Note: Descriptions are shown in the official language in which they were submitted.
- 1 - 2~92837
C~t:~ MOSAIC ~Al;!MR12~NlZ AND PRODUCTION PROCEE~ ~R~OF
BACKGROUND OF THE INVENTION
1) Field of the Invention
This invention relates to a charge mosaic mem-
brane, and more specifically to a charge mosaic mem-
brane useful for the separation of an electrolyte o~
non-electrolyte or for desalination. This invention is
also concerned with a process for the production of the
charge mosaic membrane.
2) Description of the Related Art
Conventional charge mosaic membranes, in each of
which a cationic polymer and an anionic polymer are
alternately arranged, have the function that they can
dialyze a low-molecular electrolyte therethrough but
cannot dialyze a non-electrolyte. They are hence high-
ly promising candidates for the desalination or desalt-
ing of seawater or the like and a variety of research
has been conducted thereon.
Representative charge mosaic membranes include,
for example, those produced by combining block
copolymers A-C and B-C - which have been formed by
block-copolymerizing mutually-incompatible polymers A
and B with a third polymer C, respectively - at such a
ratio as permitting the formation of a lamellar or
- 2 - 2092 83 7
cylindrical structure, followed by the introduction of
anionic groups and cationic groups; and those obtained
by forming an anionic polymer and a cationic polymer
into a mosaicked, ultrathin, planar film on a liquid-
permeable support and then allowing the cationic and
anionic polymers to epitaxially grow as layers on the
same ionic polymers of the film, respectively.
Formation of a lamellar or cylindrical structure
by making use of phase separation between two types of
block copolymers, however, involves substantial dif-
ficulty from the technical viewpoint. Because of a
limitation imposed on the mixing ratio of both the
block copolymers and the anisotropy of both the struc-
tures, it is difficult to have the polymers of the dif-
ferent types stacked as alternate layers in a cross-
section of the resultant membrane and also to c- ~ni-
cate a front side of the membrane with a back side of
the membrane via layers of each cf the two types of the
block copolymers. Moreover, a structure formed by such
phase separation is anisotropic. This makes it dif-
ficult to form the lamallar or cylindrical structure
with controlled directionality.
Subse~uent to the formation of such a structure,
cationic and anionic groups have to be introduced.
This makes the production process complex. Further, a
2~92837
limitation is imposed on the quantities of these ionic
groups to be reacted.
According to the epitaxial growth, the layer of
the respective ionic polymers is allowed to grow on a
mosaic pattern. Very strict control is therefore re-
quired upon formation of the mosaicked, ultrathin film
and also upon formation of the respective polymer
layers of the cationic and anionic polymers. This con-
ventional process is therefore not suited for the pro-
duction of a membrane of a large area.
Whichever conventional process is used, the
resulting membrane is very thin and has low strength so
that it is impossible to form a relatively thick mem-
brane having high strength and excellent performance.
SUMMARY OF THE INVENTION
An object of the present invention is to provide
a charge mosaic membrane which is useful for the
separation of an electrolyte or non-electrolyte or for
desalination.
Another object of the present invention is to
provide a simpler process for the production of such a
charge mosaic membrane.
In one aspect of the present invention, there is
thus providPd a charge mosaic membrane made of a
2~2~37
- 4
cationic polymer and an anionîc polymer. At least one
of the cationic and anionic polymers is in the form of
spheres having a diameter of 0.01-10 ~m.
In another aspect of the present invention, there
is also provided a process for the production of the
above charge mosaic membrane. The process comprises
immobilizing one of the cationic and anionic polymers
on a liquid-permeable support, filling interstices be-
tween the spheres with a monomer adapted to form the
other polymer, and polymerizing the monomer. As an
alternative, the process comprises mixing spheres of
one of the cationic and anionic polymers with a 501u-
tion of a linear polymer as the other polymer, and
casting the resultant mixture into a film. In a fur-
ther alternative, the process comprises preparing dis-
persions of spheres of the cationic and anionic
pol~mers, respectively, mixing the dispersions togeth-
er, and casting the resultant mixture into a film~ In
a still further alternative, the process comprises
casting a core-shell polymer into a film, said core-
shell polymer being formed of spheres of one of the
cationic and anionic polymers and a linear polymer
chemically bound as the other polymer on surfaces of
the spheres, and causing shells and cores to rupture.
