Note: Descriptions are shown in the official language in which they were submitted.
~, WO 93J054~13 2 ~ 9 ~ ~ ~ 3 PCr/US92/07165
"`1'
--1--
RADIC)~JF~PHI(~ .L'~
T~ç-hniçal--F- ield
This invention relates to double coated silver halide radiographic
elements of the type employed in combination with intensifying screens. More
specifically, the invention relates to a radiographic element having an ultraviolet
light -absorbing compound disposed between tne silver halide layers.
Bclc~ round Art
Photographic elements relying on silver halide emulsions for
image recording are employed in radiographic assemblies. It is known that silverhalide emulsions are more responsive to light than to X rays. Accordingly,
intensifying screens are used in radiographic assemblies. An intensifying screencontains a phosphor which absorbs X radiation and emits radiation in the visiblespectrum or in an adjacent spectral region, ultraviolet or infrared.
It is also desirable to limit patient exposure to high levels of X
radiation. Dual coated radiographic assemblies are used to allow reduced levels
2 0 of X radiation to obtain a useful radiograph. Accordingly, radiographic
assemblies can comp.rise a dual coated light sensitive radiographic element
disposed between a pair of fluorescent intensifying screens. The intensifying
screens, upon exposure to X radiation, emit radiation in the visible spectrum or in
an adjacent spectral region, e.g. ultraviolet or infrared that exposes the silver
2 5 halide emulsions and results in a recorded image in both emulsion layers.
An image sharpness lirnitation of dual coated ras~iographic
elements results from a phenomenon known as "crossover". Crossover occurs
when light emiKed by each intensifying screen passes through the film support,
exposing and giving rise to an unsharp image in the oppositely situated silver
3 0 halide emulsion layer.
A variety of techniques have been tried to reduce crossover. One
approach is to dissolve a iilter dye in one or more of the hydrophilic colloid
layers or in the support forming the radiographic element. Accordingly, in an
ultraviolet light-sensitive radiographic element, an ultraviolet light absorbing3 5 compound can be situated between the emulsion layers to reduce crossover ofultraviolet radiation. A desirable characteristic of the ultraviolet light absorbing
compound is that it exhibit minimal absorption of light at wavelengths above
wo 93/0S~43 2 (~ P~/VS92/07l6~ 1
~ , --2--
about 400 r~rn so that~the amount of yellow hue in the radiographic element is
thus minimized.
Another approach to irnprove the clarity of the exposed image by
decreasing undesirable yellow hue in a radiographic element is to add a blue dye5 tO the support to offset the yellow, thereby irnproving the contrast of the exposed
image. This approach, however, does not sufficiently offset the yellow hue, and
further improvement in decreasing the yellow hue to achieve a sharp image is
desirable.
Another prior art appoach employs T-Grain ~ emulsions to
10 achieve reduction in crossover without employing dyes and ultraviolet light
absorbers as above. It is desirable, however, to continue to provide an alternative
to radiographic elements employing T-Grain ultraviolet light-sensitive emulsions.
There is therefore still a need for irnproved ul~aviolet light absorbing compounds
for use in non-T-Grain, ultraviolet light-sensitive, radiographic elements.
The present invention solves the prior art problems noted above. It
provides ultraviolet light-absorption capability suf~lcient to substantially reduce
crossover of ultraviolet radiation to the opposite ultraviolet-sensitive emulsion
layer. It achieves a desired level of ultraviolet-light absorption withou~ irnpar~ng
an undesirable yellow hue to the photographic element. Thus, by rneans of ~his
2 0 invention ~here is provided a radiographic element that upon exposure to X
radiation provides a recorded irnage substantially free of crossover. The
inven~ion thereby plovides an exposed image that is sharper and more readable.
U.S. Pat. 3,822,131 discloses a double-sided radiographic image-
recording element having an ultraviolet absorbing material between ~he silver
2 5 halide emulsion layers to reduce crossover. The reference also discloses
inco~porating the ultraviolet absorbing material in the base in the range from
about 50 to about 2000 parts per rnillion and that the absorbers are most effective
at wavelengths of about 410 nm or less. The compounds of this patent are
expensive and difficult to prepare. Furtherrno~e, when added to the radiographic3 0 element in adequate levels to achieve maxirnum desired level of crossover ~hey
irnpart an unwanted yellow color.
U.S. Pat. 3,849,658 discloses a radiographic image-recording
elernent having an ultraviolet absorbing substance between the emulsion layers to
~educe crossover. The ultr~violet substance is incorporated in ~he support in a
3 5 range from about 50 to about 2000 parts per million or in a subbing layer in a
rang~ ~om about 25 to about 1000 parts per million. The compounds of this
patent bear no stluctural resemblance to those of the present inven~on and are
r
WO 93/05M3 2 0 9 ~1~ 3 PCr/US92/07165
.3 _
difficult and expensive to prepare, e.g. as set forth in U.S. Pat. 37420,835,
requiring a complicated multi-step synthetic procedure. Additionally, they
contribute significant yellow color to the radiographic element when added at
levels needed to ~chieve the maximum desired crossover reduction.
U.S. Pat. 4,617,374, 4,707,537, 4,749,773, 4,749,774, 4,845,187,
and 4,994,512 disclose ultraviolet light-absorbirlg compounds copolymerized
with polyester and polycarbonate compounds suitable for use as beverage bottles
and the like. The patents do not describe the use of the compounds in
radiographic elements and in the useful loadings provided by aur invention.
1 0 Some of the preferred uv absorbers employed in this invention arewithin the disclosures of the above-identified references. However, the
references do not describe the use of such compolmds in ~ ray filrns.
Fur~hermore, the references do not disclose the unexpected light-absorbing
activity of such compounds in X ray film (set forth in the experimental section of
this specification).
Research Disclos~lre, Vol. 184, August 1979, Item 1843I,
summarizes the state of the art of constructing radiographic elements, includingdual coated radiographic elements employing light-absorbing dyes to reduce
crossover. ~arch Disclosure is published by Kenneth Mason Publica~ions,
2 0 Ltd., Emsworth, Harnpshire P010 7DD, England.
Research Pi~closure, Vol. 24~, December 1984, Item 2482~,
discloses a variety of structures useful as UV absorbing compounds to reduce
crossover in radiographic film, including benzophenones, benzotriazoles,
benzylidene malonates, salicylates, oxamides, etc. The reference, however, does
2 5 not disclose the compounds of our invention. Furthermore, when compounds of
this reference are used at an adequate level to reduce crossover to achie~e
maximum irnage sharpness, excessive amounts are require~ and/or the UV
absorber imparts an objectionable yellow color.
3 0 !Brief Deslcnp~iQn~Qf the Drawings
FI~3U~E 1 is a cross-section, not to scale, of a photographic
elemeDt of the invention.
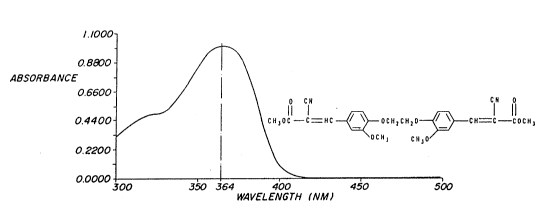
FIGUI~JE 2 is an absorbance curve of one of the uv absorbers,
3 5 Compound I, used in the islvention. Compound I has an absorption max~num (~
max~ at 356 nm in methylene chlonde in the W absorption spectrum. The
ext~nction coefficient (e) is equal to 37,105.
