Note: Descriptions are shown in the official language in which they were submitted.
2092953
1
This invention relates to a process for the
enzymatic hydrolysis of proteins and to an apparatus for
carrying out this process.
There are various known processes for the enzy-
matic hydrolysis of proteins which differ from one
another, for example, in the choice of the substrate,
the enzyme, the degree of hydrolysis and/or the required
peptide profile. In cases where, for example, a rela-
tively well-defined peptide profile, more particularly
a narrow oligopeptide profile, is required for reasons
of assimilation of the hydrolyzate by the intestinal
mucosa, known hydrolysis processes generally comprise at
least one hydrolyzate filtration or screening step.
For example, EP 226 221 describes a process for
the preparation of hypoallergenic peptides having a
molecular weight in the range from 2000 to 6000 by one
or more enzymatic protein hydrolysis steps each carried
out discontinuously in a fermentation tank and each
terminating in an ultrafiltration step.
US 4,212,889 describes a process for solubilizing
fish proteins, in which a mixture of fish flesh and
enzyme is continuously passed through an installation
comprising several hydrolysis tanks connected in series.
The problem addressed by the present invention
was to provide a hydrolysis process which, preferably
carried out continuously, would enable the efficiency of
discontinuous hydrolysis in a tank to be equalled or
even increased and which would enable a protein hydroly-
zate having a well-defined and reproducible degree of
hydrolysis and/or peptide spectrum to be obtained.
To this end, the process according to the inven-
tion for the: enzymatic hydrolysis of proteins, in which
a protein substrate is subj ected to hydrolysis with a
proteolytic enzyme, comprises a first enzymatic hydroly-
2092953
2
sis step i:n a stirred tank and a second enzymatic
hydrolysis :step in a tube. In a preferred embodiment,
the process according to the invention is carried out
continuously and the second enzymatic hydrolysis step is
carried out in a tube equipped with static mixing
elements.
Simi7.arly, the apparatus for carrying out the
process according to the invention comprises a double-
jacketed stirred hydrolysis tank which is connected
upstream to a substrate metering unit and to an enzyme
metering unit and downstream to at least one hydrolysis
tube. In a preferred embodiment, the tube is equipped
with static mixing elements.
It has been found that it is thus possible to
produce a protein hydrolyzate having a well-defined
degree of hydrolysis and/or peptide spectrum in a highly
efficient and reproducible, preferably continuous
manner.
By virtue of the process and apparatus according
to the invention, it is possible in particular to work
with a tank of relatively small dimensions which may be
filled completely without leaving any head space and
with a tube of relatively large dimensions. It is thus
possible to carry out, preferably continuously, a first
relatively ;short step, i.e. a hydrolysis initiation
step, in a relatively small tank, and a second relative-
ly long step, i . a . a hydrolysis completion step, in a
tube of relatively large volume. The reaction time,
i. e. the re:~idence time of the substrate in the total
volume represented by the sum of the tank volume and the
tube volume, can thus be controlled in a precise and
simple manner, for example by means of volumetric pumps.
If, for comparison, it is desired to carry out a
hydrolysis in a single hydrolysis tank of large dimen
sions, the residence time of a unit volume of hydroly
2092953
3
zate cannot be precisely defined. This is true of
discontinuous hydrolysis where the times required to
establish given pH and/or temperature conditions and
even to empty the tank, for example, are considerable.
However, the same is even truer of continuous hydro-
lysis, in which case is it only possible to define a
mean residence time. Even in a process of the type
described in the above-cited US 4,212,889, the residence
time can scarcely be defined any more precisely.
By contrast, it has been found that, with the
process and apparatus according to the present inven-
tion, the residence time of a unit volume of hydrolyzate
can be defined in a remarkably precise manner, the flow
of hydrolyzate through the hydrolysis tube - preferably
equipped with static mixing elements - having a very
flat front.
In t:he present specification, the degree of
hydrolysis is defined via the quantity of non-protein
nitrogen (NPN) determined as the percentage of total
nitrogen which cannot be precipitated with 13% trichlo-
roacetic aced.
The nitrogen contents are determined by the
Kjeldahl mei:hod.
The amine nitrogen (free a-NHZ) contents are
determined by reaction with ninhydrin after alkaline
hydrolysis.
The serotonin relaxation tests using tritium-
labelled exogenous serotonin (serotonin-3H) are carried
out on normal mastocytes of the peritoneal cavity of
rats by thEa method described by R. Fritsche and M.
Bonzon in Int. Arch. Allerg. Immunol. 93, 289-293
(1990).
The ELISA inhibition tests are carried out with
rabbit antibodies specific of f3-lactoglobulin (BLG),
bovine serum albumin (BSA) and casein (CAS). The sensi
,.~. 4 2092953
tivity of the method, i.e. the concentration detection
limit, is 20 ng/ml.
