Note: Descriptions are shown in the official language in which they were submitted.
20~3~33
.Z. 0050/42408
Substituted pyrido r 2 3-dlpyrLmLdLne-2,~(lH~3Hl-di~ne~
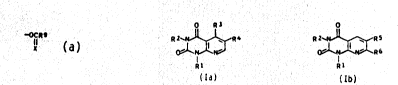
The present invention relate~ to pyrido[2,3-
d]pyrimidine-2,4(1H,3H)-dione~ of the ~ormulae Ia and Ib
O R3 0
R 2~,~R 4 R 2~R S
ol I N R6
Rl Rl
Ia rb
where
R , R are hydrogen, Cl-C4-alkyl, Cl-C4-haloalkyl or
C3-C7-cyC loalkyl;
R is hydrogen, Cl-C4-alXyl, amino, hydroxyl,
: mercapto, C1-C4-alkylthio or Cs~ or C1O-aryl
which can carry one to three of the groups
. mentioned for R1l;
R4 i hydrogen,C1-C~-alkyl,Cl-C4-alkoxycarbonyl,
. Cl-C~-(alkylthio)carbonyl, Cl-Cj-alkoxy(thio-
-. carbonyl), C1-C~-(alkylthio)thiocarbonyl,
: 15 cyano or ~m i nocarbonyl; or
. R3 and R~ together are C3-C6-alkylene, C3-Cs-alkenylene
or 1,2-phenylene;
R5 i~ hydrogen, C1-C4-alXyl, cyano, C(O)R' or
C(S)R7;
R8 i8 Cl-C4-alkyl, C8-aryl-C2-C~-alkenyl, Cg~l Clo~
or C1~-aryl, it being possible for each aryl
to carry one to three of the group~ mentioned
for R11, or i~ C~2Ra, C1-C~-alkoxycarbonyl,
Cl-C~-(alkylthlo)carbonyl, C1-C~-alkoxy(thio-
carbonyl), C1-C~-(alkylthio)thiocarbonyl,
hydroxyl, mercapto, C1-C4-alkoxy, C1-C~-alkyl-
thio, amino, Cl-C~-alkylamino, NHCXR9,
: NHSO2Rl, 2-thienyl, 3-thienyl, 2-pyridinyl,
3-pyridinyl, 4-pyridinyl, 2-indolyl,
3-indolyl, it being possible for each hetaryl
to be sub~tituted once or twice by the groups
; '
2~9~43'3
- 2 - O.Z. 0050/42008
mentioned for Rll; or
R5 and R6 together are C3-C~-alkylene, C3-C5-alkenylene
or 1,2-phenylene, each of whlch can be
substituted by Cl-C4-alkyl, Cl-C4-haloalkyl or
C3-C7-cycloalkyl or can al~o contain carbonyl
or thiocarbonyl groups;
~: R7 is hydrogen, Cl-C4-al~yl, hydroxyl, mercapto,
Cl-C4-alkoxy, Cl-C4-alkylthio, Cl-C,-alkoxy-
; carbonyl, C~-C~-(alkylthio)carbonyl, Cl-C4-
alkoxy(thiocarbonyl), Cl-C4-(alkylthio)thio-
car~onyl, amino, Cl-C~-alkylamino, di-(Cl-C4-
alkyl)amino, C~- or C1O-aryl or C5- or C10-
arylamino, it being possible for each aryl to
carry one to three of the groups mentioned -
for Rll;
Ra is halogen, hydroxyl, mercapto, Cl-C4-alkoxy,
Cl-C,-alkylthio, Cl-C~-alkoxycarbonyl, Cl-C,-
^~ (alkylthio)carbonyl, Cl-C~-alkoxy(thio-
carbonyl), C~-C,-(alkylthio)thiocarbonyl or
-OCR9
Ra i8 C1-C5-alkyl, C1-C~-haloalkyl, NR1R2 or OR1;
Rl i8 Cl-C,-alkyl, C~- or C1O-aryl which can carry
one to three of the groups mentioned for Rll;
Rll i8 halogen, hydroxyl, mercapto, Cl-C~-alkoxy,
C1-C,-alkylthio, C1-C,-alXyl, nitro or amino;
X i3 oxygen or sulfur;
with the provi~o that
a) R1 and R2 are not both H, methyl or ethyl,
b) R3 and R~ are not both H,
c) R5 and R are not both H,
d) if R1 i9 methyl, R2 i8 i-butyl and R3 i8 amino, R4 i8
not H,
e) if R1 iS methyl, R2 is H and R5 i3 phenyl, R~ i8 not
hydroxyl,
2093433
- 3 - O.Z. 0050/42008
f) if R1 is methyl, R2 is H and R~ i8 amino, R5 ~ 8 not
carboxyl or ethoxycarbonyl,
g) if Rl is H, RZ is methyl and R5 i~ H, RB L~ not
hydroxyl,
5 h) if Rl is H, R2 is methyl and R4 is ethoxycarbonyl, R6
is not methyl or amino,
i) if R5 is cyano, R~ i~ hydroxyl and Rl is n-butyl, R2
is not H,
j) if R5 is cyano, R~ is hydroxyl and R1 is n-propyl, R2
; 10 is not n-propyl,
and the agriculturally usable salts of those compounds
Ia and Ib which contain a bacic nitrogen substituent or
an acidic hydroxyl substituent.
The present invention also relates to processes
: 15 for preparing the compounds Ia and Ib and to herbicidal
agents which contain 2-(4-hetaryloxy)- or 2-(4-aryloxy)-
phenoxyacetic or -propionic acid derivatives and~or
cyclohexenone derivatives as herbicidal acti~e ingredi-
ents and compounds Ia and Ib as antidotes, and to methods
for the selective control of unwanted plant growth using
these herbicidal agents.
The clas~ of 1,3-disubstituted 2,4-dioxopyrido-
~2,3-d]pyrimidines has been known for a long time. Thus,
Arch. Pharm. 311 (1978) 406 describes pyrido[2,3-d]-
pyrimidine-2,4-diones substituted in position 7 and
l-allyl- and 1,3-dimethylpyrido~2,3-d]pyrimidine-
2,4-diones substituted in positions 6 and 7 (see also
TabLe 5). At the same time, reference is made to the
action of a few compound~ as antitumor agent~ or as
sedatives. The 3yntheses of other 5,6- and
6,7-di3ubstituted 1,3-dimethylpyrido[2,3-d]pyrimidine-
2,4-diones are described in J.Heterocycl. Chem. 22 ~1985)
345 and 1469 (~ee also Table 5).
R~3PI.A~E~S13NT S~3T
:.
.
, , ~ :. `
209 ~)l133
- 3a - O . z . 0050/42008
J. Heterocyclic Chem. 17 (1980), 235 discloses 1-~2',4'-
xylyl ) pyrido [ 2, 3-d ] pyrimidine-2, 4 ~ lH, 3H ) -dione, 3-ethyl-
l-(2~4~-xylyl)pyrido[2~3-d]pyrimidine-2~4(lH~3H)-dione~
3 -crotyl -1- ( 2 ', 4 ~ -xylyl ) pyrido [ 2, 3 -d ] pyrimidine-
2, 4 ( lH, 3H ) -dione, 3-benzyl- 1- ~ 2 ', 4 ' -xylyl ) pyrido [ 2, 3-
d ] pyrimidine-2, 4 ~ lH, 3H ) -dione, 1-propylpyrido [ 2, 3-
d ] pyrimidine-2, 4 ~ lH, 3H ) -dione, 3 -ethyl- 1-
propylpyrido [ 2, 3-d ] pyrimidine-2, 4 ~ lH, 3H ) -dione, 3 -crotyl-
l-propylpyrido ~ 2, 3-d ] pyrimidine-2, 4 ~ lH, 3H ) -dione, 3 -
benzyl-1-propylpyrido [ 2, 3 -d ] pyrimidine-2, 4 ( lH, 3H ) -dione,
1-butyl-pyrido ~ 2, 3-d ] pyrimidine-2, 4 ( lH, 3H ) -dione and 1-
crotyl-pyrido[ 2, 3-d]pyrimidine-2, 4 ~ lH, 3H) -dione .
EP-A 378 850 describes, inter alia, pyrido[2,3-
d ] pyrimidine-2, 4 ( lH, 3H ) -diones o f the f ormula
.,
R ~ NJ~z _~m
0~ N N~
Rl
R
and salts thereof for the preparation of drugs, the
variables having the following meanings:
Rh is unsubstituted or substituted heterocyclyl, aryl
or alkyl,
Rl is cycloalkyl, trif luoromethyl, unsubstituted or
2 0 substituted alkyl or aryl,
* is hydrogen, hydroxyl, cycloalkyl or unsubstituted
or ~ubstituted alkyl,
RL is unsub~tituted or substituted heterocyclyl, aryl,
hydrogen, cycloalkyl, unsubstituted or substituted
alkyl, unsubstituted or substituted amino, hydroxyl,
unsubstituted or substituted alkoxy, trialkylsilyl-
oxy, cycloalkoxy, unsubstituted or substituted aryl,
alkylcarbonyloxy, arylcarbonyloxy or unsubstituted
R13PLAC131OENT SI~BT
.' : ~ .
:.
.
2 0 9 ~ 3
- 3b - O.Z. 0050/42008
or substituted aminocarbonyloxy,
Z is -CH2-CH2- or -CH=CH-, and
R~ is 4-hydroxy-tetrahydropyran-2-on-6-yl, 4-alky1-4-
hydroxytetrahydropyran-2-on-6-yl, -CH(OH)-CH2-CH~OH~-
CH2-COORnor -CH(OH)-CH2-C(alkyl)(OH)-CH2-COORn, where
R~ is hydrogen, alkyl, phenyl-substituted alkyl, aryl
or a cation.
US 3,635,973 furthermore discloses a process for
the preparation of, inter alia, herbicidal pyrido[2,3-
d]pyrimidine-2,4(1H,3H)-diones which carry an alkyl,
aralkyl, cycloalkyl, alkenyl or alkynyl group in the
3-position.
According to US 3,836,351, pyrido~3,2-
d]pyrLmidine-2,4(1H,3H)-diones which carry an alkyl,
dLmethoxyethyl, aralkyl, cycloalkyl, pyridylalkyl,
alkenyl, tetrahydrofuryl, aryl, pyrrolidinyl,
piperidinyl, homopiperidinyl or septamethyleneimino group
in the 3-position and derivatives thereof substituted by
alkyl in the l-position are also suitable for controlling
plant growth.
The stated publications do not disclose that the
known pyrido~2,3-d]pyrimidine-2,4(lH,3H)-diones and
pyrido[3,2-d]pyrimidine-2,4~1H,3H)-diones have an
antagonistic effect on herbicides.
EP-A 387 568 discloses substituted 1,8-
naphthyridines and EP-A 368 212 naphthalene derivatives,
which are suitable as antidotes for certain cyclohexenone
derlv~tlve~.l ~
-
REPLA~EMENT S~T
.~:
~.
209~433
- 4 - O.Z. 0050/42008
///
.~ /
Cyclohexenone derivative~ of the formula XII
~ ~ ~n XII
where
Rd iS C1-C~-alkyl;
A is C~-alkylene or C~-alkenylene, it being possible
for each of these chains to carry one to three
Cl-C3-alkyl groups and/or halogen atoms, or a 3-
membered to 6-membered alkylene chain which, if
desired, i9 substituted by Cl-C3-alkyl and which
contains, as a ring member, an oxygen or sulfur atom
which i8 not directly ad~acent to the oxime-ether
moiety;
B is nitro, cyano, halogen, C1-C4-alkyl, C1-C4-alkoxy,
Cl-C4-alkylthio, Cl-C,,-haloalkyl, Cl-C"-haloalkoxy,
carboxyl, Cl-C4-alkoxycarbonyl, benzyloxycarbonyl
REPLAC~ENT SE~T
~, . .. .
- .
.
2~9 J433
. - 4a - O.Z. 0050/42008
.
and/or phenyl, it being possible for the aromatlc
radicals to carry one to three of the follow~ngz
nitro, cyano, halogen, CL-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-alkylthio, Cl-C4-haloalkyl, Cl-C4-haloalkoxy,
carboxyl, Cl-C4-alkoxycarbonyl and/or benzyloxy-
carbonyl;
,,
//
., /
REPLACEXENT S~æET
:: '
.
~ .