: , , ; ~ - . , . - . . ..
,~
, . ...... . . .. , ,., . .. ,. '. : .
'' . ., - , ~
WO ~3/(~5443 ~ ~ 4 1 ~ 3 PC~I`/US~2/0716S,"~
--4--
FlGUl~Ei3`is'an absorbance curve of ano~her uv absorber,
Compound II, used in the invention. Cornpound II has an absorption maximum
(~ max) at 364 nm in methylene chloride (~ = 45,458).
FIGURE 4 an absorbance curve of a comparison uv absorber. The
5 compound has an absorption maxirnum (~ max) at 352 nm in rnethylene chloride
( = 40,944).
FIGURE 5 an absorbance curve of a comparison uv absorber. The
compound has an absorptîon max~mum (~ max) at 377 nm in methylene chloride
(~ - 29,3~
The structural forrnulas for the compounds in FIGS. 2-5 are as
given in the FIGURES. The curves were obtained using a methylene chloride
. solution in which the concentration of the compounds was about O. l g/l.
FIGURE 6 is a graph of transmittance versus spectral wavelength
for test runs of poly (ethylene terephthalate) (PET) filrn containing specified uv
15 absorbers and for a control without uv absorber.
FIGURE 7 is a graph of percent transmittance versus spectral
wavelength for test runs of PET film containing blue dye and specified uv
absorbels and for a control without uv absorber.
FIGU~ 8 is a graph showing film speed, percent crossover, and
2 0 percent transmittance for PET film containing uv absorber used in the invention.
.~ Disclosure~Qnvention
.
This invention comprises a radiographic element comprising a
2 5 polyester support having opposed major su~faces and having an ultraviolet light-
sensitive silver halide emulsion layer on each of such surfaces. Admixed in the
support or in a layer inteIposed between the emulsion layers is an ultraviolet light
absorbing methine compound.
The methine compound is present in an amount sufficient to
3 0 reduce the average percent transmission of ultraviolet light over the range of
wavelength of from about 350 nm to about 395 nm to less than about 25 percent,
and whereby the percent transmittance of the element is at least abol1t 55 percent
at a wavelength of about 410 nm for an element thiclcness of about 0.007 inches.In another embodiment, the methine compound is present in an amount sufficient
3 5 to reduce the average percent transmission of ultraviolet light in the stated range
to less than abous 12 percent. Irnproved sharpness of the image recorded in eachsilver halide emulsion layer thus results.
.~
..
~ W0 93/05443 2 0 9~ i 3 PCI/US!12/07165
:i~ ,"
--5~
In accordance with the present invention, in one embodirnent
certain ultraviolet light absorbing methine compounds defïned below are admixed
in the polyester support material. In another emb~diment, the ultraviolet light
absorbing methine compound can be admixed in a hydrophilic colloid subbing
5 layer interposed between the silver halide emulsion layers. In one embodiment in
which the ultraviolet light absorbing methine compound is admixed in the
support, the weight proportion of the ultraviolet light absorbing methine
compound to the polyester is in the range of from about 200 to about 1600 ppm.
In another such embodiment, the weight proportion of the ultraviolet light
10 absorbing methine compound to the polyester is in the range of from about 400 to
about 800 ppm.
Best M~de For Car~ying Out The InventiQn
In a prefenred embodiment, this invention comprises a
radiographic image-recording element, comprising:
a polyester support having opposed major surfaces;
an ulsraviolet light-sensitive silver halide emulsion layer on each
of such surfaces; and
2 0 admixed in the support at least one ultraviolet light absorbing
compound as described below.
In another embodiment, the ultraviolet light absorbing compound
- is admixed in a subbing layer interposed between the silver halide emulsion
layers.
2 5 The ultraviolet light absorbing compound has the formula:
C ---H C ~0 R 1
X/ ~
OR2
wherein:
3 0 Rl and R2 are independently selected from the grouy consisting of:
alkyl ~oups having from 1 to about 20 carbon atoms, cycloaL~cyl
groups hav~ng firom about 3 to about 7 carbon atoms, and aryl groups hav~ng
from 6 to about 12 carbon atoms, aU of which may be substituted;
.
.
Wo ~3/054~3 2 0 9 ~ 1 4 3 PCr/US02/~7l65~
aL~enyl groups having from about 3 to about 11) carbon atoms;
aL~ynyl groups having from about 3 to about 10 carbon atoms,
hydrogen;
groups of the folmiula:
CN
:` --L--O ~,~C~--C/
` X1
R30
,
and groups of the formula:
OR4
-l-O~
\~< ~ C N
\
CH=C
1 0 X,
wherein L is an organic divalent linking group;
R3 and R4 are selected from the group consisting of:
aLIcyl groups having from 1 to about 20 carbon atoms, cycloaLkyl
groups having from about 3 to about 7 carbon atoms, and aIyl groups having
~m 6 to about 12 carbon atoms, all of which may be substituted;
~: alkenyl groups having from about 3 to about 10 carbon atoms;
aLkynyl ~oups having from about 3 to about 10 carbon atoms; and
hydrogen;
2 0 : X and Xl are independently selected from -CON(~s)R6, -CO2R~ and -
S02R6, wherein:
Rs is hydrogen or a substituted or unsubstituted allyl group
having ~om 1 to about 20 carbon atoms;
R6 is an alkyl ~oup having from 1 to about 20 ca~bon atoms, a
2 S Fycloalkyl group having from about 3 to about 7 carbon atoms, or an aryl group
: ~ :
`, ' ', ' " ', .
;~ WO 93/û5~13 2 0 9 ~ 3 rcr/vs92/n7165
.~..!
~7--
having ~om 6 to about 12 carbon atoms, all of which may be substituted; an
aLIcenyl group having from about 3 to about 7 carbon atoms; an alkynyl group
having from about 3 to about 10 carbon atoms; hydrogen; or a group having the
formula
NC
L Y~--C--H C~ O R 4
OR3
wherein Y is -C02-, -CON(Rs)- where Rs is as defined above, or S02-, and L"
R3, and R4 are as defined above; and
wherein the ultraviolet light absorbing compound is present in an amount
suf~lcient to reduce the average percent transrnission of ultraviolet light over the
range of wavelength of from about 350 nm to about 395 nm to less than about 25
percent, and whereby the percent transmittance of the element is at least about 55
percent at a wavelength of about 410 nm ~or an element thickness of about 0.007
15 inches. ~ another embodi nent, the ultraviolet light absorbing compound can be
employed in an amount sufflcient to reduce the average percent transmission of
ultraviolet light in such range to less ~han about 12 percçnt.
As used herein, the term "substituted alkyl" means C~-C20 aLI~yl
group substituted by one or more of halogen, phenyl, C3-C7 cycloalkyl, C1-C4
2 0 aLtcoxy, Cl-C4 aLkanoyloxy, Cl-C4 aLIcoxycarbonyl, hydroxy, am~no, carboxy,
Cl-C4 a~kylsulfonyl, Cl-C4 alkylthio, phenoxy, phenylthio, cyano, succinimido,
carbamoyl, sulfamoyl, Cl-C4 aL~coxycarbonyl and furyl. rhe term "subs~ituted
cycloaL~cyl" means C3-7 cycloalkyl groups substituted by one or more of Cl-C4
~Ikyl, Cl-C4 alkoxy, halogen, hydroxy, C1-C4 alkoxycarbonyl and the like. The
2 5 term "substituted aryl" means aryl groups conta~ning 6 to about 12 carbons
substitu~d with the substituents which may be present on the substituted aL~cyl
groups described above. The term "C1-C4 alkanoyloxy" means a C~-C4 aL~yl
group bonded to a carbonyl function which is bonded to another oxygen. For
- example, C2 alkanoyloxy is propionyloxy. The term "C1-C4 aLtcoxycarbonyl"
3 0 means Cl-C4 aL~coxy bonded to a carbonyl func~on, e.gi, the C2 aLIcoxycarbonyl
group is ethoxycarbonyl. The meaning of "reduce the average percent
transmission of ol~aviolet light over the range of wavelength of from about 350
.