The high performance liquid chromatography
analyses (HPLC tests, peptide profiles) are carried out
under non-denaturing conditions on gel based on type
TSK-62000-SWT"' silica (a product of Toyo Soda), of which
the fractionation range extends from 500 to 50,000
dalton, in a Biorad BIOSIL SEC-125T"~ column. The results
are expre:~sed in % surface distribution of the peaks
read at 2:20 nm in a 0.1 M phosphate solution + 0.4 M
NaCl at pH 6.80.
The: analyses by zone electrophoresis in poly
acrylamide~ gel (SDS-PAGE tests) are carried out by the
method described by Laemmli in Nature 227, 680 et seq.
(1970) .
The blockage of lysine is determined by HPLC and
is expressed as % blocked lysine relative to the total
lysine of the hydrolyzate.
"Static mixing elements" are understood to be
undulating crossmembers or strips of metal or plastic
which intersect or which are interlocked in one another
and which divide the space defined by the tube into a
plurality of intersecting passages progressing along the
axis of the tube. Elements of the type in question are
marketed by Sulzer A.G. of CH-8401 Winterthur, for
example under the trade-marks SMV, SMX or SMXL.
Finally, it is important to appreciate that the
tubes provided with static mixing elements are sys
tematically equipped with a double jacket even when this
is not specifically ment-Toned.
The process according to the invention may be
carried out. using any starting material rich in proteins
as the prot=ein substrate, such as flours or semolina of
oil seeds or oil seed cakes, food-quality yeasts or
bacteria, minced animal or fish flesh or milk or milk
derivative:,
2092953
for example in the form of particles in aqueous suspen-
sion or aqueous suspensions.
The protein substrate is preferably a whey
substrate containing whey proteins, more particularly a
5 sweet whey i°rom cheese production or an acidic whey from
casein production either as such or in demineralized or
lactose-free, liquid or reconstituted form.
In another preferred embodiment, the enzyme is
selected from the group consisting of trypsin, chymo
trypsin, pancreatin, bacterial proteases, fungal prote
ases and mixtures thereof.
The proteolytic enzyme and the substrate may be
mixed in a quantity of enzyme having an activity of 0.1
to 12 Anson units (AU) per 100 g substrate dry matter.
The first hydrolysis step is preferably carried
out over a period of 10 to 60 minutes at a pH and a
temperature adjusted to values favourable to the acti-
vity of the enzyme while the second hydrolysis step is
preferably carried out over a period of 1 to 8 h at a
temperature equal to or above, particularly 0 to 10°C
above, the 'temperature of the first step.
Intermediate or complementary steps may be
included, more particularly a preliminary mixing step
preferably carried out in a tube equipped with static
mixing elements; a thermal denaturing step before, in
the middle of or after the first hydrolysis step,
particularly using a heat exchanger or a tube equipped
with static mixing elements; one or more enzyme inacti-
vation steps, particularly after the second hydrolysis
step, more especially using a heat exchanger and/or a
steam injector and/or a tube equipped with static mixing
elements; a:nd/or a cooling step carried out in particu-
lar after a denaturing step, more particularly using a
heat exchanger or preferably a tube equipped with static
mixing elements for example.
2092953
6
The enzyme may be inactivated in one or preferab-
ly two steps, a first step corresponding more precisely
to autodigestion of the enzyme and a second step corre-
sponding more precisely to sterilization.
The first hydrolysis step in a tank may also be
divided into at least two parts carried out in at least
two tanks connected in series. Similarly, the second
hydrolysis step carried out in a tube may be divided
into at least two parts carried out in at least two
tubes connected in series. In the latter case, a pH
adjustment and/or an addition of enzyme may be carried
out between two successive tubes.
For the pH adjustment(s), it is preferred to use
a suitable reactant which may either be alkaline, such
as KOH, NaOH or Ca(OH)z, or acidic, such as HC1 or HP04
for example.
In one preferred embodiment of the process
according to the invention, the enzyme is a bacterial
alkaline protease, more particularly that produced by
Bacillus licheniformis and marketed by the Novo company
under the trade-mark of "Alcalase", more particularly
"Alcalase ~).6 L" or "Alcalase 2.4 L" for example.
It has been found that, with this preferred
embodiment, it was possible to obtain a hydrolysate
having a p<~rticularly high NPN and particularly reduced
al lergenic:ity .
To this end, the first hydrolysis step is carried
out at a p~H value of 7.0 to 10.0 and at 50 to 80°C and
preferably at 63 to 73°C while the second hydrolysis
step is carried out at a pH value of 6.5 to 8.0 and at
55 to 80°C' and preferably at 65 to 73°C. A thermal
denaturing step may be carried out either after the
first hydrolysis step or between the two hydrolysis
steps over a period of 30 s to 10 rains. and preferably
over a period of 4 to 6 rains. at a temperature of 80 to
n
2092953
7
120°C and preferably at a temperature of 85 to 95°C.
The enzyme may then be inactivated by an autodigestion
step carried out over a period of 10 s to 20 mins. and
preferably over a period of 2 to 8 mins. at 70 to 110°C
and preferably at 85 to 90°C, followed by a steriliza-
tion step ~~arried out over a period 5 s to 5 mins. and
preferably over a period of 30 s to 2 mins. at 110 to
150°C and preferably at 120 to 130°C.
