Note: Descriptions are shown in the official language in which they were submitted.
W092/08801 PCT/~S91/0~21
~ n~ 4~
BRIDGING ANTIBODY FUSION CONSTRUCTS
This application is a continuation-in-part of the
copending United States patent application Serial No.
07/612,110, filed November 9, 1990.
BACKGROUND OF THE INVENTION
This invention relates to therapies involving selective
destruction of cells in vivo, and more specifically, to
compositions of matter useful in the treatment of various
cancers and viral infections. In particular, this application
relates to genetically engineered-antibody fusion constructs
capable of targeting an infected cell and bringing that cell
into contact with an effector cell which can kill or
neutralize its detrimental activities.
Hormone receptors have been used as tumor-specific
markers for the delivery of cytotoxic agents to tumor cells.
For example, Pseudomonas exotoxin and di~htheria toxin have
been coupled to peptide hormones and have been shown to be
highly cytotoxic and specific for receptor-bearing cells
(Astan et al. (1989) J. Biol. Chem. 264:15157-15160; Bacha et
al. (198~) J. Exp. Med. 167:612-622).
Antibodies have been shown to mediate the lysis of tumor
cells in vitro by bridging the Fc receptor (FcR) on the
cytotoxic effector cell and the antigenic site on the target
cell (Henkart (1985) Ann. Rev. Immunol. 3:3~-58. The binding
is mediated by the variable (V) regions of the heavy (H) and
light (L) chains of the anti-tumor cell antibody and the FcR
binding site on the constant (C) region of the Ig H chain. In
an analogous manner, cytotoxic T lymphocytes have been
targeted to cells for which they have no natural specificity
SUBSTITUT' ~E~T
.. . , - . . . . -. .
. . ~ . . . -
.
WO92/08801 PCT/US91/08421
2 ~ 2
through the use of cross-linking aqents. These include several
hetero-bifunctional reagents that share the same mechanism;
they bridge a specific marker on the tumor cell surface to a
component of the T cell receptor (TCR) and in this way
activate the lytic program of the cytotoxic T lymphocyte (Lui
et al. (1985) Proc. Natl. Acad. Sci. USA 82:8648-8652; Perez
et al. (1986) J. Expt. Med. 163:166-178; Jung et al. (1986)
Proc. Natl. Acad. Sci. USA 83:4479-4483).
However, the use of heterobifunctional antibodies and
chemical cross-linking reagents may not be efficient. Because
of the random association of multiple H and L chains, only a
fraction of the resulting antibodies usually are active.
Similarly, the binding of a chemical cross-linking reagent may
disrupt or inactivate the site or protein at which the reagent
binds and hence may not enable the triggering of the effector
cells' killing or neutralizing activities.
Among the targeting approaches used to combine anti-T
cell and anti.-tumor cell specificities is the biochemical
conjugation of a peptide hormone to an antibody which
recognizes a surface antigen on a receptor-bearing cell (see,
e.g., Lui et al. (1988) Science 239:395-398). This approach
has some advantages over hetero-bifunctional antibodies.
First, the random association of the multiple ~ and L chains
is avoided, resulting in a more homogeneous preparation.
Second, the targeting of hormone receptors, relative to other
tumor-associated antigens, may lead to the preferential
killing of those cells that overexpress the hormone receptor
(i.e. the most rapidly growing cells) and thus, are the most
malignant.
Therefore, what is needed is an alternative targeting
approach involving the use of a heterobifunctional
antibody/ligand conjugate or construct that physically bridges
a receptor-bearing tumor target cell and an effector cell, and
S~IBSTITUTE S~EET
,
.
- ~. :.: . . . ':
;: '- ~
W092/08801 PCT/US91/0~21
2~9~3~2
that activates the killing mechanism. Using this ~pproach it
should be possible to confer upon a population of effector
cells an anti-tumor specificity that it does not normally have
and would lose as soon as the construct is withdrawn or
metabolized in vivo. Thus, such a construct would be useful in
an adoptive immunotherapeutic approach either alone or in
conjunction with the administr~tion of a patient~s activated
effector cells.