WO ~3/05443 2 0 9 g 1 ~ 3 Pcr/U502/07165~ ~
nm to about 395 nm tQ less tltan about [Xl percent", as is made clear from its
context and from the below Examples, the description thereof, and the
accompanying Figures, is that the transmission of ultraviolet light through the
dual coated radiographic element of the invention when averaged over the stated
5 range is less than the stated value of X.
The meaning of "whereby the percent transmittance of the element
is at least about 55 percent at a wavelength of about 410 nm for an element
thickness of about 0.007 inches" is readily understood by one skilled in the art.
The terrn does not limit the thickness of the element of the invention but rather
10 defines a characteristic of the radiographic eleme:nt, that is, other thicker elements
are also within the scope of the invention. The transmittance of an element willvary according to its thickness. The transmittance should vary in an
approximately linear manner for element thicknesses employed in the
radiographic art. Thus, an element of the invention having a thickness of about
0.007 inches (0.18 mrn) will transmit at least about 55 percent of the light of
wavelength of about 410 nm. An element of the invention than such should have
less transmittance, and a thinner element more transmittance, than abou~ 55
percent at about 410 nm.
Preferred uv absorbers are those of the above ~o~rnula wherein Rl
2 0 R2, R3 and R4 are independently selected from hydrogen; cycloaLkyl; cycloalkyl
substituted with one or two of aL~cyl, aLlcoxy or halogen; phenyl; phenyl
substituted with 1-3 of aLkyl, aLkoxy, halogen, aLkanoylamino, or cyano; straight
or branched lower aLkenyl; s~aight or branched aLIcyl and such aL~yl substitutedwith 1-3 of the following: halogen; cyano; succinimido; glutarimido;
2 5 phthalimido; phthalirnidino; 2-pyrrolidono; cyclohexyl; phenyl; phenyl
substituted with aL~cyl, alkoxy, halogen, cyano, or aLlcylsulfamoyl; vinylsulfonyl;
acrylamido; sulfarnyl; benzoylsulfonicimido; alkylsulfonamido;
phenylsulfonamido; aL~cenylcarbonylamino; groups of the formula
O
Il
~ Y
N
~ C H 2
~, WO ~3~05443 ' " PC~r/US92/07165
_g_
wherin Y is -NEI-,
N alk y I
-O-, -S-, or -CH20-; -S-R14; SO2CEI2CH2SRI~; wherein R14 is alkyl, phenyl~
phenyl substituted with halogen, alkyl, alkoxy, alkanoylamino, or cyano, pyridyl,
pyrimidinyl, benzoxazolyl, benzimidazolyl, benzothiazolyl, or a radical of the
formulae
~ 10
N--N Rls
N
-NHX3R16; -CONRIsRls; and -SO2NRIsR1s; wherein Rls is selected forn H,
aryl, alkyl, and alkyl substituted with halogen, phenoxy, aryl, -CN, cycloalkyl,15 aLt~ylsulfonyl, aL~ylthio, or aL~coxy; X3 is -CO-, -COO-, or -S02-; E~16 is selected
~r from aLkyl and aLI~yl substitu~ed with halogen, phenoxy, aryl, cyano, cycloalkyl,
a~lcylsul~onyl, alkylthio, and alkoxy; and when X3 is -CO-, R16 also can be
;; hydrogen, arnino, alkenyl, alkylamino7 dialkylan~no, arylarnino, aryl, or furyl;
allcoxy; aLkoxy substituted with cyano or alkoxy; phenoxy; or phenoxy substituted
2 0 with 1-3 of alkyl, alkoxy, or halogen.
Preferably, X and Xl are independently selected from cyano,
carbamyl, N-aLcylcarbamyl, N-aL"yl-N-arylcarbamyl, N,N-dialkylcarbarnyl, N,N-
alkylarylcarbamyl, N-arylcarbamyl, N-cyclohexylcarbamyl, aryl, 2-benzoxazolyl,
2-benzothia~olyl, 2-benzin~idazolyl, 1,3,4-thidiazol-2-yl, 1,3,4-oxadiazol-2-yl,2 5 aLkylsulfonyl, arylsulfonyl or acyl.
l'he organic lir~ing represented by L is bonded to the adjacent
oxygen atorns through non-oxo carbon atorns, e.g., unsubstituted or subs~ituted
~methylene groups, a methylidene group and an unsubstituted methylene ~roup or
a nuclear carbon atom of a carbocyclic or heterocyclic aromatic group. Thus
3 0 linking group L is selected from a wide variety of C1-Cg alkylene, C3 C8
alkenylene, C3-C$ all~mylene, C3-C7 cycloalkylene, carbocyclic and
heterocyclic arylerle and cornbin dons of such divalent groups. The alkylene
:
.. .. . . .
WO 93/OSq43 2 ~ ~ 4 1 ~ ~ `
Pcr~U~92/07165~,
--10--
linlcing groups may contain within their main chain hetero atoms, e.g., oxygen
sulfur, sulfonyl, nitrogen, substituted nitrogen, and/or cyclic groups such as C3-
C7 cycloaLI~ylene, carbocyclic arylene, or divalent aromatic heterocyclic j~roups.
Examples of aL~ylene linking groups containingr a cyclic moiety in the linking
5 chain include:
alkylene ~ alkylene
alkylene-O ~ O-alkylene
alkylene ~ alkylene
,: :
N-N
olkylene ~ ~ olkylene
alkylene ~ olkylene
alkylene ~ ~ alkylene
, ...
alkylene-N ~ N-olkylene
.
, and
:
~,, ,, ,." .~ ;", 1;" ,~",.~ ,,,", ,~ , ", ~",, ,~ ~"'"~,"~", ,,, ;,, ~ , "~
~ WO93/0~l3 2.0~ 3; P~ US92/07J~j5
f 6H5
~N~
a I ky I ene-N~N-c I ky I ene
The carbocyclic arylene g~roups may be cycloaLcylene such as 1,2-, 1,3- and 1,4-cyclohexylene, 1,2-, 1,3- and 1,4-phenylene.and 2,6- and 2,7-naphthylene.