In another preferred embodiment of the process
according to the invention, the enzyme used is a com
bination of, on the one hand, a bacterial alkaline
protease, more particularly that produced by Bacillus
licheniformis and marketed by the Novo company under the
name of "Alcalase", more particularly "Alcalase 0.6 L"
or "Alcala:~e 2.4 L", and on the other hand a pancreatic
enzyme, more particularly trypsin for example.
In this other preferred embodiment, two sub-
strates, more particularly two whey substrates, may each
be separately subjected to a separate hydrolysis with
one of the.>e two enzymes by the process according to the
invention up to a common step, preferably up to a common
sterilization step following two separate autodigestion
steps of the two different enzymes. The same substrate
may also be successively subjected to the action of one
and then 'the other of these two enzymes. This is
because it has been found that a hydrolysis product of
whey proteins, for example, obtained by this combination
can show particularly good stability in storage.
To carry out the process according to the inven
tion with a pancreatic enzyme, particularly trypsin for
example, above all in the above-mentioned combination,
the pH and temperature conditions described in EP 322
589 may be used with advantage.
The: apparatus for carrying out the process
s~
2092953
8
according t:o the invention thus comprises a double-
jacketed stirred hydrolysis tank which is connected
upstream to a substrate metering unit and to an enzyme
metering unit and downstream to at least one hydrolysis
tube equipped with static mixing elements.
In this apparatus, the tube may be vertically
arranged, its lower end being connected to the tank and
its upper e:nd opening into an outlet pipe. It may also
be arranged horizontally or in any other position. In
a preferred embodiment, it has a length of greater than
4 times its diameter.
In another preferred embodiment, the substrate
and enzyme metering units each comprise a feed vessel
connected to the hydrolysis tank by a volumetric pump.
The apparatus may also comprise a reactant
metering unit comprising a feed vessel connected to the
hydrolysis 'tank by a volumetric pump controlled by a pH
meter.
The apparatus may also comprise several tanks
connected in series instead of a single tank, more
particularly two tanks of which one may be used as a
prehydrolysis tank.
The apparatus may also comprise several hydroly
sis tubes equipped with static mixing elements connected
in series downstream of the tank by connecting pipes
which may be connected upstream to the enzyme metering
unit and to the reactant metering unit.
A tube equipped with static mixing elements may
also be provided between the enzyme, substrate and/or
3 0 reactant metering units and the tank or even between two
successive tanks where the apparatus comprises several
tanks.
The apparatus for carrying out the process
according to the invention is described in more detail
hereinafter with reference to the accompanying drawings
2092953
9
which illustrate three examples of embodiment and in
which:
Figure 1 diagrammatically illustrates a first
embodiment of the apparatus comprising a tank and a tube
equipped with static mixing elements.
Figure 2 diagrammatically illustrates a second
embodiment of the apparatus comprising a tank and
several hydrolysis tubes equipped with static mixing
elements.
Figure 3 diagrammatically illustrates a third
embodiment of the apparatus comprising two tanks and
several hydrolysis tubes equipped with static mixing
elements.
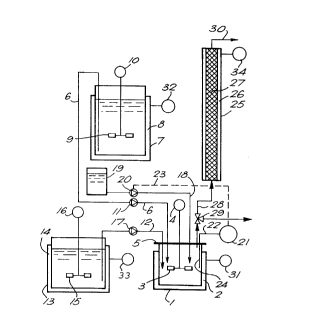
Referring to Fig. 1, the present apparatus com
prises a hydrolysis tank 1 with a double jacket 2 and a
stirrer 3 driven by a motor 4. The tank is closed in
fluid-tight manner by a cover 5 through pass various
pipes and the shaft of the stirrer 3.
The hydrolysis tank 1 is connected upstream by a
pipe 6 to a substrate metering unit 7-11, by a pipe 12
to an enzyme metering unit 13-17 and by a pipe 18 to a
reactant mei~ering unit 19-24.
The substrate metering unit comprises a substrate
feed vessel 7 with a double jacket 8 and a stirrer 9
driven by a motor 10. The vessel 7 is connected to the
hydrolysis tank 1 by the volumetric pump 11 connected to
the pipe 6.
The enzyme metering unit comprises an enzyme feed
vessel 13 with a double jacket 14 and a stirrer 15
driven by a motor 16. The vessel 13 is connected to the
hydrolysis tank 1 by the volumetric pump 17 connected to
the pipe 12..
The :reactant metering unit comprises a reactant
feed vessel 19 connected to the hydrolysis tank 1 by a
volumetric pump 20 connected to the pipe 18. The
2092953
volumetric pump 18 is controlled by a pH meter 21 of
which the measuring electrode 24 dips into the tank 1
through the cover 5 and which is electrically connected
(chain line 23) to an electronic device for controlling
5 the pump 20 (not shown).
