 690 cm~1.
Example 3a:
7-[(lR,2S,5S)-2-Benzenesulfonylamino-5-h~droxy-cyclopentyl]-
5(Z)-heptenoic acid methyl ester:
270 mg (556 ~mol) of the compound produced according to
example 3b is reacted analogously to example la and, after
working up and purification, 212 mg (556 ~mol, 100%) of the title
compound-is isolated as colorless oil.
IR (Film): 3600-3200, 3260, 3060, 2930, 2850, 1725, 1445,
1320, 1265, 1160, 1090, 1020 and 735 cm~1.
Example 3b:
7-~(lR,2S,5S)-2-Benzenesulfonylamino-5-benzoyloxy-
cyclopentyl]-5(z)-heptenoic acid methyl ester:
250 mg (724 ~mol) of the compound produced according to
example lc is reacted analogously to example lb with
. .
benzenesulfonic acid chloride and, after working up and
purification, 270 mg (556 ~mol, 77~) of the title compound is
isolated as colorless oil.
IR tFilm): 3270, 3060, 3000, 2950, 2860, 1730, 1715, 1600,
1585, 1450, 1330, 1315, 1270, 1160, 1110, 990, 1025, 910, 755,
715 and 690 cm-l.
Example_4
7-[(lR,2S,5R)-2-(Benzenesulfonylamino)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
26 mg (68 ~mol) of the compound produced according to
example 3 is reacted analogously to example 2 and, after working
up and purification, 23.6 mg (64 ~mol, 94%) of the title compound
is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.5-2.2(m, llH), 2.34(t, 2H), 3.3-
3.45(m, lH), 4.7(m, lH), 5.1(s, NH), 5.2-5.5(m, 2H), 7.45-7.65(m,
3H), 7.9(m, 2H).
Example 5:
7-[(lR,2S,5R)-2-(4-Fluorobenzylsulfonylamino)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
84 mg (210 ~mol) of the compound produced according to
example 5a is reacted analogously to example 1 and, after working
up and purification, 25 mg (66 ~mol, 31%~ of 7-[5(S)-(4--
fluorobenzylsulfonylamino)-cyclopent-1-enyl]-5(Z)-heptenoic acid
methyl ester as well as 26 mg (65 ~mol, 31%) of the title
compound are isolated as colorless oil.
-
- , . . . ~ -
~: , ,-. ~. ~. '.-: , -
.:
IR (Film): 3300, 3060, 3020, 2940, 2860, 1735, 1605, 1595,
1490, 1440, 1325, 1250, 1160, 835, 790, 735 and 700 cm~1.
Example 5a:
7-[(lR,2S,5S)-2-(4-Fluorobenzenesulfonylamino)-5-hydroxy-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
240 mg (477 ~mol) of the compound produced according to
example 5b is reacted analogously to example lb and, after
working up and purification, 175 mg (438 ~mol, 92%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3310, 3060, 3020, 2940, 2860, 1730,
1610, 1595, 1490, 1440, 1325, 1250, 1160, 835, 790, 740 and 700
cm-1
Example 5b:
7-[(lR,2S,5S)-2-(4-Fluorobenzenesulfonylamino)-5-benzoyloxy-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
250 mg (724 ~mol) of the compound produced according to
example lc is reacted analogously to example lb with 4-
fluorobenzenesulfonic acid chloride and, after working up and
purification, 243 mg (483 ~mol, 67%) of the title compou~d is
isolated as colorless oil.
IR (Film): 3270, 3060, 3000, 2950, 2870, 1730, 1710, 1590,
1570, 1490, 1450, 1335, 1270, 1165, 1150, 1090, 1025, 910, 715
and 670 cm~1.
''
.~ '
Example 6:
7-[(lR,2S,5R)-2-(4-Fluorobenzenesulfonylamino)-5-fluoro-
cyclopentyl] 5(Z)-heptenoic acid:
26 mg (65 ~mol) of the compound produced according to
example 5 is reacted analogously to example 2 and, after working
up and purification, 22.5 mg (58 ~mol, 89%) of the title compound
is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.5-2.2~m, llH), 2.35(t, 2H), 3.3-
3.45(m, lH), 4.7(m, lH), 5.15(s, NH), 5.25-5.5(m, 2H), 7.1-7.3(m,
2H), 7.9(m, 2H).
Example 7:
7-[(lR,2S,5R)-2-(Quinon-8-ylsulfonylamino)-5-fluoro-
r~
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
74 mg (171 ~mol) of the compound produced according to
example 7a is reacted analogously to example 1 and, after working
up and purification, 29 mg (70 ~mol, 41%) of 7-[5(S)-(quinon-8-
ylsulfonylamino)-cyclopent-1-enyl]-5(Z)-heptenoic acid methyl
ester as well as 19 mg (4~ ~mol, 25%) of the title compound are
isolated as colorless oil.
IR (Film): 3270, 3060, 2950, 2860, 1730, 1610, 1595, 1365,
1490, 1435, 1330, 1265, 1160, 1145, 835, 790, 735 and 700 cm~1.
~' , . , '
: :
36
~8~
Example 7a:
7-[(lR,2S,5S)-2-(Quinon-8-ylsulfonylamino)-5-hydroxy-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
339 mg (632 ~mol) of the compound produced according to
example 7b is reac~ed analogously to example la and, after
working up and purification, 259 mg (596 ~mol, 94%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3280, 3060, 3000, 2940, 2860, 1730,
1655, 1610, 1595, 1565, 1490, 1435, 1325, 1215, 1165, 1145, 1090,
835 and 790 cm~l.
Example 7b.
7-[(lR,2S,5S)-2-(Quinon-8-ylsulfonylamino)-5-benzoyloxy-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
250 mg (724 ~mol) of the compound produced according to
example lc is reacted analogously to example lb with quinon-8-
ylsulfonic acid chloride and, after working up and purification,
339 mg (632 ~mol, 87%) of the title compound is isolated as
colorless oil.
IR (Film): 3270, 3060, 2950, 2860, 1730, 1710, 1675, 1600,
1565, 1490, 1450, 1435, 1330, 1270, 1245, 1165, 1145, 1110, 1070,
1045, 1025, 900, 835, 790 and 715 cm~1.
.
:~ :
~8~
Example 8:
7-[(lR,2S,5R)-2-(~uinon-8-ylsulfonylamino)-5-fluoro-
/ cyclopentyl]-5(Z)-heptenoic acid:
~- 19 mg (44 ~mol) of the compound produced according to
example 7 is reacted analogously to example 2 and, after working
up and purification, 14 mg (33 ~mol, 77%) of the title compound
is isolated as colorless oil.
IR (Film): 3600-2400, 3290, 3060, 2950, 2860, 1710, 1610,
1590, 1360, 1490, 1435, 1330, 1265, 1160, 1145, 835, 790, 735 and
700 cm~1.
ExamPle-9:
7-[(lR,2S,5R)-2-(Benzenesulfonylamino)-5-fluoro-
cyclopentyl]-heptanoic acid methyl ester:
109 mg (284 ~mol) of the compound produced according to
example 18 is reacted analogously to example 1 and, after working
up and purification, 42 mg (115 ~mol, 40%) of 7-[5(S)-
(benzenesulfonylamino)-cyclopent-1-enyl]-heptanoic acid methyl
ester as well as 34 mg (88 ~mol, 31%) of the title compound are
; isolated as colorless oil.
IR (Film): 3370, 3060, 2930, 2860, 1725, 1440, 1325, 1160,
1090, 1070, 930, 755, 720 and 690 cm~.
, '
'
~8~
Example 10:
7-[(lR,2S,5R)-2-(Benzenesulfonylamino)-5-fluoro-
cyclopentyl]-heptanoic acid:
34 mg (88 ~mol) of the compound produced according to
example 9 is reactad analogously to example 2 and, after working
up and purification, 29 mg (78 ~mol, 89%) of the title compound
is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.0-1.35(m, 7H), 1.5-2.05(m, 8H),
2.35(t, 2H), 3.3-3.45(m, lH), 4.7(m, lH), 4.93(d, NH), 7.45-
7.65(m, 3H), 7.88(m, 2H).
Example 11
7-[(lR,2S,5R)-2-(4-Fluorobenzenesulfonylamino)-5-fluoro-
cyclopentyl]-heptanoic acid methyl ester:
120 mg (299 ~mol) of the compound produced according to
example 19 is reacted analogously to example 1 and, after working
up and purification, 44 mg (115 ~mol, 38%) of 7-[5(S)-(4-
fluorobenzylsulfonylamino)-cyclopenty-l-enyl]-heptanoic acid
methyl ester as well as 33 mg (82 ~mol, 27%) of the title
compound are each isolated as colorless oil.
IR (Film): 3370, 3070, 2930, 2860, 1730, 1665, 1590, 1490,
1450, 1435, 1330, 1290, 1230, 1160, 1090, 1115, 950, 925, 840 and
820 cm~1.
..
'~
'
.~' ' , ' , ' ,, .
39
~8~
Example 12:
7-[(lR,2S,5R)-2-(4-Fluorobenzenesulfonylamino)-5-fluoro-
cyclopentyl]-heptanoic acid:
33 mg (82 ~mol) of the compound produced according to
example 11 is reacted analogously to example 2 and, after working
up and purification, 31 mg (80 ~mol, 97%) of the title compound
is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.05-1.35(m, 8H), 1.5-2.05(m, 7H),
2.35(t, 2H), 3.38(m, lH), 4.7(m, lH), 4.95(d, NH), 7.2(t, 2H),
7.9(m, 2H).
Example 13: f~
7-[(lR,2S,5R)-2-(Q~in -8-,ylsulfonylamino)-5-fluoro-
~ o~
cyclopentyl]-heptanoic~ac~ ethyl ester
78 mg (179 ~mol) of the compound produced according to
example 20 is reacted analogously to example 1 and, after working
up and purification, 24 mg (57 ~mol, 32%) of 7-[5(S)-(quinon~8-
ylsulfonylamino)-cyclopent-1-enyl]-heptanoic acid methyl ester as
well as 19 mg (43 ~mol, 24%) of the title compound are isolated
as colorless oil.
IR (Film): 3270, 3060, 2940, 2850, 1730, 1610, 1595, 1565,
1490, 1435, 1330, 1160, 1140, 835, 795, 735 and 680 cm-1.
- . . ,
,,, ' . ': ~
81~
Example 14:
7-[(lR,2S,5R)-2-(Quinon-8-ylsulfonylamino)-5-fluoro-
cyclopentyl]-heptanoic acid:
32 mg (73 ~mol) of the compound produced according to
example 13 is reacted analogously to example 2 and, after working
up and purification, 26 mg (62 ~mol, 84%) of the title compound
is isolated as colorless oil.
IR (Film): 3600-2500, 3400, 3280, 2920, 2850, 1705, 1600,
1510, 1460, 1290, 1085, 1055, 1030 and 735 cm~1.
Example 15:
7-[(lR,2S,5R)-2-(4-toluenesulfonylamino)-5-hydroxy-
cyclopentyl]-5~Z)-heptenoic acid methyl ester:
The solution of 241 mg (612 ~mol) of the compound produced
according to example 15a is dissolved in 8 ml of anhydrous
methanol, cooled under an atmosphere of dry argon to -30C and
mixed in portions with a total of 69 mg of sodium borohydride.
It was allowed to react for 30 more minutes, excess reduction
agent is decomposed by addition of 120 ~1 of acetic acid, mixed
with water and extracted several times with dichloromethane. It
is washed with water, saturated sodium chloride solution and
dried on magnesium sulfate. The residue ob~ained after
filtration and removal of the solvent is purified by
chromatography on an 12 analytic thin-layer slabs. A mixture of
n-hexane and ethyl acetate is used as mobile solvent,
diethylether is used as eluant. 108 mg (273 ~mol, 45%) of the
title compound is isolated as polar component, as well as 48 mg
41
(121 ~mol, 20%) o~ the title compound from example la as nonpolar
component.
IR (Film): 3600-3200, 2950, 2920, 2850, 1730, 1600, 1450,
1325, 1155, 1090, 895, 815, 735 and 670 cm~1.
Example 15a:
7-[(lR,2S)-2-(4-toluenesulfonylamino)-5-oxo-cyclopentyl]-
5(Z)-heptenoic acid methyl ester.
245 mg (691 ~mol) of the compound produced according to
example la is oxidized analogously to example lf and, after
working up, 241 mg (612 ~mol, 99%) of the title compound is
isolated as pale yellow oil.
IR (Film): 3360, 2950, 2920, 2850, 1735, 1600, 1450, 1325,
1155, 1090, 900, 815, 735 and 665 cm~1.
Example 16:
7-[(lR,2S,5S)-2-(4-toluenesulfonylamino)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
108 mg (273 ~mol) of the compound produced according to
example 15 is reacted analogously to example 1 and, after working
up and purification, 9 mg (24 ~mol, 9%) of 7-[5(S)-(4-
toluenesulfonylamino)-cyclopent-l enyl]-5(Z)-heptenoic acid
methyl ester as well as 23 mg (60 ~mol, 22%) of the title
compound are each isolated as colorless oil.
IR (Film): 3250, 3010, 2960, 2930, 2870, 1730, 1600, 1~50,
1380, 1325, 1305, 1290, 1240, 1160, 1035, 925, 910, 815 and 665
cm .
'~ ,, , '
42
Exam~le 17:
7-[(lR,2S,5S~-2-(4-Toluenesulfonylamino)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
30 mg (76 ~mol) of the compound produced according to
example 16 is saponified analogously to example 2 and, after
working up and purification, 14 mg (37 ~mol, 48%) of the title
compound is isolated as colorless oil.
IR (Film): 3700-2400, 3260, 3010, 2960, 2930, 2870, 1710,
1600, 1450, 1405, 1380, 1325, 1305, 1290, 1240, 1160, 1095, 925,
910, 815, 665, 620 and 550 cm~1.
Exam~le 18:
7-[~lR,2S,5S)-2-(Benzenesulfonylamino)-5-hydroxy-
cyclopentyl]-heptanoic acid methyl ester:
132 mg (346 ~mol) of the compound produced according to
example 3a is dissolved in 5 ml of ethyl acetate, is mixed with
50 mg Pd/C (5%) and hydrogenated at 1 atm, until the
theoretically calculated amount of hydrogen has been taken up.
It is filtered, washed again and concentrated by evaporation.
109 mg (284 ~mol, 82%) of the title compound is isolated as
colorless oil.
IR (Film): 3600-3200, 3260, 2930, 2850, 1725, 1445, 1320,
1265, 1160, 1095, 1020 and 735 cm-1.
-
.
'
43
Example 19:
7-[(lR,2S,5S)-2-(4-Fluorobenzenesulfonylamino)-5-hydroxy-
cyclopentyl]-heptenoic acid methyl ester:
141 mg (355 ~mol) of the compound produced according to
example 5a is hydrogenated analogously to example 18 and, after
working up, 120 mg (300 ~mol, 84%) of the title compound is
isolated as colorless oil.
IR (Film~: 3270, 2930, 2850, 1730, 1660, 1600, 1445, 1320,
1260, 1160, 1095, 1020, 925, 840 and 820 cm~1.
~,
Example 20: ~ ~
7-[(lR,2S,5S)-2-(Quin~n-8-yl~sulfonylamino)-5-hydroxy-
e~clopentyl]-heptanoic acid méthyl ester:
149 mg (344 ~mol) of the compound produced according to
example 7a is hydrogenated analogously to example 18 and, after
working up, 142 mg (327 ~mol, 95%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-3200, 3520, 3410, 3280, 2940, 2850, 1725,
1665, 1595, 1490, 1455, 1~30, 1320, 1160, 1140, 1020, 890, 840,
790, 735 and 675 cm1.
Example 21:
7-[(lR,2S~5S)-2-(Quingn-8-ylsulfonylamino)-5-hydr
- ~--j,l.-,.. ...
cyclopentyl]-heptanoic acid:
30 mg (69 ~mol) of the compound produced according to
example 20 is saponified analogously to example 2 and, after
: ~ ,.
44
'~ 0 ~
working up and purification, 25 mg (59 ~mol, 86%) of the title
compound is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.0-l.9(m, 16H), 2.32(t, 2H), 3.4(m,
lH), 4.12(m, lH), 6.27(d, NH), 7.57(dt, lH), 7.66(t, lH),
8.06(dd, lH), 8.3(dd, lH), 8.43(dd, lH), 9.03(dd, lH).
