Note: Descriptions are shown in the official language in which they were submitted.
~ V ~ Y l
W O 92/02502 1 P ~ tGB91/01340
N-HYDROCARBYL-4-SUBSTITUTED PIPERIDINES
THEIR PREPARATION AND USE
AS CALCIUM BLOCKING AGENTS
The present invention relates to 4-substituted
piperidine derivatives, processes for their preparation,
pharmaceutical compositions containing them and their use
in therapy.
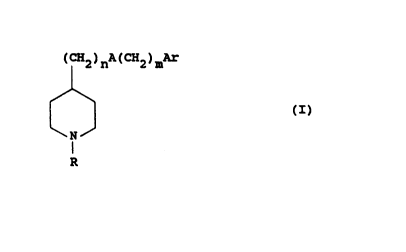
The present invention therefore provides, in a first
aspect, compounds of structure (I):
( CH2 ) nA ( CN2 ) mAr
~ (I)
R
in which
R is Cl_8alkyl(phenyl)p, C2_8alkenyl(phenyllp,
C2_8alXynyl(phenyl)p, C3_8cycloalkyl or
Cl_8alkylC3_8cycloalkyl;
p is 0 to 2;
n is 0 to 6;
A is a bond, oxygen, sulphur or NRl;
Rl is hydrogen, Cl_8alXyl or phenylCl_4alkyl;
m is 0 to 3; and
Ar is aryl or heteroaryl, each of which may be
optionally substituted;
and salts thereof.
. Suitably, R is Cl_8alkyl(phenyl)p, C2_8alkenyl-
(phenyl)p, C2_8alkynyl(phenyl)p, C3_8cycloalkyl or
Cl_8alXylC3_8cycloalkyl.
It will be understood that the alkylcycloalkyl,
alkylphenyl, alkenylphenyl and alkynylphenyl groups are
SUBSTlTlJTE 8HEET
W092/02502 2 a ~ PCT/GB9t/Ot340
- 2 -
linked to the piperidine nitrogen atom via the alkyl,
alkenyl and alkynyl moieties respectively.
Preferably R is C1_8alkyl(phenyl)p in which p is 0 or l,
i.e. C1_8alkyl, such as n-pentyl, or phenylCl_8alkyl
such as phenylpropyl, or R is C2_8alkenyl(phenyl)p where p
is 1, such as cinnamyl.
Suitably, n is O to 6; preferably n is O to 3; most
preferably n is 2 or 3.
Suitably, m is 0 to 3; preferably m is 0 or 1;
most preferably m is O.
Suitably, A is a bond, oxygen, sulphur or NRl;
preferably A is oxygen or sulphur; most preferably A is
oxygen. When A is oxygen n is preferably 2 and m is
pre~erably O.
Suitably, Ar is optionally substituted aryl or
heteroaryl; preferably Ar is optionally substituted aryl.
Suitable aryl groups include, for example,
unsaturated monocyclic and unsaturated or partially
saturated bicycIic ring systems of up to lO carbon atoms,
such as, for example, phenyl, naphthyl and
tetrahydronaphthyl. Preferred are optionally
substituted phenyl rings.
Suitable substituted phenyl rings include, for
example, phenyl rings substituted by a C1_2alkylene-
dioxy group such as a 3,4-methylenedioxy group or by 1 to
3 substituents selected from halogen, C1_4alkoxy,
nitro, SC1_4alkyl, NR2R2 (in which each R2 group
can be H or c1_4alkyl), OCF3~ C1-6alkYl'
SVBSTITIJTE SHEFT
W092/02502 PCT/GB9t/0l340
2~3~
- 3 -
trifluoromethyl, CN, optionally substituted phenyl,
optionally substituted phenylC1_4alkyl and optionally
substituted phenylCl_4alkoxy. Preferred are phenyl
rings substituted by one or two substituents, in
particular, by a single halogen, trifluoromethyl,
unsubstituted phenyl or unsubstituted phenylCl_4alkoxy
group; or by two chlorine atoms, in particular in the 3
and 4 positions of the ring.
Suitable optionally substituted phenylC1_4alkyl
groups include, for example benzyl. Suitable optionally
substituted phenylCl_4aLkoxy groups include, for
example benzyloxy groups.
Suitable substituents for said optionally
substituted phenyl, phenylC1_4alkyl and
phenylCl_4alkoxy groups include for example halogen,
Cl_4alkyl, Cl_4alkoxy, nitro and tri~luoro~ethyl groups.
Suitable heteroaryl rings include, for example,
unsaturated monocyclic and unsaturated or partially
saturated bicyclic ring systems of up to 10 carbon atoms
containing ~t least one heteroatom, such as pyridyl,
thienyl, quinolinyl, tetrahydroquinolinyl and imidazolyl
rings. The heteroaryl ring can be linked to the
remainder of structure ~I) via a carbon atom or via a
hetero atom, e.g. a nitrogen atom.
Suitable substituents for said heteroaryl rings
include, for example, 1 to 3 substituents selected from
halogen, cl_4alkyl and cl-4alkoxy
Alkyl groups present in the compounds of structure
(I), alone or as part of another group, can be straight
or branched.
SUBSTIT~JTE 5HEET
W092/02502 PCT/GB91/01340
2a~91 - 4
It will be appreciated that for use in medicine a
salt of a compound (I) should be pharmaceutically
acceptable. Examples of pharmaceutically acceptable
salts include inorganic and organic acid addition salts
such as hydrochloride, hydrobromide, sulphate, phosphate,
acetate, fumarate, maleate, citrate, lactate, tartrate,
oxalate, or similar pharmaceutically acceptable inorganic
or organic acid addition salts. other non-
pharmaceutically acceptable salts may be used for example
as intermediates and are included within the scope of
this invention.
Particular compounds of the invention include :
4-[2-(4-trifluoromethylphenoxy)ethyl~-l-pentylpiperidine
oxalate,
4-t2-(3-trifluoromethylphenoxy)ethyl]-1-pentylpiperidine
hydrochloride,
4-t2-(4-~luorophenoxy)ethyl~ pentylpiperidine
hydrochloride,
4-t2-(3~4-methylenedioxyphenoxy)ethyl]-l-pentylpiperidine
hydrochloride
4-(2-phenoxyethyl)-l-pentylpiperidine hydrochloride
4-t2-(4-phenylph~oxy)ethyl~-l-pentylpiperidine
hydrochloride,
4-t2-(4-benzyloxyphenoxy~ethyl]-l-pentylpiperidine
hydrochloride,
4-[2-(4-fluorophenoxy)ethyl]-l-cinnamylpiperidine oxalate,
4-(4-fluorobenzyloxy)-l-pentylpiperidine oxalate
4-[2-(3,4-dichlorophenoxy)ethyl]-l-pentylpiperidine
hydrochloride,
4-t2-(4-benzylphenoxy)ethyl]-l-pentylpiperidine oxalate,
4-[2-(3,4-dichlorophenoxy)ethyl]-l-cinnamylpiperidine
oxalate,
4-[2-(4-fluorophenoxy)ethyl]-l-(3-phenylpropylpiperidine
hydrochloride,
4-[2-(4-fluorophenoxy)ethyl~-l-heptylpiperidine
hydrochloride,
l-(3,3-diphenylpropyl)-4-[2-(4-fluorophenoxy)ethyl]-
piperidine oxalate,
SUBSTlTlJTE 8HE~T
092/02502 PCT/GB9t/013402 ~ 91
-- 5 --
4-t2-(3,4-dichlorothiophenoxy)ethyl]-1-pentylpiperidine
hydrochloride,
4-~2-(4-tert-butylphenoxy)ethyl]-1-pentylpiperidine
hydrochloride,
4-t2-(4-iso-propylphenoxy)ethyl]-1-pentylpiperidine
hydrochloride,
4-~2-(3,4-dichlorophenoxy)ethyl]-1-(3-phenylpropyl)-
piperidine hydrochloride, and
l-cyclopropylmethyl-4-~2-(4-fluorophenoxy)ethyl]-
piperidine oxalate.
It will be appreciated that the compounds ofstructure (I) may contain one or more asymmetric
centres. Such compounds will exist as optical isomers
(enantiomers). Both the pure enantiomers, racemic
mixture~ (50% of each enantiomer) and unequal mixtures of
the two are included within the scope o~ the invention.
Further, all diastereo~eric forms possible (pure
enantiomers and ~ixtures thereof) are within the scope of
the invention.
~ he compounds of the present in~ention can be
prepared by processes analogoùs to ~hose known in the
art. The present invention therefore provides in a
further aspect, a process for the preparation of a
compound of structure (I) which comprises:
(a) for compounds of structure (I) in which A is O, S or
N21, reaction of a compound of structure (II):
( CH2 ) nAlH
(II)
N
R
SUBSTlTUl E SHEET
W092/02502 PCT/GB91/01340
2~ 191
-- 6 --
in which R and n are as described for structure (I)and
Al is O, S or NRl, with a compound of structure
L(CH2)mAr in which m and Ar are as described for
structure (I), and L is a leaving group;
s
(b) for compounds of structure (I) in which A is O, S or
NRl, reaction of a compound of structure (III):
(CH2)nL
1 1 (III)
in which n and R are as described or structure (I) and
Ll is a group displaceable by a nucleophile, with a
compound of structure HAl(CH2)mAr where m and Ar
are as described for structure (I) and Al is as
described for structure (II); or
(c) for compounds of structure (I) in which i is NRl,
reduction of a compound of structure (IV) :
~ (IV)
N
R
in which R4 represents the group
SUBSTITUTE SHEET
W092/02502 PCT~GB91/01340
-- 7
-(cH2)nN(Rl~c(cH2)m-lAr or ~(CH2)n-lCN(R )(CH2jmAr~
and n, m, R and Ar are as described for structure (I);
5 (d) for compounds of structure (I) in which A is a bond,
reaction of a compound of structure (V) :
(~2)n+m L
~ (V)
R
(wherein R, Ll, n and m are as hereinbefore defined)
with a compound of structure XlAr in which Ar is as
described for structure (I), and Xl is an alkali metal;
~e) introduction of the group R into a compound of
~ormula (VI) :
(CH2)nA(CH2)mAr
~ (VI)
I
by reaction with a compound RL2, wherein L2 is a
leaving group;
(f) Reduction of a compound of Cormula (VII) :
SUBSTIT~TE SHEET
W092/02S02 2 ~ 3 ~ ~ 91 PCT/GB91/013~0
- 8 -
(IC~2)nA(CH2)mAr
~ (VII)
~ N J
1 5
COR
wherein R5 is Cl_7alkyl(phenyl)p, C2_7alkenyl(phenyl)p,
C2_7alkynyl(phenyl)p or Cl_7aLkylC3_8cycloalkyl;
(g) Reduction of a compound of structure (VIII):
(CH2)nA(CH2)mAr
~ (VIII)
wherein R, A, Ar m and n are as hereinbefore defined and
is a counter ion;
and optionally thereafter forming a salt.