Examples of the divalent heterocyclic g~roups include nsubstituted and
substituted ~iazines such as 1,3,5-triazin-2,4-diyl, 6-methoxy-1,3,5-triazin-2,4-
diyl and the group having the structure:
~N~
N~,~N O
. O~C H C C O C H 3
~` lo CN
,
diazines such as 2,4-pyrimidindiyl, 6-methyl-2,4-pynmidindiyl, 6-phenyl-2,4-
py~imidindiyl, 3,6-pyridazindiyl and 2-methyl-3-oxo-4,5-pyridazindiyl;
dicyanopyridines such as 3,5-dicyano-2,6-pyridindiyl and 4-phenyl-3,5-cyano-
15 2,6-pyridindiyl; qu~nolines and isoquinolines such as 2,4-quinolindiyl and 2,8-
- isoquinolinediyl; quinoxalines such as 2,3-quinoxalindiyl; and azoles such as 2,5-
thiazoldiyl, 5-methylene-2-~hiazolyl, 3,5-isothiazoldiyl, 5-methylene-3-
isothiazolyl, 1,3,4-thiadiazol-2,5-diyl, 1,2,4-thiadiæol-3,5-diyl, 2,6-
benzothiazoldiyl, 2,5-benzoxazoldiyl, 2,6-benzimidazoldiyl, 6-methylene-2-
2 0 benzothia~olyl and the group having the structure:
WO 93/05q43
2 0 9 ~ 3 ~ PCr/US92/0716~
--12--
and maleimides such as 1 me~h~13,4 maleimiclediyl and 1-phenyl-3,4-
maleimidediyl. The acyclic moieties of the linking group represented by L also
may be substituted for example, with hydroxy, alkoxy, halogen, alkanoyloxy
5 cyano, aLkoxycarbonyl, aryl, aryloxy, cycloallcyl, etc. The cyclic moieties oflinking group L may be substihlted with aLcyl as well as with the substituents
already mentioned. In addition to the possible substitution described above, thenitrogen atom of the nitrogen containing aL'cylene groups may be substi~uted, for
example, with aLcyl, aryl, alkanoyl, aroyl, aL~ylsulfonyl, or carbamoyl, e.g.,
'
1 - aryl 12 --aryl
alkylene-N alkylene, alkylene-N alkylene
INH-- aryl
aryl I = O
alkylene-N alkylene, alkylene-N --alkylene
NIH ---alkyl NH - cyc loolkyl
i I-- I ::0
alkylene-N ---alkylene, alkylene-N -alkylene
f Ikyl
alkylene-N - alkylene
.
Ln the above def initions of L, the term "alkylene" represents a straight or
2 0 branched chain divalent hydrocarbon radical which contains from one to about
W0 93/05443 2 0 ~ r/US~2/07l65
eight carbon atoms. 'llle term "carbocylic arylene" refers to phenylenc radicalsand same substituted with Cl-C4 aLtcyl, Cl-C4 alkoxy or halogen.
In one embodiment, referring to the above formula for the uv
absorber, X is -C02R6 and R6 is an alkyl group having from l to about lO
5 carbon atoms, Rl is hydrogen, an alkyl group having from 1 to about lO carbon
atoms, or a substituted alkyl group having from l to about lO carbon atoms, and
R2 is an aL"yl group having from l to about lO carbon atoms.
In another embodiment, X is -C02R6 wherein R6 is an alkyl
group having from l to about lO carbon atoms, R2 is an alkyl group having from
10 l to about lO carbon atoms, and R1 has the forrnula:
C N
--L--o~3 CH=C
~ \X,
R30
wherein Xl is C02R6 and R6 is as defined hereinabove, and R3 is an aL~cyl
15 group having from l to about lO carbon atoms.
In yet another embodiment, Rl is hydrogen, an aL~yl group having
from l to abou~ lO carbon atoms, or a substituted alkyl group having from 1 to
about 10 carbon atoms, R2 is an aL~cyl group having from 1 to about lO carbon
atoms, and X has the formula:
NC
- C 2--L--C 0 2--C = H C ~=<~ O R "
OR3
wherein R3 is an aLcyl group having from l to about lO carbon atoms, and R~ is
hydrogen, an alkyl group having from l to about lO carbon atoms, or a
2 5 substituted aLkyl group having from 1 to about l û carbon atoms.
A suitable weight proportion of the ultraviole~ light absorbing
compound to the polyester is from about 200 to about 1600 parts per million. In
" ~, . ! ,. ~ ,, ` , ' , . ' ,
WO 93/05443 2 0 9 ~ 3 PCT/US92/0716
--14--
another embodiment, the weight proportion of the ultraviolet light absorbing
compound to the polyester is frorn about 400 to about 800 parts per million.
The intensifying screens employed in the invention are capable of
emitting substantially witl~in~the ultraviolet spectral range, i.e. at about 400 nm.
5 or less. Such screens are well known in the art and are s~escribed, for exarnple, in
U.S. Pat. No. 3,822,131, issued July 2, 1974, to Cleare, and Belgian Pat.703,998,
issued Mar. 18, 1968, to Luckey.
The silver halide emulsion layel may contain varying amounts of
silver chloride, silver iodide, silver bromide, silver chlorobromide, silver
1 0 bromoiodide and the like. Conventional overcoat layers may be employed to
protect the emulsion layers from darnage such as abrasion and scratches.
; The radiographic elements can contain additional conventional
features. Referring to Rese~ch DisclQsL~re, item 18431, cited above, the
ernulsion layer units can contain stabiliærs, antifoggants, and antildnlcing agents
1 5 of the type set forth in Section II, and the overcoat layers can contain any of
variety of conventional addenda of the type set forth in Section IV. The
outermost layers of the radiographic element can also contain matting agents of
the type set out in Researçh Disclos~re, Vol. 308, Dec. 1989, Item 308119,
Section XVI. Referring further to Re~h Di~osurs, Item 308119,
2 0 incorporation of the coating aids of Section XI, and the antistatic layers of
Section xm, are each contemplated.
Referring to FIG. 1, in the assembly shown a radiographic element
100 according to this invention is positioned behveen a pair of ultraviolet light-
emitting intensifying screens 102 and 104. The radiographic element 100 is
2 5 comprised of a support 106, typically transparent or blue tinted, capable of
transmitting at least a portion of the light to which it is exposed and optional,
similarly transmissive under layer or sub layer units 108 and 110. Ln the depicted
embodiment, on the first and second opposed major faces 112 and 114 of the
support forrned by under layer units 108 and 110 are crossover reducing layers
3 0 116 and 118, respectively, each layer having adm~xed therein an ultraviolet light-
absorbing oompound of the invention. Silver halide ul~aviolet light-sensitive
emulsion layers 120 and 122 are respectively disposed on crossover reducing
layers 116 and 118.
In anotber embodiment of the invention in wbich the ultraviolet
3 5 light absorbing oompound of the invention is admLl~ed in the support, silver halide
emulsion layers 120 and I22 are disposed on faces 112 and 114 respectively
without such under layer units 108 and 110 respectively interposed therebetween.
WO 93~054~l3 2 ~ 9 ~ 1 ~ 3 PC~/US~/0,l65
--15--
In use, the assembly is irnagewise exposed to X radiation. The X
radiation is principally absorbed by intensifying screens 102 and 104, which
promptly emit ultraviolet light as a direct function of X ray exposure.
Considering first the light emitted by screen 102, the light recording, ultraviolet
5 light-sensitive, latent image-forming layer unit 120 is positioned adjacent this
screen to receive the light which it emits. Because of the proximity of screen 102
to emulsion layer unit 120 only n~inimal light scattering occurs before latent
image-forming absorption occurs in emulsion layer 120. Hence light emission
from screen 102 forms a sharp image in emulsion layer 120. However, not all of
1 0 the light en~itted by screen 102 is absorbed within emulsion layer 120. This
remaining light, unless otherwise absorbed, will reach remote emulsion layer unit
122, resulting in a highly unsharp image being formed in this remote emulsion
layer unit. Both crossover reducing layers 116 and 118 are interposed between
screen 102 and emulsion layer 122 and are capable of intercepting and attenuating
1 5 this remaining light. Both of these layers thereby contribute to reducing
crossover exposure of emulsion layer 122 by screen 102. In an exactly analogous
manner, screen 104 produces a sharp image in light recording, ultraviolet light-sensitive emulsion layer unit 122, and ultraviolet light-absorbing layers 108 and
110 similarly reduce crossover exposure of emulsion layer unit 120 by the
2 0 ultraviolet light ernitted by screen 104.
