Note: Descriptions are shown in the official language in which they were submitted.
209~23~
E443
DP~ r C~BD 80DIllM 131JIIFA~ E ~RODUC TIO~
IN CIILORIN39 DIOXTDE ~ F~MF~ 'rION
The present invention relates to the generation of
5 chlorine dioxide and decreasing the quantity o~ by-
product salt-cake which must be handled.
Chlorine d.ioxide, useful as a pulp mill bleaching
agent, is produced chemically by reduction of an acid
aqueous chlorate solution in accordance with the
equation:
Cl03-+ 2H+ + e~ ~ ClO2 + H2O ~ (1)
where the electron e~is supplied by various reducing
agents, for example, sulfur dioxide, methanol, chloride
ion or hydrogen peroxide. In many commercial processes
for effecting this reaction, the acidity for the process
is provided by sulfuric acid while the chlorate ions are
provided by sodium chlorate. The presence of these
species leads to the formation of some fo~m of sodium
sulfate as a by-product.
One particular embodiment of a commercial process is
the so-called "R~" process of the assignee of this
application, as described in U.S. Patent No. 4,081,520,
assigned to the applicant herein. Improvements in and
modifications to that process also are described in the
25 applicant's U.S. Patent~ Nos. 4,465,658, 4,473,540 and
4,6~7,969.
In that chlorine dioxide generating process, the
reaction medium is at a high total acid normality of
sulfuric acid and is maintained at its boiling point
under a subatmospheric pressure applied thereto.
Methanol is used as a reducing agent for chlorate ions,
resulting in the formation of chlorine dioxide in a
substantially pure form. The boiling nature of the
reaction medium produces steam which acts as a diluent
for the gaseou~ chlorine dioxide, so as to prevent
decomposition of the chlorine dioxide.
The sodium sul~ate by-product builds up in the
reaction medium after start-up until the solution is
:: : : .
.:
,, ~',: :
~,
2~92238
saturated with sodium sulfate, whereupon the sodium
sulfate precipitates from the reaction medium. A slurry
of the sodium sulfate is removed from the reaction
vessel, the crystalline sodium sulfate is filtered
therefrom and the mother liquor is recycled to the
reaction zone after the addition of make-up quantities of
sodium chlorate, sulfuric acid and methanol.
This process is highly efficient and rapidly
produces chlorine dioxide in commercial quantities. As
~0 may be concluded from the above equation (1), for each
mole of chlorine dioxide produced a mole of chlorate ion
and hence of sodium ion is introduced to the reaction
medium. The sodium ions combine with the sulfate ions
introduced with the sulfuric acid, to produce a sodium
sulfate, which may be sodium bi~ulfate or, more normally
under the conditions of an R-8 process, the double salt
sodium sesquisulfate, i.e., Na3H(S04)2 (or NaHS04.Na2SO4),
depending on the acidity of the solution.
Such by-product sodium sulfate and sodium
sesqui~ulfate (sometimes termed "saltcake"), generally
have been employed to make up sulfur losses in the pulp
mill. However, the adoption of high substitution of
chlorine by chlorine dioxide in the chlorination stage of
the bleach plant has led to saltcake by-product
production ~rom the chlorine dioxide generating process
~xceeding the mill make-up requirements.
In U.S. Patents Nos. 5,122,240 and 5,198,080 [E437-
R9], assigned to the applicant hereof, there is described
means for decreasing the quantity of salt-cake by-product
produced by the R8 chlorine dioxide generating process
for the same level of chlorine dioxide production.
As described therein, the portion of the by-product
sodium sesquisulfate, which cannot be used by the mill,
after making up into an aqueous solution thereof, is
acidified in an electrolytic cell while sodium ions are
removed from the solution. The acidified solution is
~, .
,, . ., "
::
. ,, , , - ,.
2~92~3~
recycled to the generator to provide a portion of the
acid therein. Commercially, this procedure is known by
the applicant's assignee as the "R9" process.
For existing chlorine dioxide generators, while this
procedure is able to decrease the quantity of salt-cake
that is required to be proces~ed by the mill or otherwise
disposed of, this result is achieved at the expense of an
increased evaporative load on the chlorine dioxide
generator. Increasing the evaporative load increases
energy requirements and operating costs are increased.
Furthermore, a substantial capital outlay may be required
to adapt existing equipment to this process.
