Note: Descriptions are shown in the official language in which they were submitted.
9.B106/MW/JLB
2090~94
TREATMENT 0~ UASTE OR OTE~R MATERIAL
This invention relates to the treatment of waste or other material
comprising sulphuric acid.
It is well known that sulphuric acid can be recovered from waste sulphuric
acid. UK patent specification No 1 288 851 discloses a process in which
spent sulphuric acid is atomised and is directed into a flame which is
formed by burning a fuel using oxygen to support combustion. The resulting
sulphur dioxide may then be used in the production of fresh sulphuric acid
by the contact process. UK patent specification No 1 602 621 discloses a
process in which a mixture of spent sulphuric acid and a liquid
hydrocarbon fuel is atomised by a first air stream, and a secondary air
stream, enriched in oxygen, is used to burn the fuel and thus generate the
necessary temperature for the thermal cracking of the sulphuric acid to
produce a gas stream containing sulphur dioxide from which a fresh
sulphuric acid can be prepared. UK patent specification No 1 092 171
discloses a process for the thermal decomposition of waste sulphuric acid
into sulphur dioxide and water vapour, wherein the thermal energy is
supplied by an exothermic auxiliary chemical reaction, comprising mixing
the sulphuric acid with the stoichiometric amount of a carbonaceous fuel,
feeding the mixture in an atomlsed form together with an at least
stoichiometric amount of a free oxygen-containing gas into a combustion
chamber, the atomised mixture being burnt under high turbulence at a
temperature of from 650 to 1000C, and the residence time of the mixture as
well as of the reaction products formed therefrom in the combustion zone
within the chamber being less than 1 second. If sulphuric acid of low
concentration ls to be decomposed and/or decomposition gases with a high
sulphur dioxide content are to be produced the carbon-containing fuel can
be partially replaced by sulphur. In this case liquid sulphur is injected,
preferably with heated air, into the combustion zone and preferably in a
flow direction opposite to the flow direction of the other reactants. The
resulting sulphur dioxide may be converted to sulphuric acid.
All these proposals share the same disadvantage: the regeneration of
sulphuric acid from a source of spent acid is economically unattractive in
that it is difficult to obtain a source of spent acid at a sufficient rate
and of a composition such that fresh sulphuric acid can be produced cheaply
~lB106/MW/JLB
209059~
-- 2 --
and in sufficient quantity to be attractive.
It has also been proposed in European patent application 195 447A to
improve a process for recovering sulphur from a feed gas having a
substantial hydrogen sulphide content (i.e. more than 60~ by volume)
wherein the gas stream is partially combusted with an oxygen-enriched
oxidant gas in a Claus reaction furnace zone, a combustion effluent is
cooled with the attendant condensation and separation of sulphur in a
condensation zone and the remaining effluent stream is further treated by
introducing a temperature moderating stream of sulphuric acid into the
reaction furnace zone to moderate the temperature of the oxygen enriched
furnace zone. A greater degree of enrichment of the oxidant gas is thereby
made possible without there being any attendant damage to the Claus
reaction furnace zone. The preferred source of sulphuric acid is spent
acid sludge from a sulphuric acid alkylation process. The improvement
thèreby provides the means for disposing of such sulphuric acid sludge.
It has further been proposed in European patent application 220 610A tosubstitute liquid sulphur for the sulphuric acid used in the process
disclosed in European patent application 195 447A. In this process the
liquid sulphur is used purely as a temperature moderator and does not
change the requirement to form sulphur dioxide.
According to the present invention there is provided a method of treating
sulphuric acid comprising decomposing the sulphuric acid in the presence of
fluid sulphur and oxygen to form a gaseous mixture comprising sulphur
vapour, water vapour and sulphur dioxide, and then reacting the sulphur
dioxide in the gaseous mixture with a reducing agent to form a sulphur
product.
The invention also provides apparatus for treating sulphuric acid
comprising a first reactor for decomposing the sulphuric acid in the
presence of fluid sulphur and oxygen to form a gas mixture comprising
sulphur vapour, water vapour and sulphur dioxide, said first reactor having
an inlet or inlets for the sulphur and sulphuric acid, and having an outlet
for said gaseous mixture, and a second reactor for reacting the sulphur
dioxide in the gaseous mixture with a reducing agent to form a sulphur
product, said second reactor having an inlet communicating with the said
91B106/MU/JLB 2 ~ 9 ~ a 9 ~
outlet of the first reactor, a inlet for reducing agent or precursor(s)
thereof, and an outlet for the sulphur product.
The method and apparatus according to the invention are particularly suited
to the treatment of waste sulphuric acid. The primary advantages offered
by the method according to the invention are those of thermal efficiency
and flexibility.
Sulphur in effect catalyses the thermal decomposition of sulphuric acidthus making possible the use of relatively low decomposition temperatures
depending upon the impurities present in the sulphuric acid. In addition,
heat for the decomposition of the sulphuric acid can be provided by the
reaction between sulphur and oxygen to form sulphur dioxide. The resulting
sulphur dioxide enters the said gaseous mixture. The key reactions that go
to form the gaseous mixture therefore comprise:
2 H2 S04 -~ 2H20 + 2S03
2 + S ~) S02 ... 2
2S03 + S -~ 3S02 ... 3
The sulphur which takes part in reaction three may exist in one of a number
of tautomeric molecular species, e.g. S2, S6 and S8 though the dimeric form
generally predominates. Reactions 1 and 3 are endothermic whereas reaction
2 is exothermic. Accordingly, it is possible for all the thermal
requirements for the decomposition of sulphuric acid to be provided by the
reactlon between sulphur and oxygen, although if desired an external source
of heat may be additionally used (particularly when commencing operation of
the method according to the invention). There is thus considerable
flexibility in operating these reactions so that they may be a net importer
or exporter of heat, and widely differing relative proportions of sulphur
dioxide and sulphur may be obtained in the resulting gas mixture.