In process (a) the reaction between a compound of
structure (II) and a compound L(CH2)mAr can take
place under conditions which depend on the nature of the
group L. For example, when L is halogen or a sulphonic
acid residue such as a tosylate or mesylate, the reaction
is carried out under standard conditions in a solvent,
optionally in the presence of a base. When a
fluoro-substituted aryl F-Ar is employed in process (a),
the reaction is effected in the presence of a strong base
SUBSTITUTE SHEET
W092/02502 2 ~ 9 1 PCT/GB91/01340
_ g _
such as sodium hydride, and in an inert organic solvent
such as dimethyl formamide. Prefera~ly the aryl group is
substituted by an activating group such as CF3 or N02.
The reaction between a compound of structure (III)
and a compound of structure HA1(C~2)mAr can take
place under conditions which depend on the nature of L1
and A. For example when L1 is hydroxy, m is 0 and Al
is oxygen or sulphur, the reaction is carried out in the
presence of diethyl azodicarboxylate and triphenyl
phosphine. Such a reaction is known as the Mitsunobu
reaction (as described in Synthesis 1981, 1).
Alternatively the leaving group Ll may be for example a
halogen atom or a sulphonyloxy group eg. methane-
sulphonyloxy or p-toluenesulphonyloxy. In this case the
reaction may be effected in the presence or absence of
solvent at a temperature in the range 0 to 200C
The reduction o~ a compound of structure (I~) can be
e fected by methods known in the art, for example using a
reducing agent such as lithium aluminium hydride.
Conveniently a compound of structure (IV) can be prepared
(for example as described below) and reduced in a
'one-pot' reaction, without isolation of compound (IV)
2S itself.
The reaction between a compound of structure (V) and
a compound of structure X1Ar can take place under
standard conditions known to those skilled in the art for
the formation of carbon-carbon bonds.
The reaction of a compound of structure (VI) with
RL2 according to process (e) may be effected in
conventional manner, for example in an organic solvent,
such as dimethyl formamide. The leaving group L2 may
be for example a halide such as bromide or chloride, an
acyloxy group such as acetoxy or chloroacetoxy or a
sulphonyloxy group such as methanesulphonyloxy or
SUBSTITUTE SHEET
W092/02502 PCT/GB91/01340
2 ~
-- 10 --
p-toluene sulphonyloxy. When L2 is a halide the
reaction is preferably carried out in the presence of a
weak base such as potassium carbonate, and when L2 is
sulphonyloxy, a strong base such as sodium hydride or
potassium t-butoxide may be employed.
Reduction of a compound of formula (VII) may be
effected using standard reducing agents such as lithium
aluminium hydride.
Reduction of a compound of formula (VIII) may be
effected for example by hydrogenation, using a noble
metal catalyst such as platinum, palladium or platinum
oxide, suitably in a solvent such as an alcohol eg.
ethanol.
The compounds of s~ructure (II) can be prepared from
the corresponding compounds in which R i5 hydrogen, by
alkylation under standard conditions. For example,
compounds o~ structure ~II) in which R is n-pentyl can be
prepared from the corresponding precursor in which R is
hydrogen by reaction with an n-pentylhalide such as
n-pentyl bromide in a suitable solvent, such as methyl
ethyl ketone, or a Cl_4alkanol such as ethanol, in the
presence of a base, such as potassium carbonate, or
dimethylformamide in the presence of an iodoalkane.
The corresponding compounds of structure (II) in
which R is hydrogen are available commercially, known in
the literature or can be prepared by standard techniques;
for example by reduction of the corresponding 4-hydroxy-
alkylpyridine.
Alternatively, compounds of structure (II) in which
Al is oxygen can be prepared by reduction of a compound
of structure (IX):
SUBSTlTlJTE 5HEET
WOg2/02502 2 ~ 3 ~ ~ 9 1 PCT/G891/01340
(CH2)nOH
(IX)
1 0 x~
in which R and n are as described for structure (I) and
X~ is a counter ion.
Compounds of structure (III) wherein Ll is OH can
be prepared as described for compounds of structure (II),
and compounds of structure (III) wherein L~ is a
halogen atom, or a mesyloxy or tosyloxy group can be
prepared from the corresponding alcohol in conventional
manner.
Compounds of structure (IV) wherein ~4 is a group
-(CH2)nN(Rl)CtCH2)m_lAr can be prepared by
reacting a compound of structure (II) wherein Al
represents NRl with an acylating agent corresponding to
the group -(CH2)mAr, for example an acid chloride
Cloc(cH2)m-lAr~
Compounds of structure (rv) wherein R4 is a group
-(CH2)n_lCN(Rl)(CH2)mAr may be prepared for
example by reaction of a corresponding compound wherein
R4 represents -(CH2)n_lCO2H or an activated
derivative thereof such as an ^id halide, ester or
anhydride, with an amine of formula HN(Rl)(CH2)m
Ar. It will be appreciated that when the acid itself is
employed, reaction with the amine should be effected in
SUBSTITIJTE SHEET
W092/02502 PCT/GBgl/01340
2~
- 12 -
the presence of a coupling agent. The carboxylic acid
may itself be prepared for example by oxidation of the
corresponding alcohol, ie. a compound of structure (II)
wherein A1 is oxygen.
Compounds of structure (V) may be prepared in
analogous manner to compounds of structure (III); where
neceæsary the chain length may be increased using methods
well known in the art.
Compounds of structure (VI) may be prepared for
example according to any of processes (a) to (d) above,
using intermediates analogous to structures (II) to (IV)
wherein R is replaced by an N-protecting group, which is
subsequently removed by methods well known in the art.
Suitable protecting groups include aralkyl groups such as
benzyl, diphenylmethyl or triphenylmethyl and acyl groups
such as acetyl, trifluoroacetyl, benzoyl,
methoxycarbonyl, ethoxycarbonyl, or benzyloxycarbonyl.
An aralkyl group such as benzyl may be cleaved by
hydrogenolysis, and an acyl group such as benzoyl may be
cleaved by hydro~ysis. It will be appreciated that where
the N-protecting group is aralkyl, the compound is of
structure (I) and this reaction sequence thus provides a
means of converting one compound of formula ~I) into a
different compound of formula (I).
A compound of formula (VII) may be prepared by
reaction of a compound of formula (VI) with an
appropriate acid derivative for example an acid chloride,
or anhydride.
A compound of structure (VIII) may be prepared using
the general methods described in processes (a) to (e)
above. In addition compounds of structure (VIII) wherein
A represents a bond may be prepared from 4-methyl
Sl,lBSTlTlJTE SHEET
W092/02502 ~ 3 ~ ~ 9; PCTtGB91/01340
- 13 -
pyridine (picoline) by reaction with a compound of
formula L(CH2)q Ar wherein L and Ar are as hereinbefore
defined and q is (m+n-l), in the presence of a strong
base such as sodium amide in liquid ammonia or an alkyl
S lithium. The resulting substituted pyridine is then
reacted with a compound RL2, as hereinbefore defined,
to give a quaternary pyridinium compound of f ormula
(VIII). Reduction of this compound according to process
(g) provides a convenient method of preparing compounds
of structure (I) wherein A represents a bond.
The compounds of the invention have been found to
exhibit high calcium influx blocking activity and as such
are expected to be of use in therapy in treating
conditions and diseases related to an accumulation of
calcium in the brain cells of mammals, in particular
humans. For example, the compounds are expected to be
o~ use in the treatment of anoxia, ischaemia including
for example stroke, migraine, epilepsy, traumatic head
injury, AIDS-related dementia, neurodegenerative diseases
such as Alzheimer's disease and age-related memory
disorders, and drug addiction withdrawal such as ethanol
addic-ion withdrawal.
In a further aspect of the invention there is
therefore provided a method of treatment of conditions or
diseases caused or exacerbated by the accumulation of
calcium in the brain cells of mammals which comprises
administering to a subject in need thereof an effective
amount of a compound of structure (I) or a
pharmaceutically acceptable salt thereof. In addition,
the present invention also provides a method of treatment
of anoxia, ischaemia including for example stroke,
migraine, epilepsy, traumatic head injury, AIDS-related
dementia, neurodegenerative diseases such as Alzheimer's
disease and age-related memory disorders, and drug
addiction withdrawal such as ethanol addiction
SUBSTITIJ~E SHEET
W~92/025~ PCT/GB91/01340
withdrawal, which comprises administering to a subject
in need thereof, an effective amount of a compound of
structure (I) or a pharmaceutically acceptable salt
thereof. The invention also provides the use of a
compound of structure (I) or a pharmaceutically
acceptable salt thereof in the manufacture of a
medicament for the treatment of the aforementioned
conditions or diseases.
In therapeutic use, the compounds of the present
invention are usually administered in a standard
pharmaceutical composition. The present invention
therefore provides in a further aspect pharmaceutical
compositions comprising a compound of structure (I) or a
pharmaceutically acceptable salt t~ereof and a
pharmaceutically acceptable carrier or excipient.
The compounds of structure (I) and their
pharmaceutically acceptable salts which are active when
gi~en orally can be formulated as liquids, for example
syrups, suspensions or emulsions, tablets, capsules and
lozenges.
A liquid formulation will generally consist of a
suspension or solution o~ the compound or pharmaceutically
acceptable salt in a suitable liquid carrier(s) for
example, ethanol, glycerine, non-aqueous solvent, for
example polyethylene glycol, oils, or water with a
suspending agent, preservative, flavouring or colouring
agent.
A composition in the form of a tablet can be
prepared using any suitable pharmaceutical carrier(s)
routinely used for preparing solid formulations.
Examples of such carriers include magnesium stearate,
starch, lactose, sucrose and cellulose.
SUBSTlTlJTE S~IEET
W092/02502 2 ~ ~ 3 ~ ~1 PCTtGB91/01340
- 15 -
A composition in the form of a capsule can be
prepared using routine encapsulation procedure~. For
example, pellets containing the active ingredient can
be prepared using standard carriers and then filled into
a hard gelatin capsule; alternatively, a dispersion or
suspension can be prepared using any suitable
pharmaceutical carrier(s), for example aqueous gums,
celluloses, silicates or oils and the dispersion or
suspension then filled into a soft gelatin capsule.