Example 22:
7-[(lR,2S,5S)-2-(4-Toluenesulfonylamino)-5-hydroxy-
cyclopentyl]-5(Z)-heptenoic acid:
47 mg (119 ~mol) of the compound produced according to
example lb is saponified analogously to example 2 and, after
working up and purification, 41 mg (107 ~mol, 90%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3470, 3260, 3000, 2940, 2870, 1710,
1600, 1445, 1320, 1160, 1095, 910, 810, 670, 570 and 550 cm~1.
Example 23:
7-[(lR,2S,5S)-2-(Quin~n-8-ylsulfonylamino)-5-hydroxy-
~ .
cyclopentyl]-5(Z~-heptenoic acid:
19 mg (44 ~mol) of the compound produced according to
example 7b is saponified analogously to example 2 and, after
working up and purification, 16 mg (38 ~mol, 85%) of the title
compound is isolated as waxy solid.
1H-NMR (CDCl3): ~ = 1.0-2.35(m, 14H), 3.43(m, lH), 4.1(m,
lH), 5.15-5.35(m, 2H), 6 47~d, NH), 7.52(dd, lH), 7.6~(t, lH),
8.04(dd, lH), 8.27(dd, lH), 8.43(dd, lH), 9.03(dd, lH).
~8~
Example 24:
7-[(lR,2S,3R~,5R)-2-Benzyloxymethyl-3-phenyl-5-fluoro-
cyclopentyl]-5(E/Z)-heptenoic acid:
33 mg (99 ~mol) of the compound produced according to
example 24a is mixed with 0.4 ml of a 5~ KOH solution, 247 ~1 of
benzyl chloride, 4 mg of tetrabutylammoniumhydrogen sulfate and
stirred intensively for 18 hours at 23C. It is poured on ice
water, extracted several times with dichloromethane, the organic
phase is dried on magnesium sulfate and excess benzyl chloride is
removed by chromatography on a 4 analytic thin-layer slabs. A
mixture of n-hexane and ethyl acetate is used as mobile solvent,
chloroform is used as eluant. The resulting residue is
saponified analogously to example 2 and, after working up and
purification, 17 mg (41 ~mol, 42%) of the title compound is
isolated as colorless oil.
IR tFilm): 3600-2~00, 3060, 3030, 2930, 2850, 1710, 1600,
1495, 1455, 1360, 1240, 1210, 1100, 970, 755, 7~5 and 700 cm~1.
Example 24a:
7-~(lR,2S,3RS,5R)-2-Hydroxymethyl-3-phenyl-5-fluoro-
cyclopentyl]-5(E/Z)-heptenoic acid methyl ester:
305 mg (532 ~mol) of the compound produced according to
example 24b is reacted analogously to example lg and, after
working up and purification, 176 mg (526 ~mol, 99%) of the title
compound is isolated as colorless oil.
IR (Film)- 3600-3200, 3030, 2920, 2850, 1730, 1450, 1435,
1370, 1150, 1045, 755 and 700 cm~1.
. . ., :.
-
, . :
:
~881~
Example 24b:
7-[(lR,2Sl3RS,5R)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
phenyl-5-fluoro-cyclopentyl]-5(E/Z)-heptenoic acid methyl ester:
742 mg (1.30 mmol) of the compound produced according to
example 24c is reacted analogously to example 1 andl after
working up and purification, 294 mg (532 ~mol, 41%) of 7-
[(4RS,5S)-4-phenyl-5-(tert.-butyldiphenylsilyloxymethyl)-
cyclopent-1-enyl]-5(E/Z)-heptenoic acid methyl ester as well as
337 mg (588 ~mol, 45%) of the title compound are isolated as
colorless oil.
IR (Film): 3070, 3030, 2960l 2930l 2860l 1735l 1600l 1590
1425, 1110, 820, 740 and 700 cm-1.
Example 24c:
7-[(lR,2Sl3RSl5S)~2-(tert.-Butyldiphenylsilyloxymethyl)-3-
phenyl-5-hydroxy-cyclopentyl]-5(E/Z)-heptenoic acid methyl ester
(A) and 7-[(lRl2S,3RS,5R)-2-(tert.-butyldiphenylsilylo~ymethyl)-
3-phenyl-S hydroxy-cyclopentyl]-5(E/Z)-heptenoic acid methyl
ester (B) :
1.97 g (3.47 mmol) of the compound produced according to
example 24d is reacted analogously to example 15 and, after
working up and purification, 742 mg (1.30 mmol, 37%) of title
compound (A) as well as 925 mg (1.62 mmol, 47%) of title compound
(B) are each isolated as colorless oil.
IR (Film) of A: 3600-3200, 3070, 3020, 2950, 2920, 2850,
1735, 1600, 1585, 1450, 1425, 1110, 820, 760, 740 and 700 cm-1.
::
~: ' ' . .. ` -
,
47
~881lS.. ~ '
IR (Film) of B: 3600-3200, 3060, 3020, 2950, 2920, 2850,
1730, 1600, 1590, 1450, 1425, 1265, 1110, 1060, 820, 740 and 700
cm~1 ~
Example 24d:
7-[(lR,2S,3RS)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
phenyl-5-oxo-cyclopentyl]-5(E/Z)-heptenoic acid methyl ester:
The solution of 11.82 g (24.0 mmol) of the compound produced
according to example 24e in 120 ml of anhydrous tetrahydrofuran
is mixed with 500 mg of copper(II)acetate, and cooled under an
atmosphere of dry argon to -78C. 3.04 ml of
trimethylchlorosilane and then 12.8 ml of a 3M solution of
phenylmagnesiumbromide in diethyl ether are added. After 45
minutes it is poured in a saturated ammonium chloride solution
and extracted several times with dichloromethane. The combined
organic extracts are dried on magnesium sulfate and the residue
obtained after removal o~ the solvent is purified by
chromatography on about 200 ml of fine silica gel. A gradient
system of n-hexane and ethyl acetate is used as mobile sol~ent.
9.78 g (17,2 mmol, 72%) of the title compound is isolated as
colorless oil.
IR (Film): 3070, 3030, 2950, 2930, 2850, 1735, 1600, 1465,
1450, 1425, 1110, 1055, 820, 780, 740 and 700 cm~1.
:
, .
48
Exam~le 24e:
7-[(lR,2S)-2-(tert.-Butyldiphenylsilyloxymethyl)-5-oxo-
cyclopent-3-enyl]-5(E/Z)-heptenoic acid methyl ester:
The solution of 5.84 g (11.5 mmol) of the compound produced
according to example 24f in 52 ml anhydrous pyridine is cooled
under an atmosphere of dry argon at 3C, 2.17 ml of
methanesulfonic acid chloride i5 instilled and stirred for 2
hours more at 3C. It is mixed with ice, diluted with water and
extracted several times with dichloromethane. The combined
organic extracts are dried on magnesium sulfate and the residue
obtained after removal of the solvent is purified by
chromatography on about 200 ml of fine silica gel. A gradient
system of n-hexane and ethyl acetate is used as mobile solvent.
4.93 g (10.0 mmol, 87%) of the title compound is isolated as
colorless oil.
IR (Film): 3070, 3040, 2950, 2930, 2850, 1735, 1705, 1585,
1425, 1110, 820, 740 and 700 cm~1.
Example 24f:
7-[(lR,2S,3R)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
(tetrayhydropyran-2-yloxy)-5-oxo-cyclopentyl]-5(E/Z)-heptenoic
acid methyl ester:
11.9 g (20.0 mmol) of the compound produced according to
example 24g is reacted analogously to example lf and, after
working up and purification, 9.39 g ~15.8 mmol, 79%) of the title
compound is isolated as colorless oil.
..
.
,, . :
, . .:
"~
49
~8~
IR (Film): 3070, 3040, 2940, 2850, 1740, 1590, 1425, 1110,
1075, 1030, 970, 820, 740 and 700 cm~1.
Example 24q:
7-[tlR,2S,3R,5S)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
(tetrayhydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(E/Z)-
heptenoic acid methyl ester:
22.4 g (47.8 mmol) of (lS,3RS,5R,6S,7R)-7-(tetrahydropyran-
2-yloxy)-6-(tert.-butyldiphenylsilyloxymethyl)-2-
oxabicyclo[3.3.0]-octan 3-ol is reacted analogously to example lj
by use of KOH-containing potassium-tert.-butanolate and, after
working up and esterification with an ethereal solution of
diazomethane, after chromatographic purification on about 1.3 l
of fine silica gel with a gradient system of n-hexane and ethyl
acetate, 21.6 g (36.3 mmol, 76~) of the title compound is
isolated as colorless oil.
IR (Film~: 3600-3200, 3070, 3040, 2940, 2850, 1730, 1595,
1425, 1110, 1075, 1025, 970, 820, 740 and 700 cm~l.
E~ample 25:
7-[(lR,2S,3RS,5R)-2-(4-Cyanobenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentylJ-5(E/Z)-heptenoic acid:
28 mg (84 ~mol) of the compound produced according to
example 24a is reacted analogously to example 24 by using 4-
cyanobenæylbromide and, a~ter working up and purification, 14 mg
(32 ~mol, 38~) of the title compound is isolated as colorless
oil.
- ~ . : , . . . :~
- . : ; . . ...
.
: ,. .
.
- 2~8gl61
IR (Film): 3600-2400, 3060, 3015, 1930, 2860, 2230, 1705,
1600, 1455, 1435, 1360, 1290, 1240, 1150, 1110, 1090, 970, 795,
755, 700 and 685 cm~1.
Example 26:
7-[(lR,2S,3RS,5R)-2-(3-Methylbenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(E/Z)-heptenoic acid:
27 mg (81 ~mol) of the compound produced according to
example 24a is reacted analogously to example 24 by using 3-
methylbenz~lbromide and, after working up and purification, 23 mg
(54 ~mol, 67~) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 2930, 2860, 1705, 1600,
1450, 1360, 1245, 1160, 1105, 1090, 970, 780, 755 and 700 cm~1.
Example 27:
7-[(lR,2S,3RS,5R)-2-(3,5-Bis-
trifluoromethylbenzyloxymethyl)-3-phenyl-5-fluoro-cyclopentyl]-
5(EJZ)-heptenoic acid:
: 29 mg (87 ~mol) of the compound produced according to
example 24a is reacted analogously to example 24 by using 3,5-
his(trifluoromethyl)benzylbromide and, a~ter working up and
purification, 28 mg (51 ~mol, 59%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 2930, 2870, 1710, 1625,
1600, 1455, 1380, 1355, 1280, 1175, 1135, 970, 885, 840, 760, 700
and 680 cm~1.
:. . : . ~ ~ .
,~, .
- ~ ;
. ~
,' . :
51
~8~
~xample 28:
7-[(lR,2S,3RS,5R)-2-(1-Naphthylme~hoxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(E/Z)-heptenoic acid:
29 mg (87 ~mol) of the compound produced according to
example 24a is reacted analogously to example 24 by using l-
bromomethylnaphtalin and, after working up and purification, 26
mg (56 ~mol, 65%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2930, 2860, 1705,
1600, 1455, 1240, 1165, 1100, 970, 803, 790, 775, 755 and 700
cm .
Example 29:
7-[(lS,2R,3RS,5S)-2-[2-(4-Fluorophenoxy)-ethoxymethyl]-3-
phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
43 mg (129 ~mol) of the compound produced according to
example 29a is reacted analogously to example 24 by using 1-
bromo-2-(4-fluorophenoxy)-ethane and, after working up and
puri~ication, 12 mg (26 ~mol, 20%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2930, 2870, 1710,
1600, 1505, 1455, 1250, 1210, 1130, 100, 1050, 830, 760, 745 and
700 cm~1.
:.
.~ , . . .
. . :
': ' '
52
Example 29a:
7-[(lS,2R,3RS,5S~-2-Hydroxymethyl-3-phenyl-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
2.36 g (4.12 mmol) of the compound produced according to
example 29b is reacted analogously to example lg and, after
working up and purification, 1.24 g (3.71 mmol, 90%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3020, 2920, 2850, 1730, 1600, 1450,
1435, 1330, 1150, 1045, 965, 755 and 700 cm~1.
Example 29b:
7-[(lS,2R,3RS,5S)-2-(tert.-Butyldipenylsilyloxymethyl)-3-
phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
Starting from (lR,3RS,5S,6R,7S)-7-(tetrahydropyran-2-yloxy)-
6-(tert.-butyldiphenylsilyloxymethyl)-2-oxabicyclo[3.3.0]octa~n-3-
ol is produced analogously to examples 24b to 24g, 7-[(4RS,5R)-4-
phenyl-5-(tert.-butyldiphenylsilyloxymethyl)-cyclopent-1-enyl]-
5(Z)-hepenoic acid methyl ester as well as the title compound.
By use of freshly sublimated-potassium-tert.-butanolate (cf.
example 24g) in each case only the Z isomer is obtained with
respect to the double bond in 5.
Example 30:
7-t(lS,2R,3RS,5S)-2-Benzyloxymethyl-3-phenyl-5-fluoro-cyclo-
pentyl]-5(Z)-heptenoic acid:
46 mg (129 ~mol) of the compound produced according to
example 29a is reacted analogously to example 24 and, after
, ~. . ~.
, , ...................................... ,: ~ . : ,
.
working up and purification, 25 mg (61 ~mol, 47~) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2930, 2860, 1710,
1600, 1495, 1455, 1360, 1240, 1210, 1100, 755, 735 and 700 cm~1.
Example 31:
7-[(lS,2R,3RS,5S)-2-(4-Cyanobenzyloxymethyl)-3-phenyl-5-
fluoro-cyclo-pentyl~-5(Z)-heptenoic acid:
43 mg (129 ~mol) of the compound produced according to
example 29a is reacted analogously to example 24 by using 4-
cyanobenzylbromide and, after working up and purification, 31 mg
(71 ~mol, 55%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 2940, 2860, 2230, 1710,
1610, 1450, 1235, 1220, 1130, 1100, 820, 760 and 700 cm~~.
Example 32:
7-[(lS,2R,3RS,5S)-2-(3-Methylbenzyloxymethyl)-3-phenyl-5-
fluoro-cyclo-pentyl]-5(Z)-heptenoic acid:
43 mg (129 ~mol) of the compound produced according to
example 29a is reacted analogously to example 24 by using 3-
methylbenæylbromide and, after working up and purification, 25 mg
(61 ~mol, 47%) of the titl~ compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2940, 2860, 1710,
1600, 1495, 1455, 1360, 1250, 1160, 1105, 1090, 780, 760 and
700 cm~1.
.
,. ., . . ~ . . ~
' ' . :, ' "
,
,, : .
54
"
2~81 61
Example 33:
7-[(lS,2R,3RS,5S)~2-(3-Cyanobenzyloxymethyl)-3-phenyl-5-
fluoro-cyclo-pentyl]-5(Z)-heptenoic acid:
43 mg (129 ~mol) of the compound produced according to
example 29a is reacted analogously to example 24 by using 3-
cyanobenzylbromide and, after working up and purification, 36 mg
(83 ~mol, 64%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2930, 2860, 2230,
1710, 1600, 1495, 1455, 1435, 1360, 1240, 1150, 1105, 1090, 795,
760, 700 and 685 cm~1.
Example 34:
7-[(lR,2S,3RS,5S)-2-[2-(4-Fluorophenoxy)-ethoxymethyl]-3-
phenyl-5-fluoro-cyclopentyl]-5(E/Z)-heptenoic acid:
33 mg (99 ~mol) of the compound produced according to
example 34b is reacted analogously to example 24 by using 1-
bromo-2-(4-fluorophenoxy)-ethane and, after working up and
purification, 17 mg (37 ~mol, 37%) of the title compound is
isolat~d as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 3000, 2930, 2870, 2850,
1710, 1600, 1505, 1455, 1250, 1210, 1140, 995, 1050, 825, 760,
745 and 700 cm~1.
: ~ ~
'; ' ~ :- ,, ,~
. ~ , . . .
~8~1
Example 34a:
7-[(lR,2S,3RS,5S)-2-Hydroxymethyl-3-phenyl-5-fluoro-
cyclopentyl]-5(E/Z)-heptenoic acid methyl ester:
586 mg (1.02 mmol) of the compound produced according to
example 34b is reacted analogously to example lg and, after
working up and purification, 335 mg (1.00 mmol, 98~) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3030, 3000, 2940, 2870, 1735,
1600, 1490, 1450, 1435, 1245, 1175, 1065, 1045, 1030, 945, 865,
760 and 700 cm~1.