In a still further alternative, the process comprises
_ 5 _ 2 09283 7
preparing dispersions of spheres of the cationic and
anionic polymers, respectively, mixing the dispersions
together, casting the resultant mixture into a film,
and filling interstices between the spheres with one of
the cationic and anionic polymers or a monomer adapted
to form one of the cationic and anionic pol~ners. When
the monomer is used, the monomer is subsequently
polymerized.
Since at least one of the cationic polymer and
the anionic polymer in the charge mosaic membrane is in
the form of spheres having a diameter of 0.01-10 ~m,
the charge mosaic membrane has been improved inter alia
in the ability to selectively separate an electrolyte
or non-electrolyte and also in mechanical strength.
The production processes according to the present in-
vention can provide the charge mosaic membrane at low
cost.
Because of the use of spheres of at least one of
the cationic and anionic polymers upon production of
the charge mosaic membrane in the present invention,
bonding of the spheres in the resulting membrane takes
placa in an isotropic manner. This has led to a sig-
nificant improvement in the dialysis of an electrolyte
through the membrane so formed. Since at least one of
the cationic and anionic polymers, that is, the
- 6 - 2~ 7
membrane-forming materials is in the form of spheres,
it is no longer necessAry to consider the direc-
tionality of the polymer phase upon production of the
membrane. By the simple process, this invention can
therefore provide a charge mosaic membrane useful for
the separation of an electrolyte or non-electrolyte or
for desalination.
BRIEF DESCRIPTION OF THE DRAWINGS
The above and other objects, features and ad-
vantages of the present invention will become apparent
from the following description and the appended claims,
taken in conjunction with the accompanying drawings, in
which:
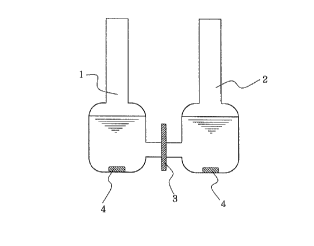
FIG. 1 is a schematic illustration of filtration
and separation of an electrolyte and a non-electrolyte
by a charge mosaic membrane according to the present
invention; and
FIG. 2 is a diagrammatic representation illustra-
ting the performance of a charge mosaic membrane, which
was obtained in Example 2, when employed for the
separation of an electrolyte and a non-el.~_trolyte.
DETAILED DESCRIPTION OF THE lNV~N'l'lON
AND PR~KKED EMBODIMENTS
_ 7 _ 2 092 8~ 7
The cationic polymer employed in the present in-
vention is preferably a polymer containing primary,
secondary or tertiary amino groups, ~uaternary ammonium
groups, or salts thereof. The anionic polymer is
preferably a polymer containing sulfonic groups, car-
boxylic groups or salts thereof. In the case of salt-
forming groups, anions such as ions of hydrochloric
acid, sulfuric acid, phosphoric acid or an organic acid
can be used for cationic groups whereas cations such as
alkali metal ions can be used for anionic groups.
Examples of the cationic polymer include poly-
vinylpyridine and quaternized products thereof; poly(2-
hydroxy-3-methacryloyloxypropyltrimethylammonium
chloride); poly(dimethylaminoethyl methacrylate),
poly(diethylaminoethyl methacrylate), and salts there-
of; copolymers of plural monomers forming the above-
ex~mplified polymers: and copolymers of monomers form-
ing the above-exemplified polymers with other monomers.
Illustrative of the anionic polymer include poly-
(2-acryloylamino-2-methyl-1-propanesulfonic acid),
poly(2-acryloylamino-2-propanesulfonic acid), poly-
methacryloyloxypropylsulfonic acid, polysulfopropyl
methacrylate, poly(2-sulfoethyl methacrylate), pol-
vinylsulfonic acid, polyacrylic acid, polystyrene-
maleic acid copolymèrs, and salts thereof; copolymers
2092837
-- 8
of plural monomers forming the above-exemplified
polymers; and copolymers of monomers forming the above-
exemplified polymers with other monomers.
To form such a polymer into spheres, various
known processes can be used including, for example, the
process in which spheres are caused to deposit from a
solution of the polymer as well as polymerization pro-
cesses such as soap free polymerization, emulsion
polymerization, suspension polymerization, reversed
phase polymerization and seed polymerization.