The method and apparatus according to the invention also offer flexibility
in that they can produce one or both of a sulphur product and a product gas
mixture comprising sulphur dioxide suitable for conversion to fresh
sulphuric acid.
There is preferably an appreciable stoichiometric excess of sulphur vapour
91B106/MW/JLB 2 ~ 9 ~ ~ 9 4
over oxygen. Typically, the amount of sulphur present during the
decomposition of the sulphuric acid is at least 150% of the amount required
for reaction with oxygen in accordance with equation 2 above.
The source of oxygen may be any gas mixture comprising molecular oxygen and
one or more gases that do not react with sulphur or sulphur dioxide, for
example nitrogen and the noble gases. Oxygen-enriched air is a preferred
source of the oxygen. Alternatively, pure oxygen may be employed.
The decomposition of sulphuric acid may take place at any temperature
within a range whose lower limit is defined by the boiling point of the
sulphur at the prevailing pressure and whose upper limit is 1500C
depending on the impurities present in the sulphuric acid. The reaction
may take place in a region in which no visible flame is present, or one in
which there is a visible flame in which the sulphur burns. The choice of
the reaction temperature typically depends on the composition of the waste
sulphuric acid. At lower temperatures within the aforesaid range, some
impurities which may be present in the waste sulphuric acid give rise to
gaseous products which if not destroyed in a downstream region can condense
as a solid at lower temperatures thus causing operating difficulties. For
example, at temperatures in the order of 500C ammonium sulphate decomposes
by one mechanism to form ammonium bisulphate which is a vapour at 500C but
which condenses at lower temperatures. Accordingly, the reaction
temperature needs to be sufficiently high for the ammonium bisulphate to
decompose lnto gaseous products (e.g. at a temperature of at least 1000C)
or its decomposition needs to be performed in a downstream catalytic
region.
The sulphuric acid is typically decomposed in a flame zone into which
sulphur, sulphuric acid and oxygen are fed and in which reactions l to 3
identified above takes place. The sulphuric acid is typically atomised
upstream of its introduction into the flame zone. Typical operating
temperatures are in the range of 600 to 1,000C. At such temperatures make
combustion of sulphur is autogenous and therefore no ignition source is
needed. If desired, the reactor in which the combustion takes place can be
pre-heated to a temperature typically in the range of 400 to 425C.
Where the composition of the waste sulphuric acid permits, the
91B106/MU/JLB
2090594
decomposition of the sulphuric acid may take place in a flameless reaction
region. The flameless reaction region may be in the same vessel as a bath
of boiling sulphur or in a vessel communicating with another vessel in
which a volume of boiling liquid sulphur is held. Preferably, the oxygen
is passed into the volume of liquid sulphur and reacts therewith to form
sulphur dioxide. The heat of reaction is typically sufficient to maintain
the liquid sulphur at or above its boiling point without the need to
provide external heating. The oxygen is preferably introduced into the
sulphur bath at high velocity to promote interfacial contact between the
gas and the liquid. If desired, mechanical agitation may be used to
enhance this contact.
Where the decomposition of sulphuric acid takes place in the same vessel as
that in which the volume of liquid sulphur is maintained, the sulphuric
acid is preferably introduced onto the surface of the liquid sulphur.
If desired, the sulphuric acid may be dried upstream of the decomposition
region. Drying may, for example, be conducted by heating the sulphuric
acid typically to a temperature in the range of 150 to 350C. The heating
may be effected by passing fluid sulphur through a heating coil immersed in
a volume of waste sulphuric acid to be dried.
The method according to the present invention typically additionally
lncludes the step of separating solids from the sulphuric acid. 5eparation
may be performed upstream of, in or downstream of the region where the
decomposition of the sulphuric acid takes place. Preferably, separatlon
takes place by causing such solids to enter a volume of liquid sulphur.
Thus, the sulphuric acid or a gaseous stream of its decomposition products
may be intimately contacted with liquid sulphur. If the decomposition of
the sulphuric acid takes placé in a vessel in which a volume of liquid
sulphur is maintained, then that volume will function as the solids
separator. If, however, the decomposition of sulphuric acid takes place in
a flame zone, then a volume or bath of liquid sulphur is preferably
employed downstream of the flame zone in order to effect the separation of
the solids.
The reducing agent preferably comprises one or more products of a reaction
between sulphur vapour and hydrocarbon. Accordingly, a gaseous or vaporous
51B106/MW/JLB
2~594
-- 6 --
hydrocarbon may be added to the said gaseous mixture produced by the
decomposition of the sulphuric acid, and the hydrocarbon then reacted
catalytically with sulphur vapour to form a mixture of hydrogen sulphide
and carbon disulphide. The hydrogen sulphide and carbon disulphide so
formed reduce sulphur dioxide in the gaseous mixture to sulphur. If
desired, the proportion of sulphur dioxide in the gaseous mixture may be
increased by introducing oxygen into the gaseous mixture intermediate the
sulphuric acid decomposition region and the reduction region whereby the
oxygen reacts with sulphur vapour to form sulphur dioxide. If removal of
particulates from the gaseous mixture is performed by means of a volume of
liquid sulphur this reaction between the oxygen and the sulphur vapour may
b~e used to raise the temperature of the gaseous mixture to a suitable value
_ the reduction reaction or reactions. Preferably, a plurality of
catalytic regions are employed to carry out the reduction reaction or
reactions. The gaseous mixture is preferably cooled between regions by the
introduction of with liquid sulphur.
The catalytic reactions are preferably performed at temperatures in therange of 600 to 800C. At such temperatures, the Claus reaction between
hydrogen sulphide and sulphur dioxide does not approach completion. In
order to separate sulphur vapour and complete the reaction between hydrogen
sulphlde ant sulphur dioxide, the gaseous mixture is, downstream of the
catalytic region or reglons, preferably contacted with an aqueous medium at
a temperature in the range of 110 to 140C and condense sulphur vapour,
while minimislng the dissolution of sulphur dioxide. The aqueous medium
preferably includes ethylene glycol or other additive effective to raise
its boiling point above the temperature at which it is contacted with the
gaseous mixture.
