Note: Descriptions are shown in the official language in which they were submitted.
2090306
TITLE: OIL AND GAS WELL OPERATION FLUID USED FOR THE
SOLVATION OF WAXES AND ASPHALTENES, AND METHOD OF USE
THEREOF
INVENTORS: DONALD A. THORSSEN AND DWIGHT N. LOREE
FIELD OF THE INVENTION
This invention relates to oil and gas well
operation fluids, particularly those used for the
removal of contaminants from wells.
BACKGROUND AND SUMMARY OF THE INVENTION
The rocks that contain oil and gas in oil
and gas reservoirs are porous and to remove the oil or
gas from the reservoirs requires that the oil or gas
move through the pores in the rock. If the pores are
blocked, then it is difficult and it may even become
impossible to remove the oil or gas from the
reservoir, with consequent economic loss to the oil or
gas well owner.
Two notorious contaminants that may block
the pores are waxes and asphaltenes. A wax is normally
defined as a hydrocarbon that is a solid at room
temperature and has 20 carbon atoms or more. An
asphaltene is an agglomerate of aromatic hydrocarbons,
and may contain bound oxygen, nitrogen and sulphur
atoms. The oil and gas in many, if not most,
reservoirs contains both waxes and asphaltenes. These
waxes and asphaltenes may be dissolved in the oil. In
some cases, however, the waxes and asphaltenes may
partially block the pores, or, as production
continues, the very action of removing oil from a
reservoir may cause waxes and asphaltenes to
precipitate out of solution and block the pores.
Also, the waxes and asphaltenes may
precipitate out of solution in the well bore itself,
-- 2090306
or in equipment used for the production of oil and gas
and reduce or block the flow of oil from the well.
The economic damage from waxes and
asphaltene precipitation can be very high, killing
some wells entirely. Consequently, a great deal of
attention has been devoted to developing cost
effective ways of preventing waxes and asphaltenes
from precipitating out of solution or of removing the
waxes and asphaltenes from an oil reservoir or well
bore.
One such attempt at a solution has been to
apply to a well a mixture of a significant proportion
of the aromatic xylene (about 45%) and a lesser
proportion of the paraffinic hydrocarbon hexane (about
30%), together with about 25% methanol. This product
is known by the name NP760 and is available from
Wellchem of Calgary, Alberta, Canada. The xylene is
intended to solvate asphaltenes and the hexane is
intended to solvate waxes. The xylene and hexane
components of the composition are each derived from
refining a feedstock and removing that particular
component from the feedstock. The result is a product
that has moderate success in solvating at least some
waxes and asphaltenes, but because the fluid is made
from a complex process, the fluid is relatively
expensive.
One difficulty with the use of hexane or
other alkanes is that they tend to cause asphaltenes
to precipitate out of solution. This in turn is
believed to increase the precipitation of waxes. How
this is believed to occur is as follows. Waxes require
nucleation sites in the oil formation or well bore to
which they can attach. Any such site will become a
nucleation site for the further accretion of waxes. In
2û9~306
time, the waxes, mixed with asphaltenes, build outward
and block the well bore or pores in the formation. In
a typical oil formation water surrounds the rock in
the formation and waxes will tend to slide off the
water and not attach to the rock. However, if
asphaltenes are present, they may attach to the rock
surface since reservoir rock contains positively and
negatively charged molecules (cations and anions)
which attract the polar asphaltenes. The asphaltenes
may then protrude beyond the water layer surrounding
the rock particle and form a nucleation site for
waxes. Hence a precondition for wax deposition is the
precipitation of asphaltenes from the oil in the
reservoir. It is the hexane that causes the
precipitation of the asphaltenes and thus the
formation of nucleation sites for waxes. The xylene is
added to solvate the asphaltenes and prevent the
formation of nucleation sites.
However, such a product, formed of an alkane
(particularly pentane, hexane and heptane) and an
aromatic, and similar products that are produced by
the steps of: (a) refining a feedstock, (b)
selectively removing hydrocarbons and (c) subsequently
mixing the selected hydrocarbons, are not believed
very effective in removing gummy layers of waxes and
asphaltenes that are typically found in oil and gas
reservoirs and well bores in relation to their cost.