Accordingly, an object of the invention is to provide a
construct that bridges an effector cell and a target cell,
thereby enabling the killing or the neutralization of that
target cell. Another object is to produce a bridging construct
that will not inactivate the killing or neutralizing
activities of the effector cell when it is bound thereto. Yet
another object is to provide an efficient and effective method
of targeting effector cells to malignant or virus-infected
cells. Still another object is to provide a method of
producing these bridging constructs.
SVMMARY OF THE INVENTION
Using the genetic approach, antibody fusions constructs
have been produced which effectively bridge a target cell,
such as a malignant or virus-infected cell, and an effector
cell. Such constructs enable treatment of malignancies and
virus infections with accuracy and efficiency.
A representative antibody fusion construct includes a
heavy chain variable region, a heavy chain constant region
having a C83 domain, and a non-immunoglobulin binding agent
which binds a surface antigen or receptor on a target cell.
The heavy chain constant region may also include other domains
such as a CH1 domain and/or CH; domain. The heavy chain variable
region, when combined with a light chain variable region,
binds to a surface antigen on an effector cell. The binding
SUBSTI~J, E ''.h'rEr
-
.. . . . .. . .
. . - : . ~
wos2/o88ol PCT/US91/OX421
~,o~3 j~ 4
agent can be a ligand or a receptor.
The term l~nonimmunoglobulin binding agent" as used herein
refers to a protein or polypeptide including ligands,
receptors, or single chain binding sites that mimic antibody
binding sites with predetermined specificity for a surface
antigen on a target cell.
The term ~'effector cell~ as used herein refers to any
cell which can neutralize or destroy the target cell with
which it has been placed in contact. The invention takes
advantage of the existence of particular surface proteins or
antigens which are specific for a particular class of effector
cells.
One preferred construct includes a heavy chain variable
region having specificity for the CD3 antigen found on the
surface of cytotoxic T lymphocytes. Other constructs embraced
by the invention have heavy chain variable regions with
specificities for a particular surface antigen on other
effector cells such as macrophages, monocutes, natural killer
cells, eosinophils, and large granular lymphocytes.
In one aspect of the invention, the non-immunoglobulin
binding agent includes a hormone or a growth factor which
binds a receptor specific for that ligand. One preferred
growth factor is an epidermal growth factor (EGF), or an
analog or fragment thereof, capable of binding the EGF
receptor found on a target cell.
In another aspect, the non-immunoglobulin binding agent
is a receptor which recognizes and binds a surface protein on
a virus-infected cell such as an HIV-infected cell. For
example, one construct includes a CD4, or an analog or
fragment thereof, which is capable of binding the gpl20
envelope protein.
SUBSTITUTE S~;~
.: . : . . . .
... .. . . .. . ,......... ..
. .
, . . . . . .
.. . .
- , ., : : ' :
W092/08801 PCT/US91/0~21
~9~3'12
In yet another aspect, the non-immunoglobulin binding
agent is a single chain binding site, as for example a peptide
sequence derived from a mammalian antibody specific for an
antigen which is characteristic of a particular target cell.
This invention also embodies nucleic acid sequences such
as DNA or RNA encoding the amino acid sequence of a bridging
antibody construct, as well as cell lines transfected with
such nucleic acid sequences which produce the aforementioned
construct. Preferred cell lines to be transfected are myeloma
and hybridoma cell lines.
In addition this invention encompasses methods of
producing the bridging antibody constructs as well as methods
of selectively killing a target cell in vivo with the use of
these constructs
The bridging antibody constructs may be prepared as
follows. Nucleic acid sequences encoding amino acid sequences
of a heavy chain variable region, a heavy chain constant
region, and a non-immunoglobulin binding agent, are linked. A
host cell is transfected with this nucleic acid and cultured
such that it expresses the construct. The host cell may be
transfected concurrently with a nucleic acid sequence encoding
a light chain variable region. The expressed hea~y chain
variable region/ligand construct and the expressed light chain
variable re~ion may then be combined to form a two or four
chain construct.
Moreover, a target cell may be selectively killed in vivo
by preparing a bridging antibody construct specific for that
target cell and for an effector cell capable of killing or
neutralizing that targe~ cell, and then administering the
construct to the circulation of a subject harboring the target
cell.