In alternative reaction schemes, the reaction with the reducing agent may
be carried out without the aid of a catalyst and/or solely wlth hydrogen
sulphide from an external source thereof. Accordingly, if desired, the
reaction of the sulphur dioxide with the reducing agent may take place as a
conventional Claus process.
It is preferred that the rate of formation of sulphur by the reduction
reaction is sufficient to meet all the sulphur requirements of the method
according to the invention. If desired, additional sulphur for use outside
9 B106/MW/JLB
2~9~9~
-- 7
the method according to the invention may be produced.
In some examples of the method according to the invention, substantially
all the sulphur dioxide content of the gas mixture is reduced to sulphur.
After separation of the sulphur, residual sulphur containing gases may be
removed in a conventional tail gas clean up process such as the SCOT
process. In other examples, sulphur dioxide is formed as an end-product
which is able to be converted to sulphuric acid by known process routes.
Methods and apparatuses according to the present invention vill now be
described by way of example with reference to the accompanying drawings, in
which:
Pigure 1 is a flow diagram of a first apparatus for treating waste
sulphuric acid;
Pigure 2 is a flow diagram schematically illustrating a Claus plant
suitable for use as part of the apparatus shown in Pigure 1.
Pigure 3 is a schematic diagram illustrating a first alternative embodiment
of a sulphuric acid decomposition reactor for use in the apparatus shown in
Plgure l;
Pigure 4 ls a schematic diagram illustrating a second alternative
embotlment of a sulphuric acid decomposition reactor for use in the
apparatus shown in Pigure l; and
Plgure 5 is a flow diagram schematically illustrating second plant for
decomposing sulphuric acid in accordance with the invention.
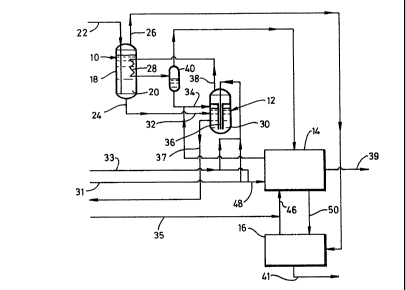
Referring to Pigure 1, the illustrated plant comprises a drier 10 for
drying waste sulphuric acid; a sulphuric acid decomposition reactor 12, a
Claus plant 14 for treating the gas mixture produced by the decomposition
reactor 12 and a SCOT plant 16 for treating the tail gas from the Claus
plant so as to form an effluent gas which can be discharged to the
atmosphere. The sulphuric acid drier 10 comprises a closed columnar vessel
18 containing a volume 20 of the waste sulphuric acid. The vessel 18 has
associated therewith an inlet pipeline 22 for a source of waste sulphuric
~ 91B106/MU/JLB
~059~
-- 8 --
acid, which pipeline 22 terminates either above or beneath the surface of
the volume 20 of the waste sulphuric acid.
The volume 20 of waste sulphuric acid in the vessel 18 is heated by
operation of a heating coil 28 immersed therein. Fluid sulphur, typically
in the gaseous state, is passed continuously through the coil 28 typically
at a temperature of about 340C, and waste sulphuric acid is fed
continuously to the drier 10.
A vapour stream consisting essentially of steam is continuously withdrawn
from the vessel 18 through an outlet 26 at the top of the vessel. Dried
waste sulphuric acid is continuously withdrawn through an outlet 24 at the
bottom of the vessel 18.
The decomposition reactor 12 comprises a closed columnar vessel 30 which
has inlets 32, 34 and 36 for dried sulphuric acid, liquid sulphur and
oxygen-enriched air respectively. A chosen volume (typically up to 6
metres deep) of liquid sulphur is maintained in the vessel 30. A stream of
oxygen-enriched air typically containing between 30 and 95~ by volume of
oxygen is continuously introduced into the bottom of the volume of liquid
sulphur in the vessel 30 at a high velocity to provide a high interfacial
area of contact between the oxygen and the liquid sulphur. The stream is
formed by mixing air taken from a pipeline 31 with oxygen taken from a
plpellne 33. If desired, mechanical agitation can be used to enhance the
lnterfaclal contact. The oxygen reacts with the liquid sulphur to form
sulphur dioxide in accordance with the reaction:
S ' 2 ~) S2
Essentially all the oxygen entering the vessel 30 is so oxidised to sulphur
dioxide. Since this reaction is exothermic, it heats the liquid sulphur in
the vessel and causes sulphur to vaporise.
A stream of dried sulphuric acid continuously withdrawn from the drier 10
is continuously introduced into the vessel 30 through the inlet 32 and
comes into contact with the liquid sulphur which is maintained at a
temperature in the range 450 to 510C. (It is an advantage of the plant
shown in Figure 1 that the temperatures in the reactor 12 are sufficiently
91B106/MW/JLB 2 ~ 9 4
low to allow the vessel 30 to be fabricated from mild steel.) At the
temperature of the reactor 12, sulphuric acid rapidly disproportionates in
accordance with the reaction:
H2S04 -~ H20 + S03
The sulphur trioxide then reacts in the vapour phase with sulphur to form
sulphur dioxide:
2S03 + S (g) -> 3S02
Thus, the sulphuric acid is decomposed into a vapour mixture comprisingsteam and sulphur dioxide. A stream of resultant gas mixture is
continuously withdrawn from the reactor 30 through the outlet 38 and
typically comprises sulphur dioxide, water vapour, sulphur vapour and
nitrogen and may include up to 50 volumes per million of oxygen. It is the
stream leaving the vessel 30 through the outlet 38 that is used to heat the
llquid sulphur in the drier 10. This stream is therefore passed through
the heat exchange coil 28 to effect this heating. Most of the sulphur
vapour in the stream is thereby condensed and the resulting gas mixture now
containing entrained droplets of liquid sulphur is passed into a pbase
separator 40 where it is separated into a gas phase comprising nitrogen,
sulphur dioxide, water vapour and uncondensed sulphur vapour and a liquid
phase comprising sulphur. A stream of the liquid phase is typically
recycled to the vessel 30
The source of the sulphuric acid may be a plant which employs sulphuricacid as a catalyst in an alkylation reaction or a plant which makes
titanium oxide by the sulphate route. As such, the sulphuric acid
typically contains organic and/or inorganic impurities, sometimes in quite
large quantities. Oxides such as silica and dissolved or precipitated
salts such as sodium sulphate and sodium chloride in the sulphuric acid
feed tend to accumulate in the volume of liquid sulphur in the vessel 30.