The waxy depositions in oil and gas formations are
complex aggregations of molecules, with many layers
and globules of different waxes and asphaltenes, which
the inventors have found are not readily removed by
simple compositions. Such products, requiring several
processing steps, tend to be expensive. Also, in some
wells such mixtures of an aromatic, alkane and alcohol
-
4 209030~
or other polar substance may increase the
precipitation of waxes and asphaltenes. Thus for
example, in the general case, stabilized C5+
condensates tend to precipitate asphaltenes, with the
future risk of wax contamination for the reasons just
mentioned. That is to say, while it is possible to
tailor a particular composition of alkanes and
aromatics to a particular well formation, such a
procedure is relatively expensive and may produce a
product that is useful for one well formation but not
for another. With the expense of the product and the
risk of actually damaging the well, the application of
such a product to a well is a venture not lightly
undertaken.
The inventors have found a composition and
a method for its use that helps to remove the
uncertainty from applying wax solvating materials to
wells, while at the same time significantly reducing
the cost of making and using the composition. The
composition is formed from a complex mixture of
aromatics and alkanes (preferably C7+ ) . The complex
mixture provides different components that solvate
different waxes and asphaltenes. Rather than using a
composition derived from selecting individual
components during refining, the composition is the
residue after lighter components (preferably
substantially all C1, C2, C3, C4 and C5) have been
removed during refining. With the appropriate
selection of the feedstock, an improved wax solvating
and asphaltene solvating composition may be derived.
The feedstock should be selected to have a
significant proportion of aromatics and alkanes. The
inventors have found that if a feedstock has a mass
percentage of trimethylbenzene higher than the mass
-- 2090306
percentage of n-decane as determined by gas
chromatography then the feedstock will have a
sufficiently complex mixture of aromatics and alkanes
for the efficient solvating of asphaltenes and waxes,
particularly after the lighter ends (C1, C2, C3, C4 and
C5 ) have been removed by distillation from the
feedstock. By a sufficiently complex mixture of
aromatics is meant aromatics other than, but not
necessarily excluding, the simple aromatics benzene,
toluene, ethylbenzene and xylene. These simple
aromatics are the aromatics normally measured in gas
chromatography since they usually yield well defined
peaks. The inventors have found that it is necessary
to have a good quantity of other aromatics, and the
presence of these other aromatics is indicated by the
quantity of trimethylbenzene.
Another indication that a feedstock contains
a suitably complex blend of aromatics and alkanes to
solvate complex gummy layers of waxes and asphaltenes
is the presence of sulphur containing compounds in the
feedstock. It is believed that sulphur is a catalyst
for the conversion of alkanes to aromatics during the
many years that the hydrocarbon deposit evolves
underground. Hence, the more sulphur, the greater the
conversion of alkanes to aromatics. Thus the presence
of sulphur is an indication that the feedstock will
have a suitable proportion of aromatics to alkanes.
Aromatic composition and alkane composition
should be in the range 30% to 70% by mass percentage
as determined by gas chromatography for a suitable
composition. However, it is not believed that such a
ratio of aromatics to alkanes is sufficient: the
composition must be suitably complex as noted above.
E
-- 2090306
5A
Further, it has been found desirable that the
feedstock be clear or have a light colour such as
~ "''"~
,;
-
6 2~90306
amber. Dark colour indicates the presence of heavy
ends (C16+) that assist in the formation of waxes. The
C16+ content of the fluid should preferably be below
2% by mass as determined by gas chromatography. If the
feedstock contains greater than 2% C16+ content, then
an additional cut may be taken to remove all or
substantially all the higher ends.
Alternatively, the fluid may be formulated
for pure asphaltene solvation. Pure asphaltene
generally occurs in only two situations in the
reservoir. In one case, pyrobitumen can be present
ingas reservoirs. This is generally believed to have
been deposited long ago when oil which had occupied
the reservoir migrated out and left the pyrobitumen
15- behind. This pyrobitumen can move during production
and plug the formation or wellbore.
Another case is in tertiary recovery using
hydrocarbon miscible solvents floods. Light
hydrocarbons in the C2 to C5 range are injected into
the reservoir to push the oil to production wells.
While these light hydrocarbons will solvate paraffinic
molecules they act to precipitate asphaltenic
molecules. Thus asphaltene will precipitate without
heavy paraffinic molecules present.