The lhydrolysis tank 1 is connected downstream to
a hydrolysis tube 25 with a double j acket 26 equipped
with static mixing elements 27 consisting of metal or
plastic crosspieces interlocked in one another. The
10 tank 1 is connected to the tube 25 by a pipe to which is
connected a three-way valve 29 designed to enable
samples of :hydrolyzate to be removed from the tank.
The 'tube 25 is vertically arranged, its lower end
being connected to the tank 1 and its upper end opening
into an outlet pipe 30.
The temperature of a fluid circulating in each of
the double jackets is regulated by a device shown
symbolically at 31 for the tank 1, at 32 for the vessel
7, at 33 for the vessel 13 and at 34 for the tube 25.
In F:ig. 2, the elements of this second embodiment
of the apparatus which correspond to the elements of the
first embodiment shown in Fig. 1 are denoted by the same
reference numerals.
In this second embodiment, the apparatus com
prises several hydrolysis tubes 25, 35, 36 equipped with
static mix~.ng elements 27, 37, 38 and connected in
series downstream of the tank 1 by connecting pipes 39,
40 connected upstream to the enzyme feed vessel 13 by
pipes 41, ~42 which rejoin the pipe 12 to which the
volumetric ;pump 17 is connected.
The connecting pipes 39, 40 are also connected
upstream to the reactant feed vessel 19 by pipes 43, 44
which rejoin the pipe 18 to which the volumetric pump 20
is connected.
In 'this second embodiment of the apparatus
2092953
11
according to the invention, a mixing tube 45 equipped
with static mixing elements is again provided between
the enzyme, substrate and reactant metering units and
the tank 1.
The various enzyme, substrate and reactant feed
vessels are connected to the tube 45 by pipes 46, 47 and
48 to which a volumetric pump 49 and the volumetric
pumps 11 and 20 are respectively connected.
In Fig. 3, the elements of this third embodiment
of the apparatus which correspond to the elements of the
first two embodiments shown in Figs. 1 and 2 are again
denoted by 'the same reference numerals.
In this third embodiment, the apparatus comprises
several hydrolysis tubes 25, 35, 36, 50 equipped with
static mixing elements and connected in series down
stream of the hydrolysis tank 1. The hydrolysis tank is
connected upstream to a second tank, in the present case
a prehydrol:ysis tank 51, by a denaturing tube 52 equip-
ped with static mixing elements.
In this third embodiment, it is the prehydrolysis
tank 51 which is connected upstream to the substrate and
reactant feed vessels 7 and 19 while the enzyme feed
vessel 13 is connected downstream by the pipes 56, 12
and 41, respectively, to the prehydrolysis tank 51, the
hydrolysis tank 1 and the pipe 39 connecting the hydro-
lysis tubes 25 and 35.
In this embodiment, inactivation tubes 53 , 54 and
a cooling tube 55 equipped with static mixing elements
and connected in series downstream of the last hydroly-
sis tube 50 are again provided.
The process according to the present invention is
illustrated by the following Examples in which parts and
percentages are by weight.
2092953
12
Example 1
The process according to the invention is carried
out in an apparatus similar to that described with
reference t~o Fig. 1, in which the hydrolysis tank has a
volume of 30 1 and the hydrolysis tube equipped with
static mixing elements has a volume of 180 1 for a
height of 3 m.
The :substrate used is a partly demineralized whey
protein concentrate having a dry matter content of 20%
and respective contents (in % based on dry matter) of
approximately 23~ proteins, 1.9% fats, 73% lactose and
1.3~ ash.
Porcine trypsin having an activity of 3 AU/g is
used as the enzyme in a quantity of 1 g enzyme to 100 g
substrate dry matter, i.e. 3 AU to 100 g substrate dry
matter.
2N K~OH is used as reactant.
The tank is first filled with substrate and,
after mixing in the enzyme, the discontinuous hydrolysis
process is initiated at pH 7.3/60°C for 15 minutes,
after which the hydrolyzed substrate has an NPN of 40~.
The process is then resumed continuously at such
a rate that the mean residence time of the substrate in
the tank is. 30 minutes and the residence time of the
hydrolyzate in the tube is 3 h. A temperature of 60°C
and a pH of 7.3 are maintained in the tank. A tempera
ture of 60°C is maintained in the tube, the pH being
allowed to float so that it falls spontaneously from
approximately 7.3 at the tube entrance to approximately
6.9 at the 'tube exit.
The lzydrolyzate has an NPN of 65% on leaving the
tube.
If, :Eor comparison, the same substrate is hydro-
lyzed discontinuously with the same enzyme in the same
enzyme-to-substrate ratio for approximately 7 h at pH
2092953
13
7.3/60°C in a 200 litre tank, a hydrolyzate having an
NPN of 60% is obtained.
Example 2
The procedure is the same as described in Example
1 except for the fact that, during three separate tests,
the useful 'volume of the tank is varied so that the NPN
obtained after the passage of the substrate through the
tank is 15, 35 and 45%, respectively.