It may be preferred to crosslink the polymer
spheres in some instances. Exemplary crosslinking
agents usable upon crosslinking them include divinyl
benzene, methylenebisacrylamide, ethylene glycol
dimethacrylate and 1,3-butylene glycol dimethacrylate
as well as tri- or tetra-functional acrylates and
methacrylates. These crosslinking agents can each be
used in a proportion of 20 parts by weight or less,
preferably 0.5-10 parts by weight, both per 100 parts
of the monomer or monomers forming the polymer.
In the present invention, it is preferred to use
the crosslinked spheres in combination with un-
crosslinked spheres. The diameter of the spheres to be
employed should be 0.01-10 ~m, with 0.02-1 ~m being
preferred.
20~2837
The charge mosaic membrane according to the pres-
ent invention is formed using spheres of at least one
of the cationic and anionic polymers. To reinforce the
membrane to be formed, it is desired to use a suitable
liquid-permeable support. Preferred examples of such a
liquid-permeable support include porous bodies such as
woven fabrics, non-woven fabrics, porous resin sheets,
sintered porous ceramic bodies, and metal meshes.
These porous bodies may have a thickness in the range
of 0.01-500 ~m, preferably of 0.1-100 ~m.
Whichever process of the present invention is
used for the production of the charge mosaic membrane,
it is essential to use at least one of the anionic
polymer and the cationic ~olymer in the form of
spheres. It is however not absolutely necessary to use
the other polymer in the form of spheres.
The charge mosaic membrane of the present inven-
tion can be produced by one or a desired combination of
the following processes:
(1) After spheres of one of the cationic and
anionic polymers are immobilized on a liquid-permeable
support, a monomer of the other polymer is filled in
interstices between the spheres, followed by
polymerization.
(2) Spheres of one of the cationic and anionic
- lo - 2~92~37
polymers are mixed with a solution of a linear polymer
as the other polymer, and the resulting mixture is cast
into a film. Incidentally, the term "linear polymer"
as used herein is employed to distinguish the linear
polymer from spheres. A polymer may therefore contain
branch cha;n~ insofar as it is not in the form of
spheres.
(3) Dispersions of spheres of the cationic and
anionic polymers are prepared, respectively. These
dispersions are then mixed and cast into a film.
~4) A linear polymer as one of the cationic and
anionic polymers is chemically bound on surfaces of
spheres of the other polymer to form a core-shell
polymer. The core-shell polymer is then cast into a
film. Shells and cores are then caused to rupture so
that the cores are joined together.
(5) Dispersions of spheres of the cationic and
anionic polymers are prepared, respectively. The dis-
persions are mixed together and cast into a film. In-
terstices between the spheres are filled with one of
the cationic and anionic polymers or a monomer adapted
to form one of the cationic and anionic polymers. When
the monomer is used, the monomer is thereafter
polymerized.
As the two type~ of polymer spheres employed
~092837
above, it is preferable to combine crosslinked spheres
with uncrosslinked spheres. After they are mixed to-
gether and cast into a film, the polymer spheres in the
resulting film are caused to rupture or deform by a
solvent, pressure or the like. This ensures succes-
sional bonding of spheres of the same ionic polymers,
resulting in a film with improved me~hanical strength.
The present invention will hereinafter be de-
scribed more specifically by the fo]lowing examples.
Example 1
Charged in a flask were 500 me of water, 3 me of
4-vinylpyridine, 0.1 ml of divinylbenzene and 0.1 g of
2,2'-azobis(2-methyl-2-methylpropinoamidine) dihydro-
chloride. They were reacted at 80~C for 5 hours under
a nitrogen gas stream, whereby an emulsion-like reac-
tion mixture was obtained.
The emulsion-like reaction mixture was then added
with 3 g of sodium chloride, ~ollowed by stirring. The
resulting mixture was then subjected to pressure ~
tration through a Millipore~ filter (product of Mil-
lipore Corporation). A solid product so collected was
washed with water and then dried, so that poly(4-vinyl-
pyridine) was obtained in the form of spheres having a
diameter of about 300 nm.
One gram of the polyvinylpyridine obtained above
- 12 - 2092337
was dispersed at a concentration of 3 wt.% in methanol
to prepare a dispersion, in which 0.2 g of methyl
iodide and 0.2 g of chloromethylstyrene were added to
achieve quaternization and also to introduce polymeriz-
able groups. They were reacted at 30~C for 40 hours,
whereby the nitrogen atom in each pyridine ring was
quaternized.