The-fluid is formulated for solvating pure
asphaltenes by increasing the temperature of the
cutpoint. This removes the C6 and C7 components which
contain a lower percentage of aromatics than the bulk
residue. The aromatics in this region are small and
not as effective as the more complex aromatics in the
remainder of the fluid.
Asphaltenes are normally colloidally
suspended in crude oil by peptizing resins (maltenes).
These peptizing resins are aromatic and polar at one
~-- 2090306
end and paraffinic or neutral at the other end. The
polar end is attracted to the asphaltene and the
nonpolar end to the crude oil. When the solid
asphaltene is completely surrounded by peptizing
resins it becomes a colloidally suspended particule
completely suspended in the crude oil.
Currently the main way of treating these
precipitations is by injecting pure xylene down a
well. Xylene is a simple aromatic with short
paraffinic side chains. The more complex aromatics in
the C8+ fluid described here with longer side chains
provide superior emulation of the maltenes that
originally suspended the asphaltene molecules than
just pure xylene.
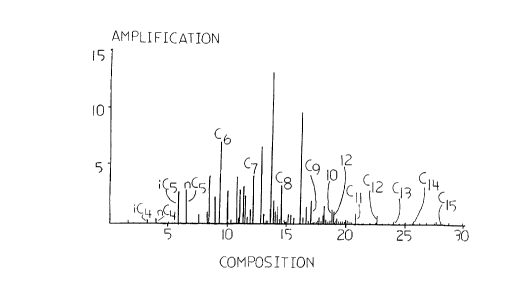
BRIEF DESCRIPTION OF THE FIGURES
Figs. 1 and 2, and tables 2 and 3, are gas
chromatographic profiles of preferred compositions of
the feedstock from which the fluid according to the
invention may be derived. Fig. 1 (Table 2) is a gas
chromatograph profile of the C5+ feed from the Wildcat
Hills plant in Alberta, Canada. Fig. 2 (Table 3) is a
gas chromatograph profile of the C5+ feed from the
Jumping Pound plant in Alberta, Canada. The
trimethylbenzene marker is indicated at lO and the n-
decane marker is indicated at 12 in each figure.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
As used in this patent document: a residual
fluid is a fluid that remains after light components
(particularly Cl, C2, C3, C4 and C5 components) of a
hydrocarbon feedstock are removed during the refining
of the hydrocarbon feedstock; a hydrocarbon feedstock
i8 a hydrocarbon fluid that has been produced directly
from an oil or gas bearing formation; sour means
-
7A 2090306
which the fluid according to the invention is derived
should
-
20903~6
sulphur containing. Cn+ indicates no greater than a
small percentage (less than 5%) of Cl, C2, ... Cn_1.
The preferred composition is a residual
hydrocarbon fluid derived from a hydrocarbon feedstock
having a complex mixture of aromatics. Complex in this
context means that there are included in the mixture
aromatics other than benzene, toluene, ethylbenzene
and xylene, such as methylethylbenzene, diethylbenzene
and propylethylbenzene, to name but a few of the
possibilities, although the mixture may also include
the simple aromatics. The hydrocarbon feedstock from
which the fluid according to the invention is derived
should contain a greater percentage of
trimethylbenzene than n-decane, in which case it is
believed that the fluid will have the desirable wax
and asphaltene solvating properties. It should be
noted that these aromatics, other than benzene,
toluene, ethylbenzene and xylene, are not readily
identifiable using gas chromatography, and so having
the trimethylbenzene peak higher than n-decane is the
manner in which the appropriate feedstock may be
identified. The feedstock is preferably but not
necessarily refined to remove essentially all C1, C2,
C3, C4 and C5 components. It is not necessary that the
feedstock have a relatively low ratio of alkanes if
the alkanes are concentrated in the lighter ends and
the lighter ends are removed by distillation. The
feedstock is preferably sour and clear or light amber,
with sulphur content exceeding 1500 ppm, and
preferably has no more than about 2% C16+. In general,
it is believed that the more sulphonated the
feedstock, the better for asphaltene solvation. If the
C16+ content is greater than 2%, a further cut should
preferably be taken to remove the higher ends.