Such solids are periodically removed from the reactor 12 in the form of a
liquid sulphur slurry through an outlet pipeline 37. They may then be
recovered by boiling the sulphur slurry in another vessel (not shown) to
produce a sulphur vapour stream with minimum entrainment of particulate
solids in the vapour. If desired, a fluxing agent such as lime may be
9~B106/MW/JLB
2~594
-- 10 --
added to the slurry so as to form a slag. If necessary, a second
purification stage can be performed by condensing and reboiling the sulphur
vapour.
A stream of the gaseous phase is withdrawn continuously from the phase
separator 40 and is then fed to the Claus plant 14 in which the sulphur
dioxide is converted to sulphur by the Claus reaction. The sulphur product
is withdrawn from the Claus plant 4 through outlet 39. The Claus plant 14
also has inlets 46 and 48 for hydrogen sulphide and oxygen-enriched air
respectively. The hydrogen sulphide is taken from a pipeline 35. The
oxygen-enriched air is formed by mixing air taken from a pipeline 31 with
oxygen taken from a pipeline 33. A tail gas containing less than S% by
volume of sulphur compounds leaves the Claus plant 14 through an outlet 50
and is further treated in the Scot plant 16 in which it is reacted with a
reducing gas to form hydrogen sulphide which is recycled to the Claus
plant. The off-gas from the Scot plant 16 is vented through outlet 41 and
typically contains less than 1000 vpm of sulphur and sulphur compounds. If
the stream of steam leaving the drier 10 through the outlet 26 is free of
organics, it may be introduced into the hydrolysis reactor (not shown) of
the Scot plant 16. Any nitric acid in this stream is reduced to nitrogen
and water vapour in the hydrolysis reactor. Any hydrochloric acid in the
stream may be absorbed and neutralised in a water wash step (not shown)
prior to an amine absorption step (not shown) performed by the Scot plant
16
A suitable Claus plant 14 for use in the plant shown in Pigure 1 is
111ustratet in Plgure 2 of the drawings. Referring to Pigure 2, a furnace
52 for conducting the Claus reaction is fitted at its upstream end with a
burner 54 having an inlet 56 for feed gas containing hydrogen sulphide and
an inlet 58 for oxygen-enriched air. The hydrogen sulphide feed stream is
typically relatively concentrated, containing at least 70% by volume of
hydrogen sulphide and hydrocarbons. It may also include other gases, for
example, carbon dioxide, hydrogen and argon.
The burner 54 also has an inlet 60 for the stream of gas mixture that is
withdrawn from the phase separator 40 of the plant shown in Figure 1 of the
drawing. The rate of supply of oxygen-enriched air in relation to the
rates of supply of the hydrogen sulphide feed stream and the gas stream
91B106/MW/JLB 2 ~ g ~ ~ 9 4
11 --
from the phase separator 40 shown in Figure 1 is such that any combustibles
other than hydrogen sulphide in the hydrogen sulphide feed stream that
react preferentially with oxygen are fully oxidised and that the proportion
of hydrogen sulphide that is burnt yields a ratio of hydrogen sulphide to
sulphur dioxide in the resulting combustion products of approximately 2:1.
Since the decomposed sulphuric acid provides some sulphur dioxide for the
Claus reaction, the hydrogen sulphide feed stream is not used as the
ultimate source of all the sulphur dioxide for the Claus reaction and
therefore a smaller proportion of the hydrogen sulphide needs to be burned
than when the hydrogen sulphide is the only source of sulphur dioxide.
Accordingly, less heat is generated per mole of hydrogen sulphide fed to
the furnace 52. The productivity of the furnace 52 can thus be maximised
by selecting a relatively high concentration of oxygen for the
oxygen-enriched air stream and a relatively high throughput of hydrogen
sulphide without creating an excessive temperature likely to damage the
refractory of the furnace 52. Typically, the maximum temperature tolerable
is in the order of 1600C.
The furnace 52 is typically substantially identical to a conventional Claus
furnace. Accordingly, the furnace has a suitable refractory lining (not
shown) and a volume sufficient for there to be a thermal reaction zone 66
contiguous with a combustion zone 64. (in addition the furnace 52 may be
provided with a baffle 65.) Reaction between hydrogen sulphide and sulphur
dioxlde ls initiated in the combustion zone 64 and continues in the thermal
reaction zone 66. The thermal reaction between hydrogen sulphide and
sulphur dioxide is endothermic and temperature drop takes place in the
thermal reaction zone 66. Accordingly, the gas typically falls in
temperature from about say 1350C in the combustion zone 64 to say 1200 to
1250C at an exit 67 from the furnace 52. The effluent gas stream is
cooled in a waste heat boiler or heat exchanger 68 to a temperature of,
say, 275 to 325C. The waste heat boiler (or heat exchanger) 68 typically
has two passes for the effluent gas stream. A major portion of the
effluent gas stream flows through both passes and is thus cooled to the
cho8en temperature of from 275 to 325C while a minor portion of the
effluent gas stream flows through only the first pass and leaves the waste
heat boiler 68 at a higher temperature, typically in the range 590 to 600C
and i9 used as will be described below.