SUSSTITUTE S~E-~
- ~. .: . :
- .:
.. : . , :
WO92/08801 PCT/~JS9l/08421
~9 ;~ 42 6
The foregoing and other objects of the present invèntion,
the various features thereof, as well as the invention itself
may be more fully understood from the following description.
BRIEF DESCRIPTION OF THE DRAWINGS
The foregoing and other objects of this invention, the
various features thereof, as well as the invention itself may
be more fully understood from the following description, when
read together with the accompanying drawings in which:
FIG. 1 is a schematic representation of one embodiment of
the bridging antibody construct of the present invention;
FIG. 2 is a diagrammatic representation of the
construction of an antibody fusion construct including the
human C71 Ig heavy chain and EGF. FIG. 2A is the restriction
map of a C~l gene fragment cloned in plasmid pBR322. FIG. 2B
shows the fusion of the C71 gene at the Sma I site to a
synthetic EGF-encoding sequence. FIG. 2C shows the sequence at
the junction of the Ig C~3 domain and the amino terminus of
EGF;
FIG. 3 is a graphic representation of EGF receptor
binding activity of the anti-CD3/EGF conjugate. The activity
is measured by comparing the abilities of the conjugate, cold
EGF, and anti-EGF receptor antibody to compete with labelled
EGF for EGF receptors on M-24 melanoma cells;
FIG. 4 is a graphic representation of anti-CD3/EGF
conjugate-induced killing of tumor cell A431 epidermal
carcinoma cells (FIG. 4A), M24 metastatic melanoma cells (FIG.
4B), and IMR-32 neuroblastoma cells (FIG. 4C), by TIL 660
cells;
FIG. 5 is a graphic representation of anti-CD3tEGF
.
WO92/08801 PCT/~S91/08421
~7J~2
conjugate-induced killing of A431 (FIG. 5A) and M24 (FIG. 5B)
cells by peripheral blood-derived cytotoxic T lymphocytes.
Killing assays were carried out as in FIG. 4;
FIG. 6 is a diagrammatic representation of the
preparation of an antibody fusion construct including the
human C~4 chain and a single chain binding site, in which FIG.
6A shows details of a VL-linker-VH sequence and FIG. 6B
illustrates an assembled expression vector pdHL2-~CD3/sca-X;
and
FIG. 7 is a graphic representation of anti-CD3/single
chain binding site conjugate-induced killing of M21 melanoma
cells by TIL 660 effector cells, using the construct described
in connection with FIG. 6.
DETAILED DESCRIPTION OF THE INVENTION
The present invention concerns bridging antibody,
constructs useful for homing an effector cell to a malignant
or virus-infected target cell. The construct includes a
conjugate of an antibody portion having a specificity for a
surface antigen on an effector cell, and a non-immunoglobulin
; binding agent complementary to receptors or ligands found on
the target cell.
The immunoglobulin portion includes a heavy chain
variable region (V~) which, when combined with a light chain
variable region (VL)r binds to a surface antigen on an effector
cell. It also includes at least a heavy chain CH3 domain
peptide-linked to the carboxy terminus of the VH domain. CH1
and/or CN2 domains may also be peptide-linked to the carboxy
terminus of the VN domain and to the amino terminus of the CH3
domain. Without the CH1 and/or the CH domains, the half-life of
the construct decreases in vivo. The immunoglobulin portion of
the construct may be chimeric in that the variable region may
SU3S, I~UTE Sl~'ET
- . . . .. . - .- . ` . ..
: ~ .
- ~ .
.
.
' . : , - : ' :
WO92/08801 PCT/~S91/08421
~09~ ~?~ 8
come from one species and the constant region from another.