2 0 9 ~ 4 3 3
- _ 5 _ O.Z. 0050/42008
and/or phenyl, it being possible for the aromatic
radicals to carry one to three of the following~
nitro, cyano, halogen, C~-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-alkylthio, Cl-C4-haloalkyl, Cl-C4-haloalkoxy,
carboxyl, Cl-C~-alko~ycarbonyl and/or benzyloxy-
carbonyl;
n is 0 to 3 or 1 to 5 when B is halogen;
R~ is C1-C4-alkoxy-C1-C6-alkyl or Cl-C4-alkylthio-Cl-C6-
alkyl;
C3-C7-cycloalkyl or C5-C7-cycloalXenyl, it being
possible for each of these groups to carry one to
three of the following: Cl-C4-alXyl, Cl-C~-alkoxy,
Cl-C4-alXylthio, Cl-C4-haloalkyl, hydroxvl and/or
halogen;
a 5-membered saturated hatarocycl~ which contains
one or two oxygen and/or ~ulfur atom~ as hetero
atoms and which can carry up to three of the follow-
ing: Cl-C~-alkyl, Cl-C~-alkoxy, Cl-C~-alkylthio and/or
C1-C~-haloalkyl;
a 6- or ?-membered heterocycle containing one or two
oxygen and/or sulfur atoms and up to two double
bonds, it being pos3ible for this ring to carry one
to three of the following: hydroxyl, halogen, C1-C~-
alkyl, Cl-C~-alkoxy, Cl-C4-al~ylthio and/or Cl-C4-
haloalkyl,
a 5-membered heteroaromatic radical containing one
or two nitrogen atoms and one oxygen or one sulfur
atom, it being possible for this ring to carry one
to three of the following: halogen, cyano, C1-C4-
alkyl, C~-C~-alkoxy, C~-C~-alXylthio, Cl-C~-haloalkyl,
C2-Cg-alkenyl, C2-C9-alkenyloxy, Cz-C6-haloalkenyl
and/or C1-C~-alkoxy-C1-C~-alkyl,
phenyl or pyridyl, it being possible for each of
: these to carry one to three of the following:
halogen, nitro, cyano, C1-C4-alkyl, C1-C~-alkoxy,
Cl-C~,-alkylthio, Cl-C~-haloalkyl, C3-C5-alkenyloxy,
C3-C5-alkynyloxy and/or -NRrRB, where
2~9J~33
- 6 - O.Z. 0050/42008
I
I
R8 i8 hydrogen, C~-C4-alkyl,C3-C~-alkenyl, C3-C~-alkynyl,
C~-C6-acyl or benzoyl whose aromatLc ring can carry
one to three of the following: nitro, cyano,
halogen, Cl-C~-alkyl, Cl-C4-alkoxy, C,-C4-alkylthio
and/or Cl-C~-haloalkyl
are described as herbicides in the earlier German
Applications DE-A 4014985 and DE-A 4014986 and are mainly
used for controlling unwanted grasses in dicotyledonous
crops and in non-graminaceous grasses. Depending on the
structure of the substituents and the application rate,
compounds from this group can also be usad for sele~ s
control of unwanted grasses in graminaceous crops such as
wheat and rice.
It i8 an ob~ect of the present invention to
provide compounds which diminish the disadvantages
associated with the use of the abovementioned herbicides
of the formula XII to an extent which is at least such
that the harvested yield of the crop plants is now
reduced negligibly, if at all.
We have found that this ob~ect is achieved by the
compounds Ia and Ib defined above.
We have also found processes for preparing
compounds Ia and Ib and methods for the combined treat-
ment of crops with the compounds Ia and/or Ib on the one
hand and wLth the herbicides XII on the other hand.
Certain derivatives Ia and Ib can be in the form
of environmentslly compatible salts. Preferred in this
connection are the al~ali metal salts, especially the
potassium or sodium salt, alkaline earth metal salts,
especially the calcium, magnesium or barium salt, mangan-
ese, copper, zinc or iron salts, and ammonium, phosphon-
ium, sulfonium or sulfoxonium salts, for example ammonium
~BPLASENBNT SHEBT
,; . .
20~433
. - 6a - O.Z. 0050/42008
-
salts, tetraalkylammonium salts, benzyltriarylammonium
salts, trialkylsulfonium salts or trialkylsulfoxon~um
salts .
R~PLAC13~1BNT S~I13E3T
., ~ , , , `
`:
,, ~
209~4~3
- 7 - O.Z. 0050/42008
salt~.
With a ~iew to the intended u~e o the compound~
Ia and Ib as crop-protection agent~, preferred ~ubstitu-
ents are the following:
5 R1 and R2 are hydrogen;
C~-C4-alkyl ~uch a~ methyl, ethyl, propyl,
1-methylethyl, butyl, 1-methylpropyl, 2-methyl-
propyl and 1,l-dLmethylethyl, especially methyl
and ethyl;
C1-C4-haloalXyl, especially C1-C2-haloalkyl such
as chloromethyl, dichloromethyl, trichloro-
methyl, chlorofluoromethyl, fluoromethyl,
difluoromethyl, trifluoromethyl, dichloro-
fluoromethyl, chlorodifluoromethyl, l-fluoro- -
ethyl, 2-fluoroethyl, 2,2-difluoroethyl, 2,2,2-
trifluoroethyl, 2-chloro-2-fluoroethyl,
; 2-chloro-2,2-difluoroethyl, 2,2-dichloro-
2-fluoroethyl, 2,2,2-trichloroethyl and penta-
fluoroethyl;
C3-C,-cycloalkyl such as cyclopropyl, cyclo-
pentyl, cyclohexyl, cycloheptyl, especially
cyclopropyl;
R3 iq hydrogen, hydroxyl, mercapto, amino;
C1-C~-alkyl a~ mentioned for Rl, especially
methyl and ethyl,
C1-C~-alkylthio ~uch aq methylthio, ethylthio,
propylthio, l-methylethylthio, butylthio,
l-methylpropylthio, 2-methylpropylthio and
l,l-dimethylethylthio;
C~- or C~0-aryl such a~ phenyl or naphthyl which
can carry one to three of the groups mentioned
` for R11, preferably nitro, fluorine and
chlorine;
R4 is hydrogen; C1-C~-alkyl aq mentioned for R1,
preferably methyl and ethyl;
C1-C~-alXoxycarbonyl ~uch as methoxycarbonyl,
ethoxycarbonyl, propyloxycarbonyl, l-methyl-
209 ~433
- 8 - O.Z. 0050/42008
ethoxycarbonyl, butyloxycarbonyl, l-methyl-
propyloxycarbonyl, ~-methylpropyloxycarbonyl,
1,l-dimethylethoxycarbonyl;
Cl-C4-(alkylthio)carbonyl such as ~methylthio)-
carbonyl, (ethylthio)carbonyl, ~propylthLo)-
carbonyl, ~1-methylethylthio)carbonyl, (butyl-
thio)carbonyl, ~l-methylpropylthio)carbonyl,
~2-methylpropylthio)carbonyl, ~l,l-dimethyl-
ethylthio)carbonyl;
C~-C4-alkoxy(thiocarbonyl) such as methoxy(thio-
carbonyl), ethoxy(thiocarbonyl), propyloxy-
(thiocarbonyl), l-methylethoxy(thiocarbonyl),
butyloxy(thiocarbonyl), l-methylpropyloxy(thio-
carbonyl), 2-methylpropyloxy(thiocarbonyl), -
1,l-dimethylethoxy(thiocarbonyl);
Cl-C~-(alkylthio)thiocarbonyl ~uch a~ (methyl-
thio)thiocarbonyl, (ethylthio)thiocarbonyl,
(propylthio)thiocarbonyl, (l-methylethylthio)-
thiocarbonyl, (butylthio)thiocarbonyl,
(1-methylpropylthio)thiocarbonyl, (2-
methylpropylthio)thiocarbonyl, (l,1-dimethyl-
ethylthio)thiocarbonyl;
R3 and R~ together are C3-C0-alkylene such as tri-,
tetra-, penta- and hexamethylene, C3-C~-alkenyl-
ene, especially C3-C~-alkenylene such a~
l-propen-1,3-ylene, 1-buten-1,4-ylene and
2-buten-1,4-ylene; 1,2-phenylene;
R5 i~ hydrogen, C~-C~-alkyl as mentioned for Rl,
preferably methyl and ethyl;
- 30 C(o)R7 and C(S)R7 where R7 i8 preferably amino,
methoxy, ethoxy, hydroxyl, methyl, ethyl,
phenyl, methoxycarbonyl or ethoxycarbonyl;
R~ is C~-C~-alkyl as mentioned for Rl, preferably
methyl and ethyl;
styryl;
C~- or C10-aryl ~uch as phenyl or naphthyl which
can carry one to three of the groups mentioned
.
209~4~3
- 9 - O.Z. 0050/42008
for Rl1, preferably chlorine, nitro and methyl;
CHzR8 where R9 i~ preferably chlorine, methoxy-
carbonyl or hydroxycarbonyl;
Cl-C~-alkoxycarbonyl a~ mentioned for R4;
cl-c4-~alkylthio)carbonyl as mentioned for R4;
C~-Cq-alkoxy(thiocarbonyl) a~ mentioned for R~;
Cl-C4-(alkylthio)thiocarbonyl a~ mentioned for
R4;
hydroxyl; amino;
C1-C4-alkoxy such as methoxy, ethoxy, propyloxy,
l-methylethoxy, butyloxy, l-methylpropyloxy,
2-methylpropyloxy and 1,l-dimethylethoxy,
particularly preferably l-methylethoxy;
C~-C~-alkylthio as mentioned for R3;
C1-C4-alXylamino ~uch as methylamino, ethyl-
amino, propylamino, l-methylethylamino, butyl-
amino, l-methylpropylamino, 2-methylpropyl-
amino, l,l-dimethylethylamino;
NHCXR~ where X is preferably oxygen, especially
methylcarbonylamino and ethylcarbonylamino;
NHSO2Rl, especially 4-toluenesulfonylamino;
2-thienyl, 3-thienyl, 2-pyridinyl, 3-pyridinyl,
4-pyridinyl, 3-indolyl, particularly preferably
2-thienyl and 3-indolyl, it being possible for
each of these groups to be substituted by one
or two of the groups mentioned for Rl1;
~ R5 and R~ together are C3-C~-alkylene such as tri- and
:i tetramethylene, C3-C5-alkenylene such as
l-propen-1,3-ylene, 1-buten-1,4-ylene and
2-buten-1,4-ylene or 1,2-phenylene, each of
which can be substituted once or twice by C1-C~-
alkyl such a~ methyl and ethyl, C1-C~-haloalkyl
such as trifluoromethyl and C3-C7-cycloalkyl
x 8uch a8 cyclopropyl, cyclopentyl and cyclo-
;~ 35 hexyl, and can also contain carbonyl or thio-
:~ carbonyl groups; 1,3-propanediylcarbonyl,
2,2-dimethyl-1,3-propanediylcarbonyl and
.
. .
.
' ' ~
.: .
- :. ... . . ...
209.~3
- 10 -O.Z. 0050/42008
1,2-phenylenecarbonyl areparticularly
preferred;
R7 is hydrogen, hydroxyl, amino;
Cl-C~-alkyl aq mentioned for Rl, e~pecially
methyl and ethyl;
C~-C4-alkoxy a~ mentioned for RB, especially
methoxy and ethoxy;
Cl-C4-alkylthio a~ mentioned for R3, e~pacially
methylthio and ethylthio;
Cl-C4-alkoxycar~onyl as mentioned for R4,
especially methoxycar~onyl and ethoxycarbonyl;
C~-C4-(al~ylthio)car~onyl as mentioned for R4;
C~-C4-alkoxy(thiocar~onyl) a~ mentioned for ~4;
C,-C4-(alkylthio)thiocarbonyl as mentioned for _
R4;
Cl-C4-alkylamino as mentioned for R6;
di-(C~-C4-alkyl)amino ~uch as N,N-dimethylamino,
~ N,N-diethylamino, N,N-dipropylamino, N,N-di-
(l-methylethyl)amino,N,N-dibutylamino,N,N-di-
(1-methylpropyl)amino, N,N-di-(2-methylpropyl)-
amino, N,N-di-(l,l-dimethylethyl)amino,
N-ethyl-N-methylamino, N-methyl-N-propylamino,
N-methyl-N-(l-methylethyl)amino, N-butyl-
N-methylamino, N-methyl-N-(l-methylpropyl)-
amino, N-methyl-N-(2-methylpropyl)amino,
N-(l,l-dimethylethyl)-N-methylamino, N-ethyl-
. N-propylamino, N-ethyl-N-(l-methylethyl)amino,
N-butyl-N-ethylamino, N-ethyl-N-(l-methyl-
propyl)amino, N-ethyl-N-(2-methylpropyl)amino~
N-ethyl-N-(l,l-dimethylethyl)amino,
N-(l-methylethyl)-N-propylamino, N-butyl-
N-propylamino, N-(1-methylpropyl)-N-propyl-
. amino, N-(2-methylpropyl)-N-propylamino,
N-(l,l-dimethylethyl)-N-propylamino, N-butyl-
: 35 N-(l-methylethyl)amino, N-(l-methylethyl)-
N-(l-methylpropyl)amino, N-(l-methylethyl)-
N-(2-methylpropyl)amino, N-(l,l-dime~hylethyl)-
'
., .
.~
;~ ,
''-'' ~'`"" ' ' ; . "' ' .
.~ . . .