Another problem which exists with re~pect to the
sodium susquisulfate by-product is its acidic nature,
which necessitates neutralization of the acid values
prior to disposal o~ unwanted quantities of the material.
With the trend towards higher chlorine dioxide
substitution for chlorine in many mills, the necessity to
neutralize increasing quantities of sodium sesquisulfate
may result in an imbalance o~ aaustic and chlorine within
the mill. The caustic demancl increases while chlorine
usage declines, resulting in increased costs to the
mills. In addition, the 1QSt acid values of the sodium
ses~uisulfate require that make-up sulfuric acid must be
fed continuously ts the chlorine dioxide generator to
-~ maintain the required acidity.
In U.S. Patent No. 5,116,595, [E439-RlOJ, assigned
to the applicant hereof, there are described procedures
whereby the acid sodium sesquisulfate is converted by
metathesis to neutral sodium sulfate and the acid values
are recovered for reuse in the chlorine dioxide
generator.
While this procedure is able to provide a neutral
by-product from the R8 chlorine dioxide generating
process while recovering acid values ~rom the salt-cake,
when the aqueous acid product o~ the metathesis process
- ,
:: , . :,
: ~ . :: ' :.
:
2~!~22~8
is used in the chlorine dioxide generator, the
evaporative load on the chlorine dioxide generator again
is increased. Commercially, this procedure is known by
the applicant's assignee as the "R10" process.
In accordance with the present invention, the
inventors have devised a scheme whereby the R8, R9 and
R10 processes are combined to achieve the individual
benefits thereof without significantly increasing the
evaporative load on the chlorine dioxide generator.
This combination process may produce the following
significant benefits:
(a) Chlorine dioxide is produced at high efficiency
from sodium chlorate, methanol and sulfuric acid by
the R~ process,
(b) The quantity of by-product sodium sulfate which
is produced may be controlled at a level desired by
a mill,
(c) The by-product sodium sulfate is provided in
neutral form, which is more easily handled and
requires no further processing, i~ required to be
disposed of,
(d) Acid values present in the by-product sodium
sulfate from the generator are reused in the
generation of chlorine dioxide,
(e) Sodium hydroxide is produced as a by-product,
which may be used elsewhere in the pulp mill without
incurring the usual penalty of coproduction of
chlorine, and
(f) The evaporative load on the chlorine dioxide
generator is not increas d to any degree beyond the
capacity of the existing equipment while obtain;ng
as much as a 70~ decrease in salt-cake production.
In one embodiment of the present invention, by
combining the metathesis operation and the electrolysis
operation on the by-product sodium ses~uisulfate from the
chlorine dioxide generator, an acid recycle stream is
- . . .
~, ~. . .
., , ., ,:
~ , :,
: '
2~2238
obtained which has a greater acidity than has heretofore
been possible and this result avoids increasing the
evaporative load on the generator.
Accordingly, in one aspect of the present invention,
there is provided a process for the production of
chlorine dioxide which comprises a plurality of steps.
Chlorate ions are reduced in an aqueous acid reaction
medium having a total acid normality of at least about 4
normal and containing sulfuric acid to form chlorine
dioxide in a reaction zone from the aqueous acid reaction
medium.
The aqueous acid reaction medium is maintained at
its boiling point under a subatmospheric pressure applied
to the reaction zsne and a by-~product acid sulfate is
-15 precipitated in the raaction zone from the aqueous acid
;reaction medium. The chlorate ions present in the
aqueous acid reaction medium usually are provided by
sodium chlorate, so that the by-product acid sulfate
usually is a sodium acid sulfate. The precipitated by-
product acid sulfate is removed from the reaction zone.
The removed by-product acid sulfate is contacted in
solid phase with an aqueous medium to effect conversion
by metathesis of the solid phase by-product acid sulfate
into solid phase neutral anhydrous sulfake and to form an
aqueous acid medium.
An aqueous solution of at least one alkali metal
salt selected from the group consisting of alkali metal
chlorate, alkali metal sulfate and mixtures of alkali
metal chlorate and alkali metal sulfate is
electrochemically acidified, while at the same time,
alkali metal ions are electrochemically removed from the
aqueous solution, so as to form an aqueous acidified
alkali metal salt solution.
The aqueous acid medium formed by the metathesis
step and the aqueous acidified solution of alkali metal
: ~. - . . . ..