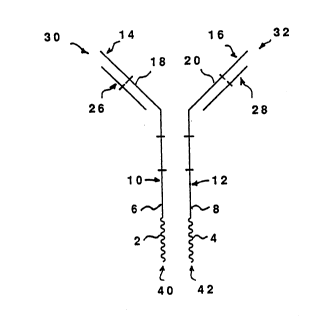
FIG. l shows a schematic view of a representative
bridging antibody construct l0. In this embodiment, ligand
molecules 2 and 4 are peptide bonded to the carboxy termini 6
and 8 of CH3 regions l0 and 12 of antibody heavy chains 14 and
16. VL regions 26 and 28 are shown paired with VH regions 18
and 20 in a typical IgG configuration, thereby providing two
antigen binding sites 30 and 32 at the amino ends of construct
l0 and two receptor-binding sites 40 and 42 at the carboxy
ends of construct l0. Of course, in their broader aspects, the
constructs need not be paired as illustrated.
A particularly useful specificity for the V~ region 26 or
28 is that for CD3, a closely associated component of the T
cell receptor found on cytotoxic T lymphocytes (CTLs). CTLs
lyse the cells to which they are targeted. The construct can
thus induce CTLs to kill tumor cells or virus-infected cells
for which they bear no specificity. Specificity for other
known surface antigens found exclusively or mostly on other
effector cells, such as monocytes, macrophages, natural killer
cells, eosinophils, or large granular lymphocytes, also may be
useful. Monoclonal antibodies to such cell surface structures
are known in the art and can be generated using known
techniques.
Binding agents include non-immunoglobulin molecules such
as ligands and receptors. Useful ligands include those
molecules complementary to receptors or surface proteins on
the chosen target cell. Useful ligands include hormones such
as melanocyte stimulating hormone (MSH), among many others.
Alternatively, the ligand may be a growth factor or other
non-immunoglobulin preferably single-chain polypeptide which
can bind to a receptor on a target cell.
One particularly useful ligand includes epidermal growth
SIJBSTITUTE SHEET
.
.
,, `,: ~ ' :
.
WO92/08801 PCT/US91/0842~
20~;~8~2
factor (EGF) because a number of malignant cells are known to
overexpress EGF surface receptors. In fact, enhanced EGF
receptor expression has been known to lead to increased
tumorigenicity. In addition, enhanced EGF receptor expression
may also serve to discriminate malignant cells from their
normal cell counterparts.
A particularly useful binding agent is a receptor such as
a CD4 which binds the gpl20 envelope protein or HIV, and also
is capable of binding the same protein expressed on the
surface of HIV-infected cells.
Other binding agents include single chain binding sites
which mimic the antibody binding site including VH and VL
domains as disclosed in U.S. Patent No. 4,946,778 (Ladner et
al.) and International Application No. PCT/US88/01737
(creative BioMolecules, Inc.), published December 1, 1988.
The binding agents may be whole native or synthetic
molecules or fragments which retain the ability to bind their
receptor. They may have the same amino acid sequence of the
native form of the ligand, or instead may be an analog of the
native form of the ligand having an amino acid sequence
sufficiently duplicative of the native sequence such that the
analog binds the native receptor on the target cell.
These constructs are produced by known recombinant DNA
technologies including the preparation of a nucleic acid
sequence encoding an amino acid sequence for the
antibody/binding agent construct, transfecting a host cell
line with that nucleic acid, and then culturing the
transfected cell line to produce the construct.
Briefly, a gene encoding the non-immunoglobulin ligand,
or fragment or analog thereof, is ligated into a plasmid
capable of transfecting a preselected host cell for
SU~STITUTF SHEET
.. , ~ .
WO92/08801 ~ PCT/US91/0~21
~- ~ 4
expression. This gene fragment may be prepared by any number
of known techniques. For example, DNA encoding the ligand may
be synthesized from the known amino acid sequence of the
ligand, or may be obtained from an established cDNA library.
The nucleic acid sequence of native EGF is known (see,
e.g., Gregory et al. (1977) J. Peptide Protein Res. 9:107-118)
and shown in SEQ ID NO:l. Alternatively, the sequence of any
number of known EGF analogs may be used (see, e.g., GB patent
application no. 2210618; and Patent Cooperation Treaty Patent
Application No. WO 89/1489A, herein incorporated as
reference).
