Note: Descriptions are shown in the official language in which they were submitted.
2~9225~
REFERENCE NO . 01-8 2 7 3
--1--
SYNCHRONIZED DONOR CELLS FOR BOVINE NUCLEAR TRANSFER
Field of the Invention
The present invention is generally directed to a
process for multiplying bovine embryos~ and is
specifically directed to an improved process for
synchronizing the cell cycle stage of donor embryonic ~ -
cells and recipient oocytes so that the overall efficiency
of the bovine em~fryo multiplication process is improved.
Background of the Invention
Embfryo multiplication by nuclear ~ransfer involves ~-
the transplantation of the living nuclei from embryonic -~
cells, or the whole embryonic cells themselves, into
recipient cells, typfically unfertilized eggs after which
the donor and recipient are fused. Such tran~fers are
made in order to increase the number of gènetically
identical embfryo~ which can be obtained from elite genetic
stock. Once a fertiliæed embryo has reached a cleavage
stage of ha~ing at lea~ two cells, it becomes practical ~ ~
~- to transfer the nuclei from such cells, or the entire ~: ~ -
f 25 cells them~elves, into recipient oocytes which have been
enucleated, to thereby create multiple genetically
j~ identical embryos from such fusions. By allowing each of
the fused nuclear tran~fer embryos to develop to a multi-
I cell ~tage, and then repeating the nuclear transplantation
procedure, large numbers of genetically identical nuclear
f transfer embryos can be created from an original donor
f embryo. While blastomeres isolated from pre-gastrulation
embryos have befen most widely used as a source of donor
: .
2~92258
-2-
nuclei, other sources of donor nuclei include bovine
embryonic stem cells and embryos from oocytes that have
been matured, fertilized, and cultured in vitro. A
limitation on the commercial use of this process, as
practiced to date, arises from the fact that there are
certain inefficiencies in the nuclear transfer process.
Not all of the nuclear fusions created result in viable
embryos. Not all viable en~ryos created by nuclear fusion
turn out to be capable of creating a viable pregnancy in
the cow resulting in a live calf. Accordingly, effort is
currently being directed toward optimizing the
efficiencies at each step in the procedure, so as to make
the overall procedure more economically practical.
The techniques of bovine nuclear transplantation are
generally described in two U.S. patents, U.S. Patent No.
4,994,384 (Prather et al.) and Patent No. 5,057,420
(Massey), both of which describe procedures for the serial
multiplication of bovine embryos. In the techniques
described in each of those pa~ents, oocytes are recovered
from the ovarie~ or reproductive tract of cows. The
oocytes are selected for proper stage, and then are
enucleated by physical aspiration through a transfer
pipette, leaving an enucleated oocyte which still retains
its external membranes. Synchronously, a donor embryo of
the proper cell staging, typically at the cleavage or
morula stage, is manipulated so that one or more cells or
blastomere~ are removed from the embryo. The donor cell ~. -
which, of course, includes its nucleus, is then inserted
into the perivitelline space of the recipient oocyte. An
electrical pulse is then applied to fuse the membranes of
the donor cell and the recipient oocyte, thu~ creating an
activated, fused single cell embryo. ~hat single cell
nuclear transfer embryo can then be cultured either ~n
vitro or in the oviduct of a mammal, until a stage in
which it can be implanted into a recipient cow. A
significant number of the fused embryos will retain
viability, can be transplanted surgically or non-
surgically into the uteri of cattle, and will result in
live births of genetically identical calves.
2~922~3
--3--
In past nuclear transplantation procedures,
individual cells within the same donor embryo were not
synchronized with respect to the cell cycle, beyond the
four-cell stage. Thus, it has only been at random that a
particular donor blastomere would be at the correct cell
cycle stage to achieve optimal development after nuclear
transfer. What is lacking in the art is both knowledge as
to which cell cycle stages are most appropriate and a
method for manipulating and synchronizing the cell cycle
stage of donor embryonic cells.