209 )~3
- 11 - O.Z. 0050/42008
N-(l-methylethyl)amino, N-butyl-N-(1-methyl-
propyl)amino, N-butyl-N-(2-methylpropyl)amino,
N-butyl-N-(l,l-dimethylethyl)amino,
N-(1-methylpropyl)-N-(2-methylpropyl)amino,
N-(1,l-dLmethylethyl)-N-(l-methylpropyl)amino
and N-(l,l-dimethylethyl)-N-(2-methylpropyl)-
amino;
C8- or C10-aryl and C6- or C1~-arylamino such as
phenyl, naphthyl or phenylamino who~e aryl
radicals can carry one to three of the groups
mentioned for R11;
R8 is halogen, especially fluorin~ and chlorine;
hydroxyl; mercapto;
C,-C4-alkoxy as mentioned for R~; -
C1-C4-alXylthio a~ mention~d fo- R3;
C1-C~-alkoxycarbonyl a~ mentioned for R4;
C1-C4-(alkylthio)carbonyl as mentioned for R~;
Cl-C~-alkoxy(thiocarbonyl) as mentioned for R~;
C1-C~-(alkylthio)thiocarbonyl as mentioned for
i 20 R~;
: -O-C-R~
.,~ 1 .
where X i~ particularly preferably oxygen and
R9 is hydrogen, methyl or ethyl;
R9 is C1-C~-alkyl as mentioned for R1, particularly
preferably methyl and ethyl;
Cl-C~-haloal~yl a~ mentioned for R~;
NR1R2, preferably amino or dimethylamino;
ORl, preferably hydroxyl, methoxy or ethoxy;
R10 i8 Cl-C~-alkyl as ment~oned for R~;
; C~- or C10-aryl such as phenyl or naphthyl which
can carry one to three of the groups mentioned
. for Rl1, especially 2-methylphenyl, 3-methyl-
phenyl or 4-methylphenyl;
Rll is halogen, especially fluorine and chlorine;
hydroxyl; mercapto; nitro; amino;
~,:
.: , :,
.: '` :
. , ; ,
209~43~
- 12 - O.Z. 0050/42008
~ Cl-C~-alkoxy as mentioned for ~, e~pecially
methoxy and ethoxy;
Cl-C~-alkylthio as mentioned for R3, e~pecially
methylthio and ethylthio;
Cl-C4-alkyl as mentioned for Rl, preferably
methyl, ethyl, propyl, 1-methylethyl and
l,1-dimethylethyl;
X is oxygen or sulfur.
Particularly suitable compounds Ia and Ib are to
10 be found in Table~ 1 to 4 (see Examples).
The novel compounds I can be obtained in a
variety of way~. Sub~tituted pyridopyrimidina~ ar~
obtained, for example, by condensing 4-aminopyrLmidines
with ethoxymethylenemalonic acid derivatives or by _
reacting 4-amino-5-formylpyrimidines with ~-diketone~
(E. Lunt and C.G. Newton in 'Comprehensive Heterocyclic
Chemistryl~, Vol. 3, Pergamon Pres~, Oxford, pp. 215-232).
Another route is the reaction of ~-dialkylamino ketones
with 4-aminopyrimidines (R. TroschUtz and H.J. Roth:
Arch. Pharm. 311 (1978), 406, 542).
The pyridinepyrimidine tsic] derivatives Ia are
obtained, for example, by reacting a 4-aminopyrimidine-
2,4(lH,3H)-dione II with a ~-chloroalkenal III in an
; inert solvent and under a protecti~e gas atmosphere.
It is advisable to carry out this reaction by
adding the alkenal III in liquid phase at room tempera-
ture over the course of 5 to 30, preferably 10 to 20, min
to the compound II.
A~ a rule, the reaction mixture i8 then heated to
; 30 from 20 to 150, preferably from 70 to 110C and left at
this for 3 to 12, in particular 5 to 7 h, before it is
cooled to room temperature. The products are generally
solid and precipitate out on cooling and can be separated
off by conventional methods. The molar ratio of compound
II to alkenal III is generally chosen in the range from
0.7sl to 1.2~1.
The pyrimidine derivatives II are generally known
.. . .
209a433
- 13 - o.Z~ 0050/42008
/
/
/
R5 is preferably hydrogen or
Cl-C,-alkyl, especially methyl or ethyl, and Rl~ is C1-C,-
alkyl, preferably methyl or ethyl.
i Another method for preparing the compounds Ib
comprises reacting a pyrimidine derivative VIIa or VIIb
in a conventional manner (see J. Heterocycl. Chem. 22
(19~5) 345) with a malonic acid derivative VIIIa, VIIIb
or VIIIc. In this case, R~ is cyano, C(o)R7 or C(S)R7, Rl5
is C~-C~-alkyl such as methyl, ethyl, n-propyl, i-propyl,
n-butyl, i-butyl, sec-butyl or tert-butyl, especially
ethyl; R~ C~-C,-alkyl a- mentioned for R15. especially
methyl; C0-aryl-C2-C,-alkenyl such as styryl;
C~-, C10- or C1~-aryl such as phenyl or naphthyl,
especially phenyl;
lt being possible for the aryl groups to carry one to
R8~LACE~BNT S~BBT
:"
',
. . .
209 ~433
- 14 - O.Z. 0050/42008
three of the groups mentioned for R11 or CH2R~.
The compounds Ib can also be prepared by resctln~
a compound Ib where R~ ls hydroxyl Ln a conventlonal
manner (see P. Màtyus, P. Soh~r and H. Wamhoff, Lleblgs
Ann. Chem. (1984) 1653) with the compound IX, which Ls
R16-Nu. In this case, R18 is C1-C4-alkyl as mentLoned for
R1, preferably i-propyl or n-butyl, and Nu is a
nucleofugic leaving group such as a halogen, preferably
iodine.
An additional method for preparin~ compounds Ib
comprises reacting a compound Ib where R~ is amino in a
conventional manner with a compound Xa, which is R9(CX)Nu,
or Xb, which is R10-SO~-Nu.
Specific examples of herbicidal cyclohexenones of
the formula XII whose tolerance by crop plants can be
improved by the compounds Ia and Ib are listed in Tables
B to R which follow (see German Applications DE-A 4014985
and DE-A-2822304).
REPLACE~ENT SHEET
.
.
.
.
2~9~433
- 15 - O. Z . 0050/42008
_ _ _
~ ~ L~ O ~
.~~ o Ut ~
E _ _ _ -- -- -- ' -- -- -- -- -- -- ~¢
-~ O O O O -- O O U~ O ` O
C: W U~ ~0 ~ ~ N ~ O --I O O ~
_____.________--~
~ ~ U
o . ~ ~ ~ ~
~' `f ______________~
m ~ ~ "~ O ~ U~ n N
~ ~ r ~ ~ ~ za
e o ,1 ~ o o ,1 ~o _1 _1 o
' 8~
E~
1 8 ~ I I O o ~ 2~, 8 æ~ I
I
U U U
e ~ c ~ ~e ~
O O O O O O S O O o o ~ o n o
o ~
.. z m m m m m m m m m m m m m m m
:'
.,
: .
209 ~33
- 16 - O. Z . 0050/42008
.
,, ~ ::
~ _ ~ ~
~ ~ E~
.
~, ~O D
Ul
.` 2;
_ ~ ~
_ ~ m
_
~q ~ U~
~ ' I Lf~
s: ~ ~ .
P~ -- U~
u ~
$ $ $ $
l l l l
o~o
,~ ~o
,~
~ C~l ~ ~
U U U U
', ' ,,
:' . ~ ' ,.,`;: ':. '. '' :. . ,
209~433
~ - 17 - O . Z . 0050/42008
_ _ _ _ _ _
O ~ O
.. ~ a
.1 ~ ~
., _ o o o o o o
. _ _ _ _ _ _ .
Ul U~
_~.~ o o o _ _ o o o ~ _ _
_ _ _ _ _ _ ~ ~ ~ _
N ~ ~ O O t~ ~ N t~ O O
a~ O ~1 ~1 o ~
_ _ _ _ _ _ _ _
U~
;~; o~ o o ~ o~ o o
~Z
a o o o o o o
o o o
i~ ~ ~ ~ ~ ~ q~ U U C~ U U U
l l l l l l l l l l l l
m
;
C
e 0~ oP~ o~
o ~ C~, P' o~ ~ oP~ ~ o
~ 3 1~1
.~
.j " o~ ~ o' ~ o' ~ ~o4 ~ oP' ~ oP'
.. .
:'
O _~
`., : : .
:;; . . . :
:
20~ 33
- 18 - O . Z . 0050/42008
.
.~ ...
X 5 3
_.N ~ ~ ~ `J N ~1
~ ~ ~ e ~
~_ _ _ _ _ _ _ _ _ _
O
. .0 l l l l l l l l l l
Z .
,~ X X X X ::: X X X :r: X
~J _ N ~ N N N ~ 01 N N N a~
_ _ _ _ _ _ _ _ _ _ _ _
~: ~ . . ... . ..... .
N
N _I I ~ N I --I
~ ~ o~ o~ g ~ ~ o~ ~ g
l l o o ~ --l ~ o o ~
o c~ ~ c ~ ~ u ~ ~ ~
~ z~ s s ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
C~ -- -- A -- -- -- -- -- .ÇI
o~,~o
Y
c c c c c c ~ c c c
. ~ O O o
s~ s~ ~ ~s~ s ~s~ ~ ~s~ ~ ~
3 ~ 3 ~ 3 3~
~ ~ ~ s ~
0~ ~ Oæ 0~
.. o
~;
: :
20~33
. ~ - 19 - O. Z . 005C/42008
;_~ _ _ __ _ _ _ _ _ _
, ~ _ _ _ _ _ _ _ _ _ _ _
. ~ o o o o _~ o o o o o o
~ l l l l l l l l l l l
~
~ o
~ a ~ U~
-- o -- -- _ _ ~ _ _ _ _
C~ U~
c a~
~ ~ l ~ ~ ~ l ~ ~ ~
J O ~ ~ ~ .C O ~ ~ ~
~: ~ J J P~ S S
O ~,Q ~ ~' _l O ~ C~ O ~ O.
U II O O ~ ~ I O O ~ ~ I O O
II O O ~ I I O O ~ I I O O
>~ I J I ~1 C ~ -I J I ~I C ~
,S~ c c ~1 u ~ ~ a~ c ~- u ~ ~ ~ c ~1 U
I IIIIIIIIIIIII~I
o~ c c c ~ b C ~ C c 1 C C C
O O O O O O O O O O O O O O
::l ~ o~ o~ o~ o~
~I ~ ~ ~ cn o
O
Z ~3 ~ ~ ~ ~ ~ ~ ~3 ~ ~ ~3 ~ 1i3
,
,.
.
20~33
- 20 - O. Z . 0050/42008
.~
_ _ _ _ ~ ~ _ _ . _ . _
~ 'J ~ ~ ~ ~ ~ ~ ~I N
~ e ~ 5
. ~ _ _ _ _ _ _ _ _ ~ _ ~ _
o _/ o o o o o o
~o l l l l l l l l l l
_ ~ ~ O
_~__ __~___ ___~___
~
_~,_ ______ _______
~Q ~
s~ ... ...... . ~
_
C J~ C
I s~ .a I
o ~ ~l l ~ ~
.,, ~ e ~ ~
o~ ~ ~ ~ o a~
O --I O ~ 0~ ~ --I O ~ P. D~
u Pl ~I o o :~ 5 0 _10 _~ O
~ W _I I O O ~ I O S O .C O
113 ~ J ~
1~1 ~ ~ ~ ~: ~1 U ~ ~ ~ U ~ U ~ ~1
-- -- .4 ~ -- -- -- -- ,~ _ _ _ _ _
E~ l l ll l l l l l l l l l l
~ ~'1~ t~ ~
b ~ o~o~ ~o~ o~
~ 2
~ ~ ~ ~ ~ ~ J~ ~ ~ ~ ~ ~
~: $ $ $ ~ $ ~ ~
~ ~ 0~ ~ Oæ 0~ 0~
U~ O _I ~ ~~ U~ ~D
Z ~Yi ~, ~Y ~ ~Yi
:` :
. .
2095~33
- - 21 - O. Z . 0050/4200S
.~ _ _
_. ~ ~ ~ ~ ~ ~ r~ ~ ~
_ ~ e ~ ~ ~ e ~
~O ~O O ~ U~
_ ~ ~ ~ O
. . . . . . . . U~
Z .
O
a~ ~ u~
~ ~ s
.
:
a ~
~ ~ ~ 3 3 Q
u ~ ~ 2 ' ',, ~,, Q Q ~ ~
v
~3 ~ ~ ~ ~ s~ ~ s~ ~r
: 3 ~ ~ ~ ~r ~ er ~ ~ ~ ~ ~ ~r ~
.- P P~
o o o o o
U o ~
~ $ ~ $ $ $ $
2 ~ S 2 2 ~ o ~ 3 ~ 0~ ?. ~ ~
~ a e ~ ~
o~
s
~ CO ~ O ~1 ~ ~ ~
o ........
Z ~
.
209~33
: - 22 - O . Z . 0050/42008
.~ I I I
_ _
. ~ . . ~
I ~ I I ~o _ _ _ _ _
oU~ I o U~ I
U~
u~
~ .
Z ~ .
U
U~
_ _ ~_ _ -- 5
_ ___________
~1 U'~ I ~ O 11~ Q O CO O ~ O C~ O CO O
. . ... ...........