-
2090306
To produce the formulation of the invention
from the feedstocks shown in Figs. 1 and 2, the
feedstocks are refined to remove substantially all of
the Cl. C2, C3, C4 and C5 components, with a small
percentage of C6, C7 and C8.
With a 120C cut, the resulting fluid, as
determined by gas chromatography, has about 0.1%
pentane, 7% hexanes, 13% heptanes, 13% octanes, 7%
nonanes, 9% decanes, 5% undecanes, 3% dodecanes, 2%
tridecanes, 1% tetradecanes, 0.6% pentadecanes, 0.3%
hexadecanes, 0.1% heptadecanes, 0.05% octadecanes, 1%
benzene, 9% toluene, 12% ethyl benzene and meta- and
para xylene, 2.6% ortho-xylene, 2.2% 1,2,4
trimethylbenzene, 0.1% cyclopentane, 2%
methylcyclopentane, 2.6% cyclohexane, 8%
methylcyclohexane and less than 0.05% of any other
constituent, including cumulatively less than 0.1%
C19+. Naphthene content is greater than about 3%.
With a 130C cut, the resulting fluid, as
determined by gas chromatography, has about 0%
pentanes, 2% hexanes, 13% heptanes, 13% octanes, 7%
nonanes, 9% decanes, 5% undecanes, 3% dodecanes, 2%
tridecanes, 1% tetradecanes, 0.5~ pentadecanes, 0.2%
hexadecanes, 0.1% heptadecanes, 0.05% octadecanes,
1.5% benzene, 12.6% toluene, 15% ethyl benzene and
meta- and para xylene, 2.4% ortho-xylene, 2.2% 1,2,4
trimethylbenzene, 0% cyclopentanes, 1%
methylcyclopentane, 1.9% cyclohexane, 7.8%
methylcyclohexane and less than 0.05% of any other
constituent, including cumulatively less than 0.1%
C19+. It will be observed that with the higher cut,
all of the remaining pentane, and more than 2/3 of the
hexanes have been removed while the aromatic content
has increased.
2090306
With a 140C cut, the resulting fluid, as
determined by gas chromatography, has about 0%
pentanes, 0.3% hexanes, 8.7% heptanes, 13.5% octanes,
6.7% nonanes, 10.7% decanes, 6% undecanes, 4%
dodecanes, 2% tridecanes, 1% tetradecanes, 0.6%
pentadecanes, 0.3% hexadecanes, 0.1% heptadecanes,
0.06% octadecanes, 0.6% benzene, 13.7% toluene, 17.9%
ethyl benzene and meta- and para xylene, 2.7% ortho-
xylene, 2.7% 1,2,4 trimethylbenzene, 0% cyclopentanes,
0.3% methylcyclopentane, 0.7% cyclohexane, 7%
methylcyclohexane and less than 0.1% of any other
constituent, including cumulatively less than 0.05%
cl9+ .
With a 150C cut, the resulting fluid, as
determined by gas chromatography, has about 0%
pentanes, 0.01% hexanes, 2.5% heptanes, 14.4% octanes,
9.5% nonanes, 14.1% decanes, 8.1% undecanes, 5.2%
dodecanes, 3.1% tridecanes, 1.8% tetradecanes, 1%
pentadecanes, 0.4% hexadecanes, 0.2% heptadecanes,
0.1% octadecanes, 0.01% benzene, 8.3% toluene, 20.3%
ethyl benzene and meta- and para xylene, 3.5% ortho-
xylene, 3.5% 1,2,4 trimethylbenzene, 0% cyclopentane,
0% methylcyclopentane, 0.07% cyclohexane, 3.7%
methylcyclohexane and less than 0.05% of any other
constituent, including cumulatively less than 0.1%
cl9+ .
With a 160C cut, the resulting fluid, as
determined by gas chromatography, has about 0%
pentanes, 0% hexanes, 0.2% heptanes, 7.9% octanes,
3~ 9.8% nonanes, 18.6% decanes, 10.8% undecanes, 6.9%
dodecanes, 4.1% tridecanes, 2.3% tetradecanes, 1.2%
pentadecanes, 0.5% hexadecanes, 0.2% heptadecanes,
0.1% octadecanes, 0% benzene, 2.2% toluene, 25.3%
ethyl benzene and meta- and para xylene, 4.2% ortho-
11 2~90306
xylene, 5.0% 1,2,4 trimethylbenzene, 0% cyclopentane,
0% methylcyclopentane, 0% cyclohexane, 0.5%
methylcyclohexane and less than 0.05% of any other
constituent, including an undetectable amount of C19+.