Several reports of efforts to optimize the cell cycle
stage of donor cell nuclei in other mammals have been
reported. Collas, P., et al., 46 Biol. Reerod. 492-500
(1992) have demonstrated that donor nuclei in early stages
of the rabbit embryo cell cycle show enhanced ability to
fuse into embryos that will mature to the blastocyst
stage. Kono, et al., 37 The ioqenoloqy 239 (1992)
demonstrated that nuclear transfer mouse embryos derived
from fusions using synchronized late-stage murine
blastomeres were better able to develop to the blastocyst
stage than embryos derived from blastomeres of other
stages.
Summarv of the Invention
The presen~ invention is summarized in that a process
for bovine nuclear transplantation includes the steps of
(1) ensuring that a majority of the donor bovine embryonic
cell3 are in metaphase, (2) allowing the embryonic cells
sufficient time post-metaphase to progress into the S-
phase of the cell cycle, (3) fusing individual donor
embryonic cells with recipient oocytes, (4) culturing the
nuclear-transferred fusion cells to the blastocyst stage,
and (5) transferring blaotG--i 3'_3 to -ecipiisnt females to
produce cloned calves. In an especially preferred
embodiment of the invention, the donor embryonic cell and
recipient oocyte are synchronized to each other, in that
at the time of fusion both are in S-phase and both are
expected to reach the next M-phase at approximately the
same time.
.
2~22~
.
It is an object of the present invention to further
develop and define a step in a bovine nuclear
transplantation process so as to increase the overall
efficiency of the process.
It is an objective of the present invention to aid in
the overall efficiency of bovine transplantation and
multiplication processes, so as to result in greater
numbers of viable pregnancies and multiplied genetically
identical calves.
It is an object of the present invention to increase
the percentage of nuclear transfer embryos that mature to
the blastocyst staqe.
Other objects, advantages, and features of ~he
present invention will become apparent from the following
specification.
Brief Description of the Drawinqs
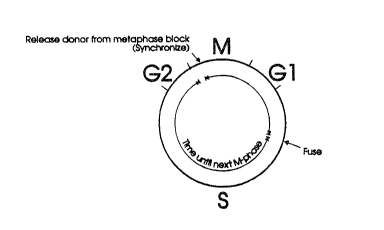
Fig. 1 schematically depicts the donor cell cycle,
indicating the ~ime of removal of the synchronizing agent,
the time of fusion and the time until next M-phase.
Fig. 2 schematically depicts the recipient oocyte
cell cycle, indicating activation time, fusion time, and
time until next M-phase.
DescriPtion of the Preferred Embodiment
In accordance with the present invention, a bovine
embryonic cell destined to be the source of a donor
nucleu~ in a nuclear transfer (NT) procedure is subjected
to synchronization in S-phase prior to nuclear
transplantation. In addition, fusion of a recipient
oocyte and a donor embryonic cell yields a higher
percentage of viable NT embryos if both are in S-phase and
if both will next enter M-phase a~ ap~roximately the sa~e
time. The present invention provide~ a method for
achieving such synchrony between donor and recipient.
The embryonic donor cells useful in the method of the
3S present invention may be obtained in a variety of ways.
Embryonic cells are often obtained by flushing embryos
from surqically recovered oviducts or may be non-
~922~8
~ 5
surgically flushed from the uterus in manners known to the
art. Embryonic donor cells may also be obtained from in
vitro maturation/in vitro fertilization/in vitro culture
(IVM/IVF/IVC) procedures. In addition, embryonic stem
cells cultured and maintained in vitro may also be used as
donor cells in the bovine multiplication procedure of the
present invention. Totipotent bovine embryonic stem cells
have been cultured to the blastocyst stage, then implanted
into recipient cows. The advent of systems to create and
culture bovine stem cells permits the serial culture of
large numbers of genetically identical donor cells. See
PCT Published Patent Application No. WO 90/03432. In
addition, bovine embryonic stem cells have been used
successfully as donor cells in nuclear transplant
experiments, yislding fetuses that have developed to at
least the 6th month of gestation. In the present
application, the term f'embryonic cell" refers to any
bovine embryonic cell that may be used as a donor cell in
nuclear transfers, notably those cells derived from bovine
embryos, from IVM/IVF/IVC procedures, and from bovine
embryonic stem cell cultures. Each cell type is useful as
a donor cell in the method of the present invention.