The nucleic acid sequence for CD4 (also known as T4) is
known (see, e.g., Maddon et al. (1985) Cell 92:93-104), and
shown in SEQ ID NO:2. In addition, the nucleic acid sequence
of any number of analogs or fragments of CD4 can be used (see,
e.g., Patent Cooperation Treaty Application Nos. WO 90/01870A
and WO 90/00566, herein incorporated as reference).
DNA encoding immunoqlobulin light or heavy chain variable
and constant regions is known and is readily available from
cDNA libraries or is synthesized biochemically (see, e.g.,
Gillies et al. (1989) J. Immunol. Meth. 125:191-202; Morrison
et al. (1984) Ann. Rev. Immunol. 2:239-256; Falkner et al.,
(1982) Nature 298:286-288; and Adams et al. (1980) Biochem.
9:2702-2710).
Host cells are transfected by any number of known
transfection techniques such as spheroplast fusion (Gillies et
al. (1989) Biotechnol. 7:799-804), and then cultured to
express the foreign DNA. The host cells transfected may be
prokaryotic or eucaryotic. However, if prokaryotic host cells
are used, the construct produced must be processed or folded
after purification from the cells. Eucaryotic host cells are
preferred, as the protein produced therein may be processed by
SUBST~r~,'JE ~:~E~
... , .. . .
. . .
;~ .. , . , . . :
..
WO 92/08801 PCI/US91/08421
2~o~2
11
the cell once it is translated. Particularly useful eucaryotic
host cells include myelomas and hybridomas such as
non-producing hybridomas (e.g., Sp2/0) and non-producing
myelomas (e.g., X63Ag8.653). These host cells may be
transfected with more than one nucleic acid sequence such as a
nucleic acid encoding the light chain variable region in
addition to one encoding the construct. Constructs synthesized
by a myeloma or hybridoma cell may be paired with a light
chain variable region or an entire light chain within the
cell.
The construct is then purified from the cytoplasm of the
host cells or from the culture media, depending on the nature
of the host cells used. Protein purification methods are
numerous and include various chromatographic methods.
Other methods of producing the construct are, of course,
possible including the preparation of an RNA sequence encoding
the construct and its translation in an appropriate Ln vivo or
in vitro system.
These genetically-engineered constructs have many uses.
For example, constructs of the invention can be used to kill
selectively a target cell in vivo. One prepares a construct
with the specificities of choice, and then administers a
therapeutically effective amount to the circulatory system of
a sub~ect harboring the target cell. The construct may be
administered in physiologic saline or any other biologically
compatible buffered solution which will not affect the ability
of the construct to bind the effector and target cells. This
solution may be administered systemically via IV or by
intramuscular injection. Alternatively, the construct may be
administered by injection directly at the site to be treated.
A truncated construct not having a C~ and/or C~ domain may be
useful for this purpose as its half-life is limited in vivo.
The construct also may be used to treat cells in vitro
SU8STITU ~ _ S~ -r I
.
. . .. . .
,
WO92/08801 PCT/~'S91/08421
2,~9 ~4~ 12
which then may or may not be returned to a subject. For
example, effector cells may be removed from a subject, treated
by incubation with the construct to bind thereto, and then
returned to the subject where the effector cell/construct
conjugate is targeted to a target cell for killing or
neutralizing.
Constructs comprising anti-T cell antibodies and peptide
hormones are useful in testing the feasibility of adoptive
immunotherapy whereby a patient's tumor-infiltrating
lymphocyte (TIL) cell line or peripheral blood-derived
cytotoxic T lymphocyte line is given an additional target
specificity. In particular, since many different tumors
overexpress the EGF receptor, the use of conjugates containing
EGF is particularly useful for many different cancers.
The ability of an EGF-containing construct to bind the
EGF receptor was examined in a competitive binding assay. FIG.
3 shows EGF receptor binding activity of a construct including
an immunoglobulin moiety with anti-CD3 specificity and EGF as
the ligand moiety. The ability of the construct (~ - ~) to
compete with labeled EGF for its receptor was measure using
M24 melanoma cells as target cell, and compared to unlabeled
EGF (o - o), unconjugated anti-CD3 antibody ( - ) and
anti-EGF receptor antibody 225 (o - o). The results are
normalized to the molar equivalents of EGF. The anti-CD3
antibody alone showed little or no inhibition activity while
the anti-CD3/EGF construct competed well with EGF for its
receptor.