Compounds of the invention may also be administered
parenterally, by bolus injection or continuous infusion.
Typical parenteral compositions consist of a solution or
suspension of the compound or pharmaceutically acceptabl
salt in a sterile aqueous carrier or parenterally
acceptable oil, for example polyethylene glycol,
poly~inyl pyrrolidone, lecithin, arachis oil or
s~same oil. Alternatively, the solution can be
lyophilised and then reconstituted with a suitable
solvent just prior to administration.
Preferably the composition is in unit dose form such
as a tablet or capsule.
Each dosage unit for oral administration contains
preferably from l to 250 mg (and for parenteral
administration contains preferably from O.l to 60 mg) of
a compound of the formula (I) or a pharmaceutically
acceptable salt thereof calculated as the free base.
The daily dosage regimen for an adult patient may
be, for example, an oral dose of between l mg and 500 mg,
preferably between l mg and 250 mg, eg.-S to 200 mg or an
intravenous, subcutaneous, or intramuscular dose of
between O.l mg and lO0 mg, preferably between O.l mg and
60 mg, eg. l to 40 mg of the compound of the formula (I)
SU~3STIT~JTE SHEEI
W092/02502 PCT/GB91/01340
2a~il3'~
- 16 -
or a pharmaceutically acceptable salt thereof calculated
as the free base, the compound being administered 1 to 4
times per day. Alternatively the compounds of the
invention may be administered by continuous intravenous
infusion, preferably at a dose of up to lOOmg per day.
Suitably the compounds will be administered for a period
of continuous therapy, for example for a week or more.
SUBSTlTlJTE SHEET
W092/02502 PCT/GB9t/0l~0
2C3~3~1
- 17 -
DATA
Ca2+ Current Measurement
Cel~ Dre~arations
Sensory neurons from dorsal root ganglia were
dissociated from 1 day old rat pups (Forda et al,
Developmental Brain Research, 22 (1985), 55-65). Cells
were plated out onto glass coverslips and used within 3
days to permit effective voltage clamp of ca2+ currents.
Solutions
The pipette (internal solution) contained in mM: CsCl,
130; HEPES, 10; EGTA, 10; MgCl2, 4; ATP, 2;
buf~ered to pH 7.2 with CsOH.
Cells were bathed in a normal Tyrodes solution
before establishment of whole cell recording when the
bathing solution was changed to one allowing isolation of
Ca2+ currents.
The external solution for recording Ca2+ channel
-- currents contained in mM: BaCl2, 10; TEA-Cl, 130; -
glucose, 10; HEPES, 10; MgCl2, 1; buffered to pH 7.3
with T~ OH. Barium was used as the charge carrier as
~ this acsists in current isolation and calciu~ dependent
inactivation of current is avoided.
Compounds were dissolved in DMSO to make a 20 mM
stock solution. At the drug concentration used the
vehicle (0.1~) had no significant effect on Ca
currents.
All experiments were performed at 21 to 24C.
Whole cell currents were recorded using ~ist EPC-7
amplifiers and stored, digitised for later analysis using
SVBSTITlJTE SHEET
W092/02502 PCT/GB9ltO1~0
18 -
PC based software similar to that described previously
(Benham & Tsien, Journal of Physiology (1988), 404,
767-784).
RESUITS
Ca2+ currents
Peak voltage gated Ca2+ channel currents of up to
l0 nA from dorsal root ganglion neurons were recorded
using lO mM Ba2+ as charge carrier. Currents were
evoked from a holding potential of -80 mV to a test
potential of O or +lO mV every lS seconds. This test
potential was at the peak of the current voltage
relationship and assessing block at this point reduced
any errors due to drifting holding potential. Some
cells showed slow rundown of current as is commonly seen
when recording Ca2+ currents. ~he rundown rate was
measured in control conditions and extrapolated through
the time o~ drug application to derive a control value to
relate the drug a~fected cùrrent to. Block by 20 ~M
drug was assessed 3 minutes a~ter drug application.
Compounds o~ the invent.cn save percentage
inhi~ition of plateau Ca2+ current in the range 30 to
2S 100%
TOXICOLOGY
The compound of Example 9 did not show any adverse
toxicological effects when administered to rats at a dose
of l0 mg/kg, i.v.
SUBSTlTlJTE SHEET
W092/02502 2 ~ ~. 3 `~ 9 ~ PCT/GB91/01340
PHARMACEUTICAL FORMULATIONS
l. Formulation for intravenous infusion
Compound of structure (I) 0.l - 60 mg
Sodium hydroxide/hydrochloric acid to pH ca 7
polyethylene glycol 0 - 30 ml
propylene glycol 0 - 30 ml
alcohol 0 - l0 ml
water to l00 ml
2. Formulation for bolus in~ection
Compound of structure (I) 0.l - 60 mg
sodlum hydroxide or hydrochloric acid to pH ca 7
polyethylené glycol 0 - 2.5 ml
alcohol 0 - 2.5 ml
wc er to 5 ml
A toxicity adjusting agent eg. sodium chloride,
dextrose or mannitol may also be added.
3. Tablet for oraI_administration
mq/tablet
Compound of structure (I) 25
lactose 153
starch 33
crospovidone 12
microcrystalline cellulose 30
magnesium stearate 2
255
SUBSTITUTE SHEET
W092/02502 PCT/GB91/Ot~O
æ~
- 20 -
EXAMPLES
Intermediate PreParations
(i) 4-(2-Hvdroxvethvl)-l-PentvlPi~eridine
A mixture of 4-(2-hydroxyethyl)piperidine (20g), 1-
bromopentane (19.2g), potassium carbon~te (21.42g) and
ethanol (400ml) was heated at reflux for 3 days. The
solution was filtered, and the solvent was removed under
reduced pressure. The residue was treated with acetone,
filtered, and the solvent was removed to give the title
compound as an oil (30.2g) which was used without further
purification.
(ii) 4-(2-Hvdroxveth~l)-l-cinnamYlPiPeridine
A mixture of 4-(2-hydroxyethyl)piperidine (16.4g),
cinnamyl bromide (25.0g), potassium carbonate (17.55g) and
ethanol (350ml) was heated at reflux for 3 days. The
solution was filtered, and the solvent removed under
reduced pressure. The residue was chromatographed on
silica gel eluted with methanol/dichloromethane to give
the title compound (12.0g) as an inpure solid which was
used without further purification.
SUBSTITUTE SHEET
W092/02502 ~ ~ 3 ~ ~ 9 1 PCT/GB91/Ot~O
(iii) 4-(3-H iroxvProPvl)-l-~entvlPvridinium bromide
A solution of 4-(3-hydroxypropyl)pyridine (27.43g), l-
bromopentane ~37.76g) and acetone ~SOml) was refluxed for
24 hours, cooled and poured into diethylether (200ml).
The oil which precipitated was collected by decantation
then washed by decantation with diethylether (5 X lOOml)
and dried at 50C O.lmmHg to give the title compound which
was used without further purification.
~iv) 4-~3-HvdroxvDroPvl)-l-Dentvl~iDeridine
A mixture of 4-~3-hydroxypropyl)-1-pentylpyridinium
bromide (8.65g), platinum oxide ~O.Sg) and ethanol (120ml)
was stirred under an atmosphere of hydrogen for 3 hours.
The mixture was filtered and the solvent removed. The
residue was dissolved in dilute sodium hydroxide ~70ml)
and extractec with dichloromethane (3 x 75ml). The
extracts were _ombined, dried over magnesium sulphate and
the solvent was removed to give the title compound as an
oil (4.68g).
v) 4-Hvdroxvmethvl-l-oentvlPvridinium bromide
A solution of 4-hydroxymethylpyridine ~25g), 1-
bromopentane ~43. g) and acetone ~50ml) was refluxed for
24 hours, cooled and poured into diethylether ~200ml)~
The oil which precipitated was collected by decantation
then washed by decantation with pentane (5 X lOOml) and
dried at 50C O.lmmHg to give the title compound which was
used witnout further purification.
SUBSTITUl-E SHEET
W092/02502 PCT/GB91/01340
~ a ~
~vi) 4-Hvdroxvmethv1-l-PentYlPiperidine
A mixture of 4-~3-hydroxypropyl)-1-pentylpyridinium
~romide ~5.2g), platinum oxide ~0.4g) and éthanol (lOOml)
was stirred under an atmosphere of hydrogen for 3 hours.
The mixture was filtered and the solvent removed. The
residue was dissolved in dilute sodium hydroxide (70ml)
and extracted with dichloromethane ~3 x 75ml). The
extracts were combined, dried over magnesium sulphate and
the solvent was removed. The residue was chromatographed
on silica gel eluted with methanol/ammonia/dichloromethane
to give the title compound as an oil ~1.35g).
(vii) 4-Hvdroxv-l-~entvl~Peridine
A mixture of 4-hydroxypiperidine ~25g), 1-bromopentane
~37.33g), potassium carbonate ~34.13g) and ethanol (400ml)
was heated at reflux for 3 days. The solution was
filtered, and the solvent removed under reduced pressure.
The residue was treated with acetone, filtered, the
solvent removed and the resulting oil distilled under
reduced pressure to give the title compound as an oil.
(18.00g, b.p. 100 C @ 0.6 mmHg.)
(viii) 4-(2-HvdroxYethvl)-1-ProDvlPi~eridine
A mixture of 4-(2-hydroxyethyl3piperidine (5g), 1-
bromopropane (4.87g), potassium carbonate (5.5g) and
ethanol (lOOml) was heated at reflux for 1 day. The
solution was filtered, and the solvent was removed under
reduced pressure. The residue was treated with acetone,
filtered, and the solvent was removed to give the title
SUBSTIT~JTE SHEEl
W092/02502 PCT/GB91/01340
2 ~
- 23 -
compound as an oil (5.lg) which was used without further
purification.
(ix) 4-(2-Hvdroxvethvl)-1-(3-~henvl)ProPvlPiPeridine
A mixture of 4-(2-hydroxyethyl)piperidine (lOg), 1-bromo-
3- ~henyl)propane (15.Bg), potassium carbonate (10.69g)
and ethanol (200ml) was heated at reflux for 24 hours.