Following exposure to produce a stored la~ent image, ~adiographic
element 100 can be removed from association with intensifying screens 102 and
104 and processed in a suitable readily available processor of a type well knownin the art.
2 5 The radiographic elements of the present invention make possible
the unique cornbination of advantages set forth above by employing an ultraviolet
light-absorbing compound of the inven~ion as described above admixed in a
orossover redueing layer or in the support.
The preparation and the properties of the radiographic element of
3 0 tlle invention is illustrated by the following examples. Descriptions and/or
preparation of some ultraviolet light absorbing ~ompounds of the invention may
be found in U.S. Pat. :Nos. 4,617,374, 4,749,774, and 4,994,512. The preparationof some of the ultraviolet light-absorbing compounds of the invention is also
illustrated by the following examples.
PR~P~13~F C~I~OUNDS
- ;, .. .,. , . . . , . ~ . ~ .
.. :: . ~ , , . . : . , .
. . ... . . . . . . . . .
,; . . . . , ,: , ~ . .
W0 93/05443 2 ~ 9 ~ I ~ 3 ~ PC~/US92/07165
~)
--16--
The intermediate`~aldehydes useful in preparing the compounds
used in the practice of the invention are l~own or are prepared by known
methods. For example, intermediate aldehydes A (below) are prepared by well-
known reactions such as those described in H. Szmant, "Organic Building Blocks
5 of the Chemical Industry"7 John Wiley and Sons, New York (1989), pp 234 and
514). In one method, catechol is monoalkylated by reacting with suitable
alkylating agents such as alkyl halides, aL~cyl sul~Ltes7 and aL~cyl phosphates7 and
the like7 to give catechol monoaLkyl ethers7 which undergo the Reimer-Tie,nan
reaction to give intermediate aldehydes A7 as follows:
OH OH OH
~3~ ~ ,-. //\,~OR
C H O
o R=a I ky I
(A)
Aldehydes A may also be prepared by reacting piperonal with HB7r
to give 3,4-dihydroxybenzaldehyde which may be alkylated with suitable
aLlcylating agents to produce A as follows:
o~ClH2 OH OH
Agent ~3/
CHO CHO
C H O
R=a I ky I
Piperonal (A)
These aldehydes A may be used directly to prepare W absorbing
2 0 compounds usefi~ the practice of the invention or may be reacted further to
., . ~ ,, ,. ~ : . .. . . . . : .. .
WO 93/0~443 2 0 9 A 1`4 ~ ` ~; Pcr/US~2/07165
--17--
produce useful, more highly substituted benzaldehydes, as in Exarnple 1 of U.S.
Pat. No~ 4,707,537 (supra), and as in the examples below.
Methods of preparation of bis-aldehydes useful in preparing bis-
methine compounds useful in the practice of the invention are described in I J.S.
Pat. No.s 4,749,774 (supra) and 4,994,512 (supra).
Methods useful in reacting the inter nediate aldehydes with the
active methylene compounds are well known and are described in the above
patents and the examples below.
~1
Preparation of Methvl 2-C~lano-3-f4-HvdroxY-3 Methoxvl2henyl2~2-Propenoat_
A mixture of vanillin (7.60 g, 0.05 mol), methyl cyanoacetate
(4.95 g, 0.05 mol), ethanol (50 rnL), piperidine (12 drops) and acetic acid (6
drops) is heated at reflux for 3 hours with stirring and then cooled. The pale
yellow product crystallizes and additional ethanol (25 mL) is added to promote
stining and ~% HCl (10 rnL) is added to neutralize any phenol salt present, The
product, methyl 2-cyano-3-(4-hydroxy-3-methoxyphenyl)-2-propenoate, is
2 0 collected by filtration, washed with ethanol and dried in air. A yield of 9.4 g
(80.6%) is obtained. The identity of the product is supported by mass
spectroscopy analysis and is shown below as compound I.
~C N
H 0 C H C
C 0 2 C H 3
CH30
f~I)
~2
3 0 ep~tion of 4-(1~01z~1Oxy)-~-~ho~v-Benzald~hvde
.~ - - - - -- . . .
WO ~3/OS~43 2 0 ~ 4 ~ ~L 3 PCI/US~2/0716~
--18--
Sodium hydroxide (2.4 g) is dissolved in water (50 rnL) by
stirring. Vanillin (7.60 g, 0.05 mol) and c~-chloro-toluene (7.60 g, 0.06 mol) are
added and the reaction mixture is heated at reflux with stirring for 16 hours. The
product is extracted from the aqueous mixture using methylene chloride (10 rnL),5 When the solvent is removed under vacuum an oil results which cIystallizes when
treated with isopropyl alcohol and stirred. Mass speetrometry supports the
following structure:
~CH20 ~ CHO
C H 3 0
Exam~le 3,
Prepa,~on ~f MethYI 3-(4-Benzy!QxY-3_MethQx,Yphenvl)-2-Cyano-2-PrQFenoate
A mixture of 4-(benzyloxy)-3-methoxybenzaldehyde (1.21 g,
0.005 mol), methyl cyanoacetate (0.50 g, 0.005 mol) methanol (10.0 rnL) and
piperidine (5 drops) is heated and stiIred at reflux for 1 hour and cooled. The
product, 3-(4-benzyloxy-3-methoxyphenyl)-2-cyano-2-propenoate is collected by
f;ltration, washed with methanol and dried in air. The yield is 1.50 g (93%) of
2 0 product having an absolption maximum at 364 nm in methylene,chloride
solution. The identity of the product is supported by mass spectrometry.
Example 4
~ation of Methvl 2-C~ano-~Hydrox~ethQxy~methoxy~hQnyll-2-
~ .
A rr~xtuxe of 4-(2-hydroxyethoxy)-3-methoxybenzaldehyde (0.98
g, 0.005 rnol), ( see Example 1 of U.S. Pat. No. 4.707,537, supra) methyl
3 0 cyanoacetate (0:50 g, 0.005 mol), piperidine (3 drops) and methanol (10 mL) is
heated and stirred at reflux for 1 hour and cooled. The pale yellow product is
, . . .
.. ' ~. , ~ . ' ,
W~ 93/OS443 2 0 9 ~ ~ q 3 1
PC~/US9~/07165
-19-
collected by filtration, washed with methanol and dried in air. A yield of 1.0 g(72%) of product is obtained which has ~e follow~ng structure as supported by
mass spectrometry:
HOCH2CH20--~CH = C
CH30 C02CH3
l~e compolmd has an absorption maximum at 365 nm in methylene chloride.