_ ~-- ~ C
_~ ~ C
~ ~ ~ C
.C ~ ~ S ~ I
O ~ I I ~ I
~1 J -- ^ S
~ S a~ C
c 2 s s ~ ~ s~
8 ~ ~ ~ 8 x
, ~ o o o o o
~1 _I O J ~ _I O S S S J S
î~ c . a~ ~ c
U
tl3 ~ ~ ~ ~ G ~r ~
~; ~
E~
: 3 ~ N ~r r ~r ~ ~ ~ ~r ~r
-I ~ ~ -1
I I I ~ ~ I
O O O
~ c
~ ~ S~
~, ~ c
~1 1 1 0 0
O O
_~ ~ S ~ ~ ?
o o O U U
8 ~ 8 ~ o ~ S 2 3 ~
.,,, .~ , ~ ~ ~ s
x ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
o o o o o o s o s o
~ h ~ 1
.: X ~ O~
a~ o _I c~l ~ ~ In u~ ~co
O .
` ,
., - . :
.
,. , ,: ~ , `.. ,. ' .. . .,. , , , ' . : .
, : . . .' : '':
209a433
- 23 - O . Z . 0050/42008
l l
U~ U~
_ _ _ _ _ _ _
_.
.~ .~
_ _ _ __ ~ _ _ _ _ _
~o o o o o ~~ o o o o
.,~ ~ .
o I I I ~ I I , , , ,
_ _ _ _ _ _ _ _ _ _ _ ~
_ _ _ _ _ _ _ _ _ _ _ _ _ _
~. C~ O 1~ ~ 1 0 L'l IJ7U'l U'~ In
,~ ...........
_i
a~ I I I I
. ~ J~
c ' .~ ,a -- _ _ _
O ~ I I
--I C ~
~ I ~ S S J S
e ~ oO~ Oa~ O 0~
~ I I I I I J J O O O O
O ~ N ~
~ ~ ~ ~ ~ ~ ~ ~ 0~ S S S~ U
3 ~ S S S .
m ~
C ~ C
:. ~ o o ~ ~ ~ o o o o ~ ~
~ æ ~s~ ~s~ ~S~ ~ ~ æ
~, 2 ~, 2 ~ 2 ~, 3 2 2 2 ~,
S~ ~ 0~ 0~ ~ S~ 0~ ~ 0
X O)
cl~ o _I ~ ~ ~r In ~D t` ~ O~ O
. ~ .
2~9 ~33
- 24 - O . Z . 0050/42008
. . . ~
_~ .~ .~~ ~ ~ ~ ~ ~
_
~ _ _ _ _ _ _ _ _ _
J~ U~ ~ ~ In u~ o o ~
~o ll l l l l l l I
C~l N ~ O O O
~
~ C~
~
. _ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~a ~ ~ O
P~ ~ In
a~
O ~ ~ I I I
~ l l ~~ ~
~ a~ ~~ s
o ~ .cP~c-, P~ ~ ~ ~ ~ ~ .a
C~ ~ OO ~ ~ ~ D~ I I I I I I
O O ~~1 ~ O 0~ 0 N N N N N N
1~ O O:1_I JJ O O O ~
o ~ rer~Ssssss
E -- ------ ---- -- -- ~
3 ~r ~ ~~ er~ ~ ~r ~ ~r .r ~ ~r ~P
1~ C ~ ~ . S
S
C C
O O O O O
o o o oo
~ s ~ ~ ~ ~ ~ 3 ~ ~ ~
S ~
Z
~ c s
oc
o ~s~ o o o o o s ~ o ~ o s
PC ~
~ ~ ~ ~ u~ ~ r~ co a~ o ,~
o ,. . . . . . . . . . . . .
.' Z ~ ~ ~ ~ ~ ~ 14 ~3 ~ ~ ~ ~ IY
,.
.
.
209~33
- 25 - O . Z . 0050/42008
__ _ _ _ . . .
_ ,~ .~ .~ ~
~:4 ~ Ei E El ~ ~ C ~
_ _ _ _ _ ~ ~ ~ _ ~ _
~o
Z .
_ _ _ _ _ _ -- _ -- -- U~ -- ~D
~ C~
:~ ~ o
-- o _ _ _ _ ~ _ _ _ _ _ _
~:4 ~ I
- ~ ~ ~
~4
o
.
o ~ 3 ~ ~ ~ s~
., U , , ,, , , o o , , o
.. , , ,, , , o o , , o
p, ~ S _l ? ~ -
u
s .C s ~ s s ~ ~P s s
,., _I
~ .C
C -- So
U
~l ~ ol
~ o~ o o o o
g~ s s s~ s
x ~
O O ~ rl O ~ ~rl ~ _I ~ _I ~ _I ~ _i
S ~ O ~ ~ ~ S ~ S ~ S ?~ S ~ S
~ -l c ~ ~ ~ ~ l ~ ~ ~ ~ ~ ~ ~ ~
~U ~ ~1 ~ ~ -- ~ ~ ~ O ~ O a) o ~ o
o~ o~ s~ o~ ~ o~ ~ s~
u~ O
o ~ ~ . ~ . . . . . .
:~
:
': :
`:
209~33
` - 26 - O. Z .0050/42008
~ t- I` 1` t` t`
_~ ;o U~
3~ X
~ ~ 5:
.,,
_ ~ _ _ _ _ _ _ _
~o ~
._ . _ . _ . . . . .
, ~ `J
Z .~
_ ~ _ ~ _ ~
~
s:4 ~ I I
~1 ~ J- O CO O D O ` O ` O ` O ` O
~ 0
o
..
J~ _ ,~ ~ _ _ _ _ _
3 ~
o o , , o o o o o
o ~ ~ o o o o o
. . ~ ~ , , , , i
. ~,
~3
m -- c~. c~, -- -- -- -- --
l l l l l l l l
~, ~, I
I I I
o o
~ ~ o~ o~
.c U
.c
o a~
s ~ 3
-I ~ ~ ~ ? ? ~ ~ ~ ?~
~ ~ I ~ I c s ~ s s s
s ~ ~ a) ~ ~
~ O ~ X ~D X ~ ~ ~ ~ ~J
:~: 0 ~C~ N St~l .C ~ ~ ~ ~ ~
'` ~ ~ 0~ 0~ ~ 0~ 0~ ~
O
U~~` ~ ~ O O O O
.~ O
'' ' ' `
., - . .
.
,. , , . - ,
, ~` `', ." ' " ' :, ' . ,
209~3~
- 27 - O. Z . 0050/4200~3
. e~
a E
o ,_
~ c o E~
. I
~r ~ ~ ~ ~ ~
o -- ~ 1~ ~ = S g ~ _
~s ~ ~. = I I I I I I I I l I ~ E I I ~
2 3~ o r~ r' ~ O C~ ( ~ o Ir~ I~ _
_ ~ ~ O
3 0 3 1--
O ~
_ r~ = = _
O ~ ~
E ~D E E
~ C~ ' o
S I~ LL ~ I~ L~
i~ 0~0 I _ _
C~: ~ S L L ~ ~ ~ ~ ~ L t~ l ~ ~ L
,~ r~ ~ ~ ~ O O ~ ~ ~ ~ O O ~ ~ ~ ~ O
L L L L `-- `-- L L L L `-- `_ L L L L ~--
A A :'~ ~ C C A :~ A A C C A ~ C
., OL L L L L L L 2 OL OL L L OL L
:~ :~, A A A ~ ~ A A ~ ~ A A ~ ~ :~ A
L L L L L L L L L L L L L L L L L
-- ~ -- ~ -- A -- A -- :~ -- ~ --
A Cl~ A ~ A 0. A ~ A ~ A C~ A C~ . A
C O C O C O C O C O C O C O C O C
W Cl: W ~ W ~ W ~ W ~ J ~ W ~ ~ L
_ ~ ~ ~ ~ ~ r~ a:~ o o _ ~ ~ ~ ~ ~ I~
:
2~9 ~33
- 28 - O . Z . 0050/42008
o~
_ _ _
_. E 5
E ~ ~ ~
.C ~ 3
o r~ ~ o ~
._ ~ ~ o o o c:l ~ ~ ~ _ ,_ ,_ a~ ~ ~
~ o
z ~ o o o 3 r~ ~ n
_ _ _ ~ 1-- o ~ o
~ 3
~ 5 ~ _ e _
_ _ _ _ _ _
U~ O O ~ o r~ o
:~ o ~ ~ ~ ~ cr
.
.,
., ___ __________ _
~, _ _ _ _ _ _ _
" ~
~1 t~7 ___~~___ _~ I ____I I ~_
C ~~ C C :~ ~, ~~ e ~" :~, ~, ~, r c ~
c t'7~ `S ~ L L~ ~1 ~ ~ L L~7 ~1 ~ ~ L L tO ~7
o ~ ~ ~ ~ o o ~ ~ ~ ~ o o ~ ~ ~ ~ o o ~ ~
.~~-LLLL--LLL L~-~-LLLL~-.-L L
,~~c_~ ~C~C~3~C ~
.,. 2 L LLLLLLL 2, L LLLLLLL L
O .~C---SC-c rc,c,c___ __ _
I ~ LLLLLLLLLL LLLLLLLLLL L
.. .
~` o
`: u o o ~c o <c o ~c o ~c o - o c o c o ~c o - o
~, ~ L L~L L~L L~L~L~L L~ L
.;~ ~
a~ ol o -- ~ ~ ~ ~ ~o r~ ~ C~ O ~
' ' :: ' ` ' ' ' ~ ;
' ,, , '
209~3~
- 29 - O. Z . 0050/42008
. .
_. ~
o _ _
r~
_ _
c: 3
CL . 2
,C ~ _
~o D u~
-- -- o r~
Z ~
_ _
_ _
~ _ ~ ~ :
U~ O O
~1 ~ O
:, ~ ~ I~
:,
.~ c ~s ~S ~S S ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~S ~S ~r ~s ~ ~ c~
'.'j
~,
:1 _ _ _ _ _ _ _ _
__II____II~
C C ~ ~ ~ C C ~ ~ C C
L L ~ ''I ~ ~ L L ~ S ~ L L ~ ~ ~ -:t L L
~ ~I O O ~ ~ 0 0 ~ ~ ~ 1 ~ O O ~ ~ ~ ~ ) O ~1 1
i' L L ~-- ~-- L L L L ~-- `-- L L L L ~ L L L S~ -- L
~ ~- ~ ~C ~ ~C~ ~C, o, ~ ~ ~ -- C ~
.... 2 .. . . ..
o .~.~.~.~.~c_CC,c__Ccccc __--_C
~,., .................. .....
U --v ~ . ~ . ~ . ~ . ~ 2 ~ . ~ . - . , . ~ . ~ . _
C~1 0 _ ~ ~ ~ ~ 'D r~ ~ cr o ~ s u~ ~ CO ~ O -~
203~33
- 30 - O . Z . 0050/42008
__
_ _
o _ _
_
E ~ ~7 3 t~ CD ~ :1 In --
S r~ _ _ _ ~ _ ~ ~ ~ ~ ~ CO _- ~ e = _ _` ~ ~ _ _
~ E ~ ' ~ E ~ ~
________ __ _____ __
Cl~ $ ~ ~ -- ~ -- o ~ ~ ol o o~ o ~ ~
:` ____ __
_ _ -- ~C ~~O ~O ~O ~ o
I I I I ~ oo o oz o o
. _ _ _ _ _ _
C C C ~ o C
L L L~--~--L L L L~-- ~-- L L
O L L L L L L L L L L L L L L ' i_ L
la S S S S ,~ ~ ~ L ~ L L L i_ L
,., . ~
., ~ _ _ __ _ _ _ _ _
~ O -- O S O S L S L ~ L ~ L
m ~ ~ ~ u~ ~,~ ~ ~ O _ ~ ~ ~~ D r~ 3~
20 9 ~433
. . . - 31 - O. Z . 0050/42008
o~
ô
E -- ; S -- -- -- -- --
. _ _ _ _ _ _ _ _ _
~_, oooo~ooo
:~ S
Il Z ~ r~
C I o O ~o C~ U~ o ~ o
_
,a ~ _ ~ _ _ _ _ =
C . _ _ _ _ _ _ -- _
~ ~ ~ ~ o ~ o ~ o ~ o
~ '- ~ - `S - .s -
'. ~
S S _ _ _ _ _ _
Z~
~s ~ ~ ~ ~ c c ~ ~ e ~ ~ ~ ~ c c
,: 12: ~1 ~1 .:t ~ L L ~) ~ ~ ~S L L ~ ~ ~ ~ L
,' ~ ~ ~ ~ O O ~ ~ ~ ~ O O ~ ~ ~ ~ O O
L L L L ~ L L L L ~-- ~-- L L L L
V V V ~ L L
C C C C C C ~ C C C ~ - C C ~ C C C
L L L L L L L L L L L L L L L L L L
.,
~. _ _ _ _ _ _ _ _ _
.~ ~ ~C L C L ~C L C C C O C o C L _~ L
,
. ~ r~ ~ ~ O ~
O O O O O O O O O O _ _ _ _ _ _ _ ~ _
21)3Y~333
- 32 - O. Z . 0050/42008
_ _ _
o E E E
r~ ,~ ,~ _ _ ~ = _ _
~ -- _ '` -- _ o 8 g
u~ c ~ ~~ E E ` ` ' ` ` `
~ ~ 3 ~
T _~J ~O ~ ~ . ~ ~1 ~ ~ ~ =
e
Z
I~ ~ ~ _ _ _ _ _ -- O O
~ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _
.; ~ _ _----_--~__=_~
. E E E -~ ~ E ~ _ E -- -- - -- ~ --
~ ~ o~ æ ~ ~ ~ g 3 g o ~ ~
l~ ~ ~
C
. ~
. o ~
o~¢~o ____~"_ _ _ _ ~_
~ ~LL~ ~ ~ ~ LL~
., ~ ~ ~ ~ O O ~ ~ ~ ~ O O ~
~ LLLL~-~-L L L L ~-~-L
.~ ~ ~ ~ ,c~
, LLLLLLL L L L LLL
.~ C S C C C C C C C C C ~ C
.~ LLLLLLL L L L LLL
~_ _ _ _ _ _
~ CL~CLO~L~ C L COC
m ~ o _
~ ~ O O O O O O O O O O _ _ _
~, . .. .
,, :
:
209~4~3
- 33 - O. Z . 0050/42008
_ = S S S
O E E E El
e~ _~ ~ ,., n ~ ~ ~ ~ ~
E c
. o o O O O O ~ ~ -- _ _
~ u7 ~ 3
s -- S S = -- ^ S E c
o
Z ~D ~ ~ E c
I o o o o u~ ~ ~ u~ I I
= ~ ~ ~ ~ ~ ~ ~ ~ o o o o o ~ o
~ ___.___________ ____
'aE . -- --~ E E E E ~ ~ E ~ -- ~ E ~ -- --
~n oooooooo~Uoooo oooo
~, o o ~ ~ cr o~ o O ~ ~ ~ ~ ~ ~ o ~ ~ ~
;
- - - - - -
:~
,j
~ - - - - - -
~1 3 ~ L L t'~ ~'7 ~ ~ L L t`l ~'7 ~ ~ ~ L
: C C C ~. ~L C ~ C ~ ~ e~ ~ C C C 3
.~ LLL~-~-LLLL~ L L LL ~
O O O O O O O O O O O O O O O O O
. L L LLLLLLLL L LL LL
:' O ~ e e e e e
.~ LLLLLLLLLLLL L LL LL
-
_ ~ L-L_LCLgeLeLO~ L ~L -
ta ~ ~ ~ ,~ , ~ O _ ~ ~ ~ u~ co 0 0
o ~
E~ Z -- s -- S s s s s s s _ s s s -- s
.
209 ~33
- 34 - O . Z . ooso/42008
, ~ E ~ ~ ~ o o 1~ r` ~`
_ _ _ _ _ -- _ _ _ _ _ -- -- o
_ _ E ~ ~
~: ~ ~ r~ ~ r~ r~ ~ ~ I~ ~ ~ O O O
= o `~ o ~ o ~ o ~ ~ ~ _ _ _ _ _
E = - ~ e . _ ~ , E E = ~ --
~ . ____________ ,~ _
1~ ~ æ c~ æ æ ~ æ ~ ~ O ~ O ~ æ o æ o O O
: ~
, Te 1.~ 1~ 1~ 1~
., Z~ ,~
O~¢fO
V
~ - - - - - -
~l s o s o - ~ s o s o
r~ CC W ~ ~ CL W CL W C~ W ~
z o o ~ ~ o ~ o c'~ o o
209~433
- 35 - O. Z . 0050/42008
_ __
~ = _
o ~r ~
_
E
C
~ _ _ ; _ _ _ _ _ _ _ _ _ -- -- -- -- _ -- _
_ ~ ` e` ~` c` ~ ` `~ e`; ~` .,` =` _,` = ~ -` ~ c~
; ~ ______~_~__ _ ____~_ _
I ~ ~ ~ ~ u~ ~ ~ ~ ~ ~ r~ I~ r~ o - o ~ o o
r ~ ~ o ~ o ~ o o o o --o --o
~ ______ _ ____--____ _ _
~ .~; e = _ _ ~ C ~ c ~' _ C _ ~ c
. _ _ _ _ _ _ ~
~ O O ~ 1-- 0 r-- O 1-- 0 r~ ~ ~ O O O O O O O O
:
:
l l-- -- ---- l l -- -- -- --
L L ~ ~ `S 3
I I I I ~ ~ I I I :
O O ~ ~ ~ ~ O O
, -- `-- L L L L `--`-- L L L ~.
~, ~ ~ - ~ 3
O O O O O O O O O 0 ~1 0
~1 _ L L L L L L L L L _ L L
.. o S ~ ,~ ~c'c ~ ~ L L L L L
~
-- ~ ~ L ~ -- C L ~ L
~ ~ ~ O _
2 o ~
E~ Z ~, ,, ,, ~ ~ ~ ~ ~ ~ ~ ~ ~
.
2û9~33
. - 36 - O . Z . 0050/42008
~: o
__ _ _ _ _ _ _ _
~ =E ~3 a a ~:
E OO
_ ~~ ~ _ ~ _ _ _ _ _ ~
c ~ ,~
._
_ _ _ _ _ _ _ _ _ _ _ _ _
~ _ _ _ _ _ _ ~ _ ~ _ ~ _
Zr~ ~ ~ O ~ o ~ o ~ o r~ r~
I ~ ~ O C o Cl~
_
-- ~ ~ O -- o --
a ~E ~ E c -- _
~ o - O O ~ O ~ O ~~ O ~~ ~ o
:~ O O O O CJ~ ~ ~ r~ C~ ~ ~ ~ o o~
.,
.~ c _ _ _ _ _ _ _ _ _ _ _
.' __ _ _
:~ LL t"l ~ ~~ L L 7 ~-~ ~ ~ L
OO ~ O O ~ O
.-- ~-- L LLL ~-- ~-- L L C L ~--
. LL L L L L L L L L L L L
. ~ ~~ A~ ~ ~
` ô ~? e e ~c - ~ c ~ '' c'` c
.~1 L L L L L L L L L L L L L
' .
O e0~ S g e g C g ~ 0~ C 0~ C
L ~ L ~ L ~I L L L
~ WC~ W C~ W
:'
~~ U~ ~O r~ ~ C~ O --
~ ,, ~ ~ ~ ~ ~ ~) ~A;
Z ~ 7
,'' .
:'.
.
:' ': ' ' ' ' ' ` ' '`' ' : ..
,
:............. ' , ' , , ' , ' ~
2~9~33
- 37 - O . Z . 0050/42008
_
. _ _ _ _ _ _ _
~ e ~ ~ = e , _ _
_.
.~ E
_ ___________ _ _ ____
~ ~` =`; e` ~` =` ~ e` ~ e` ~
Z ------------.5--~__ _ ______
I ~ -- o ~ O ~ _ ~ _ ~ ~ o o o o o O
~ ~ a ~ a ~
~ ____---- _ ____--_--____ _
~ ~ O ~ O
~ ~ ~ææ~o~æ~O~O~æ~ O~
':' _ _ ~ , Z Z
. e l l l ' ' l l ' ' ,
, ~111 ~ ~ -t `:t ~ ~ ~ ~ ~ ~
_ _ _
'' ~ ~ ~
:, I _ _ _ _ I I _ _ _
L
, O ~ ~ ~ ~ O O ~ ~ ~
-- L L L L ~ L L L
e ~ e
~0~OgggO~00~g
L L L L L t_ L L L L
O C C - C C .~ C~C
~1 L L L L L L L L L L
o ~ ~ ~
o e o C o e o e o C
~:1 L ~ L _~ L _) L~I L --~
. ~ I~ ~ ~ o _
~ ~ ~ ~ ~ ~ ~r
Z ~
.
,
,`, ~ . ' .
.
. ;
209a~33
- 38 - O . Z . 0050/42008
o
: . r ~ = ~ ~ r
_ ~ ~ ~ ~ _ ~
E C~ ~ _ ` c"o
r
~ ~D ~Cl ~ 3
._
_ -- -- ; _ -- = -- -- _ = _
_ _ _ _ _ _ _ _ _ _
O O C~ C~ O r~ o ~ _ r~ _
~I ~ ~ O C' O ~ ; ~
~ E E ~~
______~__.,_ _ _=_= _
~ ~ ~ ~ ~ ~ ~ ~ r~ ~ o ~ o ~
~i ~ ~i E ~ E ~:' ` ~ ~ ~ -- ~ -- ~
~ o O~ æ o æ , æ cO æ 3, $ ~ oO ~
C O O O L L L L L L
~ ~ ~ ~ ~ ~t -t `S ~S ~
:' _ _ _ _
.
:
., ~
I c c ~ ~. ~ ~ c e
L L t~l ~ ~ ~ L L
V V V V V V V V L
. O L L L L L L L L L
.~
_ _ _ _ _
Vl L ~ L ~ L ~ ~ ~ L
~`` ~
.' ~ . ~ o ~ t
,~ ~j O ~ ~t ~` ~ 1;~ In ~0 Ir~ ~
:' Z r~
: .
~ .
'"'
", ', ~
.: " . .`, .. ' `
` ~
209~33
- 39 - O . Z .0050/42008
~ _ _ _ _
o E E E E
o o o o ~ , S
'/ _ ~ I~ I~ r~ __ _ _ _ _ _ _
: ,_, E _ _ _ _ _ og g o~ ~ ~ ~
._~ E E ` ` ` __ _ _ _
_~ ~ ~ ~ O o ~ ~ = = -- _ _
-- -- -- -- r~ ~ _ _ _ _ _ _ _ _
e~ Z~ I~ ~ O O r--I~ ~r-- O O ,_ r~
~: , -- --o o _ _
.s ~ ~ ~ ~r
~: ~ __________________
= _ ~
~ e c E E ~' ~ E E E E E E _ -- ~; -- --
~ . __________________
U~ O O O O ~ r~ O ~ O ~ O ~ O r~ ~ r~ o o
~, ~ ~ ~ ~ O o -- -- ~ t~ o ~ o ~ o ~ _ _ ~ ~
' ~
,' = _ _
c I I I I I I
o~o ~
Y ~ ~ $ ~
C ~C ~ ~ C C
~LL~ ~ ~ ~ L_~
C C C C ~ ~ C C C C C~ ~ I C
,LLLL~-~-,L (L (L (L ~
:~ ~ ~ ~ C C ~ ~ ~ C~ ~C ,C,
O O O O O O O O O O O O O O
LLLLLLL L L L L_ _
,LLLLLLL L L L LLLL
_ _ _ _ _ _ _
S O S O S O C O C O S O S O
Y ~ ~ ~ ~ k ~ ~ ~ L ~ L ~L~L
;~l
` 2 o o o o o o o o o o __ _ _ _
E~ z; Y Y Y Y Y Y Y Y Y YY Y Y Y
. .
' ' ` ~ .
,
.... . . . . .
~ . .
.
~ .
20~4~3
- 40 - O . Z . 0050/42008
_ S S -
,_ E E E e
_ -- -- -- -- -- -- _
E ~1 ~ O O O O -- --
._ ~c ~`
_. ~ ~ ~ ~ ~ ~ ~ ~ O O
s _ _ _ _ _ _ _ _ ~ I~ _ _ _ _
Z 1~ o O o o ~ I~ O O O O
~ ~ ~ O O
__--~~~_____----_~___ --
.. . ____________~__
g'~~CrC~Oo~~OOooOO
_ _ _ _ _ _ _ _ _ _ _ _ _ _
.
-- --
~: ~ ~ ~ ~
-- -- l l -- -- -- l l --
.~ ~ ~ L L ~'7 ~1 ~ ~ L L t''l ~ ~ ~
L L ~ L L L L ~-- ~-- L L L L
L L L L L L L L L L L L L
C - ~ C C C S C C S C C ~ ~ S
._1 L L L L L L L L L L L L L t_
o O C O
, y ~ ~ ~ ~ ~L ~ O.
:, ~
.
~` .
' : . : :. .
: ' ' :' : : ' ' ::
'. ' :' :, ' ~ ' ', ' ' " :
,,:;, ' , . . .
''. :. ......... ' , ' ' ~ ' .:
209~33
- 41 - O. Z . 0050/42008
. ~ ~ S S----
_ o o ~ V ~ V
:, _ ~ ~ o o o o
_~ v ~ 1~ ~ r _, = S = ~ v
~-7 0 0 0 0 0 0 -- -- -- -- t t
~ _ _ _ _ _ _ _ _ ` ` _ _
Z ~
.-- ,-- o o o o -- _ _ ~_ _ _ o o
_ _ ~ o ~ o ~ o ~ o r~ r~ o o o o
. ~ , ~ , ~ , :~ . ~ o o ~ ~ ~ ~
r` r` ,~ r I~ ~ ~t ~t t t
-- -- -- -- -- -- -- ~ J E E 6 E -- --
~ ,,. 2 o ~`. o ~, , ~ ~
.. , ~ t ~` t 1` t 1` t -t ~ ~ ~ ~ ~ ~
~ ~ ~ ~ ~ ~ r~ r~
e-- -- ~ O ~O
_ _ _ _ _ _
I I ~
L L trl ~ t t L L ~1 ~ ~ ~,t L L
O O ~ o o
_ _ L L L L~-- ~-- L L L L ~
OOO O O O OOOOOOOO
L L L L L L LL L L L L L L
~ 1 5 1~1
L L L L L L LL L L L L L L
C _ _ _ _ _ _ _
~ C L C L ~ L C L ~C L ~ L ~ L
,.~ ~
.`', ~ O ~ O ~ ~ ~ ~ U7 ~D 1- ~ ~ ~ _ ~
,`1 Y Y Y YYYYYYYY
.