For each of the 120C, 130C, 140C, 150C
and 160C cuts, all percentages are mass fraction.
Only simple aromatics are identified. Supercritical
fluid chromatography shows that the actual aromatic
content is greater than 40%. For C5 to Cl8, the
percentage given is the sum of the peaks from the gas
chromatographic analysis. Thus, the figure for
"decanes" includes the figure for straight chain
decane. The higher cuts show increased percentages of
C8 and Cl0, and increased xylene, particularly the
150C cut. The 130C cut is preferred for wax and
asphaltene solvation. For the 120C cut, the fluid is
amber in colour with a density of 780kg/m3. Boiling
point at 1 atm is 100-300C, freezing point about -
60C, vapour pressure <15kpa, with a closed cup flaxh
point of >10C. As a flammable amd toxic liquid, this
fluid should be treated with well known safety
precautions. At the higher cuts (150C cut),
effectively all of the C6 and C7 iS removed, with
consequent increase in the xylene content to over 25%.
Such a fluid is useful for pure asphaltene solvation.
Thus a preferred composition of the
invention has less than 1% cumulatively of methane,
ethane, propane, butane and pentane; 0 to 10% hexanes;
1 to 15% heptanes; 5 to 15% octanes; 5 to 15% nonanes;
5 to 15~ decanes; 3 to 10~ undecanes; 1 to 7%
dodecanes; 0 to 5% tridecanes; 0 to 3% tetradecanes;
0 to 2~ pentadecanes; 0 to 1% hexadecanes; 0 to 1%
heptadecanes; 0 to 1~ octadecanes; 0 to 3~ benzene; 5
to 20% toluene; 10 to 35% xylenes; 1 to 5%
12 205030~
1,2,4trimethylbenzene; and cumulatively less than 2%
C16+, all of the percentages being mass fraction as
determined by gas chromatography. In another preferred
composition according to the invention, the fluid
includes less than 5% hexanes; O to 15% heptanes; O to
15% octanes; 5 to 15% nonanes; 5 to 25% decanes; 3 to
15% undecanes; 2 to 10% dodecanes; O to 5% tridecanes;
O to 3% tetradecanes; O to 2% pentadecanes; O to 1%
hexadecanes; O to 1% heptadecanes; O to 1%
octadecanes; O to 3% benzene; 5 to 15% toluene; 15 to
40% xylenes; and 1 to 896 1,2,4trimethylbenzene.
Solvation tests using the formulation
according to the invention have yielded the following
results.
Table 1
No. Location SolventContaminant %
Dissolved
1. 08-20-044-04W5 #81.0284 86.8
2. 02-09-039-07 #80.9714 98.2
3. 02-20-039-07 #80.9959 97.0
4. 10-13 (Viking) #81.0033 87.4
5. 06-10-035-06W4 #81.0280 96.2
6. 10-13 (Viking) #100.9972 63.2
7. 06-10-035-06W4 #100.9547 ~.2
8. 10-24-001-26 #81.0005 71.7
9. 8" Group Line #81.0004 ffl.6
10. 11-14-041-25 #81.0017 76.8
11. 08-14-041-25 #81.0567 935
12. 11-14-041-25 #91.0011 81.1
13. OB-14-041-25 #90.9987 97.2
14. Utikuma Keg R. #80.9536 63.0
15. Utikuma Slave #81.0221 g3.2
16. Hutton 12-18 #81.0543 83.2
17. 10-18-048-08W5 #81.1200 97.8
1~. 12-24-047-Og W4 #160.97S6 993
19. 12C-19-036-04 #80.4624 87.3@22C
20. 16-01-004-21W3 #80.2992 75.2@22C
21. Intensity Res. #80.9845 97.1
22. 05-03-055-13W5 #80.9904 96.6
23. 16-14-055-13W5 #81.0509 21.4
23a. 16-14-055-13W5 Toluene 1.0458 * 38.1
13 2090306
24. Esso Wizard Lk. 90/10 0.9813 933
25. Esso Wizard Lk. #8 0.9940 96.6
26. Esso Wizard Lk. Run 95 1.0018 913
27. Esso Wizard Lk. 90/lOXy 0.9726 97.0
28. Esso Wizard Lk. lOOXy 0.9676 965
29. 16-03-040-04W5 #8 1.0485 8~5
30. 16-03-040-04W5 #9 1.0264 96.1
31. 046-09W5 Comaplex #8 1.0545 76.1
32. 046-09W5 Comaplex #9 0.9974 ~.6