91Bl06~MW~JL~ 2 ~ 9 0 ~ 9 ~
- 12 -
The major portion of the effluent gas stream then enters a first sulphur
condenser 70 in which sulphur vapour formed by the reaction between sulphur
dioxide and hydrogen sulphide is condensed ou~ of the effluent gas stream
from the furnace 52 by cooling the gas stream to 140C. Such sulphur
condensate is then passed into a sulphur seal pit.
Downstream of the first condenser 70, the effluent gas stream consisting
essentially of hydrogen sulphide, sulphur dioxide, water vapour and any
nitrogen and carbon dioxide introduced into the furnace 52 with the feed
gas streams, is reheated at a region 72 to a temperature in the order of
220 to 250C by being mixed with the first stream taken from said minor
portion of the effluent gases. Reaction takes place between the residual
hydrogen sulphide and sulphur dioxide to form more sulphur vapour and water
vapour. This reaction takes place over a catalyst which is typically of a
conventional kind, for example, activated alumina, in a catalytic reactor
74. Since the catalytic reaction is exothermic, there is a rise in
temperature in the first catalytic reactor 74 and accordingly the gas
mixture leaving this reactor typically has a temperature in the order of
300 to 350C. If desired, the outlet temperature of the reactor 74 may be
--arranged to be higher, say in the range 350 to 400C (e.g. 375C). Such
hlgher outlet temperature tends to give improved hydrolysis of any carbon
oxy8ulphide and carbon disulphide in the gas mixture.
Prom the catalytlc reactor 74 the effluent gas stream passes through asecond sulphur condenser 76 in which sulphur is condensed out of the gas
mixture. Part of the resultance of the condensate is passed to a sulphur
seal pit 86 while the remainder is recycled to a sulphuric acid
decomposition reactor of the kind shown in Pigure 1.
Downstream of the sulphur condenser 76 the effluent gas mixture is reheated
at a region 78 from a temperature of, say, 140C to a temperature slightly
less than the inlet temperature to the first catalytic reactor 74, say, in
the range 200 to 220C. The reheating is typically effected by taking a
portion of that part of the effluent gas stream leaving the waste heat
boiler 68 which flows from the downstream end of the waste heat boiler 68
to the region 72 and mixing it with the efEluent gas from the second
sulphur condenser 76. The resulting gas mixture then passes through a
second catalytic reactor 80 where further reaction between residual
91B106/MW/JLB
2 ~ 9 4
_ 13 -
hydrogen sulphide and residual sulphur dioxide takes place to form water
vapour and sulphur vapour with the evolution of heat such that the
temperature of the gas mixture is typically raised in the order of 50C as
it passes from the inlet to the outlet of the second catalytic reactor 80.
The catalyst employed in the second catalytic reactor 80 is typically the
same as that employed in the first such reactor 74.
Downstream of the second catalytic reactor 80, the gas mixture passes
through a third sulphur condenser 82 in which sulphur is condensed out of
the gas stream. The condensed sulphur is passed to the sulphur seal pit
86. The gas stream leaving the sulphur condenser 82 may then be passed to
a conventional "tail gas clean-up plant" (not shown) which may be a SCOT
plant as shown in Figure 1 or alternatively may be a plant for operating
the Stretford or Beavon process.
Referring now to Figure 3 of the accompanying drawings, there is shown an
alternative embodiment of a sulphuric acid decomposition reactor for use in
the plant shown in Figure 1. The main difference between the reaator shown
in Pigure 3 and that shown in Figure 1, is that the vapour phase reaction
between sulphur and sulphuric acid takes place in a different vessel from
the one in which the liquid sulphur is boiled. Such a procedure is
atvantageous if impurities in the sulphuric acid are of a nature such as to
cause foaming were the acid to be introduced into a bath of liquid sulphur.
Referring to Pigure 3, a volume 102 of liquid sulphur is maintained in a
vessel 100 at a temperature at or close to its boiling point. Liquid
sulphur is continuously introduced into the vessel 100 through an inlet
104. Oxygen-enriched air or pure oxygen is introduced into the bottom of
the volume 102 of liquid sulphur in the vessel 100 through an inlet 106 at
a high velocity to provide a high interfacial area of contact bet~een the
gaseous oxygen and the liquid sulphur. If desired, mechanical agitation
may be used to enhance interfacial contact between the oxygen and the
liquid sulphur. The oxygen reacts with the liquid sulphur to form sulphur
dioxide. ~ssentially all the oxygen entering the vessel 100 takes part in
this oxidation reaction. Since the reaction is exothermic, the liquid
sulphur in the vessel is heated and thereby maintained at about its boiling
point. Accordingly, some of the liquid sulphur vaporises. A stream of a
mixture of sulphur dioxide and sulphur vapour which includes any
non-reactive gases introduced into the vessel 100 with the oxygen is
9lB106/MW/JLB
2~90~94
- 14 -
continuously withdrawn from the vessel 100 through an outlet 108 and enters
a second columnar vessel 110 through an inlet 112. Spent sulphuric acid is
introduced into the top of the vessel 110 through an inlet 114 whiah
comprises one or more atomising nozzles. Accordingly, the sulphuric acid
is dispersed within the vessel 110 in the form of a fine spray which
rapidly vaporises. The vessel 110 also has an inlet 116 through which a
stream of oxygen-enriched air or pure oxygen is continuously introduced.
The interior of the vessel 110 is typically maintained in a temperature in
the range of 450 to 900C. At such temperatures the sulphuric acid rapidly
disproportionates in accordance with the reaction:
H2S04 -~H20 + S03
The resulting sulphur trioxide then reacts in the vapour phase with sulphur
to form sulphur dioxide:
2S03 + S-~3S02.
In addltion, the sulphur vapour reacts exothermically with oxygen in the
vessel 110 to form sulphur dioxide in accordance with the equation:
S f 2 ~~ S2
Since the reaction between sulphur vapour and oxygen is exothermic, thetemperatures in the vessels 100 and 110 can typically be maintained at a
chosen values without having to provide any external heating (except at
gtart up).