The solution was filtered, and the solvent was removed
under reduced pressure. The residue was treated with
acetone, filtered, the solvent removed and th~ residue
distilled, to give the title compound as an oil (14.52g)
(b.p. 141C @ 0.2mmHg)
(x) 4-(2-HvdroxYethvl)-1-hePtvlPiPeridine
A mixture of 4-(2-hydroxyethyl)piperidine ~20g), 1-
bromoheptane (27.73g), potassium carbonate (21.39g) and
ethanol (400ml) was heated at reflux for 24 hours. The
solution was filtered, and the solvent was removed under
reduced pressure. The residue was treated with acetone,
filtered, the solvent was removed and the residue
distilled, to give the title compound as an oil ~lO.Olg)
(b.p. 110C @ O.lmmHg)
(xi) 4-~2-Hvdroxvethvl)-1-(2-ethvl)butvl~ieridine
A mixture of 4-(2-hydroxyethyl)piperidine (20g), l-bromo-
2-ethylbutane (17.9g), potassium carbonate (26g) and
ethanol (4~0ml) was heated at reflux for 4 days. The
solution was filtered, and the solvent was removed under
SUBSTlTtJTE SHEET
WO 92/02502 PCT/GB9l/01340
~,a~ 3'~ ,
- 24 -
reduced pressure. The residue was distilled, to give the
title compound as an oil (29.61g) (b.p. 102C @ 0.3mm~g)
(xii) l-CYclohexvlmethvl-4-(2-hvdroxvethvl)Piperidine
A mixture of 4-(2-hydroxyethyl)piperidine (20g),
cyclohexylmethyl bromide (27.41g), potassium carbonate
(26g) and ethanol (400ml) was heated at reflux for 4 days.
$he solution was filtered, and the solvent was removed
under reduced pressure. The residue was distilled, to
give the title compound as an oil (27g) (b.p. 165C Q
O.SmmHg)
(xiii) 4-(2-hvdroxvethvl)-1-(3-methvlbutvl)~iPeridine
A mixture of 4-(2-hydroxyethyl)piperidine (20g), l-bromo-
3-methylbutane (25.57g), potassium carbonate (26g) and
ethanol (400ml) was heated at reflux for 4 days. The
solution was filtered, and the solvent was removed under
reduced pressure. The residue was distilled to give the
title compound as an oil (23.21g) (b.p. 98C ~ O.lmmHg)
(xiv) l-~enzvl-4-(2-hvdroxvethvl)Pi~eridine
A mixture of 4-(2-hydroxyethyl)piperidine (5g), benzyl
bromide (6.15g), potassium carbonate (5.35g) and ethanol
(50ml) was heated at reflux for 24 hours. The mixture was
- poured into water (200ml) and extracted with diethylether.
The organic phase was dried over sodium sulphate,
filtered, and the solvent was removed under reduced
pressure. The residue was distilled, to give the title
compound as an oil (5.13g) (b.p. 120-130C @ O.lmmHg)
SUE~STlTlJTE SHEET
W092/02S02 PCT/GB91/Ot340
~3~
- 25 -
(xv) 4-f2-(4-FluoroPhenvl)ethvll-Pvridine
4-Picoline ~30g) was added over 30 minutes to a suspension
of sodium amide (12.56q) in liquid ammonia (150ml) and the
resulting mixture was stirred for 1.5 hours. 4-
Fluorobenzyl chloride (40ml) was then added over 15
minutes and the mixture was stirred for 3-hours. Ammonium
chloride (SOg) was added and the solvent was allowed to
evaporate. The residue was dissolved in chloroform
(300ml) and dilute sodium hydroxide ~300ml) and the
organic phase was separated, dried over magnesium sulphate
and the solvent was removed, The residue was
recrystallised from petroleum ether to give the title
compound as white needles ~25.3g), m.p. 69-70.5C
(xvi) 4-~2-(4-FluoroPhenvl)ethYll-l-PentYlpyridinium
bromide
A mixture of 4-[2-(4-fluorophenyl)éthyl)pyridine (5g), 1-
bromopentane (7.0g) and acetone (lOml) was heated at
reflux for 18 hours. The solvent was removed under
reduced pressure and the residue was recrystallised from
ethyl acetate / methanol to give the title compouna
(7.32g), m.p. 130 - 131C.
(xvii) 4-~2-(4-FluoroPhenoxv)ethvll-~i~eridine
hvdrochloride
A mixture of l-benzyl-4-~2-(4-
fluorophenoxy)ethyl~piperidine (1.50g), 10% palladium on
car~on (0.6g) and ethanol (120ml) was shaken under an
SUBSTITIJTE SHEET
W092/02502 PCT/GBg1/01340
2aC~
- 26 -
atmosphere of hydrogen at 50 p.s.i for 24 hours. The
mixture was filtered and the residue washed with ethanol.
The filtrates were combined, the solvent removed and the
residue was treated with hydrogen chloride in ether to
give a solid. Recrystallisation from ethyl acetate gave
the title compound (0.45g), m.p. 122 -123C.
Found: C, 59.58; H, 7.37; N, 5.35; Cl, 13.33%
(C13H18FNO.HCl) requires: C, 60.11, H, 7.37; N, 5.39; Cl,
13.65~
Exam~le 1
4-~2-(4-Fluoro~henoxv)ethYll-l-PentYlPi~eridine
1~ hvdrochloride
A solution of 4-~2-hydroxyethyl)-1-pentylpiperidine
(2.0q), 4-fluorophenol (1.12g) and triphenylphosphine
(2.62g) in tetrahydrofuran ~40ml) was treated with die~hyi
azodicarboxylate (1.74g) in tetrahydrofuran ~lOml). The
resulting solution was stirred at room temperature for 18
hours, the solvent was removed and the residue was
chromatographed on silica gel eluted with
methanol/dichloromethane. The resulting oil was dissolved
in ethyl acetate ~50ml) and treated with ethereal hydrogen
chloride, the precipitate was collected by filtration and
recrystallised (methanol/ethyl acetate) to give the title
compound (l.lg), m.p. 167-169 C.
Found: C, 6S.45; H, 8.90; N, 4.16; Cl, 10.75; F 5.76%.
(C18H28FNO.HCl) requires: C, 65.54; H, 8.86; N, 4.25;
Cl, 10.75; F, 5.77%.
SUBSTIT~JTE SHEET
W092to2S02 PCT/GB9l/Ol~
2 ~
- 27 -
Exam~le 2
4-~2-(3,4-MethvlenedioxvphenoxY)ethvll-l-~ent~lPi~eridine
hvdrochloride
A solution of 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.0g), sesamol (1.39g) and triphenylphosphine ~2.62g) in
tetrahydrofuran (40ml) was treated with diethyl
azodicarboxylate (1.74g) in tetrahydrofuran (lOml). The
resulting solution was stirred at room temperature for 18
hours, the solvent was removed and the residue was
chromatographed on silica gel eluted with
methanol/dichloromethane. The resulting oil was dissolved
in ethyl acetate (SOml) and treated with ethereal hydrogen
chlor~de. The precipitate was collected by filtration and
recrystallised (methanol/ethyl acetate~ to give the title
compound (0.45g), m.p. 134 - 136C.
Found: C, 64.12; H, 8.S2; N, 4.03; Cl, 10.00%.
(ClgH29N03.HCl) requires: C, 64.12; H, 8.50; N, 3.93;
Cl, 9.96%.
Exam~ie 3
4-(2-Phenoxvethvl)-l-PentvlPi~eridine hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.0g), phenol (0.94g), triphenylphosphine (2.62g) and
diethyl azodicarboxylate (1.74g). Treating the product
with hydrogen chloride gave a white solid which was
SUBSTITUTE SHEET
WO92/02sO2 PCT/GB91/013~
2 ~
- 28 -
recrystallised from methanol/ethyl acetate (0.8ag),
m.p. 158 - 159C.
Found: C, 69.10; H, 9.80; N, 4.61; Cl, 11.34%
(C18H29NO.HCl) requires: C, 69.32; H, 9.69; N, 4.49;
Cl, 11.37%
Exam~le 4
4-r2-(3-TrifluoromethvlDhenoxv)ethvll-l-DentvlPiDeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-pentylpiperidine
(2.0g), ,a,a, trifluoro-m-cresol ~1.62g),
triphenylphosphine ~2.62g) and diethyl azodicarboxylate
~1.74g). Treating the product with hydrogen chloride gave
a white solid which was recrystallised from methanol/ethyl
acetate ~0.44g), m.p. 154C.
Found: C, 59.51; H, 7.62; N, 3.80; Cl, 9.49$
(ClgH28F3NO.HCl) requires: C, 60.07; H, 7.69; N, 3.69;
Cl, 9.33%
Exam~le 5
4- r 2-(4-PhenvlDhenoxv)ethvll~ entvlDiDeridine
hYdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.0g), 4-phenylphenol (0.1.70g), triphenylphosphine
SUBSTITUTE SHEET
W092/02502 PCT/GB91/Ol~
2 `3 ~
- 29 -
~2.62g) and diethyl azodicarboxylate (1.74g). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from methano}/ethyl acetate
(0.4g), m.p. 205-206C.
s
Found: C,73.77 ; H, 8.88; N,-3.66; Cl, 9.14%
(C24H33NO.HCl) requires: C, 74.2; H, 8.8; N, 3.6;
Cl, 9.27%
Exam~le 6
4-t2-(4-Benzvloxv~henoxv)ethvll~ entvlPiPeridine
hvdrochlor~de
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(l.Og), 4-benzyloxyphenol (l.OOg), triphenylphosphine
(1.31g) and diethyl azodicarboxylate (0,87g). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from methanol/ethyl acetate
(O.lg), m.p. 168 - 169C.
Found: C, 70.42; H, 8.59; N, 3.50; Cl, 8.29~
~C25H35N02.HCl,0,5H20) requires: C, 70.31; H, 8.73;
N, 3.28; Cl, 8.20%
Exam~le 7
4-~2-(3-DimethvlaminoPhenoxv)ethVll-l-DentVl~i~eridine
dioxalate
SUBSTITU'rE SHEET
W092/02502 PCT/GB91/01340
2a~9~
- 30 -
The title compound was prepared in a similar manner tO
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.2g), 3-dimethylaminophenol (l.Sg), triphenylphosphine
(2.88g) and diethyl azodicarboxylate (1.94g). Treating
the product with oxalic acid gave a white solid which was
recrystallised from methanol/ethyl acetate ~0.2g),
m.p. 128-130C.