~m~
Prep~rationQf Elhvl 2-Cvano~ Hydroxy-4-Methoxyph~nyl-2-prQpenQa~e
A mixture of 3-hydroxy-4- methoxybenzaldehyde (isovanillin)
(1.52 g, O.ûl mol), ethyl cyanoacetate (1.13 g, 0.01 mol), ethanol (20.0 rnL) and
piperidine (S drops) is heated at reflux for 1 hour. The reaction mixture is cooled
and acidified to pH of about 4-5 with 10% HCl to produce the product which is
collected by filtration, washed with ethanol and air dried. A yield of 1.36 g
(55~) of product is obtained which collesponds to the follow~ng structure as
supported by mass spec~ome~y:
CN
CH30~--CH = C
~ \
H0 C2C2Hs
The compound has an absorption ma~irnum (~ max) a~ 354 nm in methylene
chloride solution.
2 0 9 ~ ~ ~ 3 PC~/US~2/~)7~~
-20--
~ !
Prepara~aQ~Qf Methvl 2-cya-nll-3-(3~4-Dimet-hoxyph-en-yl!2-prQRenoa~
A mixture of 3,4-dimethoxybenzaldehyde (4.15 g, 0.025 mol), I
methyl cyanoacetate (2.48 g, 0.025 mol), methanol (lO0 rnL) and piperidine (10
drops) is heated at reflux for 2 hours. The reaction mixture is then cooled and the
product collected by filtration, washed with methanol and dried in air (yield - 6.0
g, 97%). The following structure is supported by mass spectrometry: i
1 0
. . ,
CN
C H 3 0 ~ H C ~
CH30 C02CH3
-
The compound has an absorption max;imum at 361 nm in methylene chloride.
1 S l~xampl~ 7
Prep~aratinn of Ethvl 2-C~o-3-~arbethoxymethox~-3-Methoxy~henvl)-2-
~a~
2 0 A mixture of 4-carbethoxymethoxy-3-methoxybellzaldehyde (23.8
g, O.I0 mol), ethyl cyanoacetate (11.3 g, 0.10 mol), ethanol (150 mL) and
piperidine (2 mL) is heated at reflux for 4 hours. The reaction mixture is
allowed to cool, and the solid is collected by filtration, washed with ethanol and
dried in air. A yield of 31.3 g (93%) of product is obtained which has the
2 5 following structure as supported by mass spectrometry:
W0 93/OS4~3 2 0 9 ~ I ~ 3
. , Pcr/uss2/07l6s
-2~
C~HsOCCH20 <~ ~ CH = C
CHJO \CO2C2Hs
An a'osorption maximum at 355 nm is observed when the product is dissolved in
methylene chloride.
:: ~x~m~
Preparàti,on .n,f 3-r4(2 Hy~droxvethcxy!-3-MelhQxYphenYll-2-MethYlsulfQn
Propene,,N~ile
, 10
; A mixture of 4-(2-hydroxyethoxy)-3-methoxybenzaldehyde (0.98
g, 0.005 mol), (see Example 1 of U.S. Pat. No.4,707,537, supra),
methylsulfonylacetonitrile (().60 g, 0.00~ m), ethanol (10 mL) and piperidine (5drops) is heated at reflux for 1 hour, during which ~rne the product crystallizes.
15 The reaction mixtl3~ is allowed to cool and the very pale yellow product is
collected by filtration, washed with ethanol and dried in a~r. The yield of product
is 1.05 g (70.9%). Mass spectrometry supports the following structure:
.
'~
CN
HOC2H40~ CH = C~
S~2CH~
CH30
~o
n methylene chlonde d~e product has tm absolphon maximum at 362 nm.
:.
WO 93/1)5443 2 0 9 ~ t ~ 3 PCr/US!)2/~7165 "~
--22--
l~xampl~, 9
Pre~aranQn Qf 2-(~yano-3-(4-Hydrn~v-~-Mçth~xypheny~ namidç
A reac~ion mixture of vanillin (1.52 g, 0.01 mol), a-
cyanoacetamide (0.84 g, 0.01 mol), ethanol (25 mL) and piperidine (6 drops) is
heated at reflux for 1 hour and then allowed to cool to room temperature. The
reaction nixture is acidifled to pH of about 3-4 by addition of a few drops of
conce~ltrated HCl. The product which is collected by filtration, washed with
ethanol and dried in air has the following structure supported by mass
spectrometry:
.
C N
H 0 )/i3C H C
CH30 CONH2
The yield of product is 1.5 g (68.8%). In methylene chloride ~he product has an
absorp~ion maximum of 352 nm.
ExamplQ10
2 0 A reaction mixt~re of vanillin (1.96 g, 0.01 mol), 1,2-ethanediyl
bis(o~-cyanoacetate) (3.V4 g, 0.02 mol), N,N-dimethylforma nide (10 mL) and
piperidine acetate (0.2 g) is heated and s~rred at 90-95C for 1.5 hour, allowed to
cool to room temperature, and drowned into methanol (100 rnL). The pH is
adjusted to 3-4 by addition of conc. HCl and the solid product is collected by
2 5 filtration, washed with methanol and dried in ~ur. ~ yield of 2.1 g of product
having the struct~ure
H O--Ç~ C H _ C C _ H C ~ O H
CH30 Co2cH2cH2o2c OCH~
,; ; , : - .
': ' ,~"' ' ~;, ' ', . ', ' . '
-, , ,, :. ` ,
' ' ' : ': ' , ~' , ' : ' , . ,
~ W093~0~ 2Qg~1~3~ r/us~2/07~6s
I
-23-
as evidenced by NMR and mass spectrum analyses is obtained. In methylene
chloride the compound bas an absorption maximum of 365 nm and an extinction
coef~lcient of 40,490.
Example 1 1
To a solution of vanillin (4-hydroxy-3-methuxybenzaldehyde, 91.2
g, 0.60 mol) dissolved in water (500 rnL) containing sodium hydroxide (24.0 g,
0.60 mol) is added 1,2-dibromoethane (56.7 g, 0.30 mol) and the reaction mixtureis stirred and heated to reflux for 8 hours and tllen allowed to cool. The product,
4,4'-[(1,2-ethanediyl)bis(oxy)bis(3-methoxybenzaldehyde)], is collected by
filtration, washed with water and d~ied in air. A yield of 67.5 g (Gg%) of a
slightly gray solid is obtained. The identity of the product is supported by mass
15 spectroscopy analysis.
A rnixture of 4,4'-[(1,2-ethanediyl)bis(oxy)bis(3-
- methoxybenzaldehyde)] (49.5 g, 0.15 mol), methyl cyanoacetate (30 g, 0.30 mol),
N,N-dimethylformarnide (500 mL), piperidine (3 rnL) and acetic acid (1 mL) is
- heated with stirring at about 100 C. for 2 hours. The reaction rnixture is allowed
2 0 to cool to room temperature and the pale yellow solid which forms is co~lected by
filtration and washed with methanol. The crude product is reslurried in 500 rnL
of boiling methanol, collected by filtration, washed with methanol and dried in
air. A yield of 59.4 g (81%) of dimethyl 3,3'~[(1,2-ethanediyl)bis(oxy)bis(3-
methoxy-4, 1-phenylene)-bis(2-cyano-2-propenoate)] having the forrnul~:
CN~OC--C_NC~OCH,CH~o~C11=C--COCH
OCH3 CH30
~)
and designated as compound II is obtained.
3 0 Additional examples of methine compounds which may be used in
the preparation of the X ray elements of the inven~ion are set forth in the
following tables in which the compounds of each table conform to the structural
WO 93/05443 ~ 3~
2 ~ PCI`/lJS9~/07~65
~ .~
fonnula immediately preceding each such table. These compounds may be
prepared according to the procedures described above.