33. 07-11-053-26 #8 1.0292 979
34a. 16-19-071-04Chevron #8 0.9756 94.4
34b. 02-06-072-04Chevron #8 1.0014 98.7
34c. 16-19-071-04Chevron lOOXylene O.9588 ~;.7
35. 02-06-072-04Chevron lOOXylene 1.4169 933
36. Chauvco Unit No. 2 #8 0.9799 97.6
37. 06-10-035-03W5 #8 0.9548 665
38. 08-20-026-12W4 #8 1.10157 ~2
39. 03-13-044-09Amoco #8 1.0397 82.9
40. 04-22-043-08Amoco #8 0.9671 94.1
41. 02-05-043-08Amoco #8 1.0202 93.8
42. 03-13-044-09Amoco #9 0.9975 72.5
43. 04-22-043-08Amoco #9 0.9760 96.1
44. 02-05-043-08Amoco ~9 1.0372 55.3
45. Willesden Green #8 1.0127 94.1
Notes to Table 1: Wax or asphaltene amount
is listed in grams under the heading "contaminant".
The sample is indicated by the location of the well,
in the Province of Alberta, Canada, from which the
sample was derived. % dissolved is the percentage of
the original sample that was dissolved in the solvent.
It is a general indicator of the effectiveness of the
solvent on that particular composition of contaminant.
The amount of solvent used was lOOmL. #8 and #16 is
the fluid described above as the 120C cut. #9 is a
blend of NP760tm and 10% Super A Soltm, which is
available from Wellchem of Calgary, Alberta, Canada.
#10 is Petro Rep condensate having about 15% butanes,
46% pentanes, 19% hexanes and less than 1% aromatics
as determined by gas chromatography. Run 95 is 100%
FRACSOL well site operation fluid available from
Trisol Inc., of Calgary, Alberta. 90/10 is 90% of the
2090306
14
120C cut with 10% of a non-aromatic brominated non-
fluorinated hydrocarbon such as dibromomethane.
90/lOXy is 90~ of the 150C cut described above with
10% of a non-aromatic brominated non-fluorinated
hydrocarbon such as dibromomethane. lOOXy is 100% of
the 150C cut described above. lOOXylene is pure
xylene. Toluene is pure toluene. The oils from which
some of the contaminants precipitated so far as known
have the following composition: Sample 1. 8.26%
asphaltene, 11.2% wax; Sample 4. 10% asphaltene;
Sample 5. 23% asphaltene; Sample 22. 1.11% asphaltene,
4.7% wax; Sample 34c. 6.12% asphaltene, 2.9% wax;
Sample 36. 3.43% asphaltene, 3.8% wax.
These results show that the formulation of
the present invention provides comparable solvation
properties to highly refined and expensive wax
solvation products when applied to a variety of wells
without specifically formulating the composition to
the well formation.
By comparison with the product of the
present invention, so far as known, the condensate
available from other gas plants located in the
Province of Alberta is not desirable for use as a wax
and asphaltene removing fluid. Thus, for condensate
from Amerada Hess (Bearberry~, while the fluid is
clear, showing low heavy ends, the aromatic content is
too low by comparison with the light ends for a useful
feedstock. Condensate from the Can-Oxy Mazeppa plant
is dark red from the plant, which becomes black when
the lighter ends are removed, that is, when a C7+ cut
is taken, thus indicating the presence of undesirable
heavy ends. Condensate from the Burnt Timber plant has
too many heavy ends to work as a solvent, but may be
formation compatible in some wells. Condensate from
15 2090306
the Brazeau plant has too few aromatics, and too many
waxes to be useful as a solvent. Condensate from Mobil
Oil Lone Pine Creek has 6~ xylene, which might suggest
it is similar to the Jumping Pound feed (6.5% xylene).