Example 34b:
7-[(lR,2S,3RS,5S)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
phenyl-5-fluoro-cyclopentyl] 5(E/Z)-heptenoic acid methyl ester:
g25 mg (1.62 mmol) of polar compound B produced accordin~ to
example 24c is reacted analogously to example 1 and, after
working up and purification, 98 mg (165 ~mol, 10%) of 7-[4RS,5S)-
4-phenyl-5-(tert.-butyldiphenylsilyloxymethyl)-cyclopent-1-enyl]-
5(E/Z)-heptenoic acid methyl ester as well as 586 mg ~1.04 mmol,
64~) of the title compound are isolated as colorless oil.
IR (Film): 3070, 3020, 3000, 2g50, 2930, 2850, 1735, 1600,
1585, 1490, 1465, 1450, 1425, 1360, 1110, 1005, 965, 870, 760,
740 and 700 cm~1.
' - ' ': ' :
. .. .
. ~ ' ' ' ' .
56
~2~8~1
Example 35:
7-[(lR,2S,3RS,5S)-2-Benzyloxymethyl-3 phenyl-5-fluoro-
cyclopentyl]-5(E/Z)-heptenoic acid:
31 mg (93 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using benzyl
chloride and, after working up and purification, 14 mg (34 ~mol,
37%) of the title compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3020, 2930, 2850, 1705, 1600,
1490, 1465, 1100, 1070, 1030, 950, 760, 735 and 700 cm~1.
Example 36:
7-[(lR,2S,3RS,5S) 2-(3,5-Bis~
trifluoromethylbenzyloxymethyl)-3-phenyl-5-fluoro-cyclopentyl]-
5(E/Z)-heptenoic acid:
25 mg t75 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using 3,5-
bis-(trifluoromethyl)-benzyl bromide and, after working up and
purification, 27 mg (49 ~mol, 66%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-2400, 3070, 3030, 2940, 2860, 1710, 1610,
1600, 1360, 1280, 1180, 1140, 700 and 680 cml.
"
Exam~le 37:
7-[(lR,2S,3RS,5S)-2~ Naphthylmethoxymethyl)-3-phenyl-5-
~:; fluoro-cyclopentyl]-5(E/Z)-heptenoic acid:
23 mg (69 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using 1-
~'
: . .
bromomethylnaphthalene and, after working up and purification, 13
mg (28 ~mol, 41%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3020, 3000, 2930, 2850, 1710,
1600, 1505, 1455, 1250, 1220, 1100, 950, 800, 790, 780, 760 and
700 cm~1.
Example 38:
7-[(lR,2S,3RS,5S)-2-(4-Cyanobenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(E/Z)-heptenoic acid:
20 mg (60 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using 4-
cyanobenzylbromide and, after working up and purificatlon, 10 mg
(24 ~mol, 44%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 2930, 2850, 2220, 1710,
1605, 1505, 1495, 1455, 1415, 1130, 1100, 950, 820, 760 and 700
cm-1
Example 39:
7-[(lR,2S,3RS,5S)-2-(3-Methylbenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(E/Z)-heptenoic acid:
20 mg (60 ~mol) of the compound produced according to
example 34a is rea~ted analogously to example 24 by using 3-
methylbenzylbromide and, after working up and purification, 16 m~
(38 ~mol, 63%) of the title compound is isolated as colorless
oil.
, , ' :' ,,
-i .
.~ , ', " ~ .
~: ; ' , `:
58
IR (Film): 3600-2400, 3060, 3030, 2930, 2850, 1710, 1600,
1495, 1455, 1435, 1240, 1155, 1100, 1090, 950, 760 and 700 cm~1.
Example 40:
7-[(lR,2S,3RS,5S)-2-(3-Cyanobenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(E/Z)-heptenoic acid:
20 mg (60 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using 3-
cyanobenzylbromide and, after working up and purification, 17 mg
(39 ~mol, 65%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 2930, 2860, 2230, 1710,
1600, 1585, 1455, 1435, 1355, 1285, 1200, 1150, 1105, 1085, 795,
760, 700 and 685 cm~1.
Example 41:
7-[(lS,2R,3RS,5R)-2-[2-(4-Fluorophenoxy)-ethoxymethyl]-3-
phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
43 mg (128 ~mol) of the compound produced according to
example 41a is reacted analogously to example 24 by using 4-
fluorophenoxyethylbromide and, after working up and purification,
12 mg (26 ~mol, 20%) of the title compound is isolated as
colorless oil.
IR (Film): 3600-2400, 3070, 3030, 2930, 2870, 1710, 1600,
1505, 1455, 1250, 1210, 1140, 1100, 1055, 830, 760, 750 and
700 cm~1.
:', ' ''. ', ~
, , ,
59
'~ ~ 8 ~
Example 4la:
7-[(lS,2R,3RS,5R)-2-Hydroxymethyl-3-phenyl-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
3.63 g (6.34 mmol) of the compound produced acccrding to
example 41b is reacted analogously to example lg and, after
working up and purification, 2.05 g (2.04 mmol, 95%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3030, 3000, 2940, 2870, 1735,
1600, 1490, 1450, 1435, 1245, ~220, 1170, 1150, 1070, 1045, 945,
870, 760 and 700 cm-1.
Example 4lb:
7-[(lS,2R,3RS,5R)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
phenyl-5-fluoro cyclopentyl]-5(Z)-heptenoic acid:
6.15 g (10.8 mmol) of 7-[(lS,2R,3RS,5S)-2-(tert.-
butyldiphenylsilyloxymethyl)-3-phenyl-5-hydroxy-cyclopentyl]-
5(Z)-heptenoic acid methyl ester that is obtained in the course
of the synthesis described in example 29b analogously to example
24c is reacted analogously to example 1 and, after working up and
purification, 610 mg (1.1 mmol, 10%) of 7-[(4RS,5R)-4-phenyl-5-
(tert.-butyldiphenylsilyloxymethyl)-cyclo-pent-1-enyl]-5~Z)-
heptenoic acid methyl ester as well as 3.63 g (6.34 mmol, 59%) of
the title compound are isolated as colorl~ss oil.
IR (Film): 3070, 3020, 2950, 2930, 2850, 1735, 1600, 1585,
1425, 1110, 820, 760, 740 and 700 cm-1.
Example 42:
7-[(lS,2R,3RS,5R)-2-Benzyloxymethyl-3-phenyl-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
43 mg (129 ~mol) of the compound produced according to
example 41a is reacted analogously to example 24 by using benzyl
chloride and, after working up and purification, 2~ mg (56 ~mol,
43%) of the title compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 2940, 2860, 1710, 1600,
1495, 1450, 1100, 760, 740 and 700 cm~1.
Example 43:
7~ r ( lS,2R,3RS,5R)-2-(4-Cyanobenzyloxymethyl)-3-phenyl 5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid:
43 mg (129 ~mol) of the compound produced according to
example 41a is reacted analogously to example 24 by u~ing 4-
cyanobenzylbromide and, after worki~g up and purification, 21 mg
(48 ~mol, 37%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 2940, 2860, 2230, 1710,
1610, 1455, 1275, 1130, 1100, 820, 780, and 700 cm~1.
Example 44:
7-[(lS,2R,3RS,5R)-2-(3-Methylbenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid:
43 mg (125 ~mol) of the compound produced according to
example 41a is reacted analogously to example 24 by using 3-
methylbenzylbromide and, after working up and purification, 21 mg
, , ''`". ' ;. ~ :
,
61
8 ~
(49 ~mol, 38%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 2930, 2850, 1710, 1600,
1455, 1240, 1160, 1100, 950, 780, 760 and 700 cm1.
Example 45:
7-[(lS,2R,3RS,5R)-2-(3-Cyanobenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid:
43 mg (129 ~mol) of the compound produced according to
example 41a is reacted analogously to example 24 by using 3-
cyanobenzylbromide and, after working up and purification, 41 mg
(94 ~mol, 73%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3060, 3030, 2940, 2860, 2230, 1710,
1600, 1580, 1450, 143~, 1360, 1240, 1150, 1110, 1090, 950, 795,
760, 700 and 690 cm~l.
Example 46:
7-[(lR,2S,3RS,5S)-2-(2,4-Bis-
trifluoromethylbenzyloxymethyl~-3 phenyl-5-fluoro-cyclopentyl]-
5(E/Z)-heptenoic acid:
20 mg (60 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using 2,4-
bis-(trifluoromethyl)benzylbromide and, after working up and
purification, 20 mg (37 ~mol, 61~) of the title compound is
isolated as colorless oil.
'
, : '
62
IR (Film): 3600-2400, 3030, 3000, 2920, 2850, 1710, 1630,
1600, 1455, 1345, 1275, 1170, 1130, 1055, 910, 860, 840, 760 and
700 cm~1.
Example 47:
7-[(lR,2S,3RS,5S)-2-(4-Methylbenzyloxymethyl)-3-phenyl-5-
fluoro-cyclopentyl~-5(E/Z)-heptenoic acid:
20 mg (60 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using 4-
methylbenzylbromide and, after working up and purification, 15 mg
(35 ~mol, 59%) of the title compound is isolated as colorless
oil.
IR (Film): 3600-2400, 3020, 3000, 2920, 2850, 1705, 1600,
1455, 1100, 1085, 1070, 950, 800, 760 and 700 cm~1.
Example 48:
7-[(lR,2S,3RS,5S)-2-(2-Naphthylmethoxymethyl)-3-phenyl-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid (A) and
7-[(lR,2S,3RS,5S)-2-(2-naphthylmethoxymethyl)-3-phenyl-5-fluoro-
cyclopentyl]-5~E)-heptenoic acid (B):
20 mg (60 ~mol) of the compound produced according to
example 34a is reacted analogously to example 24 by using 2-
bromomethylnaphthalane and, after working up and purification, 15
mg (33 ~mol, 50~) of title compound A as well as 7 mg (~4 ~mol,
21%) of title compound B are each isolated as colorless oil.
63
~881~
IR (Film) of A: 3600-2400, 3060, 3020, 2930, 2850, 1705,
1600, 1490, 1455, 1435, 1~10, 1345, 1270, 1240, 1125, 1100, 950,
855, 820, 755 and 700 cm~1.
IR (Film) of B: 3600-2400, 3060, 3030, 2930, 2850, 1705,
1600, 1455, 1435, 1410, 1345, 1125, 1100, 1090, 1070, 970, 950,
855, 815, 755 and 700 cm~1.
Example 49:
7-[(lR,2S,3RS,5R)-2-[2-(4-Fluorophenoxy)ethoxymethyl]-3-
phenyl-5-fluoro-cyclopentyl]-5(E/Z)-heptenoic acido
31 mg (93 ~mol) of the compound produced according to
example 24a is reacted analoyously to example 24 by using 1-
bromo-2-(4-fluorophenoxy)-ethane and, after working up and
purification, 15 mg (32 ~mol, 35%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 2930, 2870, 1710, 1600,
1505, 1455, 1250, 1210, 1130, 825, 760, 740 and 700 cm~1.
Exam~le 50:
7-t(lR,2S,3RS,5S)-2-[(3RS,4S)-3-Hydroxy-4-methyl-non-l(E)~
en-6-inyl]-3-phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid
methyl ester (A) and 7-[(lR,2S,3RS,55)-2-[~3RS,4S)-3-hydroxy-4-
methyl-non-l(E)-en-6-inyl]-3-phenyl-5-fluoro-cyclopentyl]-5(E)-
heptenoic acid methyl ester (B):
41 mg (91 ~mol) of the compound produced according to
example 50a is reacted analogously to example 15 and, after
working up and puri~ication, 21 mg (46 ~mol, 51%) of title
64
compound A as well as 15 mg (33 ~mol, 36%) of title compound (B)
are each isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3030, 2970, 2930, 2850, 1735,
1600, 1495, 1450, 1240, 1125, 970, 760 and 700 cm-1.
Example 50a:
7-[(lR,2S,3RS,5S)-2-[3-oxo-4S-methyl-non-l(E)-en-6-inyl]-3-
phenyl-5-fluoro-cyclopentyl]-5(E/Z)-heptenoic acid methyl ester:
The solution of 45 mg of dimethyl-(2-oxo-3S-methyl-oct-5-
inyl)-phosphonate in 330 ~1 of tetrahydrofuran is instilled in a
suspension of 8 mg of sodium hydride dispersion (55%) in 410 ~1
of anhydrous tetrahydrofuran under an atmosphere of dry argon and
stirred for 20 minutes at 23C. It is cooled to -30C, the
solution of 70 mg (164 ~mol) of the aldehyde produced according
to example 50b is instilled in 490 ~1 of tetrahydrofuran and ~
stirred for 14 hours at -15C. It is mixed with acetic acid and
extracted several times with diethylether. The combined organic
extracts are washed with dilute sodium bicarbonate solution,
dried on magnesium sulfate and the residue obtained after removal
of the solvent is purified by chromato~raphy on a 5 analytic
thin-layer slabs. A mixture of n-hexane and ethyl acetate is
used as mobile solvent, diethyl ether is used as eluant. 41 mg
(91 ~mol, 55%) of the title compound is isolated as colorless
oil.
IR (Film): 3030, 3000, 2970, 2930, 2870, 1735, 1690, 1665,
1625, 1450, 1435, 1170, 1040, 980, 760 and 700 cm~1.
-
: .
-
:. :` ` : `
.. ..
` I : ' ` '` `
Examp~e 50b:
7-[(lR,2S,3RS,5S)-2-formyl-3-phenyl-5-fluoro-cyclopentyl]- -
5~E/Z)-heptenoic acid methyl ester:
The solution of 63 ~l of dimethylsulfoxide in 280 ~l of
dichloromethane is instilled in a solution of 34 ~1 of freshly
distilled oxalyl chloride in 690 ~l anhydrous dichloromethane
introduced under an atmosphere of dry argon at -60C, allowed to
react for 15 minutes and then mixed with the solution of 114 mg
(341 ~mol) of the alcohol produced according to example 34a in
690 ~l of dichloromethane~ It is allowed to react for 4 hours,
mixed with 107 ~l of triethylamine, poured in ice water and
extracted several times with dichloromethane. The combined
organic extracts are dried on magnesium sulfate. The residue
obtained after filtration and removal of the solvent is further
reacted without purification.
Example 51:
7-[(lR,2S,3RS,5S)-2-[3R or 3S,4S)-3-Hydroxy-4-methyl-non-
l(E)-en-6-inyl]-3-phenyl-5-fluoro-cyclopentyl]-5(E)-heptenoic
acid (A) and 7-[(lR,2S,3R5,5S)-2-[35 or 3R,4S)-3-hydroxy-4-
methyl-non-l(E)-en-6-inyl]-3-phenyl-5-fluoro-cyclopentyl]-5(E)-
heptenoic acid methyl ester (B):
15 mg (33 ~mol~ of the compound produced according to
example 50 is reacted analogously to example 2 and, after working
up and purification, 5 mg (11 ~mol, 33%) of a nonpolar component,
to which the structure of title compound A was assigned, as well
as 5.3 mg (12 ~msl, 36%) of a polar component, to which the
'
66
structure of title compound B was assigned, are each isolated as
colorless oil.
IR (Film) of A: 3600-2400, 3060, 3030, 2970, 2930, 2850,
1710, 1600, 1495, 1455, 1435, 1240, 1130, 970, 760 and 700 cm~1.
IR (Film) of B: 3600-2400, 3060, 3030, 2970, 2930, 1710,
1600, 1495, 1455, 1435, 1385, 1240, 1070, 1030, 1015, 970, 760
and 700 cm-~.
Example 52:
7-[(lR,2S,3RS,5S)-2-[(3RS,4S)-3-Hydroxy-4-methyl-non-l(E)-
en-6-inyl]-3~phenyl-5-fluoro-cyclopsntyl]-5(E/Z)-heptenoic acid:
21 mg (46 ~mol) of compound A produced according to example
50 is reacted analogously to example 2 and, after working up and
purification, 17 m~ (39 ~mol, 84%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-2400, 3080, 3060, 3030, 3000, 2970, 2930,
2870, 1710, 1600, 1495, 1455, 1435, 1405, 1345, 1320, 1240, 1035,
1010, 970, 760 and 700 cm~1.
Example 53:
7-[(lR,2S,3RS,5S)-2-[3(RS)-Hydroxy-oct-l(E)-anyl]-3-phenyl-
5-fluoro-cyclo-pentyl]-5(E)-heptenoic acid methyl ester (A) and
7-[(lR,2S,3RS,5S~ 2-[3(RS)-hydroxy-oct-l(E)-enyl]-3-phenyl-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl ester (B):
61 mg (142 ~mol) of the compound produced according to
example 53a is reacted analogously to example 15 and, after
working up and purification, 47 mg (109 ~mol, 77%) of title
, ~
. .
,
''
~7
~8~
compound A as well as 12 mg (28 ~mol, 20%) of title compound B
are each isolated as colorless oil.
IR (Film): 3600-3200, 3030, 3000, 2950, 2930, 2850, 1735,
1600, 1450, 1435, 1245, 1170, 1070, 1030, 970, 755 and 700 cm~1.
Example 53a:
7-[(lR,2S,3~S,5S)--2-[3-oxo~oct-l(E)-enyl]-3-phenyl-5-fluoro-
cyclopentyl]-5(E/Z)-heptenoic acid methyl ester:
70 mg (168 ~mol) of the compound produced according to
example 50b is reacted analogously to example 50a by use of
dimethyl-(2-oxo-heptyl)-phosphonate and, after working up and
purification, 61 mg (142 ~mol, 77%) of the title compound is
isolated as colorless oil.
IR (Film): 3030, 2950, 2930, 2850, 1730, 1690, 1670, 1625,
1450, 1430, 1165, 1070, 980, 760 and 700 cm~1.
Example 54:
7-[(lR,25,3RS,5S)-2-[3S or 3R~-3-Hydroxy-oct-l(E)-enyl]-3-
phenyl-5-fluoro-cyclopentyl]-5(E)-heptenoic acid (A) and 7~
[(lR,2S,3RS,5S)-2-[3~ or 3S)-3-hydroxy-oct-l(E)-enyl]-3-phenyl-5-
fluoro-cyclopentyl]-5(E)-heptenoic acid (B):
12 mg (27 ~mol) of compound A produced according to example
53 is reacted analogously to example 2 and, after working up and
purification, 53 mg (13 ~mol, 47%) of a nonpolar component, to
which the structure of title compound A was assigned, as well as
4.9 mg (12 ~mol, 44~) of a polar component, to which the
. , :
68
~8g~6~
structure of title co-mpound B was assigned, are each isolated as
colorless oil.
IR tFilm) of A: 3600-2400, 3080, 3060, 3030, 2950, 2930,
2860, 1690, 1600, 1495, 1455, 1305, 1260, 1035, 965, 755 and 700
cm~1 .
IR (Film) of B: 3600-2400, 3080, 3060, 3030, 2950, 2930,
2860, 1700, 1600, 1495, 1455, 1340, 1325, 1260, 1135, 1070, 1015,
965, 755 and 700 cm~1.
Exam~le 55:
7-[(lR,2S,3RS,5S)-2-[3(RS)-Hydroxy-oct-l(E)-enyl]-3-phenyl-
5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
33 mg (77 ~mol) of compound B produced according to example
53 is reacted analogously to example 2 and, after working up and
purification, 17 mg (41 ~mol, 53%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-2400, 3080, 3060, 3030, 3000, 2950, 2930,
2860, 1710, 1600, 1495, 1465, 1405, 1345, 1240, 1035, 970, 760
and
700 cm~1.
Example 56:
7-[(lS,2R,3RS,5R)-2-~(3RS,4S)-3-Hydroxy-4-methyl-non-l(E)-
en-6-inyl]-3-phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid
methyl ester:
183 mg (404 ~mol) of the compound produced according to
example 56a is reacted analogously to example 15 and, after
: ~
6g
working up and purification, 18~ mg (404 ~mol, 100%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3030, 2960, 2920, 2870, 1735, 1450,
1435, 1245, 1170, 1145, 1025, 970, 760 and 700 cm-1.
Example 56a:
7-[(lS,2R,3RS,5R)-2-[3-Oxo-4S-methyl-non-l(E)-en-6-inyl]-3-
phenyl-5-fluoro-cyclopentyl]~5(Z)-heptenoic acid methyl ester:
274 mg (561 ~mol) of the compound produced according to
example 56b is reacted analogously to example 50a and, after
working up and purification, 183 my (404 ~mol, 72%) of the title
compound is isolated as colorless oil.
IR (Film): 3060, 3030, 3000, 2970, 2930, 2870, 1735, 1690,
1670, 1625, 1450, 1430, 1370, 1170, 1040, 970, 760 and 700 cm^1.
Example 56b: -
7-[(lS,2R,3RS,5R)-2-Formyl-3-phenyl-5-fluoro-cyclopentyl]-
5(Z)-heptenoic acid methyl ester:
500 mg (1.5 mmol) of the compound produced according to
example 41a is reacted analogously to example 50b and, after
working up, 500 mg (1.5 mmol, 100%) of the titla compound is
isolated as colorless oil, that is further reacted without
purification.
':
~g8~
Example 57:
7-[(lS,2R,3RS,5R)-2-[(3RS,4S)-3-Hydroxy-4-methyl-non-l(E~-
en-6-inyl]-3-phenyl-s-fluoro-cyclopentyl]-5(z)-heptenoic acid:
184 mg (404 ~mol) of the compound produced according to
example 56 is saponified analogously to example 2 and, after
working up and purification, 132 mg (300 ~mol, 74~) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 2970, 2950, 1710, 1600,
1455, 1240, 970, 760 and 700 cm-1.
Example 58:
7-[(lS,2R,3RS,5R)-2-[3(RS)-Hydroxy-oct-l(E)-enyl]-3-phenyl-
5-fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
298 mg (695 ~mol) of the compound produced according to
example 58a is reacted analogously to example 15 and, after
working up and purifi~ation, 260 mg (603 ~mol, 87~) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3030, 3000, 2950, 2930, 2850,
1735, 1600, 1450, 1435, 1245, 1170, 1070, 1030, 970, 760 and
700 cm-1.
Example 58a:
7-[(lS,2R,3RS,5R)-2-[3-Oxo-oct-l(E)-enyl]-3-phenyl-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
270 mg (748 ~mol) of the compound produced according to
example 56b is reacted analogously to example 56a by use of
dimethyl-(2-oxo-heptyl)-phosphonate and, after working up and
: . ' , . . - .
~` :
71 ~8i~1
.
purification, 298 mg (695 ~mol, 93%) of the title compound is
isolated as colorless oil.
IR (Film): 3030, 2950, 2920, 2850, 1730, 1690, 1670, 1625,
1450, 1430, 1240, 1165, 980, 760 and 700 cm~1.
Example 59:
7-[(lS,2R,3RS,5R)-2-[3(RS)-Hydroxy-oct-l(E)-enyl]-3-phenyl-
5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
260 mg (604 ~mol) of the compound produced according to
example 58 is saponified analogously to example 2 and, after
working up and purification, 235 mg (564 ~mol, 93%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 2960, 2920, 2850, 1710,
1600, 1450, 1270, 970, 760 and 700 cm~1.
Example 60:
7-[(lS,2R,3RS,5R) 2-[(3RS,4RS)-3-Hydroxy-4-phenyl-pent-l(E)-
enyl]-3-phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl
ester:
172 mg (372 ~mol) of the compound produced according to
example 60a is reacted analogously to example 15 and, after
working up and purification, 170 my (366 ~mol, 98%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3030, 2960, 2940, 1730, I600,
1495, 1450, 1240, 1020, 970, 760 and 700 cm~1.
:~
.
r~`~
Example 60a:
7-[(lS,2R,3RS,5R)-2-[3-Oxo-4RS-phenyl-pent-l(E)-enyl]-3-
phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
274 mg (561 ~mol) of the compound produced according to
example 56b is reacted analogously to example 56a by using
dimethyl-(2-oxo-3-phenyl-butyl)-phosphonate and, after working up
and purification, 172 mg (372 ~mol, 66%) of the title compound is
isolated as colorless oil.
IR (Film): 3060, 3030, 2950, 2930, 2850, 1735, 1690, 1670,
1625, 1490, 1450, 1430, 1370, 1245, 1165, 1070, 1030, 980, 760
and 700 cm~1.
Example_61:
7-[(lS,2R,3RS,5R)-2-[3R or 3S,4RS)-3-Hydroxy-4-phenyl-pent-
l(E)-enyl]-3-phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid (A)
and 7-[~lS,2R,3RS,5R)-2-[3S or 3R,4RS)-3-hydroxy-4-phenyl-pent-
l(E)-enyl]-3-phenyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid
methyl ester (B):
170 mg (366 ~mol) of compound A produced according to
example 60 is saponified analogously to example 2 and, after
working up and purification, 24 mg (53 ~mol, 15%) of a nonpolar
component, to which the structure of title compound A is
assigned, as well as 45 mg (100 ~mol, 27%) of a polar component,
to which the structure of title compound B is assigned, are each
isolated as colorless oil.
IR (Film) of A: 3600-2400, 3060, 3030, 2960, 2940, 1710,
1600, 1500, 1450, 1240, 1020, 970, 760 and 700 cm~1.
~, . .
: ,. :
. ~g,g~l
IR (Film) of B: 3~00-2400, 3060, 3030, 2960, 2950, 1710,
1600, 1495, 1455, 1240, 1030, 1010, 980, 760 and 700 cm~1.
Example 62:
7-[lS,2R,3R,5R)-2-[(E/Z)-Diphenylmethoxyiminomethyl]-3,5-
dihydroxy-cyclopentyl]-5(Z)-heptenoic acid:
108 mg (207 ~mol) of the compound produced according to
example 62a is mixed with 1.2 ml of a mixture of acetic acid,
~ater and tetrahydrofuran (65:35:10) and stirred for 15 hours at
23C. It is concentrated by evaporation, the remaining acetic
acid is removed by repeated azeotropic distillation with toluene
and the residue is purifed by chromatography on about 20 ml of
fine silica gel. 75 mg (171 ~mol, 83%) of the title compound is
isolated as colorless oil.
IR (Film): 3700-2400, 3090, 3060, 3030, 3010, 2940, 1710
1490, 1450, 1245, 1035, 1020, 940, 745 and 700 cm-1.
ExamPle 62a:
7-[lS,2R,3R,5R)-2-[(E/Z)-Diphenylmethoxyiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl~-5(Z)-heptenoic
acid:
1.18 g (1.84 mmol) of the compound produced according to
example 62b is saponified analogously to example 2 and, after
working up and purification, 943 mg (1.81 mmol, 98%) of the title
compound is isolated as colorless oil.
.. ~ '
,
~ , : .
74
2~1 6~
IR (Film): 3600-2400, 3090, 3060, 3030, 3010, 2940, 2870,
1740, 1710, 1600, 1495, 1450, 1240, 1130, 1115, 1035, 1020, 935,
920, 870, 815, 745 and 705 cm~1.
ExamE~e 62b:
7-[lS,2R,3R,5R)-2-[(E/Z)-Diphenylmethoxyiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
The colorless solution of 1.0 g (2.18 mmol) of the compound
produced according to example 62c in 16 ml of anhydrous ethanol
is mixed with 0.4 ml of anhydrous pyridine, 588 mg of
diphenylmethoxyamine and heated for 3.5 hours under an atmosphere
of dry argon to 50C. It is concentrated by evaporation, the
residue is taken up in dichloromethane, washed with water and
saturated sodium chloride solution and the residue obtained after
drying on magnesium sulfate, filtration and concentration by
evaporation is purified by chromatography on about 50 ml of
silica gel with a n-hexane/ethyl acetate mixture. 1.18 g (1.84
mmol, 85%) of the title compound is isolated as colorless oil.
IR (Film): 3070, 3030, 3010, 2950, 2870, 1740, 1715, 1605,
1585, 1495, 1450, 1360, 1275, 1115, 1025, 940, 920j 870, ~15,
745, 715 and 705 cm~1.
- . . ; . -
;
.
,
. - . .
Example 62c:
7-[lS,2R,3R,5R)-2-Formyl-3-(tetrahydropyran-2-yloxy)-5-
benzoyloxy-cyclopentyl]-5(z)-heptenoic acid methyl ester:
9 g (19.5 mmol) of the compound produced according to
example 62d is reacted analogously to example 50b and, after
working up, 9 g (19.5 mmol, 100~) of the title compound is
isolated that is reacted without further purification.
Example 62d:
7-[lS,2R,3R,5R)-2-Hydroxymethyl-3-(tetrahydropyran-2-yloxy)-
5-benzoyloxy-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
22.4 g (32 mmol) of the compound produced according to
example 62e is reacted analogously to example lg and, after
working up and purification, 13.7 g (29.7 mmol, 93~) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3070, 2950, 2870, 1740, 1720, 1605,
1585, 1450, ~370, 1315, 1275, 1245, 1115, 1075, 1030, 870, 810
and 715 cm~1.
Example 52e:
7-[lS,2R,3R,5R)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
19.8 g (33.4 mmol) of the compound produced according to
example 62f is reacted analogously to example lh and, after
.' . ' ~ i,, , ,, , ",,,
: : :
76 ~8~
working up and puri~ication, 22.4 g (32 mmol, 96%) of the title
compound is isolated as colorless oil.
IR (Film): 3070, 3050, 3010, 2940, 2860, 1740, 1720, 1605,
1590, 1455, 1430, 1275, 1115, 1070, 1025, 825, 740 and 710 cm~1.
Example 62f:
7-[lS,2R,3R,5R)-2-(tert.-Butyldiphenylsilyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(Z)-heptenoic
acid methyl ester:
25.8 g (44.4 mmol) of the compound produced according to
example 62g is reacted analogously to example li and, after
working up and purification, 19.9 g ~33.4 mmol, 75%) o~ the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3070, 3050, 3010, 2940, 2860, 1740,
1590, 1430, 1200, 1110, 1075, 1020, 1000, 870, 825, 740 and
705 cm~1.
Example 62g:
7-[lS,2R,3R,5R)-2-(tert.-Butyldiphenylsilyloxymethyl)-3- -
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(z)-heptenoic
acid:
23.-8 g (47.9 mmol) of the compound produced according to
example 62h is reacted analogously to example lj and, after
working up and purification, 25.8 g (44.4 mmol, 93%) of the title
compound is isolated as colorless oil.
,
'
IR (Film): 3700-2400, 3070, 3050, 2940, 2860, 1710, 1590,
1430, 1390, 1260, 1200, 1110, 1075, 1045, 1020, 995, 880, 825,
7~0 and 705 cm~1.
Example 62h:
(lS,3RS,5S,6R,7R)-3-Hydroxy-6-(tert.-
butyldiphenylsilyloxymethyl)-7-(tetrahydropyran-2-yloxy)-2-
oxabicyclo[3.3.0]octane:
23.8 g (48.1 mmol) of the compound produced according to
example 62i is reacted analogously to example lk and, after
working up and purification, 23.8 g (47.9 mmol, 99~) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3070, 3050, 2940, 2860, 1740, 1590,
1430, 1390, 1375, 1355, 1240, 1110, 1075, 1020, 870, 820, 740 and
705 cm~1.
Example 62i
(lR,5S,6R,7R)-3-Oxo-6-(tert.-butyldiphenylsilyloxymethyl)-7~
(tetrahydropyran-2-yloxy)-2-oxabicyclo[3.3.0]octane:
The solution of 20.7 g (50.4 mmol) of the compound produced
according to example 62j in 400 ml of anhydrous dichloremethane
is mixed at 0C with 6.83 ml of dihydropyran, 135 mg of p-
toluenesulfonic acid and stirred for 1.5 hours under an
atmosphere of dry argon. It is diluted with dichloromethane,
washed with a saturated sodium bicarbonate solutlon, a saturated
sodium chloride solution and dried on magnesium sulfate. The
residue obtained after filtration and removal of the solvent is
.,, , , , ~ . . ..
78
~g~
purified by chromatography on about 300 ml of fine silica gel.
23.8 g (48.1 mmol, 95%) of the title compound is isolated as
colorless oil.
IR (Film): 3070, 3050, 2940, 2860, 1780, 1740, 1590, 1470,
1~30, 1390, 1375, 1355, 1245, 1170, 1110, 1075, 1035, 870, 825,
745 and 705 cm~1.
Example 62j:
(lR,5S,6R,7R)-3-Oxo-6-(tert.-butyldiphenylsilyloxymethyl)-7-
hydroxy-2-oxabicyclo[3.3.o]octane:
The solution of 1.5 g (2.66 mmol) of the compound produced
according to example 62k in 74 ml of anhydrous dimethylformamide
is mixed with 3.32 g of potassium nitrite and heated under an
atmosphere of dry argon for 16 hours to 85C. It is concentrated
by evaporation, diluted with water and extracted several times
with dichloromethane. The combined organic extracts are washed
with saturated sodium chloride solution, dried on magnesium
sulfate and the residue obtained after removal of the solvent is
purifed by chromatography on about 100 ml o~ fine silica gel.