Next, a solution of a polystyrene-polybutadiene-
polystyrene block copolymer (polystyrene content:
40 wt.%~ was coated on a glass plate and then dried to
form a film of about 100 ~m in thickness. A polyester-
made, non-woven fabric having a basis weight of 40 g/m2
and a thickness of 50 ~m was fusion-bond~d on the film
so that the non-woven fabric was filled. One side of
the non-woven fabric, said side having been unfilled,
was impregnated with the dispersion of the spherical
polymer obtained above. After the thus-impregnated
non-woven fabric was left over until it was dried to
certain extent, it was dried further at 60~C for 12
hours~
Through the unfilled side, the non-woven fabric
was impregnated further with a 10 wt.% aqueous solution
of poly(sodium styrenesulfonate). After dried at 60~C,
the non-woven fabric was washed with water and then
dried again. The whole structure was placed in water,
~0~2837
and the layer consisting of the non-woven fabric and
the membrane was peeled off from the glass plate and
the filling layer to obtain a charge mosaic membrane
according to this invention. The total amount of the
charged polymers in the membrane was 4.2 g/m2
The ability of the membrane to dialyze an elec-
trolyte and a non~electrolyte therethrough was measured
using the apparatus shown in FIG. 1. Placed in a ves-
sel 1 were a 3 wt.% aqueous solution of sodium chloride
as the electrolyte and a 0.01 mol/e aqueous solution
of acrylamide as the non-electrolyte, each in an amount
of 20 me. Purified water (40 me) was placed in a ves-
sel 2. The above membrane of the present invention was
arranged between those two vessels and the contents of
the vessels 1,2 were maintained at 25~C under stirring
for 3 days. The concentrations of the electrolyte and
non-eleckrolyte in each vessel were measursd. As a
result, 50 wt.% sodium chloride was found to have moved
between both the vessels so that sodium chloride
reached equilibrium at that concentration. on the
other hand, only about 0.2 wt.~ of acrylamide was found
to have moved. Those results indicate that the charge
mosaic membrane according to this invention has ex-
cellent property to selectively remove an electrolyte.
2092837
Example 2
~ixed were 9.7 parts of a water dispersion of
spheres of crosslinked poly(4-vinylpyridine) (solid
content: 2.15 wt.%, diameter: about 200 nm), 9.7 parts
of a water dispersion of spheres of uncrosslinked
poly(4-vinylpyridine) (solid content: 2.34 wt.%,
diameter: about 180 nm), an aqueous solution of an un-
crosslinked copolymer of sodium styrenesulfonate and
acrylamide at a molar ratio of 1:1 (solid content:
10 wt.%) and 7.4 parts of a 50 wt.% aqueous solution of
glutaraldehyde. The mixture so prepared was cast into
a film on a TEFLON~ support and dried in the air.
The membrane so formed was left over for one day
in a vaporized methanol atmosphere so that the spheres
of the uncrosslinked poly(4-vinylpyridine) were formed
into a film. The film so obtained was next left over
for 1 day in a desiccator which contained 35 wt.% of
hydrochloric acid. The film so treated was then washed
with water, dried in the air, and treated in a
diiodobutane/methanol atmosphere. ~he nitrogen atoms
of 4-vinylpyridine were then quaternized completely in
a vaporized methyl iodide/methanol atmosphere.
The charge mosaic membrane obtained as described
above remained in a film-like form even without the
liquid-permeable support. A dialysis test of the mem-
- 15 - 2~9~7
brane was conducted using a 0.05 mol/e aqueous solu-
tion of glucose and a 0.05 mol/e aqueous solution of
potassium chloride in the same apparatus as that
employed in Example 1. The thickness of the membrane
was about 150 ~m, and its dialytic performance is
diagrammatically illustrated in FIG. 2. As is clearly
envisaged from the results shown in FIG. 2, the mem-
brane according to the present invention showed good
performance for the separation o~ the electrolyte and
non-electrolyte from each other.
Incidentally, an extremely small amount of
glucose was also dialyzed in FIG. 2. A measurement er-
ror appears to be responsible for the error. Anyhow,
the electrolyte was e~ficiently dialyzed and substan-
tially no non-electrolyte was dialyzed, so that the
separation of the electrolyte and the non-electrolyte
was performed sufficiently.