The synchronization step is most preferably done by
culturing the embryonic donor cell in a culture medium
containing an optimal concentration of a cell cycle
inhibitor for a period of time sufficien~ to synchronize a
majority of the embryonic cells in a particular mitotic
phase, such as metaphase. The cell cycle inhibitor may be
any agent tha~ reversibly blocks the cell cycle at a
defined phase, such as a drug that blocks the cell in
metaphase by inhibiting the formation of spindle
microtubules.
Cell-cycle-inhibited embryonic donor cells, blocked
at a defined cell cycle phase, are subsequently released
from the cell cycle block by removing the inhibitor from
the culture medium. The donor cells are then allowed to
progress through the cell cycle into S-phase to yield,
after NT fusion, the greatest percentage increase of NT
embryos maturing to the blastocyst stage when compared to
2~2~8
, ~ --6--
NT embryos produced from fusions of non-synchronized donor
nuclei.
Less preferably, one may avoid the use of cell-cycle-
inhibiting drugs by observing the embryonic cell division
processes and noting when the embryos are in metaphase.
After visually observing cell division, it should then be
possible to culture the embryonic cells for a sufficient
length of time to assure entry into the preferred cell
cycle phase, as described below. However, this second
approach lacks the definitive and repetitive staging
accuracy of the cell cycle inhibitors and is less
preferred.
It is herein demonstrated that the greatest
percentage increase in blastocyst stage embryos is
obtained when the donor embryonic cells are in the cell
cycle phase commonly referred to as S-phase. In this
specification, S-phase is also referred to as mid-stage,
since it is in the middle of the cell cycle. S-phase is
characterized by DNA synthesis, also referred to as DNA
replication. One complete cell cycle takes approximately
20 hours in pre-gastrulation bovine embryos and other
embryonic cells. S-phase occupies the bulk of that 20
hour cycle. While the precise beginning and end points of
S-phase are imprecisely defined, bovine embryos are known
to be in S-phase from about five hours after release from
metaphase until about five hours before returning to
metaphase. S-phase is preceded by Gl-phase (herein,
early-stage), during which the cell prepares for DNA
synthe~is, and is followed by G2-phase (herein~ late-
stage), during which the cell prepares for mito~ic
division of M-phase. In M-phase, chromosomes condense and
prepare to separate into the two daughter cells. During
the metaphase portion of the M-ohase the chromosomes 1 i ne
up at the metaphase plate poised to be distribu~ed between
the daughter cells.
The synchronized embryonic cells for use within the
present invention are intended for a protocol of bovine
embryo multiplication through nuclear transplant. It is
appropriate therefore, at this juncture, to briefly
2~22~8
--7--
describe the overall bovine embryo multiplication
procedur~. Bovine ovaries are collected at the slauyhter
house, and are maintained in physiological saline for
transportation from the slaughter house to the laboratory.
Follicles ranging in size from 2 to 10 mm in diameter are
then aspirated from the bovine ovaries. The immature
oocytes contained within the follicular fluid are removed,
buffered with HEPES, and washed in hamster embryo culture
medium (HECM), described in Seshagare et al., 40 Biol.
Reprod., 599-606 (1989), and then placed into drops of
maturation medium consisting of 50 ~l of tissue culture
medium (TCM~ 199 containing 10~ fetal calf serum with
appropriate gonadotropins, luteinizing hormone (LH3 and/or
follicle stimulating hormone (FSH), and estradiol under a
layer of lightweight paraffin or silicon oil at 39C.
After twenty hours in the maturation media, the in vitro-
matured oocytes are removed and placed in HEC~ containing
1 mg/ml of hyaluronidase. The cumulus cells are removed
from the oocytes by repeated pipe~ting through very fine-
bore pipettes. The stripped oocytes are screened for polar
bodies, which are indicators of metaphase II. The
selected metaphase II oocytes are then used further in the
transplantation and multiplication procedures.