Found: C, 57.82; H, 7.63; N, 5.62%
(C20H34N20.2C2H204) requires: C, 57.83; H, 7.63; N, 5.62%
ExamPle 8
4-r2-(4-MethoxvPhenoxv)ethvl~ Dentvlpi~eridine oxalate
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(l.Sg), 4-methoxyphenol (0.93g), triphenylphosphine
(1.97g) and diethyl azodicarboxylate (1.31g). Treating
the product with oxalic acid gave a white solid which was
recrystallised from methanol/ethyl acetate (0.53g),
m.p. 119-121C.
Found: C, 63.54; H, 8.47; N, 3.69%
(Cl9H31N2 c2H2o4) requires C, 63.79; H, 8.35; N,
ExamDle 9
4-~2-(3,4-DichloroPhenoxv)ethvll-1-~entyl~i~eridine
hvdrochloride
SUBSTITUTE SHEET
WO92/02502 PCT/GB91/01~0
2 ~ 9 1
- 31 -
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-pentylpiperidine
(2.0g), 3,4-dichlorophenol (1.63g), triphenylphosphine
(2.62g) and diethyl azodicarboxylate (1.76g). Treating
the product with hydrogen chloride gave the title compound
as white prisms from methanol/ethyl acetate (1.02g),
m.p. 177 - 178C.
Found: C, 57.05; H, 7.43; N, 3.85; Cl, 27.93%
(C18H27Cl2NO.HCl) requires: C, 56.78; H, 7.41; N, 3.68;
Cl, 27.93%
ExamPle 1 0
4-~2-~4-CvanoPhenoxv)ethv~ entvlplperidine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-pentylpiperidine
~2.0g), 4-cyanophenol (l.lgg), triphenylphosphine (2.62g)
and diethyl azodicarboxylate (1.74g). Treating the
product with hydrogen chloride gave a white solid which
was recrystallised from methanol/ethyl acetate (0.9Sg),
m.p. 173 - 174C.
Found: C, 67.69; H, 8.84; N, 8.28; Cl, 10.85~
(ClgH28N2O.HCl) requires: C, 67.74; H, 8.68; N, 8.31;
Cl, 10.52%
SUBSTlTlJl'E SHEET
W092/02502 PCT/GB91/01340
49~
Example 11
4-t2-(4-Chlorophenoxv)ethYll-1-PentvlPiPeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.0g), 4-chlorophenol (1.30g), triphenylphosphine (2.62g)
and diethyl azodicarboxylate (1.74g). Treating the
product with hydrogen chloride gave a white solid which
was recrystallised from methanol/ethyl acetate (0.75g),
m.p. 185 -186C.
Found: C, 62.14; H, 8.48; N, 4.44; Cl, 20.63%
~C18H28ClNO.HCl) requires: C, 62.42; H, 8.44; N, 4.04;
C1, 20.47%
ExamDle 12
4-~2-(5,6,7,8-Tetrahvdro-2-naDthoxv)ethvll-~-
PentvlPiPeridine oxalate
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.0g), 5,6,7,8-tetrahydro-2-napthol (1.48g),
triphenylphosphine (2.62q) and diethyl àzodicarboxylate
(1.74g). Treating the product with oxalic acid gave a
white solid which was recrystallised from methanol/ethyl
acetate (0.81g), m.p. 147C.
Found: C, 68.88; H, 9.07; N, 3.40%
(C22H35NO.C2H204) requires: C, 68.71; H, 8.B9i N, 3.34%
SVBSTITUTE SHEET
W092/02~02 PCT/GB9l/Ot~O
2 ~
- 33 -
ExamPle 13
4-r2-(5,6,7,8-Tetrah~dro-l-napthoxv)ethvll-l-
5 Pentvl~iPeridine oxalate
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.0g), 5,6,7,8-tetrahydro-1-napthol tl.48g),
triphenylphosphine (2.62g) and diethyl azodicarboxylate
~1.74gl. Treating the product with oxalic acid gave a
white solid which was recrystallised from methanol/ethyl
acetate (1.14g), m,p. 162C,
~S Found: C, 68.03; H, 8.73; N, 3.40~
(C22H35NO.C2H204Ø25H20) requires: C, 67.97; H, 8.84;
N, 3.30%
ExamDle 14
4-~2-(4-Nitro-3-trifluoromethYlPhenoxY)ethYll-l-
PentvlPiPeridine hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(1.5g), 3-nitro-4-trifluoromethylphenol (1.44g),
triphenylphosphine (1.96g) and diethyl azodicarboxylate
(1.19g). Treating the product with hydrogen chloride gave
the title compound as a white solid from methanol/ethyl
acetate (0.61g), m.p. 139 - 141C.
Found: C, 53.80; H, 6.50; N, 6.45; Cl, 8.30%
SUBSTlTlJTE SHEET
W092/02502 PCT/GB91/01~0
2~8~
(ClgH27F3N203.HCl) requires: C, 53.71; H, 6.64; N, 6.59;
Cl, 8.34%
Exam~le 15
s
4-~2-(3-Fluoro~henoxv)eth~ l-pentvlDi~eridine
hvdrochloride
The title compound was prepared in a similar manner tO
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(l.Sg), 3-fluorophenol (0.84g~, triphenylphosphine ~1.97g)
and diethyl azodicarboxylate (1.31g). Treating the
product with hydrogen chlorlde gave the title compound as
a white solid from methanol/ethyl acetate ~1.21g),
m.p. 157-159C.
Found: C, 65.27; H, 8.67; N, 4.61; Cl, 10.75%
(C18H28FNO.HCl) requires: C, 65.54; H, 8.86; N, 4.25;
Cl, 10.75%
ExamDle 16
4-t2-(4-MethvlDhenoxv)ethvll-l-pentvl~i~eridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(l.SOg), p-cresol (0.81g), triphenylphosPhine (1.96g) and
diethyl azodicarboxylate (1.31g). Treating the product
with hydrogen chloride gave the tltle compound as a white
solid from methanol/ethyl acetate (1.09g),
m.p. 164 - 166C.
SVBSTlTlJTE SHEET
W092/02502 PCT/GB91/01340
~3~31
- 35 -
Found: C, 70.00; H, 9.69; N, 4.15; Cl, 10.82%
(ClgH31NO.HCl) re~uires: C, 70.02; H, 9.90; N, 4.30;
Cl, 10.88~ .
ExamPle 17
4-r2-(4-Benzvluhenoxv)ethvll-l-~entvl~i~eridine oxalate
The tltle compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-pentylpiperidine
(1.50g), 4-benzylphenol ~1.38g), triphenylphosphine
(1.95g~ and diethyl azod~carboxylate (1.31g). Treating
the product with oxal~c ac~d gave the title compound as a
~5 wh~te solid from methanol/ethyl acetate (0.377g),
m.p. 166 - 16BC.
Found: C, 70.86; H, 8.02; N, 3.07%
(C25H35NO.C2H204) requires: C, 71.18; H, 8.19; N, 3.07%
Exam~le 18
4-r2-(3-chloroPhenoxv)ethvll-l-~entvl~i~eridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(1.50g), 3-chlorophenol (0.69g), triphenylphosphine
(1.96g) and diethyl azodicarboxylate (1.31g). Treating
the product with hydrogen chloride gave the title compound
as a white solid from methanol/ethyl acetate (0.37g),
m.p. lSl - 153C.
SUBSTlTlJTE SHEET
W092/02502 PCT/GB91/01340
2a~8~9'~
- 36 -
Found: C, 62.13; H, 8.30; N, 4.05; Cl , 10.20%
(C18H28ClNO.HCl) requires: C, 62.42; H, 8.44; N, 4.04;
Cl-, 10.23%
s
ExamPle 1 9
4-8enzvl-1-pentvlpi~eridine hvdrochloride
A mixture of 4-benzylpiperidine ~3.0g), pentyl bromide
(2.84g), potassium carbonate (4.72g) and ethanol (40ml)
was heated at reflux for 48 hours. The solution was
filtered, and the solvent removed under reduced pressure.
The residue was distilled in a kugelrohr apparatusto give
an oil ~b.p. 150C @ O.lmmHg) which was treated with
hydrogen chloride to gave the title compound as a white
solid from methanol/ethyl acetate (2.06g),
m.p. 188 - 190C.
Found: C, 70.00; H, 9.69; N, 4.15; Cl, 10.82%
(ClgH31NO.HCl) requires: C, 70.02; H, 9.90; N, 4.30;
Cl, 10.88%
Exam~le 20
.
4- r2- (4-Fluorophenoxv)ethvll-l-cinnamvlPiPeridine oxalate
A solution of 4-(2-hydroxyethyl)-i-Cinnamylpiperidine
(2.94g), 4-fluorophenol (1.31g) and triphenylphosphine
(3.15g) in tetrahydrofuran (50ml) was treated with diethyi
azodicarboxylate (2.09g). The resulting solution was
stirred at room temperature for 18 hours, the solven~
SUBSTITUTE SHEET
W092/02502 PCT/GB91/01340
2 ~
- 37 -
removed and the residue chromatographed on silica gel
eluted with methanol/dichloromethane. The resulting oil
was dissolved in ethyl acetate (SOml) and treated with
oxalic acid (1.1 mole equivalents). The precipitate was
collected by filtration and recrystallised ~methanol/ethyl
acetate) to give the title compound (l.lOg), m.p. 180 C.
Found: C, 67.14; H, 6.60; N, 3.56%.
(C22H26FNO.C2H204) requires: C, 67.11; H, 6.57; N, 3.26%
ExamDle 21
4-f2-(3,4-DichloroPhenoxv?ethvl1-1-cinnamvlPi~eridine
oxalate
A solution of 4-(2-hydroxyethyl)-1-cinnamylpiperidine
(2.02g), 3,4-dichlorophenol (1.34g) and triphenylphosphine
(2.16~) in tetrahydrofuran (50ml) was treated with diethyl
azodicirboxylate (1.44g). The resulting solution was
stirred at room temperature for 18 hours, the solvent
removed and the residue was dissolved in ethyl acetate and
extracted with dilute hydrochloric acid. The aqueous
extract was basified and extracted with ethyl acetate.
The resulting organic layer was dried over magnesium
sulphate, filtered and the solvent was removed. The
residue was dissolved in ethyl acetate (50ml) and treated
with oxalic acid (1.1 mole equivalents). The precipitate
was collected by filtration and recrystallised
(methanol/ethyl acetate) to give the title compound
(0.3g), m.p. 179 - 180C.