- , ~
,.
:.,: ' ' ,
:: -:
,~, :
~9223~
salt are forwarded to the aqueous acid reaction medium in
the reaction zone.
The chlorine dioxide generating process employed
herein involves reduction of chlorate ions in an aqueous
acid reaction medium having a total acid normality of at
least about 4 normal and containing sulfuric acid, which
results in the production of an acid sulfate by-product.
The chlorate ions usually are provided by sodium
chlorate, which results in a sodium acid sulfate being
produced.
The total acid normality may range upwardly from the
at least 4 normal, generally up to about 12 normal.
Chlorine dioxide may be produced at total acid
normalities above about 9 normal, or below about 9
normal, preferably about 5 to about 9, such as about 5 to
about 7 normal or about 7 to about 9 normal~
Reduction of the chlorate ions present in the
aqueous acid reaction medium to form chlorine dioxide
generally is effected using methanol as the reducing
agent, in view of the advantages which are achieved usiny
the R8 process. Other reducing agents which produce
chlorine dioxide from chlorate ions in aqueous acid
reaction medium, such as, hydrogen peroxide or sulfur
dioxide, also may be employed.
The chlorine dioxide is generated from the aqueous
acid reaction medium maintained at its boiling point
under a subatmospheric pressure. By-product acid
sulfate, usually sodium acid sulfate when sodium chlorate
is used, precipitable ~rom the aqueous acid reaction
medium, after reaching saturation following start up, and
is removed from the reaction zone.
The removed by-product acid sulfate is contacted in
the solid phase with an aqueous medium to effect
conversion by metathesis of the solid phase by-product
acid sulPate into solid phase neutral anhydrous sulfate
and form an aqueous acid medium. The metathesis
,
" '' '' " ~" ', ,
,, ' .
, ' '
~223~
operation may be effected in any convenient manner, as
described in more detail in the aforementioned UOS.
Patent No. 5,116,595.
As described therein, the metathesis of the acid
5 sulfate, usually sodium acid sulfate, may be ef~ected
using water alone or an aqueous solution of sodium
chlorate, sodium chloride, methanol, or condensate
containing methanol and formic acid. Of these materials,
water and aqueous sodium chlorate are preferred. The
aqueous acid medium which results from the metathesis
contains acid values which are employed in the generation
of chlorine dioxide.
In addition, an aqueous solution of an alkali metal
sulfake, alkali metal chlorate or mixture thereof,
usually in the form of the sodium salt, is
electrochemically acidified while alkali metal ions are
removed therefrom to form an aqueous acidified alkali
metal salt solution, which also is forwarded to the
chlorine dioxid~ generator as an acid source thexein.
Such electrochemical acidification may be effected,
utilizing the processes described in the aforementioned
U.S~ Patents Nos. 5,122,240 and 5,198,080. The aqueous
solution which is subjected to electrochemical
acidification herein, thus, may comprises an aqueous
alkali metal chlorate solution/ usually an aqueous sodium
chlorate solution, whereby the electrochemical
acidification, which produces an acidic medium containing
chloric acid, which is forwarded to the chlorine dioxide
generator.
In one embodiment of the invention, the aqueous acid
medium formed in the metathesis step which contains some
sodium sulfate, forms at least part of the aqueous alkali
metal salt solution which is electrochemically acidified
and the resultinq acidified aqueous alkali metal salt
solution then is forwarded to the aqueous acid reaction
medium in the chlorine dioxide generator, so that the
.
. . ". ' ~, ' ,:,: : ,
.
. . ~ , .
, , ;
:,
2~9223~
acid values released in the metathesis step and formed in
the electrochemical acidification step both are recycled
to the chlorine dioxide generator in the same stream.
In this embodiment of the invention, sodium chlorate
may be added to the aqueous acid medium passed to the
electrochemical acidification step by the use of sodium
chlorate during the metathesis operation and/or by the
addition of sodium chlorate to the ePfluent stream from
the metathesis reactor as it passes to the acidification
cell. As mentioned in U.S. Patents Nos. 5,1~2,240 and
5,198,0~0, it is pre~erred to acidify an aqueous solution
of sodium chlorate and sodium sulfate.