The oocyte enucleation portion of the cloning
procedure is d~scribed in U.S. Patent No. 4,994,384, the
specification of which is hereby incorporated by
reference. Briefly, the metaphase II oocytes are either
placed in HECN containing 7.5 micrograms per milliliter
cytochalasin B for immediate enucleation or are placed in
CRlaa plu5 10~ estrus cow serum, and are enucleated later,
preferably sixteen to eighteen hours later. CRlaa medium
cor.tains 114 mM NaC1, 3.1 mM KC1, 25 mM NaHCO3, 0.4 mM
Sodium Pyruvate, 5 mM Hemicalcium Lacta~e, 1 mM Glutamir;~, -
1 ml/100 ml NEM amino acids, 2 ml/100 ml BME amino acids,
and 50 mg/ml Gentamycin.
Enuclea~ion is accomplished using a micropipette to
remove the polar body and the surrounding cytoplasm. The
oocyte can then be screened to determine which oocytes
2 ~
-8-
have been successfully enucleated. This screening is
successfully done by staining the oocytes with 1 microgram
per milliliter 33342 Hoechst dye in HECM, and then viewing
the oocytes under ultraviolet irradiation for less than 10
seconds. The oocytes that have been successfully
enucleated can then be placed in culture media, preferably
CRlaa plus 10% estrus cow serum. After enucleation, an
embryonic donor cell is placed in the peri~itelline space,
by the method also described in the above-identified
Patent No. 4,994,384.
For oocytes matured in vitro, the nuclear transfer
process is normally performed between 20 and 46 hours
after the oocyte was first placed in the maturation
medium. After the blastomere cell is transferred into the
perivitelline space of the recipient oocyte, the two cells
are fused together by electrofusion. The cells to be
fused are placed between two electrodes which are 500~
apart, referred to as the fusion chamber, which contain
Zimmerman's fusion medium. Up until the point of fusion
the donor cells and the recipient cells are maintained at
or below 3~C, a physiological temperature.
After fusion, the NT embryos thus created are then
placed in CRlaa medium plus 10% estrus cow serum at 39C
for 5 to 9 days. A de~ailed description of a process for
culturing such NT embryos in vitro is set forth in U.S.
Patent No. 5,096,822. At the end of this culture period,
the embryos can be judged for developme~tal rates. The -
embryos judged to have developed normally, either into
morulae or blastocysts, can be transferred into recipient
animals, and an offspring may be obtained after a normal
pregnancy.
The above brief description of the embryo
multiplication and nuclear trAnsnlAnt process has heen
altered in the inventive method described herein only in
the preparation of the donor embryonic cells and recipient
oocyte~. It has been found that fusions performed with
donor cells synchronized in S-phase of the cell cycle
yield a greater percentage of blastocysts for implantation
than either non-synchronized donor embryonic cells or
. .~. .
9 2 ~ 5 ~
embryonic cells synchronized in early (e.g., Gl-phase) or
late ~e.g., G2-phase) cell cycle stages. In a preferred
fusion method, the donor embryonic cells and recipient
oocytes are bokh in S-phase at the time of fusion. In a
most preferred fusion method, the donor cells and
recipient oocytes are synchroniæed in that fusion takes
place when the time-until-next-M--phase for both donor and
recipient cells is approximately equal. In other words,
it has herein been found that, a salient indicator of
success appears to be the time remaining in the cell cycle
until the next entry into M-phase, rather than the elapsed
time since the previous M-phase. It is believed by the
inventors that by synchronizing both donor and recipient
cell cycles to each other during pre-fusion DNA synthesis,
the cytoplasm and nucleus of post-fusion NT cell are
better able to coordinately proceed into G2- and M~phases.
In summary, if the donor cell is in S-phase, and also,
preferably, if the recipient cell is at the same part of
S-phase as the donor, the developmental success rate of
blastocyst development in NT fusion embryos will be better
than that of unsynchronized fusion embryos.