A gas mixture comprising sulphur dioxide and sulphur vapour is continuously
withdrawn from the vessel 110 through an outlet 118 and may then be
sub~ected to a reaction with a reducing agent comprising hydrogen sulphide
in accordance with the invention. If desired, some or all of the sulphur
vapour may be condensed from the stream withdrawn through the outlet 118
upstream of the reaction with the reducing agent.
It is not essential for the decomposition of the sulphuric acid to takeplace in a flameless reaction region (as takes place in operation of the
91B106/MW/JLB
- 15 _ 2 ~ ~ 0 3 9 ~
plants shown in Figures 1 and 3). An apparatus in which the decomposition
takes place within a flame is illustrated schematically in Figure 4 of the
accompanying drawings. The decomposition reactor shown in Figure 4
comprises a furnace 120 having a refractory lining (not shown). The
fùrnace is provided with a burner 122 having an inlet of 124 for a stream
of sulphur, which may be supplied in vapour or liquid state, and an inlet
126 for a stream of pure oxygen or oxygen-enriched air. The sulphur is
preferably fed to the burner 122 at a temperature above its ignition
temperature of 260C. No ignition source is required with such a liquid
sulphur feed provided the interior of the furnace is initially pre-heated
to a temperature greater than about 425C. The sulphur burns in a flame
zone 128 with the formation of sulphur dioxide. The furnace 120 is also
provided with an inlet 130 for sulphuric acid. There are a number of
methods well known in the art for effectively introducing sulphuric acid
into a flame zone so as to effect its decomposition. For example, the
sulphuric acid may be introduced into the flame zone through one or more
atomisers (not shown) which direct a spray or mist of sulphuric acid into
the fiame zone. The sulphuric acid is decomposed in the flame zone 128 to
water vapour and sulphur trioxide. The sulphur trioxide is then reduced by
-sulphur vapour to form sulphur dioxide. This reaction proceeds rapidly at
temperatures above 400C. The relative rates of supplying sulphur, oxygen
and sulphuric acid to the flame zone 128 are preferably selected so that
the gas mixture including combustion products leaving the flame zone 128
contains unreacted sulphur, i.e. there is a stoichiometric excess of
sulphur relative to oxygen. The gases leave the flame zone 128 of the
furnace lZ0 through an outlet 132 typically at a temperature in the range
500 to 1500C, The flame temperature is selected so as to ensure the
decomposition of substances that are gaseous at ambient temperature, say,
20C, of any vapours of substances that are normally solids. For example,
ammonium sulphate present in the waste sulphuric acid decomposes by one
mechanism to ammonium bisulphate which has a melting point of about 147C
and a boiling point of 490C. The flame temperature is thus chosen to be
sufficiently above 490C to ensure that such ammonium bisulphate decomposes
in the flame zone to gaseous products.
If the waste sulphuric acid includes organic impurities, for example
alkanes, these compounds are oxidised in the flame zone to carbon dioxide
and water vapour in accordance with the formula:
91B106/MWtJLB
2~0~9~
- 16 -
n 2n+2 + (3nl~) 2 ~) nC02 + (n+l) H20
In addition, the hydrocarbons may also react with sulphur vapour to form
carbon disulphide and hydrogen sulphide which in turn react with sulphur
dioxide to form sulphur vapour, carbon dioxide and water vapour; and with
oxygen to form carbon dioxide, water vapour and sulphur dioxide.
In the event that coke or other form of elemental carbon is produced bythermal cracking of the hydrocarbons, the temperature in the furnace is
desirably sufficiently high to remove the coke by the reaction:
Sz (g) ~ C(s) -> CS2
Alternatively, the furnace temperature can be periodically raised to decoke
the furnace
Referring to Figure 5 of the drawings, there is shown a reaction furnace
200 for the decomposition of sulphuric acid. The reaction furnace has
inlets 202, 204 and 206 for waste sulphuric acid, oxygen-enriched air and
liquid sulphur respectively. The construction and operation of the reactor
200 may be the same as that of the furnace 120 illustrated in Figure 4 of
the accompanying drawings. Accordingly, a gas mixture consisting
essentially of nitrogen, carbon dioxide, water vapour, sulphur vapour and
sulphur dioxide leaves the furnace 200 through an outlet 208. The gas
mixture may also contain any unreacted hydrocarbons that have passed
through the furnace 200. In addition, the gas mixture may contain
particulates (for example inorganic oxides and inorganic salts). The gas
mixture leaving the furnace 200 through the outlet 208 is mixed with a
stream of methane or other alkane supplied from a pipeline 209 and is then
introduced into a sulphur scrubber vessel 210 in which the gas mixture is
intimately contacted with liquid sulphur. Liquid sulphur is sprayed into
the vessel 210 through nozzles 212 at its dew point at the prevailing
pressure (typically this dew point is in the order of 380C). Solid
inorganic compounds such as silica, sodium sulphate, and sodium chloride
present in particulate form in the gas mixture accumulate in a volume of
liquid sulphur maintained in the vessel 210 and may be withdrawn therefrom
91B106/MW/JLB
~ - 17 -
through an outlet 211. The resulting contaminated sulphur may be
continuously recirculated to the furnace 200 with, preferably, the addition
thereto of a suitable fluxing ingredient such that there is produced in the
furnace 200 a slag which can from time to time be tapped off therefrom.
The scrubber vessel 210 thus provides a particularly effective means of
removing particulates from the gaseous decomposition products of the
sulphuric acid.
A resultant gas mixture free of particulates leaves the scrubber vessel 210
through an outlet 214 at its top and is mixed with a stream of
oxygen-enriched air supplied from a pipe 213. Reaction between oxygen and
sulphur vapour takes place in the gas mixture so as to raise its
temperature to a desired value suitable for catalytic reaction between
methane and sulphur vapour to form carbon disulphide and hydrogen sulphide.