In another embodiment~ the metathesis of the solid
; phase by-product alkali metal acid sulfate is effected
using the aqueous acidified alkali metal salt solution
produced in the electrochemical acidification step, to
form an agueous acid medium in the metathesis step which
is forwarded to the chlorine dioxide generator. Part of
the solid phase product neutral anhydrous alkali metal
sulfate, usually neutral anhydrous sodium sulfate, is
formed into an aqueous solution for use as feed to the
electrochemical acidification step. Sodium chlorate, or
other alkali metal chlorate, may be added to metathesis
reactor and/or to the aqueous sodium sulfate solution
forwarded to the electrochemical acidification.
The invention is described further, by way of
illustration, with reference to the accompanying
drawings, in which:
Figure 1 is a schematic flow sheet of a chlorine
dioxide generating operation provided in accordance with
one embodiment of the invention;
Figure 2 is a schematic flow sheet of a chlorine
dioxide generating operation provided in accordance with
another embodiment of the invention;
:, : ~ . , :,, .
, .~ , ,, . ~
. .
:,
.. . .. . .. . .
-: , , ,, , , ",
~ ' '
,
. . .
,
2~9223~
Figure 3 is a schemakic flow sheet of one embodiment
of metathesis operation which may be employed in the
metathesis stage in the process of Figure 1 or 2; and
Figure 4 is a schematic flow sheet of another
embodiment of metathesis operation which may be employed
in the metathesis stage in the process of Figure 1 or 2.
Figure 5 is a graphical representation of the
lowered energy requirement which is obtained using the
process of the invention at significant levels of
decrease o* sodium sulfate by-product formation, for the
process of the invention, namely the R8/R9/R10
combinations, in comparison to the combination of R8 and
R9 processes.
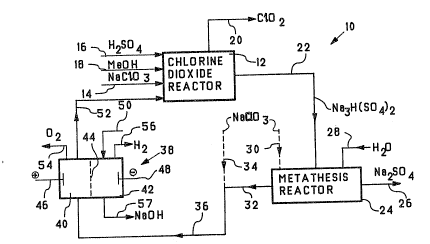
Referring to the drawings, Figure 1 illustrates an
embodiment of the application of the present invention to
chlorine dioxide production. As seen therein, a chlorine
dioxide generating operation 10 comprises a chlorine
dioxide generator 12 wherein sodium chlorate fed by line
14, sulfuric acid fed by line'16 and methanol fed by line
18 react together in an aqueous acid reaction medium to
produce chlorine dioxide. The reaction medium is
; maintained at its boiling point under a subatmospheric
pressure. Chlorine dioxide is removed from the generator
12 by line 20 in gaseous admixture with steam.
Crystalline sodium sesquisulfate is precipitated
from the reaction medium and is removed from the
generator 12 by line 22 and ~orwarded to a metathesis
reactor 24. In the metathesis reactor 24, the solid
phase crystalline sodium sesquisulfate is converted into
solid phase neutral sodium sulfate, which is removed from
the metathesis operation by line 26. The metathesis may
be carried out as described in the aforementioned U.S.
Patent No. 5,116,595 [E439]. The procedures which may be
used are described below with respect to Figures 3 and 4.
The metathesis may be effected using water fed by
line 28 or by an aqueous solution of sodium chlorate fed
, ,, ,, ~ - , , . ', ., , , ., ;
:, , : ; :, , : ~,
-:
: . . .
::
, : : , , ,. ~ ,:
: , , :
",
~223g
by line 30. In either case, there results an aqueous
acid solution of sodium sulfate in line 32. The sodium
sulfate content of this solution, in effect, is a
deadload of sodium sulfate which circulates around the
integrated operation. When sodium chlorate is not used
as the metathesizing chemical, it may be added to the
acid sodium sulfate solution by line 34. When sodium
chlorate is used as the metathesizing chemical,
additional guantities of sodium chlorate may be added by
line 34. As desired, a feed of sodium chlorate may be
omitted entirely.
The acid aqueous sodium sulfate solution in line 36
is forwarded to an electrolysis cell 38, which is
operated as described in detail in U.S. Patent No.
5,198,080 [E437-R9~ referred to above. The electrolysis
cell comprises an anode chamber 40 separated from a
cathode chamber 42 by a cation-exchange membrane 44 The
cell anode 46 and cell cathode 48 are located in the
anode compartment 40 and cathode compartment 42
respectively. Any other convenient form of cell,
including an electrolysis cell equipped with a plurality
of bipolar membranes and ion exchange membranes, may be
employed to effect the acidification.