.
., ,
. ' . ' ,
.
~' i, . '
203.)~33
- 42 - O. Z . 0050/42008
o .
~, __------_____
', ~ _ _ _ _ _ _ _ _ _ _
0, ~ cs: 3~ ~ ~ ~ ~D
C ~ ~ ~
._ _ = -- _ _, -- -- _ _
e2
. ~ _ ~ _ , _ ~ _
~1 _ ~ E _ _ ~ _ ~ _ _ _ ~. = r~ = '
U~ O ~ O CO ~ O ~ O 1~ 1~ ~ ~ ~ ~ ~ ~
o ~* o ~s ~ ~ ~ ~ ~ ~ o ~ o cr
1~
o ~ o
,~ --
~: ~ ~ ~ ~ L L t~
L L L L ~ L L L L
L L L L L L L L L L
.. . C C ,~ ,c ,~,~,c C ~ ~
: L L L L L L L L L L
; ~ ~ ~ ~ ~ ~ ~ ~ ~ ~
.' ~I_ I~
, _ _ _ _
C L ~ L C C L ~.1 L
','` .~
~' ~ . -- ~ ~ ~ ~ ~ ao ~ o
~; O O O O O O O O O O
E-l Z i i i i iii i i i
. . .
.,,
,.
.' ,, ~ .
"i i ` ; . ' `, : `
.~ .. : ` ; ' ., . :.
., .. :
" ~ , . .
209S433
:i - 43 - O. Z . 0050/42008
' _~
~= S
oE E
Eol ~ ~ E ~ E O E 'o E E
~ ~ ~ o ~ o ~ c~ ~ o o o ~ 3
.__ _ ~
o S _ S I ~ I ~o S ~ s _ _ -- _ _
cl: v~ ~i E ~ E ~ ~ E ~ oi ~ ~ i '; i i i
_ _ _ ,~ _ ,_, _ ,~ _ ,~, _ ,~ _ ,~, _ _ -- _ _ _
z ~ o ~ o ~ ~ o ~ o o ~ --
. ~. ~ ~ ~ ~ ` S S . _ ~ _ . _ ~ , , ~ `~
.. ~ ` ~ ` E ` E ` E ` E ` E ~ e
.. ~ E ~ E E ,~ E ,~ E ~ --- ~ ' -- ' -- _ - i
U~ 1~ r~ o t~ o ~ o t~l o r~ o 1~ o ~ o ~ o
o- ~ cr C~ o cr~ o ~ ~ ~ ~ cr ~ o C~ o o o
I I
L L ~ ~7 ~ ~ L L t~
t c . . . ~ o o c c c ~
~VVV~VVVVV~
o c C C C C C C C C C
.~~ . L L L L L L L L L L L L
O '` O C O C O _ O C O _ ~
~ -- V ~ L ~ L ~ L ~- L ~ L ~ ~
., ~ ~ ~ ~ W ~ W ~ W ~ ~ ~ W ~
P-l
.~ ~ . ~ o r co ~ o --
, ~ O _ _ _ _ _ _ _ _ _
,. Z ~Ji~-J~JJ~
~.`,
,,
. ,
.~ .
'
:
`
209~433
- 44 - O . Z . 0050/42008
. .~ ~ ~
. ~ _ _ _ - _
_ E _ _ _
E o o n ~ n
:. c r- r~
._ ~ _ _
_~ _ r~ _
~: .` =, _
Z _ _ _ _ r~ _
~ ~ r~ r~
:
_ _ -- ~ T -- -- _ _ _ _
E e E --` e _` --` --` _`
U~ I~ O r~ O r~ o 0
~ ~ ~ ~ ~ ~ ~ a ~ o ~
_____________ _
,, ~
C l l l l l l l l l l l l l l l l I I
_ _ _ _ _ _
~ ~ ~ ~ ~ ~.
:' e C " ~, ~, ~,, C " ~, ~, " ~ I -- _ _ _
L L ~1'~1~Lt~ L~
~ ~ ~ c c c c ~ ~ L c c c ~ ~ c c ~ c
. o o ~ ~o ~ ~ o o ~ ~ ~ ~ o o ~ ~ ~ ~
~' `- `- LLLL`-`-L L`-`-L L LL
- L L L L L L ~ L L L L
" O ~ C C ~ C C lC S C ~C - S - C - C '- C
.. . L L LLLLLLLLLLLLL L LL
. ~
O _ ,~ _ ~, _ ~, _ ~, _ " _ ~ -- _ _
: U C g CgCgCOC0C o Cg. g CO
_ ~ ~ ~ ~L~L~-~L~ L ~L
W ~ ~ ~ W ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~ C~
~ ~ ~ ~ O ~ ~ O
,.,
.,
.~ .
.,
: , :.`, :
:
209:~33
~ 45 ~ O- Z ~ 0050/42008
~ .
o
_ _ _ _ _ = ~ _ ~ _
_ _ _ _ _ _ _ _ _ _
E 0 CO
r` 1~ 3 3
_ _ _
~: Z v~ ~ E E E c 07 ~ E c
,* ~ 3 ~ ~ 2 .a~
E ~ E ~ C ~ E ~ E ~ ~ ~ E e
_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ _ ~ _
_ ~ = = = ~ ~ = = _ ~ _
1:1 ~ E -- ~ C . E ~ E ~ E ~ E ~ E ~ C ~ ~ , ~
u~ l~ r` ~ o~ ~ ~ o ~ o cr ~ ~ ~ ~ ~ o ~ o o o o o
~ ~ ~ ~ o ~ o o o o o ~ o ~ o cr o c- o o o o o
~ u ~ s s s s s s
- - - -
~ ~ - ~ ~
L L t` l ~ ~ ~ L L t'l ~ ~ 3
~' O 0 1~1 C C ~ O O C ~_
~_ ~_ L L L L~-- -- L LL L
C C ~ ~ C ~ ~ ~t,
O O O O O O O O LL
S S S S S S S S S S '- _
~,1 L L L L L L L L L LL L
~;
:~ ~ o ~
~c o c o s o s o c oc
., -- ~ ~ L ~ L ~ L ~ L~ L ~ L
':
O ~ ~ ~ ~ ~ ~ ~ ~ ~ O _
Z ~ J ~ J ~ J
209~33
- 46 - O. Z . 0050/42008
= S = S S ~:
_ _ _ _ _ _
_ ~ ~ U~
C ~ ~
._ _ S -- S -- S
~ v~ _ E E E v~ ~ ~ _
C~ o o o
~ _ . _. -- ~ S . _ ~ S ~ S ~ =
E ` _ ` E ~ ' ` = ` E
~-~ ~ ~ o ~ o ~ ~ ~ ~ o ~ o ~ o ~ o ~ o
-- . _ ~ -- . ---- ` E ` E ` E ` E
-- o l-- o ~ ~ O ~--~ O ~ ~ ~ `~ O ~ o o o
o ~ o o~ O CJ~ O O O O O ~ O ~ o ~ ~ ~ ~ o
S S
1 1 1
C S . r T ~ ~ LL
:
,, _ _ _ _
.,
.
:' I I_ _ _ _ I I _ _ _
C C~ ~ C~ ~C
I LL~LL~
._~-LLLL~-.-LLL
C -- ~ ~ ~ ~ ~C C ~ ~ :~
. C L~LLLLL~OLLLLL
1~ V
~r~ ,~ C e C C e .~ e e ~ ~ I
LLLLLLLLLLL
o
U ~ g - O C O e e e
.~ ~ ~L~L L~L~L~
~.
~ O ~
`i ~ O U~
z ~
.
209~3
- 47 - O. Z . 0050/42008
_.
~ '
`: _
.: 6 t~
`~;' o.
._ ~ ~ ~
Z E ~ v~
_ U~
_ E _ ~ ` '
E ~ E ` E
U~ o o ~ o ~ o
0~
C L L L
_ _
' l l
~ ,o ,o "
. ~ C: ~ L L
~1 C7 3
,,, o _
~ L L
~' ~ JJ~
:;
2~3~433
- 48 - O . Z . 0050/42008
~ ~ _ __ _ _ _ _ _ _
v~ Er` r~
: I CL _ __ _ ~ ~ _ _ _
~: 11 ~ E EE = ~ --~ E E -- ; I E;
. c 2 _ __ _ _ _ _ _ _ _ ~ _
~: _ I O OO O ~r~oooo oo
~: S o ~o o ~ ) o ~
c ~ ` ` ` ` ` ~` ` ~ e` ~ E
~ ______________ __
.. ~ ~ ~ ~ : I~ ~ r~ ~ o o ~ o ~ o ~ o
`'.`. l ~ r~ ~ ~ ~ 1~0 1-- 0 r~ O
,:: =
C
O ~
O~fO _ _
V _ _ _ _ ~ ~ _ _ _ _
~: ~ ~'7 ~ `S L L t~ t'~ ~t
~ O O ~
L L L L~-- ~-- L L L L
~ ~ ~ O~~C C ~
3 3 ~ L L L L ~ ~ L
~ C .~ C C C C C C C
L L'L L L L L L L L
:;
~j _ _ _ _ _
~ O ~ O C O ' O '- O
S ~ ~ L ~ L ~ L ~ L ~
_ ~ ~ ~ U~ D /~ C~ ~ O
. O O O O O O O O O --
Z ~ S S S ~ I S
. '
- 49 - O . Z . ooso/42oo9 ~) 4 3 3
. ,
::
:., ' E ~ ~ ~ ~ ~ v~
~. _ _ _ _ _ _ _ _ _
,: ~ o o ~ ~ I~I` O o
C~ o o ~
.' c r~
. ` ._
: ~ ~ E ' E E E E
Z ~ ~ ~~-1 o o ~ ~
_ _
3 ~
: ~ _ _ _ _ _ _ _ _ _ _ _ _ _
(~ _ _ _ = _ _ _ _ =
:~. ~ ____________
. . _ _
U~ ~ O ~ O r~ O l-- O ~ O ~'7 0 ~ ~
_ _ _ _ _ _
C ;r ~
_ _ _ _
~ ~ I I I I ~ ~
L ~ ~ ~ ~ ~ L L
O O ~ O O
-- ~-- LL. L L .-- `--
e ~ c ~-
3 ~ 3 3
O ' S S S S S S
v
s o s o e o s o
~ ~ ~ ~ L ~ L ~ L
S ~ L~ ~ W
_ ~ ~ ~ ~ ~O l~ ~
leC O _ _ _ _ _ _ _ _
E~ Z S S:C S S S I S
`- , - :: .. .. , : '
, -: -
203~433
- 50 - O. Z .0050/42008
: ~_ _ _ _
~ _ aE E E E =
.~ O _ _ _ _ ~ ~
O o o o ~ =
~: _ r~ c" O
3_ _ _ _ ~ ~o -- -- --
'' ~~o 0, ~ 1~` a~ ~
.~, C Z__ __, ,O _ O
- e ~ ~~ ~ ~ E
o o ~ ~ ~ ~ ~ o
O O ~ ~ O O O O ~ O ~ O O
r~ I~ ~ cO o 7 0 ~1 Cl~ c~l ~ ~ ~
C IIIII I
T
~ ~: - -
T ~ . ~) ~
' ~ ____~ ,_ _ __
1~: L L L L ~--~-- L L L L
L L L
: S S C ~ S ~ .~) S~ ~ -
L L t L L L L L
C~ I_ I~
_ _ _ __
C O S O S O SO C O
ct o
. ~ O O O o O O O Oo ~-
Z 2 Z 2 Z Z Z z z z
''
.,~' ,
. .
:~ `
'~ .
: ~ :
20~ ~433
- 51 - O. Z . 0050/42008
:::
o _ _ _ _
_ e E` e`
3 ~ ^ ~ ~ o
3. E E E E E E E =
, ~ ~ E E
~o O O ~ ~ ~ O O O O
`. _ __ __----_------__
Q _ _ _ _ E _ - = _ _ -
o o o o ~ o o o o o o o
~n oo oooooooooo
o o ~ ~ ~ C~ ~ 0 ~ ~ CJ~
C
_ _ _ _ _ _
-- --
~ C ~ ~ ~ ~ C C ~. ~ ~, ~ ~ C ~,
LL~L L ~ ~ ~ ~ L L
~ ~ ' ' ' ' o ~ ~ ~ c c o. ~ c c c !
- - L LLL~-._~L L ~LL
C C ~ S ~C, ~ ~, _, ~, C ~C
~ 3 3 L 3 LLLLLLL 3 3 LL 3 LL
C ~ C C C C C C C C S C S C _ C C C _
~ LLLLLLLLLLLLLLLLLL
_ _ _ _ _ _ _ _ _
_ ~ ~L~L~L~LCL~OL~OCOCO
Z ~ ~ ~ C~ ~ O~ ~ ~ ~ ~ ~ ~ ~ O~
~ O
E~ Z Z Z Z Z 2 Z Z Z z Z Z Z Z Z Z z z Z
J -. ' : .