By performing routine preliminary experiments on the
donor embryonic cells and activated recipient oocytes, one
may easily determine the average cell cycle duration of
each, using techniques that have been described. ;
Determination of time-until-next-M-phase is depicted for
donors and recipients respectively in Figs. 1 and 2. For
synchronized embryonic donor cells, the time-until-nex~-M-
phase is ~imply the cell cycle duration minus the time
between removal of the cell cycle inhibitor and fusion.
For oocyte matured and activated in vitro, the time-
until-next-~-phase is the estimated cell cycle duration
minus the time between activAtion and fusion. Figs. l and
2 are not meant ~o suggest that the cell cycle duration of
embryonic cells and oocytes are identical. Rather, the
figures demonstrate how to determine the time-until-next-
M-phase of either cell type. It is known that the cell
cycle duration of oocytes matured in vitro decreases with
increasing maturation time.
2 ~ ~
--10--
Oocytes used as recipient cells are developmentally
blocked at metaphase until activated. Activation occurs
in vivo at fertilization or in vitro, by a number of
methods. After activation by any method, oocytes progress
through the cell cycle toward a cell division in the next
M-phase. Of course, haploid oocytes cannot divide into
daughter cells having full complements of chromosomes.
Haploid oocytes must receive male chromosomes either by
fertilization or by nuclear transfer from a donor cell.
The donor cell synchronization process of the present
invention begins by arresting the embryonic cell cycle at
a defined phase of the cell cycle, such as metaphase,
using a cell cycle inhibitor such as Demecolcine or
Nocodazole. Removal of the inhibitor is followed by a
waiting period of experimentally-determined length
sufficient to allow the synchronized blastomeres to
advance to the mid-stage of the cell cycle. This process
yields NT donor cells which, when fused to oocytes and
allowed to develop in vitro, demonstrate increased
viabili~y typical of the present invention.
Any cell cycle inhibitor that reversibly arrests the
cell cycle at a predictable cell cycle phase and that,
upon its removal, allows the cell cycle to proceed to the
S-phase is envisioned to fall within ~he scope of the
present invention. The optimal cell cycle inhibitor
concentration for synchronizing the cells may be easily
determined by titrating the concentration used to treat
bovine embryos and choosing that which yields the highest
percentage of embryo cells synchronized at the desired
phase. Similarly, one can experimentally determine the
appropria~e time to S-phase by fusing post-inhibition
donor cells to recipient oocytes after various waiting
periods and ~han obsel-v ny -~hich -~aiti.lg paricd yl21ds l h,^
highest percentage of subsequent blastocyst formation.
Alterna~ively, one may chart the incorporation of
radiolabelled nucleotides into DNA to determine when S-
phase begins.
2 ~ 8
Example 1
Synchronization of bovine donor embryo cells
usinq Demecolcine
Demecolcine is a microtubule inhibitor known to
arrest the development of mammalian cells in the metaphase
of the cell cycle. To determine the concentration at
which the highest percentage of bovine embryo cells became
synchronized in metaphase after exposure to Demecolcine,
pre-gastrulation bovine embryos were cultured in medium
containing the three Demecolcine concentrations shown in
Table l. After 12-18 hours in medium containing
Demecolcine, the number of embryo cells arrested at the
metaphase stage was determined using a nuclear staining
assay. Nuclear material in embryo cells is visualized by
staining the cells with a nuclear dye, such as Hoechst
33342 at 1 ~g/ml in HECM, and then viewing the embryo
cells under ultraviolet irradiation for less than 10
seconds. ~he percentage of embryo cells synchronized in
metaphase varied with the concentration o Demecolcine
used to arrest the cell cycle, as shown in Table 1.
TABLE 1
, _ ~ ,
Demecolcine Number of ¦ % o cells ~-
concentration Embryos I synchronized
,~
Table 1 indicates that preferably 0.05 ~g/ml of
Demecolcine be used to arrest the development of bovine
embryo celis in mPtaphas~ ~ccord~r.gly, bovine embryos
were incubated in 0.05 ~g/ml of Demecolcine for 12-18
hours. After the Demecolcine incubation period, the
embryos were washed and placed back into culture medium
lacking the Demecolcine cell cycle inhibitor. Freed from
` the cell cycle block, the synchronized embryos then
progressed into the cell cycle.