700 mg (1.70 mmol, 64%) of the title compound is isolated as
colorless oil.
IR ~Film): 3600-3200, 3070, 3050, 2940, 2960, 1770, 1590,
1430, 1240, 1170, 1115, 1075, 825, 745, 705, 615 and
510 cm~1.
Example 62k:
(lR,5S,6R,7S)-3-Oxo-6-(tert.-butyldiphenylsilyloxymethyl)-7-
toluenesulfonyloxy-2-oxabicyclo[3.3.0]octane:
23.7 g (5.77 mmol) of (lR,5S,6R,7S)-3-oxo-6-(tert.-
butyldiphenylsilyloxymethyl)-7-hydroxy-2-oxabicyclo[3.3.0]octane
is reacted analogously to example lm and, after working up and
purification, 2.9 g (5.17 mmol, 90%) of the title compound is
isolated as colorless oil.
IR (CHCl3): 3070, 3050, 3030, 3000, 2960, 2930, 2860, 1770,
1600, 1425, 1365, 1190, 1175, 1110, 1040, 980, 960, 895, 875, 820
and 700 cm~1.
Exam~le 63:
7-[(lS,2R,3R,5R)-2-[(E/Z)-Phenylureidoiminomethyl]-3,5-
dihydroxy-cyclopentyl]-5(Z)-heptenoic acid:
99.5 mg (210 ~mol) of the compound produced according to
example 63a is reacted analogously to example 62 and, after
working up and purification, 77 mg (198 ~mol, 94%) of the title
compound is isolated as colorless oil.
~ R (Film): 3600-2400, 3380, 3260, 3010, 2950, 2860, 1710,
1670, 1600, 1590, 1500, 1450, 1240, 750 and 690 cm~1.
- ~,
" " ,,
~ '
Example 63a:
7-[(lS,2R,3R,5R)-2-[(E/Z)-Phenylureidoiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(Z)-heptenoic
acid:
345 mg (583 ~mol) of the compound produced according to
: example 63b is saponified analogously to example 2 and, after
working up and purification, 248 mg (418 ~mol, 72%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3380, 3220, 3010, 2940, 2870, 1710,
1690, 1590, 1535, 1450, 1320, 1230, 1115, 1030, 1020, 985, 870,
810, 755 and 690 cm~1.
Example 63b:
7-[(lS,2R,3R,5R)-2-[(E/Z)-Phenylureidoiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
600 mg (1.31 mmol) of the compound produced according to
example 62c is reacted analogously to example 62b by using 4-
phenylsemicarbazide and, after working up and purification, 775
mg (1.31 mmol, 100%) of the title compound is isolated as
colorless oil.
IR (Film~: 3380, 3200, 3100, 2950, 2870, 1715, 1690, 1595,
1535, 1450, 1275, 1225, 1115, 1~35, 1025, 980, 870, 815, 760, 715
and 695 cm~1.
" , ' ' ~ .
Exam~le 64:
7-[(lS,2R,3R,5R)-2-(2-Fluorobenzyloxymethyl)-3,5-dihydroxy-
cyclopentyl]-5(Z)-heptenoic acid:
50.6 mg (112 ~mol) of the compound produced according to
example 64a is reacted analogously to example 62 and, after
working up and purification, 34.9 mg (95 ~mol, 85%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2930, 2870, 1710, 1620, 1595,
1495, 1455, 1230, 1110, 1085, 1020, 995 and 760 cm~1.
Example 64a:
7-[(lS,2R,3R,5R)-2-(2-Fluorobenzyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(Z)-heptenoic
acid:
127 mg (224 ~mol) of the compound produced according to
example 64b is saponified analogously to example 2 and, after
working up and purification; 69 mg (153 ~mol, 69%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2930, 2870, 1710, 1620, 1590,
1495, 1455, 1235, 1115, 1085, 1020, 995, 870, 810 and 760 cm~1.
Example 64b:
7-[(lS,2~,3R,5R)-2-(2-Fluorobenzyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
The solution of 500 mg (1.09 mmol) of the compound produced
according to example 62d in 8.5 ml of tolene is mixed with 3.5 ml
,,, . .: .
,, ~
82
~- 2~881~
of a 25% aqueous sodium hydroxide solution, 50 mg of
tetrabutylammonium hydrogen sulfate, 1.03 g of 2-fluorobenzyl
bromide and the 2-phase system is stirred for 5 days at 23C. It
is diluted with water, and adjusted to a pH of 5 by addition of
saturated citric acid and extracted several times with diethyl
ether. The combined organic extracts are dried on magnesium
sulfate and the residue obtained after removal of the solvent is
purifed by chromatography on about 100 ml of silica gel with a
mixture of n-hexane and ethyl acetate. 137 mg (241 ~mol, 22%) of
the title compound is isolated as colorless oil.
IR (Film): 3070, 3010, 2950, 2870, 1740, 1720, 1620, 1605,
1590, 1495, 1455, 1365, 1315, 1275, 1115, 1025, 870, 815, 760 and
715 cm~
Example 65_
7-[(lS,2R,3R,5R)-2-Benzyloxymethyl-3,5-dihydroxy-
cyclopentyl]-5(Z)-heptenoic acid:
57.5 mg (133 ~mol) of the compound produced according to
example 65a is mixed analogously to example 62 and, after workiny
up and purification, 40 mg (115 ~mol, 86~) of the title compound
is isolated as colorless oil.
IR (Film): 3700-2400, 3070, 3030, 3010, 2930, 2870, 1710,
1450, 1240, 1100, 1070, 740 and 700 cm1.
,
83
~g~ul
Example 65a:
7-[(lS,2R,3R,5R)-2-Benzyloxymethyl-3-(tetrahydropyran-2-
yloxy)-5-hydroxycyclopentyl]-5(Z)~heptenoic acid:
126 mg (229 ~mol) of the compound produced according to
example 65b is saponified analogously to example 2 and, after
working up and purification, 77 mg (178 ~mol, 78%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2940, 2870, 1710,
1455, 1365, 1260, 1240, 1200, ~115, 1075, 1020, 995, 870, 810,
740 and 700 cm~1.
Example 65b:
7-[(lS,2R,3R,5R)-2-Benzyloxymethyl-3-(tetrahydropyran-2-
yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-heptenoic acid methyl
ester:
500 mg (1.09 ~mol) of the compound produced according to
example 62d is reacted analogously to example 64b by using benzyl
bromide and, after working up and purification, 134 mg (243 ~mol,
22%) of the title compound is isolated as colorless oil.
IR (Film): 3070, 3030, 3010, 2940, 2860, 1735, 1720, 1605,
1585, 1450, 1365, 1275, 1115, 1075, 1025, 870, 815, 740, 715 and
700 cm~1.
84
~81~1
Example 66:
7-[(lS,2R,3R,5R)-2-(3-Methylbenzyloxymethyl)-3,5-dihydroxy-
cyclopentyl]-5(Z)-heptenoic acid:
36.3 mg (81 ~mol) of the compound produced according to
example 66a is reacted analogously to example 62 and, after
working up and purification, 23 mg (63 ~mol, 78%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2930, 2870, 1710, 1610, 1410,
1245, 1155, 1085, 885, 785, 745 and 700 cm~
Example 66a:
7-[(lS,2R,3R,5R)-2-(3-Methylbenzyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(Z)-heptenoic
acid:
68.7 mg (122 ~mol) of the compound produced according to
example 66b is saponified analogously to example 2 and, after
working up and purification, 41.4 mg (93 ~mol, 76~) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2940, 2870, 1710, 1610, 1455,
1440, 1355, 1200, 1160, 1115, 1075, 1020, 995, 870, 780, 740 and
695 cm~1.
'
: 85
Example 66b:
7-[(lS,2R,3R,5R)-2-(3-Methylenezyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
1.3 g (2.82 mmol) of the compound produced according to
example 62d is reacted analogously to example 64b by using 3-
methylbenzyl bromide and, after working up and purification, 424
mg (751 ~mol, 27%) of the title compound is isolated as colorless
oil.
IR (Film): 3060, 3010, 2940, 2860, 1740, 1720, 1605, 1585,
1450, 1360, 1315, 1275, 1115, 1075, 1025, 780 and 715 cm~1.
~xample 67:
7-[(lS,2R,3R,5R)-2-(4-Fluorobenzyloxymethyl)-3,5-dihydroxy-
cyclopentyl]-5(Z)-heptenoic acid:
66.8 mg (148 ~mol) of the compound produced according to
example 67a is reacted analogously to example 62 and, after
working up and purification, 39.2 mg (107 ~mol, 72%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2940, 2870, 1710, 1605, 1510,
1225, 1090, 855 and 825 cm~1.
,, : :, :
86
Example 67a:
7-[(lS,2R,3R,5R)-2-(4-Fluorobenzyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl~-5(Z)-heptenoic
acid:
136 mg (238 ~mol) of the compound produced according to
example 67b is saponified analogously to example 2 and, after
working up and purification, 87.5 mg (194 ~mol, 81%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2940, 2870, 1710, 1605, 1510,
1225, 1115, 1075, 1020, 995, 855 and 825 cm-1.
Example 67b.
7-[(lS,2R,3R,5R)-2-(4-Fluorobenzyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
500 mg (1.09 mmol) of the compound produced according to
example 62d is reacted analogously to example 64b by using 4-
fluorobenzyl bromide and, after working up and purification, 144
mg (253 ~mol, 23%) of the title compound is isolated as colorless
oil.
IR (Film): 3070, 3010, 2950, 2870, 1740, 1715, 1605, 1510,
1450, 1365, 1315, 1275, 1225, 1115, 1025, 1000, 825 and 715 cm~1.
;:
87
2~8~
Example 68:
7-[(lS,2R,3S,5S)-2-[(E/Z)-Diphenylmethoxyiminomethyl]3-
hydroxy-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
72 mg (134 ~mol) of compound A produced according to example
68 is reacted analogously to example 62 and, after working up and
purification, 46 mg (101 ~mol, 76%) of the title compound is
isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3030, 3010, 2940, 2870, 1730,
1600, 1495, 1455, 1240, 1020, 935, 745 and 700 cm1.
Example 68a:
7-[(lS,2R,3S,5S)-2-[(E/Z)-~iphenylmethoxyiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-fluoro-cyclopentyl]-5(Z)-heptenoic
acid methyl ester (A) and 7-[~5R,4S)-5-[(E/Z)-
diphenylmethoxyiminomethyl]-4-(tetrahydropyran-2-yloxy)-
cyclopent-l-enyl]-5(Z)-heptenoic acid methyl ester (B):
252 mg (471 ~mol) of the compound produced according to
example 68~ is reacted analogously to example 1 and, after
working up and purification, 72 mg (134 ~mol, 28%) of title
compound (A) as well as 58 mg (112 ~mol, 24%) of the title
compound (B) are each isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3030, 3010, 2940, 2870, 1730,
1600, 1455, 1245, 1020, 935, 870, 820, 745 and 700 cm~1.
'~
' .
88
Example 68b:
7-[(lS,2R,3S,5R)-2-[(E/Z)-Diphenylmethoxyiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(Z)-hep~enoic
acid methyl ester:
The solution of 257 mg (493 ~mol) of the compound produced
according to example 68c in 10 ml of dichloromethane is mixed
with ethereal solution of diazomethane and concentrated by
evaporation after completion of the reaction. 262 mg (489 ~mol,
99%) of the title compound is isolated as colorless oil.
IR (Film): 3600-3200, 3070, 3030, 3010, 2950, 2870, 1740,
1455, 1440, 1350, 1200, 1130, 1080, 1025, 975, 910, 870, 815, 745
and 705 cm~1.
Example 68c:
7-[(lS,2R,3S,5R)-2-[(E/Z)-Diphenylmethoxyiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(Z)-heptenoic
acid:
569 mg (889 ~mol) of the compound produced according to
example 68d is saponified analogously to example 2 and, after
working up and purification, 399 mg (765 ~mol, 86%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3070, 3030, 3010, 2940, 270, 1730,
1710, 1605, 1495, 1445, 1345, 12~5, 1205, 1185, 1130, 1080, 1020,
975, 920, 870, 810, 745 and 705 cm~1.
.
:
'
,
89
2 ~ 6 ~
Example 68d:
7-[(lS,2R,3S,5S)-2-[(E/Z)-Diphenylmethoxyiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5~Z)-
heptenoic acid methyl ester:
300 mg (654 ~mol) of the compound produced according to
example 68e is reacted analogously to example 62b and, after
working up and purification, 268 mg (453 ~mol, 69%) of the title
compound is isolated as colorless oil.
IR (Film): 3380, 3210, 3100, 3010, 2950, 2870, 1735, 1715,
1690, 1600, 1535, 1450, 1360, 1320, 1275, 1115, 1070, 1~30, 970,
870, 815, 760, 715 and 695 cm~1.
Example 68e:
7-[(lS,2R,3S,5S)-2-Formyl-3-(tetrahydropyran-2-yloxy)-5-
benzoyloxy-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
1.2 g (2.61 mmol) of the compound produced according to
example 68f is reacted analogously to example 50b and, after
working up, 1.2 g (2.61 mmol, 100%) of the title compound is
isolated that is further reacted without purification.
Example 68f:
7-[(lS,2~,3S,5R)-2-Hydroxymethyl-3-(tetrahydropyran-2-
yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-heptenoic acid methyl
ester:
2.16 g (3.09 mmol) of the compound produced according to
example 68g is reacted analogously to example lg and, after
, . , . . . ~
.
.
.
,;
9o
working up and purification, 1.2 g (2.61 mmol, 85%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3060, 3000, 2940, 2860, 1735, 1710,
1600, 1585, 1450, 1355, 1310, 1270, 1110, 1070, 1025, 865, 810
and
710 cm-1.
_ample 68g:
7-[(lS,2R,3S,5R)-2(tert.-Butyldiphenylsilyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
59.4 g ~99.9 mmol) of the compound 7-[(lS,2R,3S,5R)-2(tert.-
butyldiphenylsilyloxymethyl)-3-(tetrahydropyran-2-yloxy)-5-
hydroxy-cyclopentyl]-5(Z)-heptenoic acid methyl ester producPd
according to example 29b, is reacted analogously to example lh
and, after working up and purification, 62.5 g (89.4 mmol, 90%)
of the title compound is isolated as colorless oil.
IR (Film): 3070, 3050, 3010, 2950, 2860, 1740, 1715, 1600,
1585, 1450, 1425, 1275, 1110, 1025, 970, 870, 825, 790, 710, 690,
610 and 500 cm1.
Example 69:
7-[(lS,2R,3S,5S)-2-[(E/Z)-Diphenylmethoxyiminomethyl]-3-
hydroxy-5-fluoro-cyclopen~yl~-5(Z)-heptenoic acid:
46 mg (101 ~mol) of the compound produced according to
example 68 is saponified analogously to example 2 and, after
,
~ .
~ .
91
81~1
working up and purification, 29 mg (66 ~mol, 65%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2940, 2870, 1710,
1600, 1495, 1455, 1240, 1020, 935, 745 and 700 cm-1.
Example ? -
7-[(lS,2R,3S,5R)-2-[(E/Z)-Diph~nylmethoxyiminomethyl]-3,5-
dihydroxy-cyclopentyl]~5(Z)-heptenoic acid:
128 mg (244 ~mol) of the compound produced according to
example 68c is reacted analogously to example 62 and, after
working up and purification, 84 mg (192 ~mol, 78%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3060, 3030, 3010, 2940, 1710, 1605,
1495, 1450, 1375, 1245, 1045, 1020, 935, 920, 745 and 700 cm-1.
Example 71:
7-[(lS,2R,3R,5S)-2-[(E/Z)-Phenylureidoiminomethyl]-3,5-
dihydroxy-cyclopentyl]-5(Z)-heptenoic acid:
137.6 mg (271 ~mol~ of the compound produced according to
example 71a is saponified analogously to example 2 and, after
working up and purification, 93.8 mg (241 ~mol, 89%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2~00, 3370, 3010, 2940, 1730-1680, 1600,
1540, 1450, 1375, 1240, 1115, 1045, 760 and 695 cm~1.
;;
.
Example 71a:
7-[(lS,2R,3R,5S)-2-[(E/Z)-Phenylureidoiminomethyl]-3-
hydroxy-5-benzoyloxy-cyclopentyl]-5(Z)-heptenoic acid methyl
ester:
248 mg (419 ~mol) of the compound produced according to
example 71b is reacted analogously to example 62 and, a~ter
working up and purification, 149 mg t294 ~mol, 70%) of the title
compound is isolated as colorless oil.