However, the relative lower percentage of lighter ends
means that the concentration of xylene and other
aromatics does not increase greatly if the lighter
ends are removed in accordance with the principles of
the invention. Consequently, the feed is not very
useful as a solvent. Condensate from the Husky OIl Ram
River plant has too many heavy ends, as indicated by
its dark colour, and has too few aromatics to make it
a useful feed for a solvent.
In the method of the invention, a C5+
hydrocarbon feedstock is obtained in which feedstock
the mass percentage of trimethylbenzene exceeds the
mass percentage of decane as determined by gas
chromatography; and substantially all hydrocarbons
having 1, 2, 3, 4 and 5 carbon atoms are removed,
thereby producing a residual fluid, effectively a C7+
fluid. The fluid is applied to a well as follows.
For pumping or flowing wells, the well
should be de-waxed before attempting to clean up the
formation. To clean a pumping well, an amount of the
fluid of the invention equal to about one half of the
tubing volume should be circulated in the well with a
bottomhole pump for about 24 hours. To clean the
nearby well bore formation, a squeeze volume (1.0 -
1.5 m3 per meter of perforations) of the fluid
according to the invention should be squeezed into the
formation with a clean, formation compatible fluid.
Preferably, the displacement fluid should be filtered
to remove fines. After the fluid has been squeezed
into the formation, the well should be shut in, and
16 ~o9 o306
the fluid allowed to stand for 12 hours before putting
the well back on pump.
To clean a partially plugged flowing well,
a volume of the fluid according to the invention equal
to one half o the tubing volume should be injected
down the tubing string and allowed to soak for 24
hours. The well may then be placed back on production
and tested.
To clean a completely plugged well, an
attempt should be made to solubilize the plug by
injecting a volume of the fluid according to the
invention down the tubing string. If the plug can be
solubilized, then the well should be allowed to soak
for 24 hours and the well may be placed back on
production and tested. If the plug cannot be
solubilized, then the plug may be removed by such
procedures as drilling or jetting with coiled tubing,
using the fluid according to the invention as the
jetting fluid. The well may then be placed back on
production and evaluated.
To squeeze a flowing well in which the
tubing is set in a packer, it is preferred to inject
the fluid according to the invention directly through
the perforations into the well bore using coiled
tubing. This helps to prevent well fluid entrained
solids from being re-injected into the well. If this
procedure is not viable, then an attempt may be made
to force the fluid according to the invention through
the tubing into the formation with a clean formation
compatible chase fluid. Care should be taken not to
overflush the chase fluid into the formation.
To squeeze a flowing well in which the
tubing is not set in a packer, it is preferred to
squeeze a squeeze volume of the fluid according to the
2090306
17
invention down the annulus to the perforations. The
flowline should be kept open until the resident
annulus fluid has been displaced up the tubing into
the flowline. Typical squeeze volumes are 1.0 - 1.5 m3
of the fluid according to the invention per meter of
perforations. Once the fluid is in the annulus, the
tubing valve may be closed and the fluid squeezed into
the formation with a clean formation compatible fluid
(which should not be overflushed). In either case
(with or without the tubing set in a packer), the well
may be shut in, allowed to soak and after 24 hours or
so, placed back on production and tested.
If a flowing well does not flow after
treatment, it may be desirable at that point to swab
the well.
The formulation of the present invention,
identified by Trisol Inc.ls tradename WAXSOL is
preferably pumped into the well at below fracturing
pressures. Pumping is carried out at ambient
temperature. As known in the art, since the
formulation of the invention is aromatic rich, contact
with elastomeric components in the well should be
minimized. For removal of the formulation of the
invention from the well, high (maximum) pump speeds
are recommended to aid in preventing the plugging of
downhole pumps by release of fines and scale from
downhole wax as it is dissolved.
A person skilled in the art could make
immaterial modifications to the invention described
and claimed in this patent without departing from the
essence of the invention.
2090306
17A
TABLE 2 (PART I)
RT AREA% NAME RT AREA% NAME
3.202 .07S08 iC4 14.970 .09517
3.912 .35699 nC4 15.048 .08244
5.784 2.69133 iC5 15.195 .49210
6.514 2.72246 nC5 15.370 .75489
7.492 .67313 Cyclopentane 15.510 .46649
8.340 .89997 15.759 .45354 m+p xylene
8.487 3.99820 16.000 10.08547 m+p xylene
8.875 2.17882 16.157 .54391
9.377 6.74750 C6 16.504 1.51593 oxylene
10.097 2.47707 Methyl 16.615 .14003
cyclopentane 16.742 1.95847 C9
10.340 .08951 17.068 .12964
10.752 3.93949 Benzene 17.161 .14645
10.887 .18034 17.366 .27271
11.012 2.71877 Cyclohexane 17.508 .58216
11.201 2.88368 17.822 .17800
11.428 2.21393 17.946 .79982
11.641 .47199 18.105 1.68490
11.774 1.38668 18.245 .29447
12.062 4.72465 C7 18.347 .13771
12.688 6.65060 Methyl 18.493 .12346
cyclohexane
18.660 1.32512 trimethyl
12.910 .72919 benzene
13.125 .22715 18.800 1.26158 C10
13.313 .18384 18.965 .11433
13.554 12.71929 Toluene 19.256 .53542
13.710 2.10055 19.382 .07666
13.900 1.04831 19.525 .11942
14.090 1.44920 19.616 .15763
14.287 .47715 19.746 .13944
14.505 3.13432 C8 19.856 .32040
14.716 .17785 20.044 .31498
~ 2090306
17B
TABLE 2 (PART II)
RT AREA~ NAME
20.180 .13753
20.279 .10719
20.420 .10568
20.701 .94819 Cll
20.885 .15028
21.060 .11153
21.140 .11705
21.250 .14451
21.545 .19119
21.732 .20552
21.853 .13484
21.980 .11113
22.466 .63371 C12
22.653 .05706
22.740 .11454
23.247 .06810
23.367 .09962
23.543 .12426
23.749 .08606
23.866 .06625
24.119 .28868 C13
24.306 .10446
25.387 .05999
25.668 .13514 C14
27.125 .06827 C15
2090306
17C
TABLE 3(PART I)
RT AREA% NAME RT AREA% NAME
2.341 .97890 12.101 .14885
2.618 6.86497 C4 12.358 .07718
2.816 .38619 12.588 .41137
3.697 14.33011 iC5 12.760 .53539
4.236 10.12231 nC5 12.899 .41128
5.066 .76730 Cyclopentene 13.147 .27011 m+p xylene
5.784 .93620 13.399 6.57036 m+p xylene
5.928 4.10632 13.560 .39226
6.290 1.97614 13.904 1.23017 oxylene
6.765 7.04991 iC6 14.155 1.50142 C9
7.470 2.18292 Methyl 14.471 .08211
cyclopentane 14.569 .10035
8.095 .89846 Benzene 14.774 .18688
8.240 .13707 14.917 .40929
8.371 2.14099 Cyclohexane 15.237 .13187
8.551 2.20204 15.355 .50748
8.775 1.54438 15.520 1.27618
8.995 .35825 15.663 .19968
9.129 1.05050 15.758 .09574
9.421 3.83012 C7 15.909 .10972
10.054 5.15676 Methyl 16.079 1.01203 trimethyl
cyclohexane benzene
10.274 .55886 16.224 .96784 C10
10.488 .16983 16.379 .08264
10.675 .13411 16.674 .36803
10.904 5.37600 Toluene 16.943 .07889
11.082 1.53461 17.033 .10599
11.277 .64754 17.160 .08998
11.465 1.21~31 17.275 .21482
11.660 .25546 17.469 .23662
11.739 .10947 17.602 .09868
11.895 2.41510 C8 18.130 .64582 C11
2090306
17D
TABLE 3 (PART II)
RT AREA% NAME
18.300 .09683
18.485 .07766
18.561 .08832
18.669 .09555
18.966 .12143
19.155 .13286
19.281 .11464
19.405 .07735
19.899 .48104 C12
20.168 .07798
20.974 .10136
21.553 .25211 C13
21.733 .08036
23.105 .14083 C14
24.563 .07604 C15