2~2~8
-12-
At variouc times after removal of the Demecolcine
block, embryos were harvested and individual blastomeres
were removed for use in nuclear transfers to determine the
optimal pos~-metaphase cell cycle stage for a donor
S blastomere. Blastomeres from embryos were harvested at 4-
8 hours post-metaphase (hereinafter, early-stage), at 8-14
hours post-mPtaphase (mid-stage), or at 15-20 hours post-
metaphase (late-stage). At 8-14 hours post-metaphase, the
majority of donor blastomeres were in S-phase. As a
con~rol, blastomeres from non-synchronized embryos handled
in parallel were used for transfer experiments with the
early-, mid-, and late-stage synchronized blastomeres.
Recipient oocytes removed from the follicles were
matured in vitro for 20~24 hours prior to selection of the
oocytes for metaphase II staging. The selected metaphase
II oocytes were then cultured (or matured) for an
additional 14-24 hour period at physiological temperatures
(about 39C).
All nuclear transfers were performed using entire
blastomere cells. ThC NT embryos were cultured in vitro,
as described in published PCT patent application WO
90/13627, prior to screening for blastocyst development.
Table 2 demonstrates the significant increase in the
percentage of blastocysts that develop from NT embryos
when the donor blastomeres have been synchronized and then
allowed to progreRs to the mid-stage of the cell cycle (8-
14 hours post-metaphase). In contrast, blastomeres that
have progressed from metaphase to the early- or late-
stages of the cell cycle show either a decreased ability,
or insignificantly increased ability, to develop to the
blastocyst stage when compared to non-synchronized donor
blastomeres.
~, ....
2~2~5~
-13-
TABLE 2
, -- - . . I
Percentage of Blastocysts ¦
(slastocysts Obtained/Total NT Embryos)
l ~ '
Synchronized Donor Un-s~nchronized ¦
BlastomeresDonor Blastomeres
_ I o% 5% '~
Early
(0/140) (11/235)
Mid ¦ 14%
(49/362) (25/339)
1 _ 6%
Late L (30/348) _ (27/461)
Blastocysts obtained after nuclear transplan~ of mid-
stage blastomeres were transferred to recipient cows. Of
10 cloned calves born after transfer of NT blastocysts to
maternal animals, none had either high birth weight or
congenital anomalies often associated with calves produced
by nuclear transplant techniques. Typically 2S% of all
nuclear transplant calves exhibit either high birth weight
or congenital anomaly have been reported. The data of
Example 1 suggest that by synchronizing donor blastomeres
to the mid-stage of the cell cycle certain, possibly
genetic, factors which lead to physical problems with
nuclear transplant calves may be avoided.
Example 2
Synchronization of Nocodazole-treated Bovine EmbrYos
andlCold-Culture-Activated Recipient Ooc~te~
Matured for 42 Hours In Vitro
Using a protocol similar to that of Example 1, a
second cell cycle inhibitor was employed to produce donor
cells which, when fused to recipient oocytes, yielded
embryos that developed to the blastocyst stage at an
increased frequency relative to non-synchronized donor
cells.
-14-
The cell cycle inhibitor Nocodazole, which arrests
the mammalian cell cycle in metaphase, was tested at 0.1,
1.0, and 10 ~M concentration in culture medium ~s in
Example 1 for its ability to optimally synchronize the
cells of bovine embryos. It was determined that 1.O ~M
treatment with Nocodazole for 12-18 hours proved best.
As in Example 1, bovine embryos were synchronized in
metaphase with 1.0 ~M Nocodazole. Subsequent removal of
the inhibitor from the culture medium allowed the embryos
to progress through the cell cycl~ At mid-stage (8-14
hours post-metaphase), blastomeres were harvested and used
as fusion partners for recipient oocytes. At 8-14 hours
post-metaphase, a majority of th~ blastomeres were in
S-phase.