Accordingly, the rate of addition of oxygen-enriched air is selected so as
to give a desired temperature increase in the gas mixture. The gas mixture
then enters the first stage 220 of a two stage catalytic reactor 218. The
inlet temperature for the first stage is typically in the range of 500 to
800C. The catalyst may typically be selected from charcoal, chromium,
tungsten, molybdenum, magnesium oxide, silica gel and alumina gel. Hethane
~and any other alkane) in the gas mixture reacts with the sulphur vapour
over the catalyst form carbon disulphide and hydrogen sulphide. The
resulting sulphur dioxide and hydrogen sulphide then react with sulphur
dioxide to form sulphur vapour. The chemical reactions involved are set
out below.
CH4 ~g) + 2S2 ~g) -> 2CS2 (g) + 2H2S (g)
2S2 ~g) + 2CS2 ~g) -) 2C02 (g) + 3S2 (g)
2S02 ~g) + 4H2S ~g) -~ 4H20 (g) + 3S2 (g)
These reactions take place with a net evolution of heat. The amount ofcatalyst provided, therefore, in the first stage is chosen such that the
gas mixture leaving the first stage 218 is not at so high a temperature
that the catalyst is sintered or otherwise damaged.
The gas mixture leaving the first stage 220 of the reactor 218 is reduced
in temperature upstream of the inlet to the second stage 222 of the reactor
218. The reduction in temperature is preferably effected by mixing the gas
91B106/M~/JLB 2 ~ ~ 0 5 9 4
mixture with a stream of fluid sulphur, typically in the liquid state, from
another part of the process. The gas mixture is typically thus reduced in
temperature to the same temperature at which it enters the first stage 220
of the reactor 218. The gas mixture flows into the second stage 222 of the
reactor 218 and the same reactions as take place in the first stage occur.
A gas mixture substantially free of hydrocarbons and carbon disulphide but
still containing hydrogen sulphide leaves the second stage 222 of the
reactor 218 and is then cooled in a heat exchanger or waste heat boiler 224
to a temperature of about 170C, that is the temperature at which the
viscosity of sulphur is at a minimum.
The thus-cooled gas mixture is then fed into a packed liquid-vapour contact
column 2Z8. The column 228 performs two main functions: first, it enables
residual hydrogen sulphide to be substantially entirely consumed by
reaction with sulphur dioxide to form sulphur and water; second it acts as
a sulphur condenser. In the column 228, an ascending gas phase is
intimately contacted with a descending liquid phase comprising an aqueous
medium at a temperature of about 120C. The aqueous medium typically
comprises a solution of ethylene glycol and water. Typically, the solution
contains at least 95% by weight of ethylene glycol. The addition of such
ethylene glycol ensures that a temperature at which the sulphur can
contense ant be relatively non-viscous is maintained in the column 228. A
gas mlxture essentially free of sulphur vapour and hydrogen sulphide then
leaves the top of the column 228 through the outlet 230 at a temperature in
the order of 120C. A liquid phase comprising water, ethylene glycol and
condenset sulphur vapour is withdrawn from the bottom of the column 228
through an outlet 232, and is then cooled in a heat exchanger 234 and the
resultant cooled liquid passed into a separator 236 at a temperature of
about 120C and separated therein, by virtue of the difference in specific
gravity between liquid sulphur and water, into a lower layer of liquid
sulphur and an upper layer of aqueous medium including dissolved ethylene
glycol. The liquid sulphur is withdrawn from the bottom of the separator
236 through an outlet 237 and is used to provide the coolant for the
catalytic reactor 218 and the liquid sulphur feed for the scrubber vessel
210. Any excess liquid sulphur may be taken as a separate product via an
outlet 239. The aqueous medium is recycled from the separator 236 to the
top of the column 228 being mixed with make up aqueous medium comprising
water and ethylene glycol from an inlet pipe 241.
91B106/MW/JLB 2 ~ 9 0 ~ 9 4
_ 19 -
In comparison with the operation of the plant shown in Figure 1, that shown
in Figure 5 consumes less oxygen for a given throughput of waste sulphuric
acid and conducts the reduction of sulphur dioxide generated from the waste
sulphuric acid at a lower temperature.
One of the main advantages of the method according to the invention is the
relative flexibility with which it can be operated to give varying amounts
of sulphur dioxide and sulphur products. This flexibility is illustrated
by the following examples of the operation of the plant shown in Figure 5
of the drawings. In each example, the letters A to P represent .he
following streams.
A - feed sulphuric acid to the furnace 200.
B - oxygen-enriched air feed to the furnace 200.
C - liquid sulphur feed to the furnace 200.
D - gas mixture leaving the furnace 200 through the outlet 208.
P - methane feed to the gas mixture leaving the furnace 200.
P - gas mixture fed to the scrubber 210.
-G - fresh sulphur feed to the scrubber 210 (excluding recycle)
H - gas mixture leaving the scrubber 210
I - oxygen stream that is mixed with gas mixture leaving the scrubber 210.
J - feed to the catalytic reactor 218 (all oxygen assumed to be fully
reacted).
K - gas mlxture leaving secont stage 222 of catalytic reactor 218.
L - gas mixture leaving heat exchanger 224.
M - gas mlxture leaving column 228.
N - fresh aqueous eed to the column 228 (excluding recycle)
O - sulphur product (excluding recycle to other parts of the process)
P - sulphur quenchant introduced between stages 220 and 222 of the reactor
218.
In Example 1 below, the streams B and I are pure oxygen, the catalytic
reactor 218 employs 0.768 kg of catalyst (silica gel) per kg/hr feed of
waste sulphuric acid and operates at a pressure of 0.2 bar gauge. In this
example, a resulting product stream (M) comprising sulphur dioxide, water
vapour, and carbon dioxide with a trace of methane IS produced.