Hydrolyzates having respective NPN's of 59, 63
and 66~ are thus obtained at the tube exit.
For comparison, an NPN of 60~ is obtained in
approximately 7 h under the same conditions discontin-
uously in a tank, i.e. at pH 7.3/60°C with a substrate
having a dry matter content of 20% and a quantity of
enzyme having an activity of 3 AU per 100 g substrate
dry matter.
In other words, it is possible by the present
process continuously to obtain an NPN higher than that
which would be discontinuously obtained if the substrate
had an NPN above 35% at the tube exit.
Example 3
The ;procedure is the same as described in Example
1 except that a pH value of 7.8 as opposed to 7.3 and a
temperature of 55°C as opposed to 60°C are maintained in
the tank.
A hydrolyzate having an NPN of 70% is obtained at
the tube exit.
Example 4
The ;process according to the invention is carried
out using an apparatus similar to that described with
reference to Fig. 2.
A whey protein concentrate having a dry matter
2092953
14
content of :33~, including 7.5% proteins, is used as the
substrate.
The enzyme used is a bacterial alkaline protease
produced by Bacillus licheniformis and marketed by the
Novo company under the name of "Alcalase 2.4 L" which
has an activity of 2.4 AU/g. This enzyme is used in a
total quantity of 4 to 8.6% based on protein, i.e. 2.2
to 4.7 AU per 100 g substrate dry matter.
2N Kc~H is used as the reactant.
After the process has been suitably initiated, it
is resumed continuously. The throughput of substrate
and the dimensions of the tube and the tank are deter-
mined in such a way that the residence times of the
substrate or the hydrolyzate are, respectively, 5 to 10
minutes in a preliminary mixing tube equipped with
static mixing elements preceding the tank, 5 to 8
minutes in a thermal denaturing tube equipped with
static mixing elements connected in series between the
preliminary mixing tube and the tank, 25 to 40 minutes
in the tank (first hydrolysis step), 15 to 25 minutes in
a first tube A equipped with static mixing elements
following the tank (tube A of the second hydrolysis
step), 15 to 25 minutes in a second tube B equipped with
static mixing elements (tube B of the second hydrolysis
step), 0 to 100 minutes in a third tube C equipped with
static mixing elements (tube C of the second hydrolysis
step), 5 to 20 minutes in an inactivation tube equipped
with static mixing elements connected in series to the
remainder o:E the tube C and 5 to 15 minutes in a cooling
tube equipped with static mixing elements.
The total quantity of enzyme is divided into 4
parts, namely a first part of 5 to 15% of the total
mixed with t:he substrate in the preliminary mixing tube,
a second part of 30 to 40% of the total mixed with the
substrate in the tank, a third part of 20 to 30~ of the
2092953
total mixed with the substrate in the tube A and a
fourth part of 20 to 30% of the total mixed with the
substrate i:n the tube B.
The ;pH value of the substrate is adjusted to 7.3
5 as far as the tube B from which the pH is left to float.
The temperature is adjusted to 75°C in the
preliminary mixing tube, to 85°C in the thermal denatur
ing tube, to 70°C in the tank, to 71°C in the tube A and
the tube B, to 80-105°C in the activation tube and to 2
10 8°C in the cooling tube.
The :hydrolyzate thus produced is collected after
the cooling tube.
Example 5
15 The present process is carried out using an
apparatus of the type similar to that described with
reference to Fig. 3.
A whey protein concentrate having a dry matter
content of 28%, including 7% proteins, is used as the
substrate.
Alcalase 2.4 L is used as the enzyme in a total
quantity of 2 to 6% based on protein, i.e. 1.2 to 3.6 AU
per 100 g substrate dry matter.
2N K~~H is used as the reactant.
After the process has been suitably initiated, it
is resumed continuously. The throughput of substrate
and the dimensions of the tubes and the tanks are
determined in such a way that the successive steps take
place as follows.
In a preliminary mixing tube equipped with static
mixing elements preceding a prehydrolysis tank, 33% of
the total quantity of enzyme is mixed with the substrate
at pH 8.7/10°C.
A first phase of the first hydrolysis step is
carried out in the prehydrolysis tank for 15 minutes at
2092953
16
65°C.
In a thermal denaturing tube equipped with static
mixing elements connected in series between the pre-
hydrolysis tank and a hydrolysis tank, the temperature
is increased to 92°C for 5 minutes followed by cooling
to 65°C.
In t:he hydrolysis tank, the remaining 66% of the
total quantity of enzyme is added and a second phase of
the first hydrolysis step is carried out over a period
of 45 minutes at pH 7.4/65°C.
In three tubes equipped with static mixing
elements and connected in series downstream of the tank,
the second :hydrolysis step is carried out over a period
of 195 minutes at 65°C, i.e. for 65 minutes in each
tube. The pH is adjusted to 7.5 at the entrance of each
tube and then floats.
In an inactivation tube equipped with static
mixing elements connected in series after the three
hydrolysis tubes, the enzyme is autodigested for 5
minutes at 87°C.
In a steam injection heating unit connected in
series after the inactivation tube, the hydrolyzate is
sterilized for 1 minute at 125°C.
The :hydrolyzate is then collected after cooling.
Example 6
The present process is carried out using an
apparatus similar to that described with reference to
Fig. 1, in which the hydrolysis tank has a volume of 2.8
1 and the hydrolysis tube equipped with static mixing
elements ha;s a volume of 11.6 1 for a length of approxi-
mately 5 m.
A whey protein concentrate having a dry matter
content of 33%, including 7.5% proteins, is used.
Alcalase 2.4 L is used as the enzyme in a total
2092953
17
quantity of 6.3% based on protein, i.e. 3.4 AU per 100
g substrate dry matter.
2N KOH is used as reactant.
The tank is first filled with substrate and,
after mixing in the enzyme, the discontinuous hydrolysis
process is initiated for 25 minutes at pH 7.3/70°C.
The process is then resumed continuously at such
a rate that the total residence time of the hydrolyzate
in the apparatus is 240 mins. (47 mins. in the tank and
193 mins. in the tube). A temperature of 70°C and a pH
of 7.3 are maintained in the tank. A temperature of
70°C is maintained in the tube, the pH being allowed to
float so that it falls spontaneously from approximately
7.3 at the tube entrance to approximately 6.72 at the
tube exit.
Samples are taken for analysis at the tube exit
at times of 0, 60, 120 and 180 minutes counting from 240
minutes afi~er the start of the continuous process.
These samples have the pH values and the amine nitrogen
contents shown in Table I below where the corresponding
quantity of KOH used to keep the pH at 7.3 in the tank
is also shown.
Table I
Time pH Amine nitrogen KOH
(mins.) (%) (g/h)
0 6.71 0.26 124
60 6.72 0.25 123
120 6.73 0.26 125
180 6.71 0.26 125
The quantities of KOH indicated in g/h correspond
to an average consumption of 44.375 g per 1 of the tank
for a residence time of 47 minutes.
2092953
18
It can be seen from Table I that the characteris-
tics of the hydrolyzate hardly vary irrespective of the
time the samples are taken for analysis from the tube
exit. It i:: also possible to verify by zone electropho-
resis in polyacrylamide gel (SDS-PAGE method) that the
advantageous peptide profile of these samples, mostly
small peptides, also remains remarkably constant.
For comparison, the same substrate is subjected
to enzymatic: hydrolysis with the same enzyme in the same
enzyme-to-substrate ratio discontinuously for 47 minutes
in a 2 litre tank at 70°C and at a pH kept at 7.3.
After these first 47 minutes, the pH is left to float.
Samples are taken for analysis after 47 minutes counting
from the bs~ginning of hydrolysis and then at various
times up to and beyond 240 minutes. These samples have
the pH value's and amine nitrogen contents shown in Table
II below.
Table II
Time pH Amine nitrogen
(mins.) (%)
47 7.30 0.21
67 7.0 0.22
140 6.77 0.24
197 6.75 0.27
240 6.72 0.26
300 6.68
360 6.65
The quantity of KOH used to keep the pH at 7.3
during the first 47 minutes is 45.5 g per litre of the
tank.
It can be seen from Table II that the charac-
2092953
19
teristics o7. the hydrolyzate obtained discontinuously in
a tank vary rapidly, again after the time of 240 minutes
corresponding to the continuous residence time in the
apparatus used in Example 6.
This demonstrates one of the advantages of the
process according to the invention in which there is no
danger of development of the product comparable with
that occurring during the time required to empty the
tank in a discontinuous process.
Example 7
The present process is carried out using an
apparatus similar to that described with reference to
Fig. 1, in 'which the hydrolysis tank has a volume of 5
1 and the hydrolysis tube equipped with static mixing
elements ha:~ a volume of 9.6 1 for a length of approxi-
mately 5 m.
A whey protein concentrate having a dry matter
content of :33%, including 7.5% proteins, is used as the
substrate.
Alca:lase 2.4 L is used as the enzyme in a total
quantity of 8% based on protein, i.e. 4.4 AU per 100 g
substrate d:ry matter. Of these 8%, 2% are used in the
tank and 6% are added at the tube entrance.
2N KOH is used as reactant.
In two separate tests, the tank is first filled
with substrate and, after mixing in the enzyme, the
discontinuous hydrolysis process is started at pH 7.3
and at two different temperatures of 72.5°C and 74°C for
40 minutes.
Each process is then resumed continuously at such
a rate that the total residence time of the hydrolyzate
in the apparatus is 116 minutes (40 minutes in the tank
and 76 minutes in the tube). Respective temperatures of
72.5°C and '74°C and a pH of 7.3 are maintained in the
2092953
tank for each of the two tests. A temperature of 72°C
is maintained in the tube, the pH being allowed to
float.
The hydrolyzates thus obtained corresponding to
5 the temperatures of 72.5°C and 74°C in the tank have
respective NPN's of 97.2% and 91.4% and necessitated the
use of respective quantities of 205 g/h and 198 g/h KOH
to keep the pH at 7.3 in the tank. In addition, zone
electrophoresis in polyacrylamide gel (SDS-PAGE method)
10 shows that they have a relatively narrow peptide pro-
file.
For comparison, the same substrate is subjected
to enzymati~~ hydrolysis with Alcalase 2.4 L in a total
quantity of 4% based on protein, i.e. 2.2 AU per 100 g
15 substrate dry matter, continuously for 200 minutes in a
5 liter tan)H; at 70°C and at respective pH values of 6.4,
6.8, 7.3 and 7.8 in four separate tests.
The lhydrolyzates thus obtained have NPN's of 80
to 83% and necessitated the use of respective quantities
20 of KOH (in g/h) of 33.4, 50.1, 60.2 and 77.2 to keep
their pH values at 6.4, 6.8, 7.3 and 7.8. In addition,
they have respective amine nitrogen contents of 0.17,
0.20, 0.21 and 0.22%. The SDS-PAGE test shows that they
have a relai~ive broad peptide profile.
This demonstrates another advantage of the
process according to the invention insofar as it is
possible to obtain a product having a high degree of
hydrolysis .and a relatively narrow peptide profile by
comparison with a product obtained by continuous enzy-
matic hydro:Lysis in a tank which has a lower degree of
hydrolysis <~nd a relatively broad peptide profile.
Example 8
The present process is carried out using an
apparatus similar to that described with reference to
-- 2092953
21
Fig. 1, in which the hydrolysis tank has a volume of 2.8
1 and the hydrolysis tube equipped with static mixing
elements has a volume of 11.6 1 for a length of ap-
proximately 5 m.
A whey protein concentrate having a dry matter
content of :33%, including 7.5% proteins, is used as the
substrate.
Alca:Lase 2.4 L is used as the enzyme in a total
quantity of 7% based on protein, i.e. 3.8 AU per 100 g
substrate d:ry matter. Of these 7%, 2% are used in the
tank and 5% are added at the tube entrance.
2N K()H is used as the reactant.
The tank is first filled with substrate and,
after mixing in the enzyme, the discontinuous hydrolysis
process is :started at pH 7.8/70°C for 25 minutes.
The process is then resumed continuously at such
a rate that the residence time of the hydrolyzate is 45
minutes in 'the tank and 170 minutes in the hydrolysis
tube, i.e. a total of 215 minutes. A temperature of
70°C and a pH of 7.8 are maintained in the tank. A
temperature of 70 ° C is maintained in the tube, the pH
being allowed to float so that it falls spontaneously
from approximately 7.8 at the tube entrance to approxi-
mately 6.67 at the tube exit.
In an inactivation tube equipped with static
mixing elements and connected in series with the exit of
the hydroly~;is tube, the hydrolyzate is inactivated for
18 minutes at 90°C. In a cooling tube equipped with
static mixing elements connected in series downstream of
the inactivation tube, the hydrolyzate is cooled to
ambient temperature.
At the exit of the cooling tube, samples are
taken for analysis at times of 0, 60, 120, 180 and 240
minutes counted at the exit of the hydrolysis tube from
215 minutes after the start of the continuous process.
2092953
22
These samples have the pH values, amine nitrogen con-
tents, lysine blockages and NPN's shown in Table III
below where the corresponding quantity of KOH used to
keep the pH at 7.8 in the tank is also shown.
Table III
Time pH Amine KOH Lysine NPN
nitrogen blockage
(mins.) (%) (g/h) (%) (%)
0 6,.67 0.26 125 16.3 95
60 6..68 0.25 124 16.2 96
120 6..67 0.26 128 16.3 94
180 6..67 0.27 124 16.2 95
240 6..67 0.26 127 16.1 96
It can be seen from Table III in the same way as
from Table I of Example 6 that the characteristics of
the hydroly~:ate hardly vary irrespective of the time at
which the samples are taken for analysis at the exit of
the hyrolysis tube.
The peptide profile and the hypoallergenic
properties of the product obtained under the conditions
of the present Example are also examined by subjecting
it to the HPLC, ELISA and serotonin-3H tests of which the
results are set out in Tables V, VI and VII below.
For comparison, 160 kg of the same substrate are
subjected to discontinuous enzymatic hydrolysis with the
same enzyme in a total quantity of 7% based on protein
in a tank at, 70°C. Of these 7% of enzyme, 2% are used
for a first hydrolysis phase for 45 minutes at pH 7.8,
after which the pH is left to float. After 60 minutes,
the remaining 5% enzyme are added and hydrolysis is
continued at: 70°C and at a floating pH up to and beyond
X092953
23
215 minutes.
20 kg hydrolyzate are removed after 120 minutes
counting from the beginning of hydrolysis. Further
quantities of 20 kg are taken after 150, 180, 200, 250,
300 and 360 mins. A sample is taken for analysis after
215 minutes' hydrolysis.