,
.
::
W~ 93/054'~3
2 0 ~ 3 P~T/US~2,07l65
--25-- i
TABLE 1
NC\ ,~
C=HC }~OR,
OR2
Exr.
-- ~ -C~3
13 -CO2C6Hl I-C4H9-n -CH3
14
--C02CH2CH=CH2 --CH2CH--CH2 -CH3
1 s -co2cH2cH2oH -C6H5 -c~3
16 -co2cH2c6H5 -CH2C6H5 ~ 2C6HS
] 7 -co2cH2cH2cN C6H 11 -CH3
18
--Co2cH2c -CH -C~t2C6~~ -H
19 -C02CH2CH(C2H5)C4Hg-n -(C~32)1 ICH3 -CH3
20 -CO2C6H5 -(CH2)18c~t3 -CH3
22 -CO2C5Hg -c~2cH2oH -C6Hl I
-CO2cH3 --C H 2 C H ~ C H 2 -CH3
23
-CO2CH3 -c~t3 --C H 2 C aC H
24 -C02CH3 -CH2C6H4-P~02C}~3 -ct~3
25 -C02CH3 -~6H4-P-C02CH3 -~H3
26 C02CH2CH2CI -CH2CH2OCoCH3 -CH3
27 -CO2CH2c~2Oc6H5 ~H2~H2oc6Hs -CH3
28 -CO2CH2C}~2CN -C~t2C~2Ct -CH3
29
-C02C112CH(OH)CH20H --CU~CN~N~ -CH3
o
30 -SO2CH3 -CH2OcO2c2Hs ~H3
31 -SO2C6H~ -CH2CH2OCOCH3 ~3
32 -SO2C6H4-P C~ CH2c6H4-3~o2~3 -CH3
33 -SO2C~ p OCH3 . -H . -cH2cH2oH
34 ~ONE12 ~H2CH20H
35 ~ON~C6Hs -CH2OCO2C~H~ CH3
36 -CONHC6~l I CH2CH2~H2(~H2OH CH3
37 ~NHc~2c6Hs ~H~C}~2QC0(~3 -CH2C~120COCH3
38 -C02CEI3 C6H4-~cH3 ~13
39 -C02CH3 ~H3 CH2c6H4-}~co2cH3
SUB5mUTE SHEE7
2 0 9 ~ PC~`/US92/07165
-2~
TABLE 2
C=HC (~--O--L--O ~ CH=C~
O R 2 O R 3
EXP. ~
-CO2CH-3 - - -CH2CH2 -`- R2~R3
41 -CO2cH3 (-CH2-)4 -C~H5
42 -CO~CH3 (-CH2-)8 - -H
43 -CO2C2H5 -CH2CH2OCH2CH~- -C4Hg-~
~ -co2(cH2)llcH3 CH~C6H4-P-CH2- -CH3
-co~cH2c6Hlo-pCH~OH -cH2c(c~3)cH2- -CH~
47 -Co2cH2cH2oc6Hs -CH2CH2sO2cH~cH2- -CH2C6H5
~: C02CH2C = CH -CH2CH2N(sO2cH3)cH2cH2- -CH2C6HII
48 -CO2CH2cH2OcH2cH2oH -cH2cH2scH2cH2- -C2H5
49 -CO2CH2CH2scH2cH2OH -cH2c6Hlo-~cH2- -CH3
-Co2cH2c(cH3)cH2oH CH2CH2OC6H4-~OcH2cH2 -CH3
51 -co~cH2c~(cH3)cH2oH -CH2CH2OCO(CH2)4OCOCH2CH- -CH3
2 -CO2CH2CH(CH3)2 -CH2CH2OCOC6H4-m-cO2cH2cH2- -CH3
53 -SO2CH3 -cH2cH2oc~(cH~)2ococH2cH2- -CH3
54 -SO2c6Hs -CH2cH(o~cH2- -CH3
-SO2C6H3-3~4~iCl -CH2CH(OcOcH3)cH2- -CH3
56 -SO2C4H9-n -CH2CH(CH2O~- -CH3
* 57 -CONH2 -CH2CH2CH(O~CH2cH2- -CH3
58 -CONHC6H5 -cH2cH(o~cH2- -CH3
, 59 -CONHC6H4-~OCH3 -CH2CH2- -CH2CO~C2H5
-CO~(CH3)C6H5 (-CH2-)4 -c~2c6H4-p-co2cH3
62 -CON(C2H5)C6HIl -cH2c6H4-p~H2- -CH2COOH
- C O N H C H 2 C H _ C H ~ -CH2CH2CH(O~C~2- -CH3
63 -coNHc7Hl3 -CH2CH2cH(Oco~c2Hs)cH2- -CH3
64 -coN~c6H4-o~cH3 -CH2CH2CH(OH)CH2- -CH3
-CONHC6H3-2-OCH3-S-CH3 ~H2CH(O~CH2- -CH3
66 -CONHC3H5 -CH2CH(~C~l2- -CH2C6H
67 -CONH~ H4-p~oc6Hs -CH2CH(OH~CH2- -CH3
SlJE~STlTUTE 5HEE~
., .. , . ., , , . , . ~ .. . . .
WO 93/05~43 2 ~ 9 ~ 1
3~cr/usg2/o7l6
--27--
~ ~ a~ a~
z
â~ ~ ~' a~
z
\c~/
~ ~ I ~ :
$~ ~ e ~ ~ $ ~ I ~ $
z ~ ~ O t~
.
~ .
~ .
. . . .
WC) ~3/05443 ~ O ~
1 ~ 3` pcr/~s~2/o7~
-2
. ~ a, ~ a
o ~ .. ,.'
J
/ ~ ~ u $o o o O o 0 8
i, ~ 1
# Iv a, ~ ~ ~ 3 a, mr' a ~ a,
a~ 3 ~39
:;:
~ : ~ Z a~ o _ ~ ~
, .
.;,:, :', ., ., ,. . , . . .' :. ., .: . ': . ' : :
WO 93/05443 '2 ~ 4 3 P~/IJS92/07165
5~ 2~
~ 1
C ~
-
~:
O C~l
C~ V
X ~q r~ q ~ U
' '
,'
K~?0~;8 ~ ~Z8~ ~
~ k1
- ~ Z ~
... ... . .
WO 93/0~43 2 0 9 ~ 3
P(~/US~/07165~
,
~:
X ~ C:
\c~/ D~^ U U, U ~ U, O ~ U ~,
11
I
., o ~ ~
~ { a ~,
T'
¦ ¦ ;
C ~ A ~
~ 0 rl $ 8 ~ $
o o o ~ ~ ~ o o o _ _
~ ~ .
:: :
WO 93/OSM3 2 0 9 ~il 4 3
~$ , ~. P~r~Uss2/o7l6s
--31--
_ ~ U ~, U ~ , U
` /
U U ~~ U ~ U U U
~ I
O ~ /~
.
X~ ~ o ~ ~
:
WO 93/~5443
~ O` ~ 3 PCr/~J~2/07165, f
- 3 2 - C~;
a ~a
.....
~.................. .,,
~o .
oo 2 >~
o~æ~, æ sæ æ
~c
a~ 3 a~ 3
jJ
'~ ~
Z ~ o _
W~ 93/054~3 2 o~g ~ ~ 9 ~ ; PCI'/US~2~07165
.~ .