The aqueous acid solution in line 36, which may be
in the form of a slurry, if desired, is fed to the anode
compartment 40 while an electrolyte is fed to the cathode
compartment by line 50. As a current is applied between
the anode and cathode, several reactions occur
simultaneously. At the anode 46, water is electrolyzed
to oxygen and hydrogen ion, as follows:
H2~ ~ ~~2 + 2H~ + 2e-
while at the cathode 48 water is electrolyzed to hydrogen
and hydroxyl ion, as follows:
e~+ H2O ~ ~H2 + OH-
At the s~me time, sodium ions in the aqueous solution orslurry of a mixture of sodium sulfate and sodium chlorate
,: :
,, , ~, , .:
. .
~223~
11
migrate under the influence of the applied current from
the anode compartment 40 across the cation-exchange
membrane 44 to the cathode compartment 42. In effect,
therefore, the electrolytically-produced hydrogen ions
replace the sodium ions in the anode compartment 40 and
the transferred sodium ions are available to combine with
the electrolytically-produced hydroxyl ions in the
cathode compartment 42.
As noted earlier, the sodium sulfate contained in
the solution ~eed in line 36 to the cell 38 can be
considered to be a deadload circulating via the generator
12 in a closed loop, so that the overall reaction in cell
38 may b~ represented, as follows:
xNaCl03 + 3H20 ~ (x-2) NaCl03 + 2HCl03 + 2NaOH
+ ~o2 + H2
where x is the molar amount of sodium chlorate which is
processed. Alternatively, the process may be represented
as~
Na3H ( S04) 2 + H20 ~ 2NaHSO4 + NaOH
The acid produced is in addit.ion to the acid already in
the stream 42 as a result of metathesis.
The further acidified stream which results from the
anode compartment is forwarded by line 52 to the chlorine
dioxide generator 12, thereby providing acid and chlorate
ions for the chlorine dioxide generating process.
The sodium sulfate removed from the system by lin~
26 corresponds to the proportion of the sulfuric acid and
sodium chlorate reactants fed to the chlorlne dioxide
generator from external sources, namely by line 16 for
sulfuric acid and by unconverted sodium chlorate in line
52 as well as in line 14.
Oxygen is vented from the anode compartment 40 by
line 54 while hydrogen is vented from the cathode
compartment by line 56. Alternatively, any other anodic
reaction producing hydrogen ions can be employed, for
example, hydrogen gas oxidation, as described in one
. . .. . :: ,
, ~ ..... .
. :
.. :
.
2~9223~
12
embodiment of the procedures described in afor~mentioned
U.S. Patent No. 5,198,080 [E437-R9]. The sodium
hydroxide solution formed in the cathode compartment i5
removed therefrom by line 57. If desired the sodium
hydroxide solution may be cycled through the cathode
compartment until a desired concentration is achieved.
The useful sodium hydroxide solution contains sodium
which would otherwise have formed sodium sulfate. This
sodium hydroxide is employed in the purifying and
bleaching operations effected in the bleach plant o~ the
pulp mill. The gaseous by-products, namely hydrogen and
oxygen also can be utilized in the pulp mill.
Referring now to Figure 2, this Figure illustrates
a further embodiment of the application of the present
invention to chlorine dioxide production from a chlorine
dioxide generator 12'. Elements of the arrangement which
are in common with Figure 1 have been designated by the
same numerals primed and will only be described as
necessary.
In this embodiment, a portion of the solid phase
neutral sodium sulfate rasulting from the metathesis
reactor 24' is ~orwarded by line 58 to a dissolving tank
60, wherein the sodium sulfat~ is dissolved in water fed
by line 62 to ~orm an aqueous solution thereof. Sodium
chlorate also may be fed to the dissolving tank 60 by
line 64, as well as a portion of the recycled acid medium
in line 65. The resulting aqueous solution of sodium
sulfate, optionally containing sodium chlorake, is
forwarded by line 66 to the anode compartment 40' of the
cell 38', for acidification as described above with
respect to Figure 1.
In this case, the acidified solution of sodium
sulfate, optionally containing sodium chlorate, is
forwarded by line 68 ko the metathesis reactor 24' to be
employed as a source of water therein. Optionally, a
portion or all o~ the acidified solution in line 68 may
. .