The dialysis experiment shown in FIG. 2 was con-
ducted under atmospheric pressure so that a long time
was needed for the dialysis of the electrolyte. The
dialysis time can however be shortened significantly by
forming the membrane ~h; nn~r or applying a pressure
Example 3
A charge mosaic membrane was produced in a
similar manner to Example 2 except that a half of the
20~2g37
- 16 -
sodium styrenesulfonate in the uncrosslinked 1:1 (by
molar ratio) copolymer of sodium styrenesulfonate and
acrylamide in the aqueous solution was replaced by
crosslinked spheres of sodium styrenesulfonate-styrene-
S acrylamide-divinylbenzene (molar ratio: 50/30/10/10) in
an amount sufficient to make the total molar amount of
sodium styrenesulfonate equal to that of the acryl-
amide.
The thickness of the membrane was about 200 ~m
and its dialytic performance was similar to that of the
membrane of Example 2.
Example 4
A. Preparation of polyt4-vinylpYridine~ microgel
Ten parts of 4-vinylpyridine, 1 part of divinyl-
benzene t 0.2 part of 2,2'-azobis(2-methyl-2-methyl-
propinoamidine) dihydrochloride and 500 parts of water
were placed in a l-e flask, followed by polymeri~ation
at 70~C for 7 hours in a nitrogen atmosphere.
The polymerization mixture so obtained was in the
form of an emulsion-like liquid. The polymerization
product was purified by dialysis. As an alternative,
the polymerization product can be purified by first
subjecting the polymerization mixture to pressure fil-
tration and then washing with water the polymerization
product so collected. After drying, the polymerization
2o~2837
- 17 -
product was successfully redispersed in water and
methanol, respectively. The particle size of the
polymer spheres so obtained was about 150 nm when
dried, about 200 nm when redispersed in water, and
about 500 nm when redispersed in methanol.
B. Pre~aration of linear poly(sodium sulfonate)
Sodium styrene sulfonate (12 parts), 4 parts of
acrylamide, 0.5 part of 2,2'-azobis(2-methyl-2-methyl-
propinoamidine) dihydrochloride, 0.8 part of diallyl
malonate, 1 part of crotonaldehyde and 100 parts of
water were placed in a flask and the reacted at 70~C
for 10 hours in a nitrogen atmosphere. The resulting
polymer was purified by reprecipitation in acetone-
water and then dried at room temperature. The
molecular weight of the polymer so obtained was about
44,000 (gel permeation chromatography; GPC). The
polymer contained an amidino group at one end.
C. Preparation of core-shell ~olymer
one part of the linear poly(sodium sulfonate)
prepared above under B. was dissolved in 10 parts of
water, in which 0.5 parts of sodium bicarbonate were
dissolved. The solution so prepared was stirred for 5
hours. One part of epibromohydrin was added to the
solution, followed by a reaction at 45~C for 10 hours.
The reaction mixture was purified by dialysis.
-
2~92837
- 18 -
Bromine in the reaction product, i.e., the
polymer so obtained was ionized with an alkali and
quantitated. The molecular weight of the polymer was
about 40,000 when one bromine atom was bonded to one
end of the polymer. This finding is substantially con-
sistent with the results by the GPC analysis. An
aqueous solution of the polymer was prepared at a con-
centration of 5 wt.% in terms of solid. Forty parts of
the solution were taken and then mixed with 10 parts of
the 10 wt.~ methanol solution of the poly(4-vinyl-
pyridine) microgel described above under A. The
resulting mixture was reacted at room temperature for
24 hours, and the reaction product was collected by
filtration, washed with water and then dried under
reduced pressure.
By quantitation of the aldehyde and observation
by a transmission electron microscope (TEM), the linear
polymer B was found to exist only on surfaces of
spheres of the poly(4-vinylpyridine) and one backbone
~o of the linear polymer B was found to exist per 10
pyridine units. If desired, the nitrogen atom of each
pyridine ring can be completely quaternized with methyl
iodide or the like.
In place of the poly(4-vinylpyridine) spheres in
Example 2, the core-shell polymer obtained as described
~0~2837
-- 19 --
above was used. The core-shell polymer was mixed with
an aqueous solution of the linear poly~sodium sul-
fonate) in an amount corresponding to sulfonic acid
which was equivalent to the poly(4-vinylpyridine) in
the core-shell polymer. The resulting mixture was cast
into a film and, after having been treated as in Exam-
ple 2, the resulting membrane was spread over a porous
stainless steel support and press-bonded under a pres-
sure of 200 kg/cm2. The charge mosaic membrane ob-
tained as described above showed still better desalting
effect when an aqueous solution of salt, as a solution
to be dialyzed, was pressurized.