~ ~ ~ .
.~
,
,' ~
~ . T ~5
'_` ' qC: I ~
.' ~
~0
, ~
$ ' ..
..'' .. ..
~`
; ~ ~
. '
~,~ ~ b~ ~
~ ~ ~ ,
:::
.. . .,; .... ,, . ., . . , " , .. .. . .
i - , . ., ` , ; .. . ;. ,... " ~, ,. . . ' ..... ;
`......... , .. ,.. ,- .. -, .
~V0 93/05443 2 ~9 ~ 1 4 ~ PCr/US~2~07165~ ~
-3~-
~x~mples 1~4-143
Preparation of polyester sllpport with uv absorber:
A 7 mil thick clear poly (ethylene terephthalate) ("PET") support
is prepared as a control, and 7 mil thick PET supports for f~s of the invention
5 are prepared as follows:
Poly (ethylene terephthalate) granules (I.V. between about 0.55
and about 0.70 dl/g=about 0.63 as measured in a 60:40 percent by weight solutionof phenol and chlorobenzene at 25 C) are vacuum dried and dry blended with
800 ppm by weight of a uv light-absorbing compound (identified below). The
blend is dried overnight (16 hours) in a vacuum oven at 110 C. After drying, the
material is melt blended and extruded into 7 rnil film on a 1 inch Killion extruder
(25 to 1 L/D).
Filrn support 1: Control PET 7 mil thick film sample (containing no uv absorbing1 5 compound).
Film support 2: contains the compound of EX. 1 hereinafter identified as
"Compound I).
FIG. 2 is the characteristic absorption curve of compound I.
Film support 3 contains the compound of EX 11 hereinafter identified as
"Compound II".
FIG. 3 is the characteristic absorption curve of compound II.
2 5 ~lm support 4 (Comparison) contains an ultraviolet light-absorbing compound
` ~ having the structure
., ~
NO2 ~CN
(~C H=~ )~ 0
3 0 tm)
FIG. 4 is the characteristic absorption curve of compound m.
. ~.. - .... ~ . . .. ... . .. . . ..
WO 9~/05M3 2 0 9 ~ 1 4 3 P~r/usg2/o7l6~ ¦
--35--
Pilrn support S (Comparative example)
contains an ul~aviolet .light-absorbing eompound of the invention having ~he
structure
.
[~ ,CN
~` ~CH=~o
. ~ .
FIG. S is the characteristic absorption curve of compound IV.
Additional 7 mil thick PET film supports are also prepared with
the above compounds and also containing 250 ppnn by weight of a blue dye, 1,4-
bis(2,6-diethylanilino)-anthraquinone, admixed with each such PET SUppOIt.
Transmission spectra are obt~ined for each sample using standard
techniques. The results are shown in FIGs. 6 and 7 and Table 9 as ~ollows:
TAB1~3 9
EXAMP~ NO~: 7.mil P:E~TFilm Suppo~r~No.: ontainin~ 800 ppm of Compound:
134 l None (control)
135 2 II
136 3
137 4 III
138 5 IV
139 6None (control) + 250 ppm blue dye
140 7II ~ 250 ppm blue dye
141 8I + 250 ppm blue dye
142 9m -~ 250 ppm blue dye
143 10IV -~ 250 ppm blue dye
WO 93/0~43 2 0 9 4
P~r/aJs~2/07l~js,~,~
, --36--
Colorirnetly~d~lta is obtained us~ng standard techniques and the
results are shown in Table 10, below. The terrn "B*" is the Tone value.
TABLE ~
Fi]m Supp~ort B*
0.45
2 1.11
3 1.07
4 5.13
1.92
6 -16.20
7 -15.50
8 -lS.70
9 -10.40
-12.70
PIG.S 6 and 7 of percent tr~nsmission for the same weight
proportion of each uv absorber in the PET support, with and without 2S0 ppm by
weight of 1,4-bis(2,6-diethyLanilino)-anthraquinone blue dye, show that the
SUppOltS containing compound I and compolmd II exhibit better absorption in the
ultraviolet range of about 350 nm to about 395 nm than does compound m at the
same weight propor~on of 800 ppm. The suppor~s containing compounds I and II
also show less unwanted absoIption in the visible range above 400 nm. As a
result, the poly (ethylene terephthalate) suppo~s containing compounds I and ~I
can employ a lower weight percent of uv absorber than a support containing
compound m for a similar amount of uv absorption. A support containing an
t ultravioIet light-absorbing compound of the invention therefore exhibits less
undesirable yellow hue than a support containing compound m.
~e colorirnetry results in Table 10 can be interpreted in that the
more negative the value of the tone (B*), the colder is the tone and hence the
2 0 mo~ desirable is the support. A "Just Noticeable Differençe" tJND) is 0.7. Thus
Ln Table 10 there is no noticeable difference between support 2 and 3, and the
good tone of the support con~aining compound I is not noticeably different than
the support containing compound II which also exhibits good tone. When the
colonmetry results of ~tlm supports 2 and 3, containing compounds I and II,
2 5 re~ectively, a~e compared to film supports 4 and S, respectively, it is noted tha~
the film supports which contam the prior art compounds have significantly more
.~ .
WO 93~0~3 2 0 9 ~1~ 3 ~ PCr/US~2/0716~
--37--
objectionable yellow color, i.e. a more positive value of B*. The sarne results are
obtained even when the blue toner dye is added (film supports 7 and 8 as
compared to film supports 9 and 10, respechvely7 in Table 10).
5 ~m~9~L
7he 7 mil PET suppor~s of EXS. I and l l respectively containing
cornpound I and compound Il are each coated with a standard silYer halide
emulsion layer on each side of the support and are each assembled into a
radio~raphic element of the invention. Each elen-lent is tested for stated loadings
1 0 of absorber compound for speed, percent crossover, and percent transmittance in
the wavelength ran~es of 350-395 nrn and 400-430 nm and the results shown in
Table 11, below, and in FIG. 8. Table 11 shows the percent transmittance in the
stated wavelength ranges for the stated absorber loadings. FIG. 8 graphs speed,
percent crossover, and percent transrnittance at 380 nm.
Both radiographic elements exhibit good crossover reduction while
maintaining good film speed, and exhibited good ~ansmittance, especially in the
range of about 400 ppm to about 800 ppm.
TABLI~ 11
Element containing Loading (ppm) Percent Percent
Compound: Transmittance Transmittance
(350-395 nm) (400-430 nm)
200 39.00 74.42
4~0 21.83 73.54
800 5.95 63.32
1600 1.03 56.17
2 200 39.3~ 75.66
2 40Q 23.48 71.51
2 800 ~.00 62.51
2 1600 2.40 57.61
SlJBSTlTlJTE SHFFr
~, . . ~ . . . .
WO 93/05443 2 0 9 ~ PCr/1)5!~2/07165~;
--38--
Industrial Appli~abilit~r
The radiographic irnage-recording element of the invention
provides a substantially yellow-free image,recording material useful in
5 medical and other applications. The erx?osed, de veloped image is easier to
read because it provides better cont;st and a sharper image than other
radiographic elements. This in turn should lead to improved information-
collection from the imaged material, as for example improving diagnostic
capability in medical applications.
This invention has been described above with particular
reference to preferred embodiments. A skilled practi~oner familiar with the
detailed description above, can make many substitutions and modifications
without departing from the scope and spirit of the appended claims.