IR (~ilm): 3600-2400, 3380, 3070, 3010, 2950, 2870, 1740-
1680, 1600, 1540, 1450, 1360, 1315, 1275, 1175, 1115, 1070, 1030,
940, 760, 715 and 695 cm~1.
~xample 7lb:
7-[(lS,2R,3R,5S)-2-[(E/Z)-Phenylureidoiminomethyl]-3-
(tetrahydropyran-2-yloxy)-5-benzoyloxy-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
300 mg (654 ~mol) of the compound produced according to
example 68e is reacted analogously to example 62b and, after
working up and purification, 268 mg (453 ~mol, 69%) of the title
compound is isolated as colorless oil.
IR (Film): 3380, 3210, 3100, 3010, 2950, 2860, 1735, 1715,
1690, 1595, 1530, 1450, 1315, 1275, ~115, 1025, 970, 870, 810,
755, 715 and 695 cm~1.
93
1 6 1
Example 72:
7-[(lS,2R,3S,5S)-2-(3-Methylbenzyloxymethyl)-3-hydroxy-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
201 mg (435 ~mol) of the compound produced according to
example 72a is reacted analogously to example 62 and, after
working up and purification, 89 mg (235 ~mol, 54%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3200, 3010, 2940, 2860, 1735, 1610, 1440,
1360, 1250, 1220, 1155, 1100, 1090, 780, 745 and 695 cm~1.
Example 72a:
7-[(lS,2R,3S,5S)-2-(3-Methylbenzyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-fluoro-cyclopentyl]-5(Z)-heptenoic
acid methyl ester (A) and 7-[(5R,4S)-5-(3-methylbenzyloxymethyl)-
4-(tetrahydropyran-2-yloxy)-cyclopent-1-enyl]-5(Z)-heptenoic acid
methyl ester (B):
800 mg (1.7~ mmol) of the compound produced according to
example 72b is reacted analogously to example 1 and, after
working up and purification, 201 mg (436 ~mol, 25%) of title
compound A as well as 212 mg (480 ~mol, 28%) of title compound B
are each isolated as colorless oil.
IR (Film): 3600-3200, 3010, 2940, 2850, 1730, 1610, 1440,
1360, 1250, 1225, 1155, 1095, 870, 815, 780, 745 and 695 cm~1.
:
' ' ' ': '
'
94
U l
Exam~le 72b:
7-[(lS,2R,3S,5R)-2-(3-Methylbenzyloxymethyl)-3-
(tetrahydropyran-2-yloxy)-5-hydroxy-cyclopentyl]-5(Z)-heptenoic
acid methyl ester:
1.23 g (2.75 mmol) of the compound produced according to
example 68f is reacted analogously to example 64b and, after
working up and purification, 1.18 g (2.56 mmol, 93%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3300, 3010, 2940, 2860, 1740, 1610, 1595,
1440, 1355, 1250, 1200, 1155, 1115, 1075, 1020, 975, 905, 870,
815, 780, 745 and 695 cm~1.
Example 73:
7-[(lS,2R,3S,5S)-2-(3-Methylbenzyloxymethyl)-3-hydroxy-5-
fluoro-cyclopentyl] 5(Z)-heptenoic acid:
30.7 mg (81 ~mol) of the compound produced according to
example 72 is saponified analogously to example 2 and, after
working up and purification, 19.8 mg (54 ~mol, 67%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2930, 2860, 1710, 1610, 1590,
1455, 1440, 1360, 1240, 1155, 1100, 1085, 780, 745 and 695 cm~1.
Exam~le 74:
7-[(lS,2R,5S)-2-(3-~ethylbenzyloxymethyl)-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
76 mg (148 ~mol) of the compound produced according to
example 74a is reacted analogously to example 11 and, after
.
,' ~
2~88~1
working up and purification, 36.4 mg (106 ~mol, 72%) of the title
compound is isolated as colorless oil.
IR (Film): 3010, 2930, 2850, 1730, 1610, 1590, 1455, 1410,
1355, 1240, 1160, 1100, 1090, 780, 755 and 695 cm~1.
Example 74a:
7-[(lS,2R,3S,5S)-2-(3-Methylben~yloxymethyl)-3-
toluenesulfonyloxy-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid
methyl ester:
58 mg (162 ~mol) of the compound produced according to
example 72 is reacted analogously to example lm and, after
working up and purification, 76 mg (148 ~mol, 92%) of the title
compound is isolated as colorless oil.
IR (Film): 3050, 3010, 2950, 2920, 2860, 1735, 1600, 1435,
1360, 1190, 1175, 1095, 975, 945, 885, 815, 780, 745 and 665 cm~
Example 75:
7-[(lS,2R,5S)-2-(3-Methylbenzyloxymethyl)-5-fluoro-
cyclopentyl]-5(~)-heptenoic acid:
36 mg (106 ~mol) of the compound produced according to
example 74 is saponified analogously to example 2 and, after
working up and purification, 8.6 mg (26 ~mol, 25%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2920, 2850, 1705, 1610, 1590,
1455, 1410, 1355, 1240, 1155, 1105, 1085, 780, 755 and 695 cm~1.
,- ,, ~ . .,
,~ ' "' ` .
:.
'. ,
i~
,, ' ' ''
96
2 ~
Example 76:
7-[(lS,2R,3S,5R)-2-(3-Methylbenzyloxymethyl)-3,5-dihydroxy-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
87.2 mg (189 ~mol) of the compound produced according to
example 72b is reacted analogously to example 62 and, after
working up and purification, 63.4 mg (168 ~mol, 89%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-3100, 3010, 2950, 2930, 2860, 1735, 1610,
1440, 1360, 1245, 1160, 1100, 780, 745 and 695 cm~1.
Example 77:
7-[(lS,2R,3S,5R)-2-(3-Methylbenzyloxymethyl)-3,5-dihydroxy-
cyclopentyl]-5(Z)-heptenoic acid:
53 mg (14~ ~mol) of the compound produced according to
example 76 is saponified analogously to example 2 and, after ~
working up and purification, 40 mg (110 ~mol, 79%) of the title
compound is isolated as colorless oil.
IR (Film): 3600-2400, 3010, 2930, 2860, 1710, 1610, 1590,
1455, 1430, 1405, 1355, 1245, 1155, 1100, 780, 745 and 6~5 cm~1.
Example 78:
~ -[(lR,2S,5R)-2-[3-(4-Fluorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid methyl ester:
The solution of 80 mg (max. 136 ~mol) of the amine produced
according to example 78a in 1 ml of dichloromethane is mixed with
21 ~1 of 4-fluorophenylisocyanate and stirred for 2.5 hours at
23C. It is purified by chromatography on a three analytic thin-
97
, . .
layer slab. A mixture of n-hexane and ethyl acetate is used as
mobile solvent, ethyl acetate is used as eluant. 25 mg (66 ~mol,
48%) of the title compound is isolated as colorless oil.
IR (Film): 3250-3450, 3050, 2950, 2850, 1730, 1640, 1595,
1555, 1515, 1490, 1430, 1325, 1235, 835, 735 and 705 cm~1.
Example 78a:
6-[(lR,2S,5R)-2-Aminomethyl-5-fluoro-cyclopentyl]-4(Z)-
hexenoic acid methyl ester:
The solution of 747 mg (2.77 mmol) of the compound produced
according to example 78b in 24 ml of tetrahydrofuran is mixed
with 874 mg of triphenylphosphine and heated for 3.5 hours to
50C under an atmosphere of dry argon. Then it is mixed with 3
ml of water and refluxed for 1 hour. It is concentrated by
evaporation, taken up with dichloromethane, dried on magnesium
sulfate and, after filtration and removal of the solvent, 1.62 g
(max. 2.77 mmol) of amine contaiminated with triphenylphosphine
and triphenylphosphinoxide is isolated, that is further reacted
without purification.
Example 78b:
6-[(lR,2S,5R)-2-Azidomethyl-5-fluoro-cyclopentylJ-4(Z)-
hexenoic acid methyl ester:
The solution of 934 mg (3.04 mmol) of bromide produced
according to example 78c in 67 ml of dimethylformamide is mixed
with 839 mg of sodium azide and heated for 4 hours under an
atmosphere of dry argon to 60C. It is poured in ice water,
. .
.
'.'' ' ''' '
98
~8816~
extracted several times with diethyl ether, the organic phase is
dried on magnesium sulfate and the residue obtained after
filtration and removal of the solvent is purified by
chromatography on about 200 ml of fine silica gel by using a
gradient system of n-hexane and ethyl acetate. 747 mg (2.77
mmol, 91%) of the title compound is isolated as colorless oil.
IR (Film): 2950, 2870, 2100, 1735, 1435, 1360, 1245 and
1160 cm~1.
Example 78c:
6-~(lR,2S,5R)-2-Bromomethyl-5-fluoro-cyclopentyl]-4(Z)-
hexenoic acid methyl ester:
The solution of 743 mg (3.04 mmol) of alcohol produced
according to example 78d in 22 ml of acetonitrile is mixed with
2.05 ml of collidine, 5.05 g of tetrabromomethane, 4.0 g of
triphenylphosphine and stirred for 17 hours at 23C under an
atmosphere of dry argon. It is concentrated by evaporation and
the residue obtained is purified by chromatography on about 200
ml of fine silica gel by using a gradient system of n-hexane and
ethyl acetate. 934 mg (3.04 mmol, 100%~ of the title compound is
isolated as colorless oil.
IR (Film): 2950, 2860, 1735, 1435, 1360, 1245, 1220, 11~0
and 950 cm~1.
.-.
,
99
Example 78d:
6-[(lR,2S,5R)-2-Hydroxymethyl-5-fluoro-cyclopentyl]-4(Z)-
hexenoic acid methyl ester (A) and 6-[(5S)-5-hydroxy-cyclopent-1-
enyl]-4(Z)-hexenoic acid methyl ester (B):
3.69 g of the substance mixture produced according to
example 78e is reacted analogously to example lg and, after
working up and purification, 943 mg (4.20 mmol, 40%) of title
compound B as the nonpolar component, as well as 758 mg (3.10
mmol, 30%) of title compound A as the polar component are each
isolated as colorless oil.
IR (Film) of A: 3200-3600, 3050, 2950, 2870, 1735, 1435,
1360, 1165, 1040, 945 and 875 cm~1.
IR (Film) of B: 3200-3600, 3050, 2940, 2850, 1735, 1435,
1360, 1200, 1160 and 1025 cm~1.
Example 78e:
6-[(lR,2S,5R)-2-tert.-Butyldiphenylsilyloxymethyl-5-fluoro-
cyclopentyl]-4(z)-hexenoic acid methyl ester and 6-[(5S)-5-
(tert.-butyldiphenylsilyloxym~thyl)-cyclopent-1 enyl]-4(Z)-
hexenoic acid methyl ester:
5.0 g (10.4 mmol) of the compound produced according to
example 78f is reacted analogously to example 1 and, after
working up and purification, 3.81 g of a mixture of both title
compounds is isolated as colorless oil.
:,
~ , , ''
,~ , .
100
Exam~le 78f:
6-~lR,2S,5S)-2-tert.-Butyldiphenylsilyloxymethyl-5-hydroxy-
cyclopentyl]-4(Z)-hexenoic acid methyl ester:
62.6 g (max. 58.3 mol) of the compound produced according to
example 78g is mixed analogously to example 68b and, after
working up and purification, 26.8 g (55.7 mmol, 96%) of the title
compound i5 isolated as colorless oil.
I~ (Film): 3600-3200, 3070, 3040, 2950, 2930, 2850, 1735,
1585, 1460, 1425, 1240, 1160l 1110, 1005, 820, 740 and 700 cm~1.
Example 78g:
6-[(lR,2S,5S)-2-tert.-Butyldiphenylsilyloxymethyl-5-hydroxy-
cyclopentyl]-4(Z)-hexenoic acid:
23.1 g (58.3 mmol) of the compound produced according to
example lk is mixed analogously to example lj by using
carboxypropyltriphenylphosphonium bromide and, after working up,
62.6 g of the title compound is isolated as crude product, that
is further reacted without purification.
Example 79:
6-[(lR,2S,5R)-2-[3-(4-Fluorophenyl)ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
25 mg (66 ~mol) of the compound produced according to
example 78 is saponified analogously to example 2 and, after
working up and purification, 21 mg (57 ~mol, 87~) of the title
compound is isolated as colorless oil.
'' ~ ,. .
101
1H-NMR (CD30D): ~ = 1.4-2.4(m,12H), 3.12-3.35(m,2H),
4.74(m,lH), 5.4-5.55(m,2H), 6.32-7.04(m,2H), 28-7.38~m,2H).
Example 80:
6-[(lR,2S,5R)-2-[3-(4-Phenylphenyl)ureidomethyl]-5-fluoro-
cyclopentyl]-4(z)-hexenoic acid methyl ester:
80 mg tmax. 136 ~mol) of the compound produced according to
example 78a is reacted analogously to example 78 by using 4-
phenyl-phenylisocyante and, after working up and purification, 25
mg (57 ~mol, 42%) of the title compound is isolated as colorless
oil.
IR (Film): 3200-3450, 3030, 2950, 2860, 1735, 1645, 1590,
1550, 1485, 1310, 1265, 1230, 835, 765, 735 and 700 cm~1.
Example 81: .
6-[(lR,2~,5R)-2-[3-(4-Phenylphenyl)ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
25 mg (57 ~mol) of the compound produced according to
e~ample 80 is saponified analogously to example 2 and, after
working up and purification, 14 mg (33 ~mol, 58~) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.5-2.4(m,12H), 3.17-3.35(m,2H),
4.78(m,1H), 5.4-5.55(m,2H), 7.22-7.~(m,9H).
.
. ~: , ;, ~ .. ..
.:
~,,' ~ , ' ,
. .
102
Example 82:
6-[(lR,2S,5R)-2-[3-(4-Methylphenyl)ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid methyl ester:
80 mg (max. 136 ~mol) of the compound produced according to
example 78a is reacted analogously to example 78 by using 4-
methylphenylisocyante and, after working up and purification, 28
mg (74 ~mol, 55%) of the title compound is isolated as colorless
oil.
IR (Film): 3250-3450, 3050, 2950, 2860, 1730, 1640, 1600,
1555, 1515, 1260, 815, 735 and 705 cm~1.
Example 83:
6-[(lR,2S,5R)-2-[3-(4-Methylphenyl)ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
28 mg (74 ~mol) of the compound produced according to
example 82 is saponified analogously to example 2 and, after
working up and purification, 14 mg (39 ~mol, 52%) of the title
compound is isolated as colorless oil.
1H-NMR (CD~OD): ~ = 1.45-2.4(m,15H), 3.12-3.34(m,2H),
4.76(m,lH), 5.4-5.55tm72H), 7.06(d,2H), 7.21(d,2H).
Example 84:
6-[(lR,2S,5R)-2-[3-(3-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid methyl ester:
- 80 mg (max. 136 ~mol) of the compound produced according to
example 78a is reacted analogously to example 78 by using 3-
chlorophenylisocyante and, after working up and purification, 25
"'
,:
. .
.
,
103
-" ~088~1
mg (63 ~mol, 46%) of the title compound is isolated as colorless
oil.
IR (Film): 3200-3450, 3040, 2950, 2860, 1730, 1650, 1590,
1550, 1480, 1435, 1420, 1300, 1265, 1230, 1165, 775, 735, 700 and
680 cm1.
Example 85:
6-[(lR,2S,5R)-2-[3-(3-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
25 mg (63 ~mol~ of the compound produced according to
example 84 is saponified analogously to example 2 and, after
working up and purification, 10 mg (26 ~mol, 41%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.45-2.4(m,12H), 3.15-3.35(m,2H),
4.76(m,lH), 5.4-5.57(m,2H), 6.9-6.98(m,lH), 7.15-7.24(m,2H),
7.57(s,lH).
Example 86:
6-[(lR,2S,5~)-2-[3 Phenyl-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z) hexenoic acid methyl ester:
80 mg (max. 136 ~mol) of the compound produced according to
example 78a is reacted analogously to example 78 by using
phenylisocyante and, after working up and purification, 24 mg (66
~mol, 49%) of the ti~le compound is isolated as colorless oil.
IR (Film): 3200-3450, 3050, 2950, 2860, 1730, 1645, 1595,
1550, 1495, 1435, 1310, 1230, 1170, 750, 735 and 695 cm-1.