Found: C, 60.07; H, 5.67; N, 2.92; Cl, 14.79%.
S~JBC i ITtJTE 5HEET
WO 92/02502 PCr/GB9l/01340
~, 3 3 ~
-- 38 --
~C22H25C12NO.C2H204) requires: C, 60.01; H, 5.67;
N, 2.92; Cl, 14.76%
ExamPle 22
s
4-f3-~4-FluoroPhenoxv)ProPvll-l-Pentylpiperidine
hvdrochloride
The title compound was prepared in a similar manner to
10 example 1 f~om 1-pentyl-4-(3-hydroxypropyl)piperidine
(2.0g), 4-fluorophenol (l.OSg), triphenylphosphine (2.46g)
and diethyl azodicarboxylate (1.63g). Treating the
product with hydrogen chloride gave a white solid which
was recrystallised from methanol/ethyl acetate (1.32g),
15 m.p. 148 - 150C.
Found: C, 65.94; H, 9.2g; N, 4.15; Cl, 10.32%
(ClgH30FNO.HCl) requires: C, 66.36; H, 9.09; N, 4.07;
Cl, 10.31%
ExamDle 23
4- r 3-(4-E~enzYloxvphenoxv)Propvll-l-Dentvl~i~eridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 1-pentyl-4- (3-hydroxypropyl)piperidine
(2.0g), 4-benzyloxyphenol (1.88g), triphenylphosphine
(2.46g) and diethyl azodicarboxylate (1.48g). Treating
30 the product with hydrogen chloride gave a white solid
which was recrystallised from acetonitrile (1.48g),
m.p. 163 - 164C.
SUBSTlTlJTE SHEET
W092/02502 PCT/GB91/01340
2~g/~3 ~
- 39 -
Found: C, 72.43; H, 8.91; N, 3.31; Cl, 8.06%
(c26H37No2.Hcl1 requires: C, 72.28; H, a.86; N, 3.24;
Cl, 8.21%
ExamPle 24
4-(4-FluoroPhenoxv)methvl-l-~entvlPiPeridine hvdrochloride
~he title compound was prepared in a similar manner to
example 1 from 1-pentyl-4-hydroxymethylpiperidine tl.lg),
4-fluorophenol (0.69g), triphenylphosphine ~1.63g) and
die~yl azodicarboxylate (1.08g). Treating the product
wit.. oxalic acid ga~e a white sol~d which was
recrystallised from methanol/ethyl acetate ~0.25g),
m.p. 111 -112C.
Found: C, 60.25; H, 7.56; N, 3.88%
(Cl~H26FNO.C2H204Ø5H20) requires: C, 60.3; H, 7.72;
N, 3.70%
ExamPle 25
4-(4-Fluorobenzvlox~ -Dentyl~i~eridine oxalate
A solution of 4-hydroxy-1-pentylpiperidine (2.0g) in
dimethylformamide (25 ml) was treated with sodium hydride
(0.012 mole) and then stirred for 1 hour when 4-
fluorobenzyl chloride (1.43 ml) was added and the mixture
was stirred for 3 days. Water (100 ml) and
dichloromethane (100 ml) were added and the organic layer
was separated, washed with water (2 x 100 ml), and dried
SUBSTITUTE SHEET
WO 92/02502 PCr/GB91/01340
-- 40 --
over magnesium sulphate. The solvent removed and the
residue was chromatographed on silica gel eluted with
methanol/dichloromethane. The resulting oil was dissolved
in ethyl acetate and treated with oxalic acid ~1.1 mole
5 equi~ralent). The precipitate was collected by filtration
and recrystallised (methanol/ethyl acetate) to give the
title compound (0.2g), m.p. 124 - 125 C.
Found: C, 61.71; H, 7.69; N, 3.94%.
(C17H26FNO.C2H2O4) requires: C, 61.77: H, 7.64; N, 3.79%
Exam~le 26
4-Benzvloxv-1-~entvl~i~eridine oxalate
Substitut1on of benzyl bromide (2.0g) for 4-fluorobenzyl
chloride, in the procedure described in example 25, gave
the title compound,as a white solid on recrystallisation
from methanol/ethyl ac~tate yield (0.2g),
20 m.p. 119 - 121C.
Found: C, 64.63; H, 8.11; N, 4.14%
(C17H27NO.C2H2O4) requires: C, 64.98; H, 8.32; N, 3.99%
Exam~le 27
4-(4-FluoroPhenoxv)-1-~entvl~iPeridine oxalate
The title compound was prepared in a similar manner to
example 1 from 4-hydroxy-1-pentylpiperidine (2.0g), 4-
fluorophenol (1.31g), triphenylphosphine (3.15g) and
diethyl azodicarboxylate (2.09g). Treating the producz
SUBSTITUTE SI-IEET
WO 92/02502 PCr~GB91/0l340
-- 41 --
with oxalic acid gave a white solid which was
recrystallised from methanol/ethyl acetate (0.65g),
m.p. 164C.
Found: C, 60.91; H, 7.56; ~, 4.06%
(C16H24FNO-C2H2O4) requires: C, 60.83; H, 7.37; N, 3.9496
Exam~le 28
4-(3,4-MethvlenedioxvPhenoxY)-l-~entYlDiPeridine oxalate
The title compound was prepared in a similar manner to
example 1 from 4-hydroxy-1-pentylpiperidine (2.0g),
sesamol (1.66g), triphenylphosphine ~3.15g) and diethyl
azodicarboxylate ~2.09g). Treating the product with
oxalic acid gave a white solid which was recrystallised
from methanol/ethyl acetate (0.65g), m.p. 164C.
Found: C, 59.76; H, 7.22; N, 3.72%
~C17H25NO3.C2H2O4) requires: C, 59.83; H, 7.14; N 3 67%
Exam~le 29
4-~2-~4-Fluoro~henoxv)ethvll-l-~ro~YlPiDeridine oxalate
The title compound was prepared ~n a similar manner to
example 1 from 4- ~2-hydroxyethyl)-1-propylpiperidine
~1.85g), 4-fluorophenol (1.12g), triphenylphosphine
(2.62g) and diethyl azodicarboxylate (1.74g). Treating
the product with oxalic acid gave a white solid which was
recrystallised from methanol/ethyl acetate ~0.3g),
m.p. 109-112C.
SUBSTlTlJTE SHEET
W092/02502 PCT/GB91/0 W 0
~ a 3~
Found: C, 59.66; H, 7.46; N, 3.B0%
(C16H24FNO.C2H204ØSH2O) requires: C, 59.50; H, 7.43;
N, 3.~6%
S
ExamPle 30
4- r2- (4-FluoroPhenoxv)ethvl1~ 3-PhenvlProPvl)piperidine
hvdrochloride
~0
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-(3-
phenylpropyl)piperidine (2.47g), 4-fluorophenol (1.12g),
triphenylphosphine (2.62g) and diethyl azodicarboxylate
(1.74g). Treat~ng the product with hydrogen chloride gave
a white solid which was recrystallised from methanol/ethyl
acetate (0.65g), m.p. 111-113C.
Found: C, 68.04; H, 7.72; N, 3.83, Cl, 9.11%
(C22H28FNO.HCl.O.SH20) requires: C, 68.23; H, 7.75;
N, 3.60; Cl, 9.04%
ExamPle 31
4-~2-(4-FluoroPhenoxv)ethv~ -heDtvlDiPeridine
hvdrochloride
The title compound was prepared in a similar manner tO
example 1 from 4-(2-hydroxyethyl)-1-heptylpiperidine
(2.27g), 4-fluorophenol (1.12g), triphenylphosphine
(2.62g) and diethyl azodicarboxylate (1.74g). Treating
the product with hydrogen chloride gave a white solid
SUBSTITI.JTE SHEET
WO92/02S02 PCT/GB9l/Ot~0
~33~91
- 43 -
which was recrystallised from methanol/ethyl acetate
(l.lg), m.p. 139-141C.
Found: C, 66.71; H, 9.32; N, 4.05; Cl, 10.08%
(C20H32FNO.HCl) requires: C, 67.10; H, 9.29; N, 3.91;
Cl, 9.90%
Exam~le 32
4-~2-(3,4-MethvlenedioxvPhenoxv)ethvll-l-hePtvlPiPeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-heptylpiperidine
(2.27g), sesamol (1.38g), triphenylphosphine (2.62g) and
diethyl azod~carboxylate (1.74g). Treating the product
with hydrogen chloride gave a white soild which was
recrystallised from methanol/ethyl acetate 10.65g),
m.p. 129-231~.
Found: C, 65.61; H, 8.85; N, 3.71; Cl, 9.26%
(C21H33NO3.HCl) requires: C, 65.69; H, 8.93; N, 3.65;
Cl, 9.23%
ExamPle 33
4-i2-(4-FluoroPhenoxv)ethvl1-1-(2-ethvl)butvlPiPeridine
oxalate
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-(2-
ethyl)butylpiperidine (2~97g)~ 4-~luorophenol (1.08g),
SUBSTITUTE SHEET
W092/02502 PCT/GB91/01340
2~83~
- 44 -
triphenylphosphine (2.62g) and diethyl azodicarboxylate
~1.74g). Treating the product with oxalic acid gave a
white solid which was recrystallised from methanol/ethyl
acetate (0.65g), m.p. 13?-138C.
Found: C, 63.24; H, 8.26; N, 3.58%
(ClgH30FNO.C2H2O4) requires: C, 63.46; H, 8.11; N, 3.52%
ExamPle 34
4-~2-(3,4-MethvlenedioxvDhenoxv)ethvll-1-(2-
ethvl)butvlPiperidine oxalate
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl~-1-(2-
ethyl)butylpiperidine (2.25g), sesamol (1.32g),
triphenylphosphine (2.62g) and diethyl azodicarboxylate
(1.74g). Treating the product with oxalic acid gave a
white solid which was recrystallised from methanol/ethyl
acetate (1.25g), m.p. 133-134C.
Found: C, 62.05; H, 7.88; N, 3 . 39%
(C20H31NO3.C2H2O4) requires: C, 62.39; H, 7.85; N, 3.31%
ExamPle 35
l-Cvclohexvlmethvl-4-r2-(3,4-
methvlenedioxvPhenoxv)ethvllPiPeridine hvdrochloride
The title compound was prepared in a similar manner ro
example 1 from 1-cyclohexylmethyl-4-(2-
hydroxyethyl)piperidine (2.25g), sesamol (1.38g),
SUBSTITUTE SHEET
W092/02502 PCT/GB91/01340
2~3~491
triphenylphosphine ~2.62g) and diethyl azodicarboxylate
~1.74g). Treating the product with hydrogen chloride gave
a white solid which was recrystallised from methanol/ethyl
acetate ~1.72g~, m.p. 177~178C.