Oocytes used a~ recipients in Example 2, were
prepared by culturing the recipient oocytes at a
temperature in the range of 24-26C during the maturation
period. This cold culture step, relative to the normal
bovine physiological temperature of 39C (used in Example
1), has been shown to increase the survival of developing
NT embryos to the blastocyst stage.
Cold culture is believed to serve as a form of pre-
fusion activation of the recipient oocyte. Cold culture
releases the oocyte cell cycle from the M-phase block that
is typically observed in unactivated oocytes. Activation
methods other than cold culture are also useful for
increasing efficiency of the protocol. In vivo, oocytes
are activated at the time of fertilization. Whatever the
cause of the activation phenomenon, it has been used
herein to increase the efficiency of a practical bovine
embryo multiplication protocol.
Cold culture may be performed at any temperature
significantly below physiological temperatures (e.gO,
~elow 30C and above freezing.) Further, the duration of
cold culture may vary from as little as approximately 2
hours to 24 hours or more. All recipient oocytes in
Example 2 were cold shock cultured, whether they were
recipients of Nocodazole-treated embryonic cells or
control cells.
~ 22~8
-15-
In Example 2, recipient oocytes were matured for
approximately 42 hours before cold culture activation.
Oocytes that have matured for this length of time have a
cell cycle of about 10 hours duration. Cold shock
activation for 2 hours provides a recipient oocyte for
fusion that is in S-phase and about 9 hours away from its
next M-phase. The donor embryonic cells used in Example
2, with a cell cycle time of about 20 hours, were cultured
for about 10 hours after removal of the Nocodazole, to
provide a donor cell that is in S-phase and about 10 hours
away from its next M-phase.
Blastomeres obtained from mid-s~age embryos and fused
to activated recipient oocytes yielded NT embryos having
an enhanced ability to mature to the blastocyst stage Ln
vitro when compared to NT embryos produced using non-
synchronized donor blastomeres. NT embryos produced from
synchronized blastomeres reached the blastocyst stage 27%
of the time while NT embryos derived from non-synchronized
donors reached the blastocyst stage only 18~ of the time.
The NT data of this example demonstrate that
development is better when the donor nucleus and the
recipient oocyte are both in S-phase (or mid-stage) and
would both reach the next mitotic division at the same ~-
time.
Example 3
SYnchronization of NocodAzole-treated Bovine Embryos
and Cold-Culture-Activated ReciPient OocYtes
Matured for 24 Hours_In Vitro
Recipient oocytes were matured in vitro as described
above for 20-28 hours before activation. Fusion with
synchronized donor cells was performed approximately 6
hours post-activation. Oocytes that have matured in vitro
for 20-28 hours have a cell cycle time of approximately 24
hours. Therefore, these activated recipient oocy~es were
approximately 18 hours from next metaphase.
To demonstrate the benefit of donor-recipient
synchronization, bovine blastomere cells in two different
portions of S-phase were used as donors in fusions with
the above-described activated oocytes. Bovine embryo cells
2~2~8
~ -16-
were blocked in metaphase with 1.0 ~M Nocodazole and were
then allowed to proceed into the cell cycle ~or S or 10
hours post-removal of the metaphase block. At five hours,
the donor blastomeres had passed through G1-phase and were
s in early S-phass. The donor blastomeres were
~pproximately 15-18 hours away from next metaphase. By 10
hours, the blastomeres were in mid-S~phase and were about
10 hours away from next metaphase.
Fusions were performed as detailed above. Table 3
details the results of fusions with donor cells that were
either synchronized or unsynchronized with recipient
oocytes with respect to the next metaphase. It is
apparent from the data that when the time to next
metaphlse is similar, the percentage of NT embryos that
develop to the blastocyst stage is markedly higher.
TABLE 3
I ~_ r l
¦ Synchronized Synchronized
Oocyte~ Blastomeres Percentage of
_ - _ Blastocysts
Time ¦ Time Time Time ¦ Obtained
since ¦ until since until ¦ (Blastocysts~
last ¦ next last next ¦ Total NT
meta- ¦ meta~ meta- meta- ¦ Embryos)
L
`: