Note: Descriptions are shown in the official language in which they were submitted.
2~3~
HOECHST AKTIENGESEI.LSCHAFT HOE 92/F 087 Dr. ~E/PP
Desc.ripl:ion
Salts of 2-benzoylcyclohexanediones, selective herbi~idal
agents, process for their preparation, and their use for
controlling weed~
EP-A 137 963, EP-A-186 118, EP-A 274 634, ~P-A 298 680
and VS-4,780,127 disclose 2-benzoylcyclohexanedione
deri-iatives which are described as agents for controlling
a broad range of mono- and dicotyledon weeds.
WO 91/05470 discloses 2-benzoylcyclohexanedione deriva-
tives which are selectiv2 in rice plants when applied at
low dosage rates of active substance.
JP-A-3063-248 describes ammonium salts of 2-(2,4-di-
chlorobenzoyl)cyclohexanedione and 2-(2-nitro-4-chloro-
~nzoyl)cyclohexanedione which have a better ~electivity
in rice when applied at do~age rates from 32 to 63 gha
than 2-t2-nitro-4-chlorobenzoyl)c:yclohexanedione. The~e
ammonium salts are highly phytotoxic at dosage rates
above 63 g/ha. At the ~ame time, a broad action again~t
weeds i~ only reached at dosage rAtes from 125 to
250 g/ha.
Surprisingly, it has now been found that salts of speci-
fically ~ubstituted cyclohexanedione derivatives have an
outstanding selectivity in rice and an excellent broad-
range action against the harmful plants which occur
typically in rice species. The broad ran~e of selectivity
in rice allows even higher dosage rates of active ~ub-
~tance to be used, resulting in a very broad control of
weeds. Moreover, it has been found that the~e salts can
be used highly selectively a~ainst some important harmful
plants in other crops such as cereals and maize. The 2-
benzoylcyclohexanediones on which they are based, in
contrast, are not sufficiently effectiv2 in important
useful crops such as maiæe and rice again~t the harmful
2~3 1~
plants which are typical in these ~ 80 that in ~ome cases
only synergistic mixtures allow the 2-benzoylcyclo~
hexanedione derivatives ~o be used adequately (cf.
EP-A-274,634; WO 91/05469).
The compounds according to the invention control, in
particular, perennial weeds which can be f ound in rice
fields and which are frequently difficult to control, for
example Sagittaria spec., Cyperus serotinus, Scirpus
maritimus, Eleocharis 6pec. and Scirpus juncoides, as
well as a broad range of annual weeds.
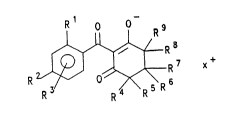
The present invention therefore relates to salts of
2-benzoylcyclohexanediones of the formula I
R ~ 7 x+ (I)
in which
Rl is halogen, (Cl-C4)alkoxy, (C~C4)alkyl, ( Cl-C4 ) halo-
alkyl, -NO2, -CN or S~O~Rl;
R2 and R3 independently of one another are hydrogen,
halogen, (C1-C4)alkyl, (Cl-C4)alkoxy, (Cl-C4)halo-
alkoxy, (Cl-C4)haloalkyl, -CN, -NO2, -S(O~m-Rll,
-NRl2Rl3, NRl4-co-Rl5, _co_R16;
R4, R6, R7, R8 and R9 independently of one another àre
hydrogen or t Cl-C4 ) alkyl;
R5 is hydrogen, ( Cl-C4 ) alkyl or -CO-O-( C~-C4 ) alkyl;
R10 is ( Cl-C4 ) alkyl, ( C~-C4 ) haloalkyl or (C~-C4~alkoxy;5 Rl1 is (Cl-C4~alkyl, (C~-C~)haloalkyl, phenyl, benzyl or
_NRl7RlB;
2~31~
Rl2 and Rl3 independently of one another are hydrogen or
(C~-C4)alkyl;
R14 is hydrogen or ( Cl-C4 ) alkyl;
R1~ is (C1-C4)alkyl;
Rl~ is hydrogen, (C1-C4)alkyl, (cl-c4)haloalkyl or
( C~-C4 ) alkoxy;
R17 and R1~ independently of one another are hydrogen or
~ C~-C4 ) alkyl and
n and m independently of one another are 0, 1 or 2,
and X+ is a metal cation equivalent such as, for example,
an alkali me~al cation or alkaline earth metal cation
equivalent, or, i~ appropriate, a ~ubstituted ammonium or
phosphonium ion of the formula II
R20
R19 y+ R21 (I~)
R22
in which Y i~ nitrogen or phosphoru~,
R1~ is hydrogen, ~C~-C20)alkyl, ((7-C?n~alk Y 1 7~
alkoxy, it being possible for the last-mentioned
radicals to be optionally monosu~stituted or poly-
substituted by halogen, alkoxy, alkylthio or mono-
or dialkylamino;
RZ, R2l and R22 independently of one another are hydrogen,
(Cl-C20)alkyl, (C~~C~Q)alkenyl or (C~-C~o)aU~nyl, it
being possible for these aliphatic radicals to ~e
monosubstituted or poly3ub~tituted by halogen,
amino, alkylamino or dialkylamino or to be inter-
rupted once or more than once by heteroatom~ or
heteroatom groups such as oxygen, sulfur, -N~- or
-N(C1~C1~ yl; hy~(C1-C2~)alh~l, hydroxy(C2-C20)aIkenyl or
-alkynyl, ~Cl-Cl2~alkylsulfonyl-(C~ 2)al~Yl, ~3-Clz)-
cycloalkyl or tC3~Cl2)cycloalkenyl, it being po~si~le
~J ~
for these cycloaliphatic radicals to be monosub-
stituted or polysubstituted by halogen, amino,
alkylamino or dialkylamino, ~C~-Cl2)alXyl~ulfonyl,
hydroxyl or (C~-C1a)alkoxy and/or to be intPrrupted
once or more than once by heteroatoms or heteroatom
groups ~uch as oxygen, sulfur, -N~-
or-N(C1-C12)alkyl, or R2D, R21 and R22 independently of
one another are a radical of the formula
( R 2 3
~(CH2)q ~
in which p and q are 0, 1 or 2 and R23 i~ hydrogen,
( Cl-Cl2 ) alkyl, ( Cl-Cl2 ) alkoxy, ( Cl-C12 ) alkylthio~
(Cl~Cl2)alkylsulfonyl, CN, NO~, halogen, (C~-C~2)halo-
alkyl, (C~-C 12 ) haloalkoxy, ~mino, (Cl-Cl2)alkylamino
or tcl-cl2)dialkylamin
or, in the event that Y is N, the radicals R20 and R
together with the nitrogen atom form a cycloali-
phatic four- to twelve-membered ring sy~tem which i~
optionally monounsaturated or polyunsa~urated and
which is optionally monosubstituted or polysubsti-
tuted by halogen, (Cl-Ca)alkyl, (C3-C8)alkenyl
(Cl-Ca)alkoxy, amino, (Cl-Ca)alkylamino, (Cl-C~)dial~
kylamino, phenyl, benzyl or hydroxyl and/or inter-
rupted once or more than once by heteroatoms or
heteroatom groups such as oxygen, sul~ur, ~NH- or
-N~C1-C1z)alkyl;
or a sulfonium or ~ulfoxonium equivalent of the
formula III
-- 5 --
()r
I
Rl9 S~ R21 ( III )
I
R~
in which R19, R20 and R2l have the abovementioned
meaning and r i~ 0 or 1,
and tautomeric forms thereof, with the exception of thoqe
compounds in which R1 i8 chlorine or nitro, R2 is chlorine
and R3 to ~9 are hydrogen.
Alkyl radicals are to be understood as meaning radicals
with the stated number of carbon atoms. The radicals can
be straight-chain or branched. The most common radicals
are, for example, methyl, ethyl, n-propyl, isopropyl,
n-butyl, isobutyl, sec.-butyl or tert.-butyl. Halogen is
to be understood as meaning fluorine, chlorine, bromine
or iodine. Haloalkyl radicals can be monosubstituted or
polysubstituted by halogen, i.e. they can also be per-
halogenated.
Preferred compounds of the abovementioned ~ormula (I) arethose in which
Rl is fluorine, chlorine, bromine, iodine, methsxy,
nitro, cyano or -S(0) nRl;
R2 and R3 independently of one another are hydrogen,
fluorine, chlorine, bromine, iodine, methyl, meth-
oxy, trifluoromethoxy, difluoromethoxy, cyano,
nitro, trifluoromethyl, S02R~ NR12Rl3
-N(CH3)-Co-Rl5 or ~C0-0-~Cl-C4)-alkyl and
R4, R5, R6, R7, R8 and R9 independently of one another ~re
hydrogen or methyl and the remaining radicals h ve the
abovementioned meanings.
2 ~ 9 31~ ~
Particularly preferred compounds of the formula (I) are
those in which
R2 and R3 independently of one another are hydrogen,
fluorine, chlorine, bromine, -N(CH3~ 2 ~ methoxy,
nitro, -SO2CH3, -SO2C2H5, -SO2CH2Cl, -SO2N(C~3)2,
trifluoromethyl or difluoromethoxy.
Particularly suitable compounds of th~ form~la (I)
according to the invention are ~he compounds in which
2-t2-chloro-4-methylsulfonylbenzoyl)-1,3-cyclohexane-
dione,
2-~2-chloro-4-ethylsulfonyl~enzoyl)-1,3-cyclohexanedione,
2-(2-nitrobenzoyl)-4,4-dimethyl-1,3-cyclohexanedione,
2-(2~chloro-4-ethylsulfonylbenzoyl)-5,5-dimethyl-1,3-
cyclohexanedione,
2-~2-nitro--4-chlorobenzoyl)-4-(1-methylethyl) 1,3-cyclo-
hexanedione,
2-(2-nitro-4-chlorobenzoyl)-4,4-d~methyl-1,3-cyclohexane-
dione,
2-(2-chloro-4-methylsulfonylberlzoyl)-4,4-dimethyl-6-
methyl-1,3-cyclohexanedione,
2-(2-nitro-4-methylsulfonylbenzoyl)-4,4-dimethyl-1,3-
cyclohexanedione,
2-(2-nitro-4-di~luoromethoxybenzoyl)-1,3-cyclohexanedione
or
2-(2-nitro-4-difluoromethoxybenzoyl)-4,4-dimethyl-1,3-
cyclohexanedione are uset as a benzoylcyclohexanedione
component.
The compounds of the formula (I) can exist in various
tautomeric structures ~keto-enol tautomerism), the
formula I describes only one of the tautomeric forms
which are possible:
2~331~
R~ ~ R
I Ia
~7 :~-
Ib
The invention furthermore relates to a process for the
preparation of compounds of the formula I, which com-
prises replacing, in compounds of the formula IV
R2 ~ (IV)
which also exist in various tautomeric structures,
the acidic hydrogen atom by a cation of the formula X~
which is suitable for use in her~icides, by reacting them
with the inorganic or organic bases on which the radical
X+ is based.
Solvents which are generally used are protic or aprotic
solvents such as, for sxample, water, msthanol, ethanol,
isopropanol, n-butanol, dimethylformamide, dimethyl
sulfoxide, N-methylpyrrolidone, acetonitrile, acetone,
'
2~3 1~
-- 8 --
methyl isobutyl ketone, dioxane, tetrahydrofuran or
mixture~ of theRe ~olvent~, and the process is carried
out at temperatures between -25C and ths reflux tempera-
ture of the ~olvent, in particular between 0C and 60C.
The sequenc~ in which the reactant~ are added i~ not
important. The educt~ are generally employed in ~toichio-
metric amounts, depending on the valency of the cation.
Bases which are suitable for preparing the ~alts accord-
ing to the invention are, for example, alkali metal
carbonates such as potassium carbonate, alkali metal
hydroxides and alkaline earth metal hydroxide~, ammonia,
ethanolamine, triethylamine, diethylamine, piperidine,
morpholine, diethanolamine, diallylamins, tri-n-propyl-
amine, decylamine and dodecylamine.
The 2-benzoylcyclohexanedione derivatives of the form-
ula (I) have been disclosed, ~ee, for example,
EP-A-137,963, 186,118, 274,634 and 298,680, all of which
have been mentioned above; UOS~ Patent 4,780,127,
WO 91/13548 and EP 92,103,664.6, or they can be prepared
~y the methods described in these publications.
The present invention furthermore relates to a proces~
for the selective control of harmful plants in Grops o
useful plants such as cereals, maize and, in particular,
in rice crops, which comprises applying an effective
amount of one or more compound~ of the formula I to the
area under cultivation containing harmful plants and crop
plants or their seeds. Active substance concentrations
which are suitable are, for example, 0.001 to 0.5 kg/ha,
preferably 0.01 to 0.25 kg/ha, in particular 0.02 to
0.20 kgtha, in rice crops and 0.01 to 2 kg/ha, preferably
0.05 to 1.0 kg/ha, in cereal~ or maizeO
The compounds employed according o the invention have an
excPllent herbicidal activity against a broad range of
economically important monocotyledon and dicotyledon
~ Q~
~ ~t9 ~3
_ ~ _
harmful plants. ~he active substances also act
efficiPntly on perennial weeds which produce shoots from
rhizomes, root stocks and other perennial organs and
which are difficult to control. In this context, it does
not matter whether the ~ubstance~ are applied pre-plant-
ing, preemergence or post-emergence. Application pre-
emergence and/or early post-emergence i8 preferred.
Specifically, examples may be mentioned of 80me repre-
sentatives of the monocotyledon and dicotyledon weed
flora which can be controlled by the agents according to
the invention, without this mention being intended as a
restriction to certain species.
Examples of monocotyledon weed species on which the
active substance acts effectively are, from the annual
group, Cyperus species and, from amongst the perennial
species, perennial Cyperus species, Scirpus ~pecies and
Eleocharis species.
The active substances to be employed according to the
invention effect an outstanding control on weeds which
occur under the specific agronomical conditions of rice
such as, for example, Sagittaria, Alisma, Eleocharis,
Scirpus, Cyperus etc..
In the case of the dicotyledon weed ~pecies, the spectrum
of action extends, for example, to Rotala, Sphenoclea,
Eclipta, Potamogeton, ~eteranthera, Aeschynomene and
Ammania.
In addition, the active substance acts on weed species
which can be found mainly in other crops such as cereal~
and maiza: examples of weed species on which the active
substance acts effectively are, from among~t the mono-
cotyledon weed species, for example, Avena, Lolium,
Alopecurus, Ph~laris, Echinochloa, Di~itaria, Setaria a~
well as Cyperus species from the annual group and, from
amongst the perennial specie~ Agropyron, Cynodon,
10 - ~
Imperata a8 well a~ Sorghum and al~o perennial Cyperus
BpeCies. In the case of the dicotyledon weed specie~, the
spectrum of action extends ~o species ~uch as, for
example, Galium, Viola, Veronica, Lamium, St~llaria,
~maranthus, 5inapi~, Ipomoea, Matricaria, Abutilon and
Sida from amongst the annuals as well as Convolvulus,
Cirsium, Rumex and Axtemisia in ~he case of the perennial
weed~.
If the compounds employed according to the invention are
applied to the 80il ~urface before germination, then the
weeds grow until they have reached the cotyledon stage
but then their grow~h stops and, eventually, after three
to four weeks have elapced, they die completely.
If the compounds of the formula (I) are applied post-
emergence to the green parts of the plants, growthsimilarly ~tops drastically a very ~hort time after the
treatment and the weed plants remain at the growth stage
at the point of time of applica'tion, Qr they die com-
pletely after a certain time, B0 that, in th.i~ manner~
competition by the weeds, which is harmful to the crop
plants, is eliminated by the use of the compound~ of the
formula ~I) according to the invention at a very early
point in time and in a sustained manner.
Although the compounds employed according to the inven-
tion have an outstanding herbicidal activity a~ainstmonocotyledon and dicotyledon weeds, crop plants of
economically important CXOp8 such as, for example, wheat,
barley, rye, maize, sugar beet, cotton and 80ya, and, in
particular, rice crops of sown or transplanted rice, are
not damayed at all, vr only to a negligible extent. For
these reasons, the compounds of the formula 5I) are
highly suitable for selectively controlling harmful
plant9 in areas used for agricultural crop production~
preferably in cereals, maize and rice.
2~c~3~ ~5
The application rates of the compounds of the formula ~I)
depend on the environment in terms of climate and ~oil
and on the ~pecific harmful plants, the crops and the
varieties in question, for example rice varieties. Late
pre-emergence or early post-emergence application is
particularly preferred.
The compounds of the formula (I) can be f~rmulated in
various ways, depending on the prevailing biological
and/or ~hemico-phy~ical parameters. Examples of possible
formulations which are suitable ar~: wettable powders
(WP), water-soluble powder~ (SP), emulsifiable concen-
trate~ (EC), aqueous solutions or concentrates (SL),
emulsions (EW) such as oil-in-water and water-in oil
emulsions, sprayable solutions, capsule suspensions (CS),
dispersions on an oil or water basi~, suspoemulsions,
suspension concentrates (SC), dusts (DP), o:Ll-miscible
solutions (OL), seed-dressing agents, granules (GR) in
the form of microgranules, spray granules, coated gran-
ules and adsorption granules, granules for soil applica-
tion and for broadca~ting, wate~r-dispersible granules
(WG), ULV formulations, microcapsules or waxes.
These individual formulation types are known in principle
and described, for example, in:
Winnacker-Kuchler, ~'Chemische Technologie" [Chemical
Technology], Volume 7, C. ~auser Verlag ~unich, 4th Ed.
1986; van ~alkenbur~, "Pesticides Formulations" Marcel
Dekker, N.Y., 2nd Ed. 1972-1973; K. Narten3, "Spray
Drying Handbook", 3rd Ed. 1979, G. Goodwin Ltd. London.
The necessary formulation auxiliaries such as inert
materials, urfactants, solvents and other additives are
also known and described, for example, in: Watkins,
"Handbook o~ Insecticide Dust Diluents and Carriers", 2nd
Ed., Darland Books, Caldwell N.J.; ~.v. Olphen,
"Introduction to Clay Colloid Chemistxy"; 2nd Ed., J~
Wiley & sonB~ N.Y.; Mar~den, "Solvents Guide", 2nd Ed.,
3 1 ~ ~
- 12 -
Inter cience, N.Y. 1950; McCutcheon~s ~Detergents and
Emulsifiers Annual", ~C Publ. Corp., Ridgewood N.J.;
Sisley and Wood, ~Encyclopedia of Surface Active Ag~nts",
Chem. Publ. Co. Inc., N.~ 64; Schonfeldt,
"Grenzflàchenaktive ~thylenoxidaddukte" [Surface-active
Ethylene oxide adduct6], Wis~ Verlagsgesell., Stuttgart
1976; Winnacker-Kuchler, ~Chemi~che Technologie" tChem-
ical Technology], Volume 7, C. ~auser Verlag, Nunich,
~th Ed., 1986.
Based on these formulations, it iB also possible to
prepare combinations with other pesticidally active
substances, such as other herbicides, fungicide~ or
insecticides, or else safeners, fertilizers and/or growth
regulators, for example in the form of a readymix or a
tank mix.
Wettable powders are preparations which are uniformly
dispersible in water and which, besides the active
substance, also contain wetting agents, for example
polyoxethylated alkylphenols, polyoxalkylated fatty
alcohols, polyoxalkylated fatty~ amines, alkane- or
alkylsulfonylbenzenesulfates, and dispersing agents, for
example sodium ligninsulfonate, sodium 2,2'-dinaphthyl-
methane-6,6'-disulfonate, sodium dibutylnaphthalenesul-
fonate and also sodium oleoylmethyltaurate, in addition
to a diluent or inert substance.
Emulsifia~le concentrates are pr~pared by dissolving the
active substance in an organic solvent, for example
butanol, cyclohexanone, dimethylformamide, xylene and
also higher-boiling aromatic~ or hydrocarbons, wi~h the
addition of one or more emulsifier~. Emulsifiers which
can be used are, for example. calcium salts of alkylaryl-
sulfonic acids, such as calcium dodecylbenzsnesulfonate
or non-ionic emulsifiers, such as fatty acid polyglycol
esters, alkylaryl polyglycol ethers, fatty alcohol
polyylycol ethers, propylene o~ide~ethylene oxid~
2~3~5
- 13 -
cond~nsation products, alkyl polyethers, ~orbitan fatty
acid e~ters, polyoxyethylene sorbitan fatty acid esters
or polyoxyethylene sorbitol esters.
Dusts are obtained by grinding the active substance with
finely divided solid substances, for example talc or
natural clays ~uch a8 kaolin~ bentonite, pyrophyllite, or
diatomaceous earth.
Granules can be produced either by spraying the active
substance onto adsorptive, granulated inert material or
by applying active substance concentrates onto the
surface of carriers such as sand, kaolinites or of
granulated inert material, by means of binder~, ~or
example polyvinyl alcohol, sodium polyacrylate or alter-
natively mineral oils.
Suitable active substance~ can also be granulated in the
manner which iB conventional Eor the production of
fertilizer granules, if desire~d in a mixture with
fertilizer~.
As a rule, water-disper~ible granules are prepared by the
customary methods ~uch as spray drying, fluidized-be~
granulation, disk granulation, mixing with high-speed
stirrers and extrusion without 601id inert material.
The agrochemical preparations generally compri~e 0.1 to
99 percent by weight, in particular 0.1 to 95% by weight,
of active ~ubstance of the formula (I~.
The active substance concentration in wettabla powders
i6, for example, about 10 to 90~ by weight; the remainder
to 100% by weight is composed of conventional formulation
components. In th case of emulsifiable concentrates, the
active substance concentration can be about 1 to 90%,
preferably 5 to 80%, by weight. ~ormulations in the form
of dusts contain 1 to 30, preferably in most cases 5 to
X~13 ~ ~
20% by weight of active substance, ~prayable ~olutions
about 0.05 to 80, preferably 2 to 50% by weight. In the
case of water-dispersible granules, the active sub~tance
content depends partly on whether the active compound i8
liquid or solid and on which granulation auxiliarie~,
fillers etc. are u~ed. It i8 generally between 1 and 95,
preferably between 10 and 80% by weight in the case of
the water-dispersible granules.
In addition, the act.ive substance formulations mentioned
comprise, if appropriate, the adhesives, wetting agents,
dispersing agents, emulsifiers, penetrants, pr2serva-
tives, anti-freeze agents, solv~nts, fillers, carriers,
colorants, antifoam agents, ~vaporation inhibitors and p~
and viscoslty regulators which are conventional in each
case.
A. Formulation example~
a) A dust is obtained by mixin~ 10 parts by weight of
a compound of the formula (I) and 90 part~ by weight
of talc as inert ~ubstanc~3 and comminuting the
mixture in a hammer mill.
b) A wettable powder which is readily disper~ible in
water is obtained by mixing 25 parts by weight of a
compound of the formula (I), 64 parts by weight of
kaolin-containing quartz as inert substance,
2~ 10 parts by weight of potassium ligninsulfonate and
1 part by weight of ~odium oleoylmethyltaurate a~
wetting and dispersing agent, and grinding the
mixture in a pinned disk mill.
c) A di~persion concentrate which is readily di~pers-
ible in water i8 obtained by mixing 20 parts by
weight of a compound of the formula (I) with 6 parts
by weight o~ alkylphenol polyglycol ether
(~Triton X 207), 3 parts by weight of isotride~anol
~3~5
- 15 -
polyglycol ether (8 E0) and 71 parts by weight of
paraffinic mineral oil (boiling range, for example,
approx. 255 to above 277C) and ~rinding the mixture
in a ball mill to a finene~s of below S micron
d) An emulsifiable concentrate i~ obtained from
15 parts by wPight of a compound of the formula (I),
75 parts by weight of cyclohexanone as ~olvent and
10 part~ by weight of oxethylated nonylphenol as
emulsifier.
e) Water-dispersible granules are obtained by mixing
parts by weight of active substance of the
formula (I),
10 part~ by weight of calcium ligninsulfonate,
5 parts by weight of sodium lauryl sulfate,
3 parts by weight of polyvinyl alcohol and
7 parts by weight of kaolin,
grinding the mixture in a pinned disk mill and
granulating the powder in a ~Eluidized bed by ~pray-
ing on water as granulation llqu~d.
~0 f) Water-disparsible granules are also obtained by
homogenizing and precomminuting
25 parts by weight of active ~ubstance of the
formula (I),
5 parts by weight of ~odium 2,2'-dinapthylmethane-
6,6'-disul~onate,
2 parts by weight of sodium oleoylmethyltaurate,
1 part by weight of polyvinyl alcohol,
17 part by weight of calcium carbonate and
50 parts by weight of water
in a colloi~ mill, subsequently grinding the mixture
in a bead mill, and atomizing and drying th~
~ 3~3
- 16 -
resulting suspension in a sE)ray tower by means of a
Gingle-sub~tance nozzle~
B. Chem-cal examples
1. Isopropylammonium ~alt of 2-(2-chloro-4-methyl~ul-
fonylbenzoyl)-1,3-cyclohexanedione
1.6 g (0.0049 mol) of2-t2-chloro-4-methylsulfonyl)~
1,3-cyclohexanedione and 0.42 mol ~0.048 mol) of
isopropylamine are combined at room temperature in
20 ml of dichloromethane and, after 2 hour~, the
mixture i6 evaporated. 1.9 g ~100 % of theory) of
the isopropylammonium salt of 2-(2-chloro-4-methyl-
sulfonylbenzoyl)-1,3-cyclohexanedione of melting
point 112C are obtained.
2. Sodium salt of2-(2-nitro-4-difluoromethoxyben20yl)-
4,4-dimethyl-1,3-cyclohexanedione
2.13 g (0.006 mol) of 2-(2-nitro-4-difluoromethoxy-
benzoyl)-4,4-dimathyl-1,3-cyclohsxanedione in 50 ml
of methanol are combined, at room temperature, with
0.24 g (0.006 mol) NaOH dissolved in 5 ml of H20.
After 1 hour, the mixture i8 evaporated. 2.35 g
(99 % of theory) of the sodium salt of 2-(2-nitro-
~-difluoromethoxybenzoyl)-4,4-dLmethyl-1,3-cyclohex-
anedione of melting point 160C are obtained.
The compounds listed in Table 1 are obtained
analogously:
2 ~
~ 17 ~
_= =--_ _ e = _
~ N ~ _ ¦~ ~ Y~ l
= ~ T T I I = T
a~a: ~: I I I :1: I I
+ _ -~ _ _ :~ _ _
x 1~ I __ I :C I I I
1~: I __ O O O I I
o ~~ ~T ~ ~
~C I T I I T :C; I
NC O ~U~ T u~ O
~ _ I O ~ O Z oN o
D w O _ C~J _ _ SD t~
E~
2 ~ ,s
- la-
= - = == - - :- - - =
~_ o ~ a:~ ~ r-
_ _ . N _------ T - ----
cna: I I I I I I I I I I I :~:
. __ . _ _
I I I I I :I: I I T T I
: I I I I I I I I I I t~
_ _ _ _
:r: I :C I I I I I :~: O ) I
Ul~r I I I I I I I ~.) I :1: I
~ __ _ _ ____ _
I I I I I I t~ I S~) I
1:1: :1: ~: S I :1: I I T 1 I ~
~ oN oN ~ ~ ~~ o o O ~~ ~ V l l
L~= _ _ O ~,._ N C~) ~ Lf~ OE~ .- _ ,_
0 5
-- 19 --
r -~ =~ =~ _ = =__ =
3 0
~r
_
a'a: I I I T I I t.) :IC :i:
__ _ _ _ _ _ _
c~ I I I X t.~) O C~ I I
_ __ _ _ .
~ I $ I I I I I T
_ .
U'a: I I I I I I I I
_ ._ ___
ul~r I I I I O I t:\ O
-- I . _
I I I I ~ ~ t )
. _ _ _
"'a: T I X ~ I :C I :1: T
_ . - _ _ .
C~~ O" oN ~o~N ~ ~æ ~'~ oN ~~
_ _ . .
-a: ~ ~ ~ ~ ~ o ~ ~ ~
_ _ _ _ _ __
~ _ ~ ___ ~ ~n æ ._ ~
~3~`.3
- 20 -
C. Biological examples
1. Pre-emergence effect on weeds
SePds or rhi20me pieces of monocotyledon and dicoty-
ledon weed plants were placed in sandy loam soil in
plastic pots and covered with soil. The active
substances of the formula (I) which were fQr~ulated
in the form of wettable powders or emulaion concen-
trates were then applied to the surface o~ the 80il
cover in the form of aqueous suspensions or emul-
sion~ at an application rate of 600 to 800 1 of
water/ha ~converted), at various dosage rates. After
the treatment, the pots were placed in gre nhouse
and kept under good growth conditions for the weeds.
After the test plants had emerged, the damage to the
plants or the negative effect on plant emergence was
scored visually after 3 to 4 week~ by comparison
with untreated controls.
The damage to tha weed plants, or the compatibility
with the crop plants, wa~ scored using a key in
which the effectivene~s i8 expre~sed by ~igures from
0 to 5. The rigures denote:
0 = no effect
1 = 0 to 20 ~ effect or damase
2 = 20 to 40 ~ effect or damage
3 ~ 40 to 60 % effect or damage
4 = 60 to 80 ~ effect or damage
5 - 80 to 100 ~ effect or damage
The active substances employed according to the
invention showed a good herbicidal pre-emergence
activity against a broad ran~e of gras~ weeds and
broad-leaf weeds, while the rice plants ~uffered
little damage, or none at all. ~ome re~ults ar~
compiled in Table 2.
2~3~
- 21 -
Table 2: Pre-emergence effect
Ex. Dosage ~ idal activity
No. rAte (kg
of a. 9 ./ha) STME C~SE SIAL hOMU ECCR AVSA
1 1.25 5 5 5 5 5 4
. _
2 1.~5 5 5 5 5 5 5
_ _ ____ _
3 1.25 5 5 5 5 5 4
_ _
~ 1~25 5 5 5 5 5 5
_
1.25 5 5 5 5 5 5
_
6 1.25 5 5 5 5 5 5
_ _ _
7 1.25 5 5 5 5 5 5
_
8 1.25 5 5 c; 5 5 4
_ _ _ _
9 1.25 5 5 ~j 5 5 5
_ _
1.25 5 5 c, 5 5 5
11 1.25 5 5 5 5 5 5
_ _
12 1.25 5 5 5 5 5 5
30 ~ - ~ _ ~ _ ____~
Abbreviations:
STME = Stellaria media
CHSE = Chrysanthemum segetum
SIAL = Sinapis alba
LOMU = Lolium multi~lorum
ECCR = Echinochloa crus-galli
~VSA = Avena sativa
a.~. - active substance
2 ~ 3 ~
- 22 -
2. Post~emergence effect on weeds
Seeds or rhizome pieces of monocotyledon and dicoty-
ledon weed plants were pl ced in sandy loam 80il in
plastic pots and grown in the greenhouse under good
growth condition~. Three weeks after sowing, the
test plants are treated in the three-leaf ~tage.
The active substances of the formula (I) which were
formulated as wettable powders or emulsion concen-
trates are sprayed, at various dosage rates, onto
the gxeen parts of the plants at an application rate
of 300 to 600 l of water/ha (converted) and, after
the test plants had remained in the greenhouse for
approx. 3 to 4 weeks under optimum growth condi-
tions, the effect of the preparations was scored
visually by compari~on with untreated controls. The
agents according to the invention al~o showed a good
herbicidal po~t-emergence activity against a ~road
range of economically important grass weeds and
broad-leaf weeds (cf. Table 3).
2 ~ s
- 23 -
Table 3: Post-emergence activity
~_
Ex. Dosage ~erbicidal activity
No. rate (kg
of a.s./ha) STME C~S~ SIAL ECCR
1 1.25 5 55 5
2 1.25 5 55 4
3 1.25 5 5-5 5
4 1.25 4 55 5
1.25 5 55 4
6 1.25 5 55 5
7 1.2~ 4 55 5
8 1.25 5 55 5
_ _
9 1.25 5 55 5
._ . _
1.25 5 55 5
1.25 5 - 55 5
12 1 25 I S 5- 5 - 5
3. Effect on weeds and ~electivity in rice
Weeds encountered specifically in rice growing as
well as rice plants were grown in waterlogged soil.
The active sub~tance~ o the ormula (I~ formulated
in the form of we table powder~ or emul6ion
~ ~ 9 3 ~ ¢ ~
- 24 -
concentrates were then applied to these plant~ at
various do~age rates in the fo~m of aqueous ~uspen-
sions or emulgiong at an application rate of 600 to
800 1 of water~ha (converted). After the treatment,
S the pot~ were placed in ~he ~reenhouse. After the
test plants had emerged after 3 to 4 weeks, they
were scored visually by comparison with untreated
controls.
The active substances employed according to the
invention showed a good herbicidal pre-emerg~nce
activity against a broad range of grass weeds and
broad-leaf weeds, while the rice plants suffered
little damage, or none at all. Some xesults are
compiled in Table 4.
J3~5
Table 4
Pro- Dosage Herbicidal activity
duct. rate ~kg
S No. of a.s./ha) S~PY ELAC CYMO ORSA-V
1 o l25 5 5 5
2 o 125 5 5 55
_
6 o 215~ 5 5 5
8 o 2125 55 55 55
_
9 0.25 5 5 . 2
0.12 5 4 5 0
_ _
0.25 5 5 5
0.12 5 4 ~ 0
11 o0 2152 5 45 s5 0
_
12 ~ S S _
Abbreviations:
SAPY = Sagittaria pygmaea
~LAC = Eleocharis aciculariR
CYMO = Cyperus monti (= serotinus)
ORSA-V = Oryza sativa (planted)