20~5~3
- 52 - O. Z . 0050/42008
. ,
,. _ ~_ ____
:..................... . E e ~ v
O ~ ~ ~ r7
!,.~ E_ _ -- -- -- -- -- _
.- ,cE E E E ~ ~ ~
CO U~ ~ U') ~ o o o o
:', Z ~o ~ ~ ~ ~ 1' 1~ 1~ r~ ,~
~,~ I ~ ~ o o o o ~ ~ ~
-- -- o ~ o ~ _ _ _
E E
o o ~ ~ o
. ~ g Cl
_ _ _ _ _ _
:~ C -- -- ~ I ~O D 'D ~D
;~ ~
'
, ~ _ _ _ _
~ $
:~,;, C C ~ ~ ~ ~ C C
~ L L t'l ~'1 ~ ~ L L
C C c c ~ c c
.
~0 _ ~
~ L ~ L '- C o
:, Z ~ ~ . W C~ Li.l
; .
.,1 ~3 ~ o ~
ZZZ Z Z2ZZ
'~'' '' : ' ''
:'
; ~ :
2~9~33
.
: - 53 - O. Z . 0050/42008
. '
. , --
r~
~ e E
_ ~
^' EiE E = _ e e` e` ~`
:~' o E ~ ~ E E ~ ~ ~
._ _ _ _ ` ` `
o _ ~ ~ ~ ~ _ _
e~ z E E E E ~ ~ = e -- e
O o ,~
~ _ _ _ _
c ~ _ _ _ _ _ _
3:1~ ~E e E E E e
~ æ æ ~ ~ æ æ ~ ~ æ æ ~ ~ -
~. _ _
o~o ~ coo~
o o o o o o o o o o o o o o o o o o
L L L L L L L L L L L L L L L L L
L L L L L L L L L L L ~ L L L L L
-- ~
~LO~0~C~0 CO COCOCOCOCO
l-- ~ ~ o -- ~ ~
o o o o o o o o o -- -- -- -- -- -- -- -- --
. .
.: .
2~9~33
- 54 -O . Z . O0S0/42008
r_ = S S S = S~ S ' S
. o E E e E `` E E` ` ~i ~ ~ ~
. o ~ I~ I~ r-- r-- ~ ~ ~ ~ o o ~ o
~ ~ o o o I ~
: _ ~ _ ~ _ _ ~ ~ r` r` -- -- o o -- -- -- --
~ CL = _ S ~
~ ,c E E E E ~ ~ o o ~ ~
~ _____ _ ~_,~______
: ~: o S ~ = S I I -- ~ ~
~ _ oooo~~ ~oo~oooo__
= _ _ _ -- o o o o -- -- o o _ _ _ -- I_
_ -- S ~ ~ s ~ S ` _ ~ -- -- o
~ E E E E ` ` ` ` E; ~ ~; -- -- =
~ __________~
= -- =
. . . ..~ E ~ E ~ e
. _ _ -- -- -- -- ~ 3 ~ ~
~ U~ OOOO1~ r~OO~OOoo1--.-- O
c.~
:: s _ _ _ _
s ~
., s
~' o ~ _ _ _ _ _ _
z~ cc ~, ~. ~ ~ :~ ~.
s r
o~o _ _ _ _ e ~ c ,, ~, ,, ,, ! c
I ~
'~ ~ ~ ~1 ~ ~ L L ~ t`~ ~ t L L ~ ~ 3 3 $_ t_
r ~CCCCL ~ cccc~cccc~
O
~: L L L L ~-- ~-- L L L L ~-- ~-- L L L L ~
~ C S ~ ~ S ~ ~ ~ S C
L L Lg L L L L L L L L L L OL
SSSCS - CSSSSSSCSSSS
1~ L L L L L L L L L L L L L L L L L L
.,.
_ _ _ _ _ _ _ _ _
:,' ~ S O S O -- O S O C O S O S O S O _ O
~ ~: L L ~ L ~ ~ ~ ~ Ll ''
,~ IL1
1~l ~ o o _ ~ ~
:; ~ O O O O O O ~ O O O O _ _ _ _ _ _ _ -- --
., .
209a433
- 55 - O.Z. 0050/42008
- Herbicidal active ingredient~ and antldote
compound~ can be applied together or separately after
emergence to the leave~ and ~hoots of the crop plants and
the unwanted gra~ses. The antidote is preferably applied
at the same tLme as the herbicidal actLve ingredient.
Separate application with the antidote being applied
fir~t, and then the herbicidal active ingredient, to the
field is also posqible. This may entail the herbicidal
active ingredient and antidote being formulated together
or separately in a form which can be suspended, emulsi-
fied or dissolved for spraying.
Also Lmportant in practice is treatment of the
seeds of the crop plants with the antidote before sowing.
The herbicidal active ingrPdient is then applied alone in -
a conventional manner.
The amounts of an antidote which are required
vary when herbicidal (hetaryloxy)- or aryloxyphenoxy-
acetic acid derivatives XI are used in different crops.
The ratios of amounts can be varied within wide limits.
They also depend on the structure of the (hetaryloxy)- or
aryloxyphenoxyacetic acid derivative and the particular
crop. Suitable ratios of antidote to herbicidal active
ingredient are from O.Olsl to 10:1, preferably from 0.1:1
to 4:1, by weight.
The amounts of an antidote required for the
cyclohexenone derivative XII vary for different crops.
The ratios of the amounts of a cyclohexenone derivative
~II and a compound Ia or Ib can be varied within wide
li~its. They depend on the structure of the cyclohexenone
derivatLve XII and of the compound Ia or Ib and on the
particular crop. Suitable ratios of antidote to herbi-
cidal active ingredient sre from 0.01:1 to 10:1, prefer-
ably 0.25:1 to 4:1, by weight when applied either to-
gether or ~eparately.
The novel herbicidal agent~ can contain, be~ide~
the compound Ia or Ib a~ antidote and a herbicide from
the group of (hetaryloxy)- or aryloxyphenoxyacetic acids
: .
. .
2~3~33
- 56 - O.Z. 0050/42008
XI or of cyclohexenones XII, other the herblcidal or
~- growth-regulating active ingredients with a different
chemical structure, with retention of the antagoni~tic
effect.
The agents according to the invention or, in the
case of separate application, the herbicidal actLve
ingredients or the antidote are applied, for example, in
the form of directly sprayable solutions, powders,
suspensions, including high-percentage aqueous, oily or
other suspensions, or dispersions, emulsions, oil disper-
sions, pastes, dusting or broadcasting agents or gran-
ules, by spraying, atomizing, dusting, broadcasting or
watering. The application forms depend entirely on the
purposes for which they are used.
Suitable for preparing directly sprayable solu-
tions, emulsions, pastes and oil dispersions are mineral
oil fractions of medium to high boiling point such as
kerosine or diesel oil, also coaltar oils, and oils of
vegetable or animal origin, aliphatic, cyclic or aromatic
hydrocarbons, eg. benzene, toluene, xylene, paraffin,
tetrahydronaphthalene, alkylated naphthalenes or deriva-
tives thereof, eg. methanol, ethanol, propanol, butanol,
chloroform, tetrachloromethane, cyclohexanol, cyclohexan-
one, chlorobenzene, isophorone, highly polar solvents
such as dimethylformamide, dimethyl sulfoxide, N-methyl-
pyrrolidone and water.
Aqueous application forms can be prepared from
emulsion concentrates, pastes or wettable powders or
oil dispersions by adding water. To prepare emulsions,
pastes or oil dispersions, the herbicidal active
ingredient and/or antidote can be homogenized, as such
or dissolved in an oil or solvent, using wetting agents,
adhesion promoters, dispersants or emulsifiers, in water.
However, it is also possible to prepare concentrates,
which are suitable for dilution with water, from herbi-
cidal active ingredient and/or antidote, wetting agent,
adhesion promoter, dispersant or emulsifier and,
209a433
.
- 57 - O.Z. 0050/42008
possibly, solvent or oil.
Suitable surfactants are alkalL metal, alkaline
earth metal and ammonium salts of ligninsulfonic ac~d,
naphthalenesulfonic acid, phenolsulfonic acid, alkylaryl
sulfonates, alkyl sulfates, alkylsulfonates, al~ali metal
and alXaline earth salts of dibutylnaphthalene~ulfonic
acid, lauryl ether sulfate, fatty alcohol sulfates, fatty
acid alkali metal and alkaline earth metal calts, salts
of sulfated hexadecanols, heptadecanols, octadecanols,
salts of sulfated fatty alcohol glycol ethers, products
of the condensation of sulfonated naphthalene and naph-
thalene derivatives with formaldehyde, products of the
condensation of naphthalene or of naphthalenesulfonic
acids with phenol and formaldehyde, polyoxyethylene
octylphenol ether, ethoxylated isooctylphenol, octyl-
phenol, nonylphenol, alkylphenol polyglycol ether,
tributylphenyl polyglycol ether, alkylaryl polyether
alcohols, isotridecyl alcohol, fatty alcohol/ethylene
oxide condensates, ethoxylated castor oil, polyoxyethy-
lene alkyl ether, ethoxylated polyoxypropylene, laurylalcohol polylglycol [sic] ether acetal, sorbitol ester,
lignin sulfite waste liquors and methylcellulose.
Powders and dusting and broadcasting agents can
be prapared by mixing or grinding together herbicidal
active ingredient and/or antidote with a solid carrier.
Granules, eg. coated, impregnated or homogeneous
granuies, can be prepared by binding the active ingredi-
ents to solid carrier~. Examples of qolid carrier~ are
mineral earths such as silica gel, silicic acids, sili-
cates, talc, kaolin, attapulgite, limestone, chalk, bole,loe~, c18y, dolomite, diatomaceous earth, calcium and
magnesium sulfates, magnesium oxide, ground plastics,
fertilizers such as ammonium sulfate, ammonium phosphate,
ammonium nitrate, ureas and vegetable products such as
cereals flour, bark meal, wood meal and nutshell meal,
cellulose powder and other solid carriers.
The formulations contain from 0.01 to 95% by
-"- ' ''
2 0 9 ~ ~3 3
- 58 - O.Z. 0050/42008
weight of herbicidal active ingredient and antldote,
preferably from 0.5 to 90% by weight. The appllcatlon
rates for herbicidal acti~e ingredient are from 0.2 to
5 kg/ha active substance.
The preparation of the novel compounds Ls
explained in detail by the following examples.
PREPARATION EXAMPLES
EXAMPLE 1
5-Ethyl-1,3,6-trimethylpyrido[2,3-d]pyrimidine-2,4-
(lH,3H)-dione
O Et
Me~
Me
11.6 g (75 mmol) of 4-amino-1,3-dimethylpyrimidine-
2,4(lH,3H)-dione were introduced into 100 ml of absolute
dimethylformamide under nitrogen at room temperature.
Then, over the course of 15 min, 13.2 g (100 mmol) of
3-chloro-2-pentene-2-carbaldehyde were added dropwise.
The reaction mixture was then heated to 95C over 30 min
and kept at this temperature for 6 h. It was subsequently
cooled to room temperature, and the product was removed
by filtration. Drying resulted in 15.6 g (67~) of yellow
crystalline product (active ingredient example 1.006).
` The products listed in Table 1 were prepared
correspondingly:
. . ' ' ' .
~ ~ .
209~'133
: - 59 - O.Z. 0050/42008
TAB~E 1
0 R3
Me`N~R 4
l~le
Example No. R3 R4M.p.
1.001 Ph H145C
1.002 3-NO2-C6H4 H244-248C
1.003 4-Cl-C6H4 H165C
1.004 Me Ms15~-156C
1.005 Me Et90C
1.006 Et Me120-123C
1.007 Ph Me172C
1.008 -(CH2)3- 134C
1.009 -(CH2)~- 148-150C
1. 010 - (CH2) 5- 145C
1.011 -tCH2)0- 165C
EXA~PLE 2
1,3-Dimethyl-7-(3-nitrophenyl)pyrido~2,3-d]pyrimidine-
2,4(lH,3H)-dione
M~`~N 2
Me
A mixture of 6.2 g (40 mmol) of 4-amino-1,3-
dimethylpyrimidine-2,4(lH,3H)-dione and 11.6 g (45 mmol)
of 3-nltro-~-dimethylaminopropiophenone hydrochloride in
100 ml of glacial acetic acid was refluxed for 3 h. After
addition of 2 ml of concentrated hydrochloric acid, the
mixture was ~tirred at the same temperature until the
su~pension was colorless. Cooling and filtration resulted
in 8.9 g (78~) of cry~talline product (acti~e ingredient
example 2.001).
:
;
.
~ ' .
.
" .
209~433
- 60 - O.Z. 0050/42008
The products listed in Table 2 were prepsred ~n
a sLmilar manner:
TABLB 2
o
R 2`NJ~R 5
O~N~N~R 6
Rl
S Example No. R1 ~ R5 R6 M.p.