Found: C, 66.01; H, 8.44; N, 3.85; Cl, 9.39%
(C21H31NO3.HCl) requires: C, 66.04; H, 8.44; N, 3.67
Cl, 9.28%
Exam~le 36
4-r2-(4-Fluorophenoxv)ethvll-l-cvclohexvlmethvlPiperidine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 1-cyclohexylmethyl-4-(2-
hydroxyethyl)piperidine (2.25g), 4-fluorophenol (1.08g),
triphenylphosphine (2.62g) and diethyl azodicarboxylate
(1.74g). ~reating the product with hydrogen chloride gave
a white solid which was recrystallised from methanol/ethyl
acetate (0.98g), m.p. 178 - 180C.
Found: C, 67.68; H, 8.85; N, 4.12; Cl, 9.87%
(C20H30FNO.HCl) requires: C, 67.68; H, 8.78; N, 3.94;
Cl, 9.96%
ExamPle 37
1-(3-Methylbutyl)-4-~2-(3,4-methYlene-
dioxvPhenoxv)ethyllpiDeridine hvdrochloride
SUBSTITUTE~ SHEET
w092/02502 PCT/GB91/Ot34~
~3~'~9~
- 46 -
The title compound was prepared in a similar manner to
example 1 from l-(3-methylbutyl)-4-(2-hydroxyethyl)-
piperidine (2.0g), sesamol (1.38g), triphenylphosphine
(2.62g) and diethyl azodicarboxylate (1.74g). Treating
S the product with hydrogen chloride gave a white solid
which was recrystallised from methanol/ethyl acetate
(0.17g), m.p. 16B-169C.
Found: C, 63.95; H, 8.50; N, 4.05; Cl, 10.17%
(ClgH29N03.HCl) requires: C, 64.12; H, 8.50; N, 3.94;
Cl, 9.96%
Exam~le 38
1-Benzvl-4-f2-(4-fluoro~henoxv)ethvll-1-~iPeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 1-benzyl-4-(2-hydroxyethyl)piperidine
(3.83g), 4-fluorophenol (1.96g), tripnenylphosphine
(4.61g) and diethyl azodicarboxylate (2.78g). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from methanol/ethyl acetate
(2.42g), m.p. 175 -176C.
Found: C, 68.48; H, 7.22; N, 3.92; Cl, 10.07~
(C20H25FNO.HCl) requires: C, 68.66; H, 7.20; N, 4.00;
Cl, 10.13%
SUBSTITUTE SHEET
W092/02502 PCT/GB9l/01340
2 ~
- 47 -
ExamPle 39
4-~2-~4-Fluorophenoxv)ethYll-1-(2-Phenvlethvl)-PiPeridine
hYdrochloride
A mixture of 4-[2-(4-fluorophenoxy)ethyl]-piperidine
hydrochloride (0.57g) and sodium hydride (80% in oil)
(0.146g) in dimethylformamide (lOml) was stirred under
nitrogen until effervesence had subsided. 2-Phenylethyl
bromide (0.3ml) was added and the mixture stirred for 48
hours. The mixture was poured into water (50ml) and
extracted w~th ether. The ether phase was washed with
d~lute hydrochloric ac~d and the resulting precipitate
collected by filtration. Recrystall~sation from water
~ave th¢ tltle compound (0.228g) m.p. 210-212C
Found: C, 69.61; H, 7.48; N, 3.96; Cl, 9.77%
(C21H26FNO.HCl) requires: C, 69.12; H, 7.73; N, 3.84; Cl,
9.72%
Exam~le 40
4-l2-(4-FluoroPhenoxv)ethvll-1-(4-~henvlbut~ i~eridine
hvdrochloride
The title compound was prepared in a similar manner to
example 39 starting from 4-[2-(4-fluorophenoxy)ethyl]-
piperidine hydrochloride (l.Og), sodium hydride (80% in
oil) (0.3g) and 4-phenylbutyl chloride (0.649g) in
dimethylformamide (20ml) and recrystallising the product
from ethyl acetate/methanol, yield (0.39g), m.p. 166-
168C.
SU STlTlJTE SHEET
WO 92/02502 PCI'/GB91/01340
2~8~
- 48 -
Found: C, 70.20; H, 8.00; N, 3.87; Cl, 8.91%
(C23H30FNO.HCl) requires: C, 70.48; H, 7.97; N, 3.57; Cl,
9. 05%
ExamPle 41
1-~3,3-DiPhenvlProPvl)-4-r2-(4-fluoroDhenoxy)eth
PiPeridine oxalate
A mixture of 4-[2-(4-fluorophenoxy)ethyl]-piperidine
hydrochloride
(2.0g), 3,3-diphenylpropane-1-ylmethanesulphonate (2.Z3g)
and sodium hydride ~80% in oil) (0.58g) in
dlmethylformamide ~40ml) was stirred at 60C u~der
nitrogen for 48 hours. The mixture was poured into water
(200ml) and extracted with ether. The ether phase was
treated with dilute hydrochloric acid and an oil
precipitated. The oil was separated and dissolved in
dichloromethane. The dichloromethane solution was washed
with dilute sodium hydroxide solution, dried over sodium
sulphate and the solvent removed. The residue was
dissolved in ethyl acetate and treated with oxalic acid
when the title compound crystallised. Yield ~0.963g), m.p.
160-161C
Found: C, 70.96; H, 6.75; N, 2.83%
(C28H32FNO.C2H2O4) requires: C, 70.98; H, 6.90; N, 2.66%
ExamPle 42
4-~2-(4-FluorothioPhenoxv~ethvll-l-DentvlDiPeridine
hvdrochloride
SUBSTITVTE SHE~T
W092/02502 PCT/GB9l/01340
2 ~ 3 1
- 49 -
The title compound was prepared in a slmilar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.00g), 4-fluorothiophenol (1.28g), triphenylphosphine
~2.62g) and diethyl azodicarboxylate (1.74g). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from ethyl acetate to give the
title compound as white plate crystals (0.33g), m.p.169-
165C.
Found: C, 62.41; H, 8.47; N, 4.09; Cl, 10.17%
(C18H28FNS.HCl) requires: C, 62.49; H, 8.45; N, 4.05; Cl,
10.25%
Exam~le 43
4-[2-(3,4-DichlorothioPhenoxv)ethY11-1-~entvlPiPeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.009), 3,4-dichlorothiophenol (1.79g),
triphenylphosphine (2.62g) and diethyl azodicarboxylate
(1.74g). Treating the product with hydrogen chloride save
a white solid which was recrystallised from ethyl acetate
to give the title compound as a white crystalline solid
(0.77g), m.p.158-159C.
Found: C, 54.41; H, 7.11; N, 3.48; Cl , 8.89%
(C18H27C12NS.HCl) requires: C, 54.48; H, 7.11; N, 3.53;
Cl , 8.93%
SUBSTlTlJTE SHEET
W092/02S02 PCT/GB91/01340
~a8~
- 50 -
ExamPle 44
l-Pentvl-4-~3 ~h nYlProPyl)piDeridine hvdrochloride
A mixture of 4-(3-phenylpropyl)piperidine (Sg), 1-
bromopentane (7.42g), potassium carbonate (lOg) and
ethanol (125ml) was heated at reflux for 1~ hours. The
solution was filtered, and the solvent was removed under
reduced pressure. The residue was dissolved in
dichloromethane and the dichloromethane solution washed
with dilute sodium hydroxide solution, dried over sodium
sulphate and the solvent removed. The residue was treated
with hydrogen chloride in ether to give a solid.
Recrystal~isat~ an from ethyl acetate gave the tltle
compound (4.19g), m.p. 188-189C.
Found: C, 72.56; H, 10.29; N, 4.58; Cl, 11.44%
~ClgH31N.HClØ25~20) requires: C, 72.56; H, 10.36; N,
4.45; Cl, 11.27%
Exam~le 45
4-r2-(4-~luoro~hen~l)ethYll-l-~entvlPiPeridine
hvdrobromide
A mixture of 4-[2-(4-fluorophenyl)ethyl]-1-
pentylpyridinium bromide (3.0g), platinum oxide (0.6g) and
ethanol (lOOml) was shaken under an atmosphere of hydrogen
for 15 minutes. The mixture was filtered and the filtrate
was evaporated to dryness. The residue was recrystallised
from methanol/ethyl acetate to give the title compound.
m.p. 173-174C.
SIJBSTlTlJTE SHEFr
W092/02502 PCT/GB91/01340
2 ~
- 51 -
ExamPle 46
4-f2-(4-NitroPhenoxv)ethvll-l-PentvlPiPeridine
hvdrochloride
S A mixture af 4-(2-hydroxyethyl)-1-pentylpiperidine (2.5g),
sodium hydride (60% in oil) (0.42g) and dimethylformamide
(20ml) was heated at 50C for 1.5 hours. 1-Fluoro-4-
nitrobenzene (2.14ml) was added and the mixture was
stirred at 50 for 5 hours. The mixture was cooled,
poured into water and extracted with dichloromethane. The
dichloromethane extracts were dried over magnesium
sulphate and the solvent removed. The residue was
chr matographed on silica gel, with
mèthanol/dichloromethane as eluent,and the product was
treated with hydrogen chlor~de to give a yellow solid
which was recrystallised from ethyl acetate to give the
title compound as a yellow crystalline solid (0.937g),
m.p.174-176C.
Found: C, 60.35; H, 8.15; N, 7.85; Cl , 9.70~
(C18H28N03.HCl) requires: C, 60.58; H, 8.19; N, 7.85; Cl,
9.93%
ExamDle 47
.
4-~2-(2-FluoroPhenoxv)ethvll-l-PentvlDiPeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(1.5g), 2-fluorophenol (0.84g), triphenylphosphine (1.96g)
and diethyl azodicarboxylate ~1.19ml). Treating the
product with hydrogen chloride gave a white solid which
SUBSTITUTE 8HEET
W092/02S02 PCT/GB91/01340
- 52 -
was recrystallised from ethyl acetate/methanol to give the
title compound as a white crystalline solid (0.87g),
m.p.150-152C.