. .
,~. . .
2~2238
13
be forwarded by line 72 to the chlorine dioxide generator
12'. The acid stream resulting from the metathesis
reaction, cont~;n;ng acid from the sodium sesquisulfate
and formed in the cell 38', is forwarded to the chlorine
dioxide generator 12' by line 70.
In both the embodiment of Figure 1 and that of
Figure 2, the combined effects of the metathesis and the
electrolytic acidification produces an acid stream for
feed to the chlorine dioxide generation operation which
does not increase the evaporative load on the generator,
at least up to a significant decrease in the proportion
of sodium sulfate produced per mole of chlorine dioxide.
The metathesis operation effected in the embo~;r-nt
of Figures 1 and 2 may comprise one of the procedures
illustrated in Figures 3 and 4. In the case of Figure
3, the metathesis is carried out using aqueous sodium
chlorate solution, while, in the case of Figure 4,
metathesis is carried out using water. As seen in Figure
3, crystalline sodium sesquisulfate, removed in slurry
form with spent reaction medium from the chlorine dioxide
generator, is forwarded by line 110 to a first filter 112
wherein the crystalline material is separated from spent
reaction medium, which is recycled by line 114 to the
chlorine dioxide generator. If wash water is desired to
assist in separating the crystalline material from
entrained reaction medium, it may be fed to the filter
112 by line 116.
The crystalline sodium sesquisulfate is forwarded
from the filter 112 by line 118 to a metathesis reactor
120, which preferably takes the form of one or more
stirred tanks. To the metathesis reactor 120 is fed an
aqueous sodium chlorate solution by line 122 of
sufficient concentration and temperature to effect
metathesis conversion of the crystalline sodium
sesquisulfate to crystalline anhydrous neutral sodium
sulfate, with release of acid into the sodium chlorate
/
.. A . ...
, ' ,. "'
" " . " ', ~ . ' '
~9223~
14
solution. The resulting slurry is forwarded by line 124
to a second filter 126 for separation of the solid phase
sodium sulfate, which is recovered as a product by line
128.
If desired, wash water may be fed by line 129 to the
filter 126 to assist in freeing the solid phase from
entrained sodium chlorate-containing liquid. The
filtrate contains sodium chlorate, sulfuric acid and
dissolved sodium sulfate and is recovered by line 130.
If desired, a portion of stream 130 may be recycled and
used as at least part of the wash water feed in line 116
to the filter 112, to decrease further volume of water
fed to the chlorine dioxide generator.
Turning now to Figure 4, the same elements as in
Figure 3 are designated by the same reference numbers in
Figure 4 primed and referred to only as necessary. In
this embodiment, the solid phase crystalline
ses~uisulfate is contacted with water in line 132 in the
metathesis reactor 120' to effect metathesis of the
sodium sesquisulfate to form neutral anhydrous sodium
sulfate. Part of the filtrate from the filter 126' may
be recycled by line 134 to the first filter 112', so as
to provide wash water employed therein to free the sodium
sesquisulfate from spent reaction medium, or may be fed
;: 25 to the metathesis reactor 120' via line 136.
The invention is illustrated further by the
following Example:
Example
The steam-to-chlorine dioxide ratio (lb/lb) was
calculated for various levels of reduction of overall
salt cake (sodium sesqui-sulfate) for a chlorine dioxide
generator operating at a total acid normality of about 8
N with feeds of 620 g/l sodium chlorate, 20% methanol
feed and 93~ H2S04 feed sufficient to produce chlorine
dioxide at an efficiency of about 98% to compare an
: operation using the combination of R8 and R9 processes,
.
- , ,,
, : ,, :, , ~ ' , .
, .,
" 2~223~
as described in the aforementioned U.S. Patent No.
5,1g8,080 and an operation as described above with
respect to Figure 1. The results of these calculations
were plotted graphically and appear in Figure 5.
As may he seen from this Figure, above a level of
decrease in salt cake production of about 25%, there is
a significant saving in steam requirement.
In summary of this disclosure, the present invention
provides a novel chlorine dioxide generating procedure
which enables a significant decrease in by-product sodium
sulfate to be achieved without significantly increasing
the evaporative load on the generator by integrating the
R8, R9 and R10 processes. Modi~ications are possible
within the scope of this invention.
,, '': . ' :: ; ., : . '
..
, . , ~