9iB106~MW/JLB 2 ~ ~ ~ 5 9 4
- 20 -
In Example 2 below, the streams B and I comprise 30% by volume of axygen
and 70% by volume of nitrogen. The catalytic reactor 218 employs 2.281 kg
of silica gel catalyst per kg/hr of sulphuric acid feed and operates at a
pressure of 0.2 bar gauge. In this example, both a product stream M
comprising sulphur dioxide and a product stream 0 comprising sulphur are
produced.
In Example 3, the streams B and I each consist of 20% by volume of oxygen
and 80% by volume of nitrogen. The catalytic reactor 218 employs 4.827 kg
of silica gel catalyst per kg/hr of sulphur acid feed and operates at 0.2
bar gauge pressure. A sulphur product stream 0 is produced.
In Example 4, the streams B and I consists solely of oxygen. The catalytic
reactor 218 employs 0.833 kg of silica gel catalyst per kg/hr of waste
sulphuric acid feed and operates at a pressure of 0.2 bar gauge. A sulphur
product (stream 0) only is produced.
In all the Examples the stream N consists of water (mol fraction 0.8) and
ethylene glycol (mol fraction 0.2). In addition, in each example, the
reactor 218 achieves 99.9% conversion of methane.
91B106/MW/JLB
20~059~
- 21 -
Example 1
Stream A B C D E
T,C 25 25 382 800 25
Kilograms/Hr
N2 - - - - ~
02 - 491
CH4 - - - - 157
H2S
H20 26 - - 247
S02 - - - 1,777
H2S041,000
S - - 1,273 711
C02 - - - 81
N-C8 26
Total 1,052 491 1,273 2,816 157
-
TtroecamF670 1G20 38H2 25 600
Kilograms/Hr
N2 - - - ~ ~
02 - - - 65
CH4 157 - 157 - 157
H2S
H20 247 - 247 - 247
S02 1,777 - 1,777 - 1,907
H2S04
_ S 711 1,523 961 - 896
C02 81 - 81 - 81
To~al 2,973 1,523 3,223 65 3,288
Stream 750 175 120 2N5 120
Kilo~rams/Hr
N2
02
CH4
H2S 332 332
H20 425 425 2,651 2,050
S02 965 965 653
H2S04
S 1,249 1,249 - - 194
C02 512 512 512
Total 3,483 3,483 3,816 2,050 194
.
91B106/MW/JLB
2~9~
- 22 -
Example 2
Stream A B C D E
T,C 25 25 368 800 25
Kilograms/Hr
N2 - 1,353 - 1,353
02 - 663 - - -
CH4 - - - - 259
H2S
H20 26 - - 247
S02 - - - 2,121
H2S041,000
S - - 1,582 848
C02 - - - 81
N-C8 26
Total 1,052 2,016 1,582 4,650 259
Stream F G H I J
T,C 676 120 368 25 600
Kilograms/Hr
N2 1,353 - 1,353 281 1,634
02 - - - 138
CH4 259 - 259 - 259
H2S
H20 247 - 247 - 247
S02 2,121 - 2,121 - 2,396
H2S04
S 848 2,166 1,433 - 1,295
C02 81 - 81 - 81
Total 4,909 2,166 5,494 419 5,912
Stream K L M N 0 P
T-~C 750 175 120 25 120 120
Rilo~rams/Hr
N2 1,634 1,634 1,634
02
CH4
H2S 525 525
H20 552 552 9,899 9,069
S02 820 820 327
H2S04
S 1,856 1,856 - - 163 268
C02 793 793 793
Total 6,180 6,180 12,653 9,069 163 268
-
91B106/MW/JLB 2 ~ 9 9 5 9 4
- 23 -
Example 3
Stream A B C D E
T,C 25 25 361 800 25
Kilograms/Hr
N2 - 3,081 - 3,081
02 - 880
CH4 - - - - 380
H2S
H20 26 - - 247
S02 - - - 2,555
H2S041,000
S - - 1,973 1,022
C02 - - - 81
Total1,o5226 3,961 1,973 6,986 380
S,trOcamF681 120 3H61 25 600
Kilograms/Hr
N2 3,081 - 3,081 844 3,925
02 - - - 241
CH4 380 - 380 - 380
HH220 247 _ 247 _ 247
S02 2,555 - 2,555 - 3,03
H2S04
S 1,022 2,982 2,031 - 1,7gO
C02 81 - 81 - 81
Total 7,366 2,982 8,375 1,085 9,461
p
Tt,~rcam75Ko l7L5 120 N25 120 120
Kllo~rams/Hr
N2 3,925 3,925 3,925
02
CH4
H2S 775 775
H20 691 69120,102 19,001
S02 729 729
Hs2SO42,545 2,545 _ _ 327 331
C02 1,125 1,125 1,125
Total 9,790 9,79025,152 19,001 327 331
-
51B106/MW/JLB 2 0 ~ ~ 5 9 4
_ 24 -
Example 4
Stream A B C D E
T,C 25 25 377 800 25
Kilograms/Hr
N2 - - - - -
02 - 492
CH4 - - - - 241
H2S
H20 26 - - 247
S02 - - - 1,779
H2S04 1,000
S - - 1,275 712
C02 - - - 81
Total 1,052 492 1,275 2,819 24l
Stream F G H I J
T,C 620 120 377 25 600
Kilograms/Hr
N2
02 _ _ _ 73
CH4 241 - 241 - 241
H2S
H20 247 - 247 - 247
S02 i,779 - 1,779 - 1,92g
H2S04
S 712 1,496 933 - 860
C02 81 - 81 - 81
Total 3,060 1,496 3,281 73 3,354
Stream K L M N 0 P
T,C 750 175 120 25 120 120
K~lograms/Hr
NZ - - - - - -
02
CH4
H2S 560 560
H20 492 492 2,058 1,269
S02 527 527
H2S04
S 1,443 1,443 - - 327 410
C02 743 743 743
Total 3,765 3,765 2,801 1,269 327 410
-