The hydrolyzates corresponding to the various
removals and samples are immediately inactivated (for 18
minutes at 90°C in a heat exchanger) and then cooled to
l0 ambient temj?erature (in a heat exchanger) and analyzed.
They have i~he pH values, amine nitrogen contents (%
based on powder containing 97% dry matter), lysine
blockages and NPN's shown in Table IV below.
Table IV
Time pH Amine Lysine NPN
nitrogen blockage
(mins.) (%, powder) (%) (%)
60 7 ,. 56
120 6..97 0.60 15.3
150 6,.87 0.63 17.2 90
180 6..82 0.66 18.2 94
200 6..80 0.68 18.3 95
215 6..80 0.68 18.9 95
250 6..79 0.69 19.4 96
300 6..77 0.71 19.5 96
360 6..76 0.74 19.6 97
It can be seen from Table IV in the same way as
from Table :CI in Example 6 that the characteristics of
the hydrolyzate obtained discontinuously in a tank vary
rapidly again after the 215 minutes corresponding to the
continuous residence time in the apparatus used in
2092953
24
Example 8.
This confirms one of the advantages of the
present process in which there is no danger of develop-
ment of the product comparable to that which occurs
during the time required to empty the tank in a discon-
tinuous process.
The peptide profile and the hypoallergenic
properties ~of the product obtained under the conditions
of the above Comparison Example are also examined after
215 minutes by subjecting the product to the HPLC, ELISA
and serotonin-3H tests of which the results are set out
in Tables V, VI and VII below.
Analogous tests are carried out on the product
obtained continuously in a tank under the conditions
corresponding to pH 7.3 presented for comparison with
Example 7, the results also being set out in Tables V,
VI and VII :below.
Table 0 Peptide profile (HPLC test)
Product Percentage of peptides in the ranges
acc. to within the molecular weight limits
expressed in kDalton
>14 14-6 6-3.5 3.5-1.0 < 1
Example 8 5 7 9 30 49
Comparison 4 7 10 31 48
(disc. tank)
Comparison 21 13 9 24 33
( cont . tank;
The test results set out in Table V clearly
illustrate the fact that a hydrolyzate continuously
2092953
obtained by the process according to the present inven-
tion can have a peptide profile at least as narrow and
centred on ithe small peptides as a hydrolyzate obtained
for compari:aon in a discontinuous tank whereas a hydro-
5 lyzate obtained for comparison in a continuous tank has
a much broader peptide profile displaced towards the
large peptides.
Table VI ELISA inhibition test
Product Residual antigenicity expressed in
acc. to ug antigen per g protein
BLG BSA CAS
Example 8 53 20 150
Comparison 41 7 141
(disc. tank;)
Comparison 111 > 1000 319
( cont . tank;)
Table VII Serotonin-3H relaxation test
Product Residual antigenicity expressed in
acc. to ug of BLG equivalent for the relaxation
per g protein equivalent
Example 8 20
Comparison 5
(disc. tank;l
Comparison 50
( cont . tank;~
2092953
26
The test results set out in Tables VI and VII
illustrate 'the fact that a hydrolyzate obtained by the
process according to the present invention can be at
least as hypoallergenic as a hydrolyzate obtained for
comparison p_n a discontinuous tank whereas a hydrolyzate
obtained for comparison in a continuous tank is not
hypoallergenic.
Example 9
The ;present process is carried out in the same
way as described in Example 7 under conditions corre-
sponding to a temperature of 72 . 5 ° C in the tank. The
tube is divided into nine segments. Samples are taken
between two successive segments as the continuous
hydrolysis progresses after the discontinuous initiation
phase. Samples are taken at the tube exit at the same
intervals when the duration of the continuous hydrolysis
reaches the time corresponding to the residence time of
the product in the tube.
The amine nitrogen content of the samples is
determined. Each of these contents is divided by the
equilibrium content towards which the hydrolyzate tends.
On a system of coordinates, the quotients obtained are
plotted as ordinates while the quotients of the sampling
times divided by the residence time of the hydrolyzate
in the apparatus are plotted on the abscissa.
A sic~moid curve is obtained, crossing from the
abscissa 0.8, intersecting the vertical of the abscissa
1.0 at two thirds of its maximum value and reaching its
maximum value, in other words touching the horizontal of
the ordinate: 1.0, at the vertical of the abscissa 1.2.
For comparison, the test is carried out in the
same way except for the fact that the tube used is empty
and, in addition, has the same dimensions as the tube
equipped with static mixing elements.
2092953
27
Samp7les are taken under the same conditions, the
same quotients are established and the corresponding
curve is drawn in the same way.
A sigmoid curve is obtained, crossing from the
abscissa O.E>, intersecting the vertical of the abscissa
1.0 at half' its maximum value and only reaching its
maximum value, i.e. 1.0, beyond the vertical of the
abscissa 1. F3 .
This demonstrates another two advantages of the
process according to the invention, namely on the one
hand the rapidity with which steady-state conditions can
be established and, on the other hand, the homogeneity
of the hydrolyzate on leaving the apparatus.