A population of TIL cells derived from a patient with a
malignant melanoma was used as a source of activated T-cells
for testing a genetically engineered anti-T cell/EGF
construct. These cells had little or no cytolytic activity
against the tumor targets against which they were tested. In
the presence of very low concentrations of the conjugate,
- ,, . ' -
.
-
, ' '- . ' `~ ' ' -
- ~ .
WO92~08801 PCT/~S91/0~21
2 a~ .~3 (53 !l 2
cells expressing EGF receptor were killed readily. This
activity was seen at concentrations (10-12 to 10-1lM) that were
significantly lower than the KD for EGF binding to its receptor
2 x 10lM~l)
A second cytotoxic T lymphocyte line, derived from
peripheral blood and specific for autologous Epstein Barr
Virus (EBV)-transformed cells but having no specificity for
tumor cells, also can be induced to kill the tumor cells.
These lymphocytes have been maintained in culture for an
extended time in the presence of IL-2 and stimulated bimonthly
with mitomycin C-treated autologous EBV-transformed B cells.
The ability of these cells to kill EGF receptor-bearing tumor
cells over an extended period has not diminished, thus making
this EBV-specific cytotoxic T lymphocyte system generally
useful for testing hormone constructs.
The specificity of a construct of the present invention
was examined by testing the activity of the anti-CD3 antibody
alone or in combination with unconjugated EGF. The results
which foll:~ clearly demonstrate that the two need to be
physically linked for activity.
The epidermal carcinoma cell line, A431, expresses a very
high number (2 x 106/cell) of EGF receptor on its cell surface,
and this overexpression has been correlated with its ability
to form tumors in nude mice (Santon et al. (1986) Cancer
Res.46: 4701- 4705). The a~ility of the anti-CD3/EGF construct
to mediate`the killing of labeled A431 cells by a human TIL
cell line (TIL 660) in a 4 hour chromium release assay was
tested, and the results are shown in FIG. 4A. 5lCr-labeled
targets were incubated for four hours with the indicated
amount of construct and varying ratios of effector cells. The
amount of released radioactivity was used to calculate the
percent of target cell lysis.
SlJBSTlTUT_ S~:EET
... . . . ,, . .. .... ~ . . :
. . : . `. . :
.. . . ..
,, , ` . ~ , ~.
,, ~ - ~ - . . : -
. .
.. ` ~ .
WO~2/08X01 PCT/~S91/08421
2~a3 ~ 14
The parameters that were varied in the first studies were
the effector cell-to-target cell (E:T) ratio and the
concentration of the construct. No killing of the A431 targets
was seen in the absence of the construct, demonstrating that
the TIL 660 line has no specificity for these cells.
Significant levels of lysis were seen with concentrations of
construct as low as O.l ngJml (6 x lO-l3M), and this killing
increased as a function of construct concentration or
effector-to-target ratio. Very little additional killing was
seen at concentrations above 25 ng/ml (l.5 x lO-10M).
Exactly the same results were obtained when the
constructs were made with the human C71 or C74 H-chain genes.
The C74 H chain was used for the construct because of its
inability to fix human complement.
Additional tumor cell lines were tested for their
susceptibility to TIL cell lysis in the presence of the
anti-CD3 JEGF constructs. These include a human metastatic
melanoma line (M24) expressing a moderate level of EGF
receptor, as well as a neuroblastoma line (IMR-32) that is
very sensitive to lysis in an ADCC assay (lysis by Fc
receptor-bearing cells in the presence of an anti-tumor
antibody) but expresses little or no detectable EGF receptor.
The results are shown in TABLE l.