. .
,. . '
104
Example 87:
6-[(lR,2S,5R)-2-[3-Phenyl-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
24 mg (66 ~mol) of the compound produced according to
example 86 is saponified analogously to example 2 and, after
working up and purification, 16 mg (46 ~mol, 70~) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.45-2.4(m,12H), 3.12-3.35(m,2H),
4.75(m,1H), 5.4-5.55(m,2H), 6.96(t,lH), 7.2-7.38(m,4H).
Example 88:
6-[(lR,2S,5R)-2-[3-(3,4-Dichlorophenyl)-ureidomethyl]-5-
fluoro-cyclopentyl]-4(Z)-hexenoic acid methyl ester:
80 mg (max. 136 ~mol) of the compound produced according to
example 78a is reacted analogously to example 78 by using 3,4-
dichlorophenyliso~yante and, after working up and purification,
26 mg (60 ~mol, 44%) of the title compound is isolated as
colorless oil.
IR (Film): 3500, 3200-3450, 3010, 2950, 2860, 1730, 1650,
1585, 1540, 1470, 1375, 1265, 1230, 1130, 1025, 815, 735 and
700 cm1.
Example 89:
6-t(lR,2S,5R)-2-[3-(3,4-Dichlorophenyl)-ureidomethyl]~5-
fluoro-cyclopentyl]-4(Z)-hexenoic acid:
26 mg (60 ~mol) of the compound produced according to
example 88 is saponified analogously to example 2 and, after
' ~ ~
~: :
105
~8~
working up and purification, 15 mg (36 ~mol, 60%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.5-2.4(m,12H), 3.16-3.35(m,2H),
4.75(m,1H), 5.4-5.55(m,2H), 7.21(dd,1H), 7.35(d,1H), 7.71(d,1H).
Example 90:
6-[(lR,2S,5R)-2-[3-(4-Nitrophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid methyl ester:
100 mg (max. 170 ~mol) of the compound produced according to
example 78a is reacted analogously to example 78 by using 4-
nitrophenylisocyante and, after working up and purification, 32
mg (79 ~mol, 46%) of the title compound is isolated as yellow
oil.
IR (Film): 3200-3450, 3010, 2950, 2870, 1730, 1700, 1670,
1610, 1600, 1550, 1500, 1330, 1300, 1230, 1175, 1110, 850, 735
and
700 cm~1.
Example 91:
6-[(lR,2S,5R)-2-[3-(4-Nitrophenyl~-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
32 mg (79 ~mol) of the compound produced according to
example 90 is saponified analogously to example 2 and, after
working up and purification, 31 mg (79 ~mol, 100%) of the title
compound is isola~ed as yellow oil.
1H-NMR (CD30D): ~ = 1.5~2.4(m,12H), 3.18-3.35(m,2H),
4.76(m,1H), 5.4-5.55(m,2H), 7.58(d,2H), 8.14(d,2H).
, . ................................................... .
', ~ , ' ' ` `
' '
106 ~ ~ g~,
Example 92:
6-[(lR,2S,5R)-2-[3-(4-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid methyl ester:
100 mg (max. 170 ~mol) of the compound produced according to
example 78a is reacted analogously to example 78 by using 4-
chlorophenylisocyanate and, after working up and purification, 38
mg (101 ~mol, 59%) of the title compound is isolated as colorless
solid.
IR (KBr): 3200-3450, 3030, 2960, 2850, 1735, 1650, 1590,
1545, 1470, 1420, 1375, 1265, 1230, 1160, 780, 735 and
700 cm-1.
Example 93:
6-[(lR,2S,5R)-2-[3-(4-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
38 mg (101 ~mol) of the compound produced according to
example 92 is saponified analogously to example 2 and, after
working up and puri~ication, 33 mg (86 ~mol, 85%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.45-2.4(m,12H), 3.15-3.35(m,2H),
4.76(m,lH), 5.4-5.55(m,2H), 7.21(dd,2H), 7.35(dd,2H).
Example 94:
6-[(lR,2S,5R)-2-[3-(4-Aminophenyl)-ureidometh~1]-5-fluoro-
cyclopentyl]-4(z)-hexanoic acid:
The solution of 15 mg (38 mmol) of compound produced
according to example gl in 1 ml of ethyl acetate is mi~ed with 5
107 i~ 8~
mg of palladium on carbon (10%) and hydrogenated at 1 at
hydrogen. After absorption of the theoretical amount of
hydrogen, it is filtered, concentrated by evaporation, and the
residue chromatographically purified on an analytic thin-layer
slab. A mixture of dichloromethane and ethanol is used as mobile
solvent, a mixture of trichloromethane and isopropanol is used as
eluant. 13 mg (36 ~mol, 94%) of the title compound is isolated
as waxy solid.
lH-NMR (CD30D): ~ - 1.2-2.0(m,14H), 2.26(t,2H), 3.1-
3.3(m,2H), 4.76(m,1H), 6.71(d,2H), 7.08(d,2H).
Example 95
6-[(lR,2S,5R)-2-[3-(4-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-hexanoic acid:
16 mg (44 ~mol) of the compound produced according to
example 93 is reacted analogously to example 94 and, after
working up and purification, 16 mg (42 ~mol, 94%) of the title
compound is isolated as colorless solid.
1H-NMR (Acetane d6): ~ = 1.25-l.9(m, 14H), 2.28(t,2H), 2.5-
3.5(s,1H), 3.18(m,1H), 3.32(m,1H), 4.77(m,1H), 5.99(t,1H),
7.21(d,2H), 7.5(d,2H), 8.08(s,1H).
Example_96:
6-[(lR,2S,5R)-2-[(2-Nitrophenyl)-sulfonylaminomethyl3-5-
fluoro-cyclopentyl]-4(Z) hexenoic acid methyl ester:
147 mg (max. 250 ~mol) of the compound produced according to
example 78a is reacted analogously to example lb by using 2-
10~
~8~
nitrobenzenesulfonic acid chloride and, after working up andpurification, 54 mg (126 ~mol, 50~) of the title compound is
isolated as colorless oil.
IR (Film): 3200-3400, 3100, 3010, 2950, 2920, 2860, 1730,
1590, 1540, 1435, 1415, 1360, 1165, 1070, 855, 780, 740 and
655 cm-1.
Example 97:
6-[(lR,ZS,5R)-2-[(2-Nitrophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-4(Z)-hexenoic acid:
54 mg (126 ~mol) of the compound produced according to
example 96 is saponified analogously to example 2 and, after
working up and purification, 48 mg (116 ~mol, 92~) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.4-2.4(m,12H), 2.99(dd,lH),
3.15(dd,lH), 4.7(m,lH), 5.33-5.5(m,2H), 7.38-7.48(m,3H), 8.03-
8.1(m,lH).
Example 98:
6-[(lR,2S,5R~-2-[(4-Methylphenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-4(Z)-hexenoic acid methyl ester:
147 mg (max. 250 ~mol) of the compound produced according to
example 78a is reacted analogously to example lb by using tosyl
chloride and, after working up and purification, 83 mg (209 ~mol,
84%) of the title compound is isolated as colorless oil.
IR (Film): 3200-3400, 3010, 2950, 2870, 1730, 1600, 1435,
1330, 1285, 1160, 1090, 1075, 815 and 665 cm~1.
,.
; . . : : ~ ': ' '
.
lOg
~881~1
Example 99:
6-[(lR,2S,5R)-2-[(4-Methylphenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-4(Z)-hexenoic acid:
83 mg (209 ~mol) of the compound produced according to
example 98 is saponified analogously to example 2 and, after
working up and purification, 69 mg (180 ~mol, 86%) of the title
compound is isolated as colorless solid.
IR (Film): (CD30D): ~ = 1.4-1.55(m,1H), 1.63-2.38(m,11H),
2.42(s,3H), 2.77(m,1H), 3.92(m,1H), 4.7(m,1H), 5.33-5.5(m,2H),
7.38(d,2H), 7.72(d,2H).
Example 100:
6-[(lR,2S,5R)-2-[(3,4-Dichlorophenyl)-sulfonylaminomethyl]-
5-fluoro-cyclopentyl]-4(Z) hexenoic acid methyl ester:
147 mg (max. 250 ~mol) of the compound produced accordin~ to
example 78a is reacted analogously to example lb by using 3,4-
dichlorobenzenesulfonic acid chloride and, after working up and
purification, 81 mg (179 ~mol, 72%) of the title compound is
isolated as colorless oil.
IR (Film): 3090, 3010, 2950, 2850, 1730, 1565, 1450, 1380,
1170, 1140, 1095, 1030, 885, 825, 780, 735, 710, 675 and 625 cm~
.
110
~8816~
Example 101:
6-[(lR,2S,5R)-2-[(3,4-Dichlorophenyl)-sulfonylaminomethyl]-
5-fluoro-cyclopentyl]-4(Z)-hexenoic acid:
81 mg (179 ~mol) of the compound produced according to
example 100 is saponified analogously to example 2 and, after
working up and purification, 68 mg (155 ~mol, 87%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.4-2.4(m,12H), 2.83(dd,lH),
2.97(dd,lH), 4.7(m,lH), 5.35-5.5(m,2H), 7.73(m,2H), 7.98(d,lH).
Example 102:
6-[(lR,2S,5R)-2-[(4-Fluorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-4(z)-hexenoic acid methyl ester:
147 mg (maxO 250 ~mol) of the compound produced according to
example 78a is reacted analogously to example lb by using 4-
fluorobenzenesulfonic acid chloride and, after working up and
purification, 56 mg (139 ~mol, 56%) of the title compound is
isolated as colorless oil.
IR (Film): 3200-3400, 3010, 2950, 2860, 1730, 1600, 1490,
1430, 1330, 1235, 1160, 1090, 840 and 670 cm~1.
Example 103:
6-~(lR,2S,5R)-2-[(4-Fluorophenyl)-su~fonylaminomethyl]-5-
fluoro-cyclopentyl]-4(z)-hexenoic acid:
56 mg (139 ~mol) of the compound produced according to
example 102 is saponified analogously to example 2 and, after
. .;
' . ~'`: ~
lll
working up and purification, 51 mg (132 ~mol, 95%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.4-2.36(m,12H), 2.8(dd,lH),
2.95(dd,1H), 4.7(m,1H), 5.33-5.5(m,2H), 7.3(m,2H), 7.9(m,2H).
Example 104:
6-[(lR,2S,5R)-2-[Phenylsulfonylaminomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid methyl ester:
147 mg (max. 250 ~mol) of the compound produced according to
example 78a is reacted analogously to example lb by using
benzenesulfonic acid chloride and, after working up and
purification, 51 mg (133 ~mol, 53~) of the title compound is
isolated as colorless oil.
IR (Film): 3200-3400, 3060, 3010, 2950, 2860, 1730, 1445,
1325, 1160, 1095, 755, 720 and 690 cm~1.
Example 105:
6-[(1~,2S,5R)-2-[Phenylsulfonylaminomethyl]-5-fluoro-
cyclopentyl]-4(Z)-hexenoic acid:
51 mg (133 ~mol) of the compound produced according to
example 104 is saponified analogously to example 2 and, after
working up and purification, 43 mg (116 ~mol, 88%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.4-2.4(m,12H), 2.78(dd,lH),
2.93(dd,lH), 4.7(m,lH), 5.33-5.5(m,2H), 7.52-7.65(m,3H~,
7.86(m,2H).
,
.
:,
112 ~ ~8
Exam~le 106:
6-[(lR,2S,5R)-2-[(4-Chlorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-4(Z)-hexenoic acid methyl ester:
147 mg (max. 250 ~mol) of the compound produced according to
example 78a is reacted analogously to example lb by using 4-
chlorobenzenesulfonic acid chloride and, after working up and
purification, 60 mg (143 ~mol, 57%) of the title compound i5
isolated as colorless oil.
IR (Film): 3200-3400, 3090, 3010, 2950, 2870, 1730, 1585,
1470, 1435, 1330, 1160, 1090, 1010, 830, 750 and 620 cm~l.
Example 107:
6-[(lR,2S,5R)-2-[(4-Chlorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-4(Z)-hexenoic acid:
60 mg (143 ~mol) of the compound produced according to
example 106 is saponified analogously to example 2 and, ~ter
working up and purification, 45 mg (111 ~mol, 78%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.4-2.4(m,12H), 2.8(dd,lH),
2.95(dd,1H), 4.7(m,1H), 5.33-5.5(m,2H), 7.58(d,2H), 7.82(d,2H~.
Exam~le 108:
6-[(lR,2S,5R)-2-[(4-Methylphen~l)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-hexanoic acid:
20 mg (52 ~mol) of the compound produced according to
example 99 is reacted analogously to example g4 and, after
- , , , ~ ; ,. , - - .
, ~ "
113
~881~1
working up and purification, 20 mg (52 ~mol, 99%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.2-l.9(m,14H), 2.28(t,2H), 2.42(d,3H),
2.76(dd,lH), 2.92(dd,lH), 4.68(m,lH), 7.48(d,2H), 7.72(d,2H).
Example 109:
5-[(lR,2S,5R)-2-[Phenyl-sulfonylaminomethyl]-5-fluoro-
cyclopentyl]-hexanoie acid:
19 mg (51 ~mol) of the compound produced according to
example 105 is reacted analogously to example 94 and, after
working up and purification, 19 mg (51 ~mol, 100%) of the title
eompound is isolated as eolorless oil.
1H-NMR (CD30D): ~ = 1.2-I.9(m,14H), 2.28(t,2H),
2.77(dd,lH), 2.92(dd,lH), 4.7(m,lH), 7.52-7.66(m,3H), 7.85(m,2H).
Example 110:
6-[(lR,2S,5R)-2-[(4-Chlorophenyl)-sulfonylaminomethyl]-5-
fluoro-eyelopentyl]-hexanoie aeid:
19 mg (47 ~mol) of the eompound produeed aecording to
example 107 is reaeted analogously to example 94 and, after
working up and purifieation, 17 mg (42 ~mol, 89%) of the title
eompound is isolated as eolorless oil.
1H-NMR (CD30D): ~ = 1.2-l.9(m,14H), 2.29(t,2H),
2.81(dd,lH), 2.95(dd,lH), 4.72(m,lH), 7,58(d,2H), 7,82(d,2H).
:
114
Example 111:
6-[(lR,2S,5R)-2-[~4-Fluorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-hexanoic acid:
19 mg (49 ~mol) of the compound produced according to
example 103 is reacted analogously to example 94 and, after
working up and purification, 19 mg (49 ~mol, 100%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.2-1.96(m,14H), 2.28(t,2H),
2.78(dd,lH), 2.93(dd,lH), 4.72(m,lH), 7,3(m,2H), 7,9(m,2H).
Example 112:
6-[(lR,2S,5R)-2-[3-(4-Fluorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-hexanoic acid:
10 mg (27 ~mol) of the compound produced according to
example 79 is reacted analogously to example 94 and, after
working up and purification, 10 mg (27 ~mol, 100%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.25-2.0(m,14H), 2.26(t,2H), 3.1-
3.32(m,2H), 4.76(m,lH), 6.97(m,2H), 7,31(m,2H).
Example 113:
7-[(lR,2S,5R)-2-[(4-Chlorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
129 mg (max. 245 ~mol) of the compound produced according to
example 113a is reacted analogously to example lb by using 4-
chlorobenzene sulfonic acid chloride and, after working up and
: ` ' :
'~ ~
115
~8~
purification, 49 mg (113 ~mol, 46%) of the title compound is
isolated as colorless oil.
IR (Film): 3200-3400, 3010, 2950, 2860, 1730, 1585, 1435,
1330, 1160, 1090, 830, 750, 735, 700 and 620 cm~1.
Example 113a:
7-[(lR,2S,5R)-2-Aminomethyl-5-fluoro-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
416 mg (1.47 mmol) of the compound produced according to
example 113b is reacted analogously to example 78a and, after
working up and purification, 776 mg (max. 1.47 mmol, about 50%)
of the title compound is isolated as colorless oil.
Example 113b:
7-[(lR,2S,5R)-2-Azidomethyl-5-fluoro-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
502 mg (1.56 mmol) of the compound produced according to
example 113c is reacted analogously to example 78b and, a~ter
working up and purification, 416 mg (1.47 ~mol, 94%) of the titlP
compound is isolated as colorless oil.
IR ~Film): 2950, 2870, 2100, 1735, 1435, 1360, 1245 and
1160 cm-1.
.