S Found: C, 65.14; H, 8.87; N, 4.30; Cl , 10.82%
(C18H28FNO.HCl) requires: C, 65.54; H, 8.86; N, 4.25; Cl,
10.75%
ExamDle 48
4-f2-(4-tert-Butvl~henoxv)ethYll-l-Pentvl~i~eridine
hvdrochloride
The t~tle compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylp~perid~ne
(1.5g), 4-tert-butylphenol (1.127g), triphenylphosphine
(1.96g) and diethyl azod~carboxylate (1.19g). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from ethyl acetate/methanol to
give the title compound as a white crystalline solid
(1.23g), m.p.l89-191C.
Found: C, 71.67; H, 10.50; N, 3.88; Cl , 9.68%
(C22H37NO.HCl) requires: C, 71.8; H, 10.41; N, 3.81; Cl,
9.63%
Exam~le 49
l-Pentvl-4-~2-(4-trifluoromethoxv~henoxv)ethvllPi~eridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-pentylpiperidine
SUE3STlTlJTE SHEET
W092/02502 PCT/GB9t/01340
~ ~J ~
(1.5g), 4-trifluoromethoxyphenol (1.335g),
triphenylphosphine (1.9-6g) and diethyl azodicarboxylate
(1.19ml). Treating the product with hydrogen chloride
gave a white solid which was recrystallised from ethyl
acetate/methanol to give the title compound as a white
crystalline solid (O.95g), m.p.154-156C.
Found: C, 57.29; H, 7.31; N, 3.52; Cl , 8.59%
(C1gH28F3NO2.HC1) requires: C, 57.64; H, 7.38; N, 3.54;
Cl, 8.96%
Exam~le 50
4-~2-(4-iso-Pro~vlPhenoxv)ethvll-1-PentvlPiDeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-pentyipiperidine
(1.5g), 4-isopropylphenol (1.02g), triphenylphosphine
(1.96g) and diethyl azodicarboxylate (1.19ml). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from ethyl acetate/methanol to
give the title compound as a white crystalline solid
(1.21g), m.p.185-187C.
2~
Found: C, 71.35; H, 10.23; N, 4.05; Cl , 10.08%
(C21H35NO.HCl) requires: C, 71.26; H, 10.25; N, 3.96; Cl,
10.02%
SUBSTITVTE 5HEET
W092/02502 PCT/GB91/01~0
2 ~
- 54 -
Exam~le 51
4-~2-(3-iso-Pro~vl~henoxv)ethvll-1-PentYlPiPeridine
hYdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
~1.5g), 3-isopropylphenol (1.02g), triphenylphosphine
(1.96g) and diethyl azodicarboxylate (1.19ml). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from ethyl acetate/methanol to
gi~e the title compound as a white crystalline solid
(O.Sg), m.p.l66-168C.
Found: C, 71,40; H, 10.30; N, 3.97; Cl , 10.00%
(C21H35NO.HCl) requires: C, 71.26; H, 10.25; N, 3.96; Cl,
10.02%
ExamPle 52
4-r2-(3-tert-ButvlPhenoxv)ethvll-~-~entvlDiDeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(1.5g), 3-tert-butylphenol (1.127g), ~riphenylphosphine
(1.96g) and diethyl azodicarboxylate (1.19g). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from ethyl acetate/methanol to
give the title compound as a white crystalline solid
(0.80g), m.p.171-173C.
Found: C, 71.80; H, 10.57; N, 3.88; C1 , 9.67%
SUE3STITUTE SHEET
.
W092/02502 PCT/GB91/013~
2 ~
- 55 -
~C22H37NO.HC11 requires: C, 71.8; H, 10.41; N, 3.81; Cl,
9.63%
ExamPle 53
4-~2-(2-~henvl~henoxv)ethvll-1-PentvlPi~eridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(l.Sg), 2-phenylphenol (1.28g), triphenylphosphine (1.96g)
and diethyl azodicarboxylate (1.19g). Treating the
product with hydrogen chloride gave a white salid which
was recrystallised from ethyl acetate/methanol to give the
title compound as a white crystalline solid (1.05g),
m.p.175-177C.
Exam~le 54
l-Pentvl-4-r2-(4-trifluoromethvl~henoxv)ethvll-~i~eridine
oxalate
A mixture of 4-(2-hydroxyethyl)-1-pentylpiperidine (2.0g),
sodium hydride (60% in oil) (0.4g) and dimethylformamide
(20ml) was refluxed 1.5 hours. 4-Fluoro-
trifluoromethylbenzene (1.64ml) was added and the mixture
was refluxed for 18 hours. The m~xture was cooled, poured
into water and extracted with ether. The ether extracts
were dried over magnesium sulphate and the solvent was
removed. The residue was chromatographed on silica gel
with methanol/dichloromethane as eluent and the product
was treated with oxalic acid to give solid. This was
SUBSTlTlJTE SHEET
W092/02502 PCT/GB91/01340
9 ~
- 56 -
recrystallised from ethyl acetate/methanol to give the
title compound. (O.Sg), m.p.101-103C.
Found: C, 57.76; H, 7.00; N, 3.27%
S (Cl9H28F3NO C2H204 0 1 H20) requires: C, 57.9; H, 6.9; N
3.2%
ExamPle 55
4-l2-(3,5 Dichloro~henoxv)ethvll-l-~entvlPiDeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(2.0g), 3,5-dichlorophenol (1.63g), triphenylphosphine
(2.62g) and diethyl azodicarboxylate (1.74g). Treatlng
the product with hydrogen chloride gave a white solid
which was recrystallised from ethyl acetate/methanol to
give the title compound as a white crystalline solid
~l.lg), m.p.l68-170C.
Found: C, 56.80; H, 7.40; N, 3.64; Cl , 9.33; Cl, 27.92%
(C18H27Cl2NO.HCl) requires: C, 56.78; H, 7.41; N, 3.68;
~l-, 9.30; Cl, 27. 93%
ExamDle 56
4-~2-(3,4-Dichloro~henoxv~ethvll-l-heDtvlDiDeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-heptylpiperidine
(2.27g), 3,4 dichlorophenol (1.63g), triphenylphosphine
SURSTITVTE 8HEET
W092/02502 PCT/GB91/01340
2~d~1
- 57 -
12.62g) and diethyl azodicarboxylate (1.74g). Treating
the product with hydrogen chloride gave a white solid
which was recrystallised from ethyl acetate/methanol to
gi~e the title compound as a white crystal}ine solid
(0.7g), m.p.l38-139C.
Found: C, 58.87; H, 7.B8; N, 3.50; Cl , 8.68; Cl, 26.00%
(C20H31C12NO.HCl) requires: C, 58.76; H, 7.89; N, 3.43;
Cl-, 8.68; Cl, 26~01%
Exam~le 57
4-~2-(3,4-Dichloro~henoxv)ethvll-1-~3-
phenvlPro~y~Lp-iperidine hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-~2-hydroxyethyl)-1-(3-phenylpropyl?-
piperidine (2.47g), 3,4-dichlorophenol ~1.63g),
triphenylphosphine ~2.62g) and diethyl azodicarboxylate
~1.74g). Treating the product with hydrogen chloride gave
a white solid which was recrystallised from ethyl
acetate/methanol to give the title compound as a white
crystalline-solid (0.75g), m.p.l37-138C.
Found: C, 61.24; H, 6.45; N, 3.36; Cl , 8.7%
(C22H27C12NO.HC1Ø1 H20) requires: C, 61.56; H, 6.34; N,
3.27; C1, 8.30%
SUBSTIT~JTE SHEET
WO92/02502 PCT/GB91/01340
- 58 -
Exam~le 58
1-Cyclo~ro~ylmethvl-4-~2-(4-fluoroPhenoxv)ethvll-
PiPeridine oxalate
The title compound was prepared in a similar manner to
example 41 from 4-[2-(4-fluorophenoxy)ethyl]-piperidine
hydrochloride (2.0g), bromomethylcyclopropane ~2.0ml) and
sodium hydride (80% in oil) (0.58g) in dimethylformamide
~40ml). Treating the product with oxalic acid in ethyl
acetate gave a solid which was recrystallised from ethyl
acetate to give the title compound. Yield, ~0.963g) m.p.
129-132C
Found: C, 61.95; H, 7.05; N, 3.91%
(C17~24FNO.C2H2O4) requires: C, 62.11; H, 7.13; N, 8.81%
Exam~le 59
1-(3,3-Di~henvlpro~-2-envl)-4-t2-(4-fluoroDhenOxY)ethvll-
Piperidine 1-(3,3-DiPhen~l~roP-2-envl)-4-~2-(4-
fluorophenoxv)ethvll-Piperidine oxalate
Methanesulphonyl chloride ((0.46ml) was added to a
solution of 1,1-diphenyl-2-hydroxymethylethylene (1.14g)
in tetrahydrofuran ~20ml). The mixture was stirred for 1
hour when 4-[2-(4-fluorophenoxy)ethyl]-piperidine (1.42g)
and triethylamine (0.8ml) was added. The mixture was
stirred under nitrogen for 48 hours then heated at reflux
for 8 hours. The mixture was poured into water (200ml)
and extracted with ether. The ether phase was dried over
SUBSTITUTE SHEET
W092/02502 PCT/GB9l/Ot~O
_ 59 _
magnesium sulphate, filtered and the solvent removed. The
residue was chromatographed on silica gel with
methanol/dichloromethane as eluent and the product was
treated with oxalic acid to give solid. This was
recrystallised from ethyl acetate/methanol to give the
title compound. (0.6B7g), m.p.l74-176C.
Found: C, 70.84; H, 6.34; N, 2.93%
(C28H30FNO.C2H204) requires: C, 71.27; H, 6.38; N, 2.77
Exam~le 60
4 ~2-(2-3enzvlphenoxv)ethvll-l-PentvlPiPeridine
hvdrochloride
The title compound was prepared in a similar manner to
example 1 from 4-(2-hydroxyethyl)-1-pentylpiperidine
(1.5g), 2-hydroxydiphenyl methane (1.38g),
triphenylphosphine (1.96g) and diethyl azodicarboxylate
(1.19g). Treating the product with hydrogen chloride gave
a white solid which was recrystallised from ethyl
acetate/methanol to give the title compound as a white
crystalline solid ~1.65g), m.p.ll9-120C.
Found: C, 73.32; H-, 8.94; N, 3.61; Cl, 8.70%
(C25H35NO.HClØ3H20) requires: C, 73.59; H, 8.89; N,
3.43; Cl, 8.69%
SUBSTITIJTE SHEET