'
W092/08801 PCT/~S91/0~21
2 U ~ 2
TABLE I
l25I_EGF Bound
- Cell Line(pq/2 x 105 cells
A431 (epidermal carcinoma) 236.8
M24 (metastatic melanoma) 34.1
IMR-32 (neuroblastoma)0.72
The killing of these cell lines by the TIL 660 effectors
was found to be directly related to the expression of EGF
receptor (FIGS. 4B and 4C). The M24 line expresses EGF
receptor, although ten-fold less than A431 cells, and is
killed almost as well at low conjugate concentrations. The
killing of A431 cells increased at higher concentrations of
the conjugate (greater than 1.5 ng/ml~ whereas the killing of
M24 cells did not. This difference may reflect the saturation
of M24 cell receptors at the lower concentration. The
neuroblastoma line, IMR-32, does not express EGF receptor and
was not killed by TIL 660 cells in the presence of the
anti-CD3/EGF conjugate (FIG. 4C).
.,
As shown in FIG. 5, a second cytotoxic T lymphocyte line,
W-1, which is derived from peripheral blood and is both CD3+
and CD8~, also killed the EGF receptor-bearing A431 (FIG. 5A)
and M24 (FIG. SB) cells very efficiently in the presence but
not in the absence of the construct.
The specific lysis of the A431 and M24 tumor cell lines
was measured in the presence or absence of the conjugate, as
well as its component parts. Four hour cytotoxicity assays
were carried out using an effector (TIL 660 cells)-to-target
ratio of 50:1 with the indicated additions. Values represent
the amount of lysis obtained in a particular reaction
expressed as the percentage of that obtained with the
anti-CD3/EGF construct. The results are shown in TABLE 2.
SuBsfl~ur~ SHET
;-. . - . ~ . . .. -..... . ..
. . ` . . . - ..
. . , . . . .. .. .. . -
-. . . .. . . ..
- .. . . . : : .. :
WO 92/08801 PCI~U~i91J08421
16
~ 3r~ 4~
TABLE 2
% Maximum Lysis of Cell
Line:
Additions A431__ M24
None
EGF (0.5 ng/ml) O O
Anti-CD3 (5 ng/ml) O O
EGF + Anti-CD3 l O
Anti-CD3/EGF (S ng/ml) 100 100
Construct + Anti-CD3
(0.5 ~g/ml) 71 48
Construct + Anti-CD3
(10 ~g/ml) 10 lS
Neither EGF alone, anti-CD3 antibody alone, nor EGF in
combination with anti-CD3 antibody were able to mediate
cytotoxic T lymphocyte killing of the tumor targets.
Concentrations of antibody that were 100-fold higher also did
not significantly increase the specific lysis above background
levels. Clearly, physical linkage of the antibody and EGF is
required for killing activity since only the construct was
able to mediate the lysis of the EGF receptor-bearing targets.
Some inhibition of killing activity is possible with a
100-fold excess of anti-CD3 antibody. Since this represents
only 0.5 ~g/ml, it is possible that there may still be CD3
molecules available for binding. When the concentration was
increased to 10 ~g/ml, significant inhibition was observed.
SUBSTITUTE SHEET
, . . . .. . . . . . .
.. - . . ..
., . -. ~ . ; ~ - . . .
, . . . .; ~ :. . ~ :
'' ' ~ ' :
:
WO92/08801 PCT/US91/0~21
2~8'~2
17
The invention may be better understood from the following
nonlimiting Examples, in which are described the preparation
of bridging antibody fusion constructs using non-
immunoglobulin binding agents chosen first from ligands
adapted from the proteins EGF and CD4 and then from a single
chain binding site adapted from the mouse anti-human melanoma
antibody 9.2.27.
EXAMPLE 1
Constructs Utilizinq Liqand Non-immunoqlobulin Bindinq Aaents
1. Plasmid Construction
An EGF gene fragment was synthesized from the known
protein sequence described in Gregory et al. (J. Peptide
Protein Res. (1977) 9:107-118), herein incorporated as
reference. FIG. 2 and SEQ ID NO:l shows the nucleic acid
sequence synthesized and its corresponding amino acid
sequence. A CD4 gene fragment (nucleic acid numbers 145-1266)
encoding the extracellular domain including the variable-like
region (amino acid numbers 1-94) and the joining-like region
(amino acid numbers 95-109) was synthesized as described in
Maddon et al. (Cell (1985) 92:93-104), herein incorporated as
reference. The entire amino acid sequence including the
transmembranous and cytoplasmic domains of the protein, along
with its corresponding nucleic acid sequence, is shown in FIG.