116
~ ~ g ~
Example 113c:
7-[(lR,2S,5R)-2-Bromomethyl-5-fluoro-cyclopentyl]-5(Z)-
heptenoic acid methyl ester:
410 mg tl.59 ~mol) of the compound produced according to
example 113c is reacted analogously to example 78c and, after
working up and purification, 502 mg (1.56 mmol, 98%) of the title
compound is isolated as colorless oil.
IR (Film): 2950, 2870, 1735, 1435, 1360, 1245, 1225, 1170
and 950 cm~l.
Example 113d:
7-[(lR,2S,5R)-2-Hydroxymethyl-5-fluoro~cyclopentyl]-5(Z)-
heptenoic acid methyl ester (A) and 7-[(5S)-5-hydroxy-cyclopent-
1-enyl]-5(Z)-heptenoic acid methyl ester (B):
3.75 g of the substance mixture produced according to
example 113e is reacted analogously to example 78d and, after
working up and purification, 941 mg (3.95 mmol, 38%) of title
compound B as nonpolar component as well as 820 mg (3.17 mmol,
31%) of title compound A as polar component are each isolated as
colorless oil.
IR (Film) of A: 3200-3600, 3050, 2950, 2870, 1735, 1435,
1360, 1165, 1040, 945 and 875 cm~1.
IR tFilm) of B: 3200-3600, 3050, 2950, 2860, 1735, 1435,
1360, 1200, 1160 and 1025 cm~1.
117
~8~6~
Example 113e:
7-[(lR,2S,5R)-2-tert.-Butyldiphenylsilyloxymethyl-5-~luoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester and 7-[(5S)-5-
ttert.-butyldiphenylsilyloxymethyl)-cyclopent-1-enyl]-5(Z)-
heptanoic acid methyl ester:
5.08 g (10.3 mmol) of the compound produced according to
example li is reacted analogously to example lg and, after
working up and purification, 3.75 g of a mixture of both title
compounds is isolated as colorless oil.
Example 114:
7-[(lR,2S,5R)-2-[(4-Chlorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid:
49 mg (113 ~mol) of the compound produced according to
example 113 is saponified analogously to example 2 and, after
working up and purification, 47 mg (122 ~mol, 99%) of the title
compound is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.35-2.2(m,12H), 2.38(t,2H),
2.93(t,2H), 4.79(m,lH), 5.35-5.58(m,3H), 7.48(d,2H), 7.79(d,2H).
Example 115:
7-[(lR,2S,5R)-2-[(4-Fluorophenyl)-sul~onylaminomethyl]-5-
fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl ester:
129 mg (max. 245 ~mol) of the compound produced ac~ording to
example 113a is reacted analogously to example lb by using 4-
fluorobenzene sulfonic acid chloride and, after working up and
'
118
~ ~ 8 8 ~ ~ 1
purification, 50 mg (120 ~mol, 49%) of the title compound is
isolated as colorless oil.
IR (Film): 3200-3400, 3060, 3010, 2940, 2860, 1730, 1590,
1490, 1435, 1330, 1235, 1165, 1150, 1090, 840, 735, 700 and
670 cm~1.
Example 116:
7-[(lR,2S,5R)-2-[(4-Fluorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-5(æ)-heptenoic acid:
50 mg (120 ~mol) of the compound produced according to
example 115 is saponified analogously to example 2 and, after
working up and purification, 44 mg (110 ~mol, 91%) of the title
compound is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.35-2O2(m,12H), 2.38(t,2H),
2.g2(t,2H), 4.77(m,lH), 5.35-5.58(m,3H), 7.2(m,2H), 7.79(m,2H~. -
Example 117:
7-[(lR,2S,5R)-2-[3-(4-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
129 mg (max. 245 ~mol) of the compound produced according to
example 113a is reacted analogously to example 78 by using 4-
chlorophenylisocyanate and, after working up and purification, 60
mg (146 ~mol, 60%) of the title compound is isolated as colorless
oil.
IR (Film): 3200-3400, 3040, 2950, 2850, 1730, 1650, 1595,
1545, 1470, 1420, 1375, 1260, 1225, 1160, 785, 735 and 700 cm~1.
, ~
119
~881&1
Example 118:
7-[(lR,2S,5R)-2-[3-(4-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
60 mg (146 ~mol) of the compound produced according to
example 117 is saponified analogously to example 2 and, after
working up and purification, 42 mg (106 ~mol, 72%) of the title
compound is isolated as colorless oil.
lH-NMR (CDCl3): ~ = 1.38-2.2(m,12H), 2.36(t,2H), 3.2(t,2H),
4.78(m,lH), 5.35-5.48(m,2H), 5.58(t,lH), 7.15-7.25(m,4H),
7.49(s,1~).
Example 119:
7-[(lR,2S,5R)-2 [3-(4-Nitrophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid methyl ester:
129 mg (max. 245 ~mol) of the compound produced according to
example 113a is reacted analogously to example 78 by using 4-
nitrophenylisocyanate and, after working up and purification, 53
mg (126 ~mol, 51%) of the title compound is isolated as colorless
oil.
IR (Film): 3200-3400, 3080, 3010, 2950, 2860, 1730, 1700,
1670, 1610, 1600, 1550, 1500, 1325, 1300, 1230, 1175, 1110, 8~0,
735 and 700 cm~1.
.
120
Example 120:
7-[(lR,2S,5R)-2-[3-(4-Nitrophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-5(Z)-heptenoic acid:
53 mg (126 ~mol) of the compound produced according to
example 119 is saponified analogously to example 2 and, after
working up and purification, 44 mg (105 ~mol, 83%) of the title
compound is isolated as yellow oil.
1H-NMR (CDC13): ~ = 1.32-2.15(m,12H), 2.29(t,2H),
3.12(t,2H), 4.72(m,lH), 5.25-5.4(m,2H), 5.5(t,lH), 7.08-
7.15(m,4H), 7.4(s,lH).
Example 121:
7-[(lR,2S,5R)~2-[(E/Z)-3-(3,4-Dichlorophenyl)-
ureidoiminomethyl]-5-fluoro~cyclopentyl]-5(Z)-heptenoic acid
methyl ester:
55 mg (max. 199 ~mol) of the ~ompound produced according to
example 121a is reacted analogously to example 62b by using 4-
(3,4-dichlorophenyl)semicarbazide hydrochloride and, after
working up and purification, 75 mg (164 ~mol, 82%) of the title
compound is isolated as colorless oil.
IR (Film): 3360, 3200, 3100, 2940, 1730, 1690, 1580, 1520,
1470, 1390, 1290, 1220, 1130, 1025, 950, 875, 815, 740 and
690 cm-1.
... '
,,' ' ~'' . ,,; ,
121
~81~1
Example 12la:
7-[(lR,2S,5R)-2-Formyl-5-fluoro-cyclopentyl]-5(Z)-heptenoic
acid methyl ester:
410 mg (1.59 mmol) of compound A produced according to
example 113d is reacted analogously to example 50b and, after
working up, 440 mg (max. 1.59 mmol) of the title compound is
isolated as colorless oil, that is further reacted without
purification.
Example 122:
7-[(lR,2S,5R)-2-[(E/Z)-3-(3;4-Dichlorophenyl)-
ureidoiminomethyl]-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
38 mg (83 ~mol) of the compound produced according to
example 121 is saponified analogously to example 2 and, after
working up and purification, 28 mg (63 ~mol, 76%) of the title
compound is isolated as colorless oil.
1H-NMR (CDCl3): ~ = 1.62-2.5(m,14H), 2.77-2.88(m,0.25H),
4.78(m,lH), 5.2-5.3(m,-0.25H), 5.42-5.58(m,1.75H), 6.52(d,0.25H),
7.21(d,0.75H), 7.23-7.4(m,2H), 7.72(dd,1H), 8.02(s,0.75H),
8.28(s,0.25H), 9.62(s,0.75H), 10.01(s,0.25H).
Exam~le 123:
7-[(lR,2S,5R)-2-[(E/Z)-3-(4-Chlorophenyl)-
ureidoiminomethyl]-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid
methyl ester:
165 mg (max. 596 ~mol) of the compound produced according to
example 121a is reacted analogously to example 62b by using 4-(4-
:: ' ' ` :
:
..
122 ~8~
chlorophenyl)semicarbazide-hydrochloride and, after working up
and purification, 198 mg (467 ~mol, 78%) of the title compound is
isolated as colorless oil.
IR (Film): 3370, 3200, 3100, 2940, 2870, 1730, 1685, 1590,
1525, 1400, 1310, 1225, 1090, 950, 825 and 740 cm~1.
Example 124:
7-[(lR,2S,5R)-2-[(E/Z)-3-(4-Chlorophenyl)-
ureidoiminomethyl]-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
60 mg (142 ~mol) of the compound produced according to
example 123 is saponified analogously to example 2 and, after
working up and purification, 50 mg (122 ~mol, 86%) of the title
compound is isolated as colorless oil.
lH-NMR (CDC13): ~ = 1.62-2.S(m,14H), 2.77-2.88(m,0.25H),
4.76(m,lH), 5.2-5.3(m,-0.25H), 5.42-5.58(m,1.75H), 6.52(d,0.25H),
7.2(d,0.75H), 7.28(m,2H), 7.43(m,2H), 8.02(s,0.75H),
8.24(s,0.25H), 9.64(s,0.75H), 9.98(s,0.25H).
Example 125:
7-[(lR,2S,5R)-2-[3-(4-Nitrophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-heptanoic acid:
22 mg (56 ~mol) of the compound produced according to
example 120 is reacted analogously to example 94 and, after
working up and purification, 18 mg (44 ~mol, 79~) of the title
compound is isolated as yellow oil.
1H-NMR (CD30Dj: ~ = 1.25-2(m,16H), 2.23(t,2H), 3.12-
3.36(m,2H), 4.76(m,lH), 7,58(d,2H), 8.13(d,2H).
..
..,~ ` :
'
123
~881~1
Example 126:
7-[(lR,2S,5R)-2-[3-(4-Chlorophenyl)-ureidomethyl]-5-fluoro-
cyclopentyl]-heptanoic acid:
20 mg (52 ~mol) of the compound produced according to
example 118 is reacted analogously to example 94 and, after
working up and purification, 20 mg (50 ~mol, 96%) of the title -
compound is isolated as colorless solid.
lH~NMR (CD30D): ~ = 1.25-2(m,16H), 2.23(t,2H), 3.12-
3.32(m,2H), 4.75(m,1H), 7,21(d,2H), 7.35(d,2H).
Example 127:
7-[(lR,2S,5R)-2-[4-Fluorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-heptanoic acid:
22 mg (55 ~mol) of the compound produced according to
example 116 is reacted analogously to example 94 and, after
working up and purification, 19 mg (47 ~mol, 86%) of the title
compound is isolated as colorless solid.
1H-NMR (CDCl3)~ 2(m,16H), 2.35(t,2H), 2.83-
3.08(m,2H), 4.73(m,lH), 5.08(t,lH), 7.2(m,2H), 7.89(m,2H).
Example 128:
7-[(lR,2S,5R)-2-[4-Chlorophenyl)-sulfonylaminomethyl]-5-
fluoro-cyclopentyl]-heptanoic acid:
23 mg (56 ~mol) of the compound produced according to
example 114 is reacted analogously to example 94 and, after
working up and purification, 20 mg (48 ~mol, 85%) of the title
compound i= isolated as colorless solid.
.:
124
~881~1
1H-NMR (CDCl3)~ l.g5(m,16H), 2.3(t,2H~, 2.76-
3(m,2H), 4.68(m,lH), 5.02(t,lH), 7.42(d,2H), 7.73(d,2H).
Example 129:
7-[(lR,2S,5R)-2-[~E/Z)-2-(4-Fluorophenylsulfonyl)-
hydrazonomethyl]-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl
ester:
165 mg (max. 596 ~mol) of the compound produced according to
example 121a is reacted analogously to example 62b by using 4-
fluorobenzenesulfonylhydrazide and, after working up and
purification, 225 mg (525 ~mol, 88%) of the title compound is
isolated as colorless oil.
IR (Film): 3180, 2950, 2860, 1730, 1710, 1590, 1490, 1435,
1365, 1320, 1235, 1170, 1155, 1090, 835 and 670 cm~1.
Example 130:
7-[(lR,2S,5R)-2-[(E/Z)-2-(4 Fluorophenylsulfonyl)-
hydrazonomethyl]-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
60 mg (140 ~mol) of the compound produced according to
example 129 is saponified analogously to example 2 and, after
working up and purification, 52 mg (125 ~mol, 90%) of the title
compound is isolated as colorless oil.
1H-NMR (C~Cl3): ~ = 1.54-2.5(m,14H), 4.8(m,lH~, 5.22-
5.56(m,lH3, 7.1-7.23(m,3H).
;
.. , ' :
125
Exam~le 131:
7~ R,2s~5R)-2-[(E/z)-2-(4-Methylphenylsulfonyl)-
hydrazonomethyl]-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid methyl
ester:
55 mg (max. 199 ~mol) of the compound produced according to
example 121a is reacted analogously to example 62b by using 4-
toluenesulfonylhydrazide and, after working up and purification,
78 mg (192 ~mol, 97%) of the title compound is isolated as
colorless oil.
IR (Film): 3200, 3000, 2950, 2870, 1730, 1710, 1595, 1435,
1360, 1320, 1160, 1090, 1030, 930, 810, 705 and 670 cm~1.
Example 132:
7-[(lR,2S,5R)-2-[~E/Z)-2-(4-Methylphenylsulfonyl)-
hydrazonomethyl]-5-fluoro-cyclopentyl]-5(Z)-heptenoic acid:
39 mg (96 ~mol) of the compound produced according to
example 131 is saponified analogously to example 2 and, after
working up and purification, 33 mg (84 ~mol, 88%) of the title
compound is isolated as colorless solid.
1H-NMR (CDCl3): ~ = 1.55-2O2tm,12H), 2.32(t,2H~,
2.42(s,3H), 4.77(m,lH), 5.23-5.55(m,2H), 6.69(d,0.lH),
7.13(d,0.9H), 7.3(d,2H), 7.8(d,2H).
12
Example 133:
7~ R~2s~5R)-2-[(E/z)-2-(4-Fluorophenylsulfon
hydrazonomethyl]-5-fluoro-cyclopentyl]-heptanoic acid:
37 mg (89 ~mol) of the compound produced according to
example 130 is reacted analogously to example 94 and, after
working up and purification, 14 mg (34 ~mol, 38%) of the title
compound is isolated as colorless oil.
1H-NMR (CD30D): ~ = 1.0-1,75(m,15H), 2.08-2.22(m,3H),
4.62(m,lH), 7.04(d,lH), 7.21(m,2H), 7.82(m,2H).
Example 134:
7-[(lR,2S,5R)-2-[(E/Z)-2-(4-Methylphenylsulfonyl)-
hydrazonomethyl]-5-fluoro-cyclopentyl]-heptanoic acid:
19 mg (49 ~mol) of the compound produced according to
example 132 is reacted analogously to example 94 and, after
working up and purification, 9 mg (23 ~mol, 47%) of the title
compound is isolated as colorless oil.
1~-NMR (CD30D): ~ = 1.12-1.34(m,16H), 2.16 2.32(m,3H),
2.92(s,3H), 4.7(m,lH), 7.12(d,lH), 7.37(d,2H), 7.76(d,2H).
Example 135:
7-[(lR,2S,5R)-2-[(E/~)-3-(4-Chlorophenyl)ureidoiminomethyl]-
5-fluoro-cyclopentyl]-heptanoic acid (A) and 7-[(lR,2S,5R)-2-[3-
(4-chlorophenyl)ureidoiminomethyl]-5-fluoro-cyclopentyl]-
heptanoic acid (B):
20 mg (49 ~mal) of the compound produced according to
example 124 is reacted analogously to example 94 by using PtOz
,
.
- ,
127 ~8~
and, after working up and purification, 11 mg (27 ~mol, 54%) of
title compound A as nonpolar component as well as 8 mg (19 ~mol,
39%) of title compound B are each isolated as waxy solid~
1H-NMR (CD30D) of A: ~ = 1.27-2.12(m,15H), 2.23(t,2H),
2.43(m,lH), 4.78(m,2H), 7.22(d,lH), 7.27(d,2H), 7.5(d,2H)
1H-NMR (CD30D) of B: ~ = 1.25-2(m,16H), 2.26(t,2H),
2.74(dd,lH), 2.95(dd,lH), 4.76(m,lH), 7.27(d,2H), 7.45(d,2H)
:
,:- .,
' ~, ' ' , ~ ' ~ ' '
::