;
~` 2.001 ~e Me H 3-NO2-C5H~ 261C
2.002 Me Me H -CH=CH-Ph 100-110C
2.003 Me Me H 2-Thienyl 186-188C
10 2.004 ~e Me H 3-Indolyl 210-215C
2.005 Me Me Me 4-Cl-C6H~ 235-244C
2.006 Et H H 4-NO2-C6H~ > 250C
EXAMPLE 3
1,3-Dimethyl-6-methoxycarbonyl-7-methoxycarbonylmethyl-
pyridot2,3d]pyrimidine-2,4(lH,3~)-dione tsic]
n
Me ~ C02Me
CH2C02Ma
M~
A su~pen~ion of 4.85 g (20 mmol) of 5-dimethyl-
aminomethylenepyrimidine and 3.48 g (20 mmol) of dimethyl
acetonedicarboxylate in 100 ml of absolute ethanol was
cooled to 0C. 2.4 g (24 mmol) of triethylamine were
added dropwise over the course of 20 min, and the mixture
was then ~tirred at room temperature for 2 h. It was then
cooled to -15C, and the precipitate was filtered off and
dried under reduced pressure. This resulted in 3.81 g
2S (59~) of product (active ingredient example 3.008).
The compounds listed in Table 3 can be prepared
in a similar manner:
:
--: . : . .. ....
.. , ' :. ~ ' . ~ '' ` `
. , . ' ' .
209~433
- 61 - O. Z . 0050/42008
TABL~ 3
R 2`N~,R 5
oll~NJ~R6
R I
Example Rl R2 R5 R6 M.p.
No .
. .
3.001 Me Me CONH2 NH2 >250C
3.002 Me Me COHH2 Me >250C
3.003 Me Me CONHPh Ph ~250C
3.004 Me Me CO2Et CH2C189- 90C
3.005 Me Me COzEt Ph 155-157C
3.006 Me Me CO2Et CO2Et>250C
3.007 Me Me CO2Me NH2 >250C
3.008 Me Me C02~e CH2CO2Me164- 166C
3.009 Me Me C02H Me >250C
3.010 Me Me CO2H OH 263-266C
3.011 Me Me C02H CH2CO2H>250C
3.012 Me Me C02H Ph 217-219C
3.013 Me Me COMe Me 153C
3.014 Me Me COPh Ph >250C
3.015 Me Me COCO2Et Me >250C
3.016 Me Me COCO2Et Ph >250C
-OC
3.017 Me Me ~ >250C
3.018 Me Me -C-(CH2) 3 - >250C
3.019 Me Me -C-IClH2-cMe2-cH2- 181 - 192 C
O
: 3. 020 Et H C0Mc Me >250C
3.021 Et H CO2Et Me 250C
3.022 Et H CO2Et NH2 >250C
3.023 Et H CO2Et OH >250C
3.024 Et H C2~H2 Me 215C
3.025 Et H CO2H NH2 >250C
3.026 cyclo-Pr Et COMe Me >250C
; 3.027 cyclo-Pr Et CO2Et Me 240C
3.028 cyclo-Pr Et CN NH2 >250C
, 3.029 cyc l o-Pr Et CONH2 Me 83C
'` 3.030 cyclo-Pr Et CO2Et NH2 >250C
3.031 cycto-Pr Et CONH2 NH2 >250C
3.032 cyclo-Pr Et CO2MO OH 148-150C
:' :
.:.
: , ` ' ''~ - :
.' : `, ' ' . ` ' ' ' ' .:
` : `: .. .
209 ~433
- 62 - O.Z. 0050/42008
- EXAMPLE 4
1,3-Dimethyl-7-ethoxycarbonyl-8-ethylcarbonylaminopyrido-
[2,3-d]pyrimidine-2,4(lH,3H)-dione [~lc]
Me~N~CO ~E t
OJ~N~NH I I E t
Me o
A mixture of 5.60 g (20 mmol) of ethyl 7-amino-
1,3-dimethyl-2,4(1H,3H)-dioxopyrido[2,3-d]pyrimidine-6-
carbo~ylat2 and 2.86 g (22 mmol) of propionic anhydride
in 30 ml of propionic acid was refluxed for 24 h. Cooling
and filtration resulted in 5.47 g (82%) of product
(active ingredient 4.002).
The compounds listed in Table 4 were prepared in
a similar manner:
TABLE 4
~. .
.. O
Me~C02Et
O'l~R 6
., M~
:~ 15 Example No. R~ M.p.
.,
4.001 -NHCOMe 148C
4.002 -NHCOEt 131-134C
4.003 -~SO ~ ~250-C
4.004 -OCHMe2 112C
:Compounds Ia and Ib which are di~closed in the
literature or prepared by literature methods and which
- are likewi~e able to increa~e the tolerance by crop
plants of the herbicidal acti~e ingredient~ of the
Sormulde XI and ~II are ll~ted ln T~ole 5 whlch ~ollowe.
'
209~3
- 63 - O.Z. 0050/42008
TABL~ 5
O R3 o
Me~R 4 Me~NJ~R 5
oJ~ I N~J C~NJ~N~R6
Me Me
la la
Example R3 R' R5 - ~6 Llterature
5.001 OH C02Et - - 1
5 . 002 NH 2 CN - - *
5 . 003 OH H - - *
5 . 004 - - H Ph 2
5 . 005 - - H 4-Me-C6H4 2
S . 006 - - Me Ph 2
-CH2-(o-c6H4) 2
5.008 - ~ -(CH2) 2-(-C6H4) 2
5 . 009 - - - (CH2) 3- (o-COH4) 2
5,010 - - CN NH2 3
5.011 ~ ~ C02Et NH2 3
5.012 - - C02Et OH 3
. 5.013 - - C02Et Me 3
5.01~ - - C02H NH2 3
5. 015 ~ ~ -- C -- N - C -- N -- 3
5.016 - _ ~ C -- NH ~ 6 -- NH -- 3
S
1) G.L. Ander~on, J. Heterocyl. [~ic] Chem. 22 (1985)
1469.
2) R. TroschUtz, H.J. Roth, Arch. Pharm. 311 (1978)
` 406.
3) R. Hirota, Y. Ritade, S. Senda, J. Heterocycl. Chem.
22 (1985) 345.
~) prepared in a ~imilar manner to 1).
` Example~ of the biological a~-tion
The effect of variou3 repre~entative~ of the
:. . .
;; . ~ . . . :
209S433
- 64 - O.Z. 0050/42008
novel herbicidal agent~ or combinations thereo, composed
of herbicide and antidote, on the growth of wanted and
unwanted plants compared with the herbicldal actLve
ingredient alone was demonstrated by the followlng
examples from glasshouse tests:
The plants were grown in plastic flowerpots with
a capacity of about 300 cm3 containing loamy sand with
about 3.0% humus as substrate. The seeds of the te~t
plants were sown shallowly, keeping the species separate,
and were moistened. The pots were then covered with
transparent plastic covers until the seeds had uniformly
germinated and the plan~ ~ad star. d to grow.
For the post-emergence treatment, the test plants
were grown to a height of from 3 to 20 cm, depending on -
- 15 species, and only then treat2d. The herbicidal agents
were suspended or emulsified in water as dispersant and
were sprayed using finely distributing nozzles.
The example~ of cyclohexenone herbicides XII were
fH3 OH ~N-oC2Hs
C2HS-S-~ ~ H ~ B.15
n-C 3H7
,~
~ ~{~(CH2)2C~=C ~ I
S Y ~ \ E.34
(~ C2H5
' The~e herbicidal active ingredient-~ were each added as
commercial formulation (100 g/l emulsion concentrate) to
the preparation of the particular antidote and thus
applied together with the latter.
For comparative test~, active ingredients B.15
and F.34 were each applied with a mixture of 80% by
volume cyclohexanone and 20% by volume ethoxylated castor
oil (formulation without antidote).
All the antidotes were prepared for the post-
emergence treatment in a mixture composed of 80% by
- volume cyclohexanone and 20% by volume ethoxylated castor
::`
209~33
- 65 - O.Z. 005~/42008
oil containing 10% by weight active ingredlent.
- List of test plants:
Abbreviation Lat. name English name
S LOLMU Lolium multLflorum Italian ryegras~
SETVI Setaria viridi~ Green foxtail
TRZAW Triticum aestivum Winter wheat
ZEAMX Zea mays Indian corn
The test vessels were placed in a glasshouse with
the warm-loving species at 18 to 30C and those from
temperate clLmatic zone~ at 10 to 25C.
The agents were suspended in water as carrier and
dispersant and were applied to run-off to the plants
using a mobile sprayer with finely di~persing nozzles.
- 15 The tests la~ted 3 to 5 week~ during which the
plants were tended and their reaction to the individual
treatments was recorded.
The damage caused by the chemical agents was
assessed on a scale-from 0 to 100% by comparison with the
untreated control plants. On this scale, 0 means no
damage and 100 means complete destruction of the plants.
The following Tables 6 to ll document the
antidote action of novel pyridot2,3-d]pyrimidine-
2,4(1H,3H)-diones Ia and Ib.
The antidote compounds distinctly improve the
tolerance of the herbicides B.15 and E.34 by graminaceous
crop plants.
TA3LE 6:
Improvement in the tolerance of herbicide B.15 (sethoxy-
dim) by corn and wheat on adding an antidote compound
with post-emergence application; glasshouse test
~:.
.
' ;.
.
. .
. ~', '` '',, . ~' '' ,'
2~9~433
- 66 - O.Z. 0050/42~08
Application ra~e ~kg/ha] Test plsnts and d~mage ~%]
Anti- Herbicide Antidote Crop plant Unwanted plant
dote Indian Winter Lolium
No. corn wheat mLltiflorum
- _
- 0.015 80 - 9~
3.001 0.015 0.06 lS - 98
- 0.06 - 40 100
1.004 0.06 0.25 - 0 100
Table 7:
Improvement in the tolerance of herbicide E.34 by corn
and wheat on adding an antidote compound with po~t-
emergence application; glasshouse test
Application rate [kg/ha] Te~t plant~ and damage [%]
15 Anti- Herbicide Antidote Crop plant Unwanted plant
: dote Indian Winter Lolium
No. corn wheat multiflorum
- 0.25 85 80 100
1.006 0.250.75 15 20 90
.
Table 8:
Improvement in the tolerance of herbicide E.34 by corn on
;~ adding an antidote compound with po~t-emergence applica-
tion; glasshouse test
Application rate lkg/ha] Test plants and damage [%]
Anti- Herbicide Antidote Crop plant Unwanted plant
dote Indian Setaria
No. corn virdis
- 0.125 - 75 100
; 1.001 0.1250.375 30 100
Table 9s
Improvement in the tolerance of herbicide E.34 by corn on
: adding a pyrido~2,3-d]pyrimidin having an antidote
.~ 35 ef~ect; post emergence application in the greenhouse
2~9~3~
- 67 - O.Z. 0050/42008
Application rate [kg/ha] Test plants and ~Am~ge [%]
Anti- Herbicide Antidote Crop plant U~wanted plant
dDte Corn LixisSetar~a
No. variety viridis
_ _
- 0.25 - 100 100
3.008 0.25 0.25 30 100
3.00S 0.25 0.25 20 100
3.004 0.25 0.25 30 100
3.006 0.25 0.25 0 100
1.007 0.25 0.25 20 95
1.006 0.25 0.25 30 100
` 1.010 0.25 0.25 10 95
- 0.125 - 85 100
5.011 0.125 0.125 50 100
5.013 0.125 0.125 30 100
3.001 0.125 0.125 15 100
3.008 0.125 0.125 20 98
3.005 0.125 0.125 10 100
3.004 0.125 0.125 20 95
~: 3.006 0.125 0.125 0 98
1.007 0.125 0.125 10 90
1.006 0.125 0.125 15 85
1.008 0.125 0.125 10 90
3.016 0.125 0.125 30 90
1.010 0.125 0.125 0 9o
.~,
Table 10
Improvement in the tolerance of the herbicide ~.34 by
: corn and wheat on atding a pyrido~2l3-d]pyrimidine having
; 30 an antidote effect; postemergence application in the
~ greenhouse
~ . .
~. :
209~4~3
- 68 - O.Z. 0050/42008
Application rate [kg/ha] Te~t plants and damage ~%]
Anti- Her~icide Antidote Crop plant Unwanted plant
dote Corn Spring Setaria
No. I,l~ ~ wheat v~id~
variety Star
- 0.06 - 60 90 100
5.011 0.06 0.06 20 - 100
3.013 0.06 0.06 0 - 75
3.001 0.06 0.06 0 _ 90
3.009 0.06 0.06 20 _ 85
- 3.003 0.06 0.06 20 _ 80
3.005 0.06 0.06 o - 80
3.004 0.06 0.06 0 65 85
3.006 0.06 0.06 0 10 90
: 1.007 0.06 0.06 10 20 80
1.009 0.06 0.06 10 65 90
3.016 0.06 0.06 10 50 95
Table 11
Improvement in the tolerance of herbicide E.34 by wheat
by adding a pyridot2,3-d~pyrimidine having an antidote
effect; postemergence application in the greenhouse
Application rate [kg/ha] Test plants and damage [%]
Anti- Herbicide Antidote Crop plant Unwanted plant
2S dote Spring wheat Setaria
No. Star viridis
- 0.03 - 85 75
3.008 0.03 0.03 lO 60
3.005 0.03 0.03 lO 45
3.004 0.03 0.03 40 75
3.006 0.03 0.03 0 40
;..... .