3 and in SEQ ID N0:2.
The EGF or CD4 gene fragment was ligated to an engineered
SmaI site at the 3' end of the human C71 gene. This is shown
schematically in FIG. 2. An XhoI site was placed to the 3'
side of the EGF coding sequence for litigation to a fragment
containing the 3' untranslated region and poly A addition
signal from the mouse Ig CK gene.
'' ; ' ' - ' :. ' - -
' ' - '- ~ ' " . . ~' , '. ' " ;
: . . , ~ . .. ~ - , .
- : . . , -
. .: , . , : . . .
W092/08801 PCT/~'S91/0~21
v region cassettes encodlng the H and L chain variable
regions of the mouse anti-CD3 antibody, OKT3 (ATCC number CRL
8001), were constructed from cloned cDNAs as described by
Gillies et al. (J. Immunol. Meth. (1989) 125:191-202), herein
incorporated by reference. The cassettes were inserted into
the chimeric antibody expression vector pdHL2 to give
pdHL2-CD3. The modified H chain, to which EGF or CD4 was
fused, was inserted into the pdHL2-CD3 plasmid as a HindIII to
EcoRI fragment. A second construct was made by replacing the
HindIII to NsiI fragment of the C71 gene with the
corresponding fragment of the C74 gene. In both cases the
lysine residue, normally found at the carboxy terminus of Ig H
chains, was omitted from the fusion proteins.
2. Cell Culture and Transfection
Mouse hybridoma cells (Sp2/0 Agl4, ATCC No. CRL 1581)
were maintained in Dulbecco~s Modified Eagle's medium (DMEM)
and transfected as described by Gillies et al. (Biotechnol.
(1989) 7:799-804). Human tumor cell lines A431 (epidermal
carcinoma, ATCC number CRL 1555), M24 (metastatic melanoma,
originally obtained by D.C. Morton, UCLA, and provided by
Ralph Reisfeld, Scripps Clinic), and IMR-32 ~neuroblastoma,
ATCC number CCL 127) were maintained in RPMI 1640 containing
10% FBS. The human tumor-infiltrating lymphocyte (TIL) line
660, derived from a human melanoma patient, was cultured in
AIM V medium (GIBCO) containing IL2 (Hoffmann-LaRoche) as
described by Reilly et al. (J. Immunol. Meth. (1990)
126:273-279). Greater than 90~ of the cells were CD3+ and CD8+
when examined by fluorescence microscopy.
Transfectants secreting human antibody determinants were
identified by ELISA, and their culture supernatants were
tested further for anti-CD3 reactivity by their ability to
stain TIL 660 cells in the presence of a fluorescenated
anti-human Ig antiserum. Both the chimeric and conjugated
antibody constructs were found to stain these cells as well as
S~BSTITUTE S~EFT
.
~: . - .
. ;: .: ;
.
092/08801 PCT/US91/0~21
~ O ~ a (~
the original mouse antibody (OKT3, Ortho Diagnostic Systems).
3. Protein Purification
Chimeric antibody, antibody/EGF constructs, and
antibody/CD4 constructs were purified by affinity
chromatography using protein A Sepharose (Repligen). Cell
culture medium was used as a source of material for the
purification. Electrophoretic analyses of the purified
proteins showed that they were both fully assembled into
antibody molecules and that the conjugated H chain migrated as
would be expected for the fusion of the Ig and EGF sequences.
4. EGF ComPetitive Bindinq AssaY
M24 melanoma cells (2 x 105 cells in a final volume of 0.1
ml) were mixed on ice in Hank's balanced salt solution
containing 0.1% BSA and 20 mM HEPES together with l25I-EGF (10
ng/ml final concentration, Amersham) and varying
concentrations of cold competitor (either EGF, antibody or
antibody conjugate). After a 2 hour incubation at 4C, cells
were washed three times by centrifugation, and the
cell-associated radioactivity was counted. A non-specific
background, determined by incubation with a 200-fold excess of
cold EGF, was subtracted from all data points. The results
were expressed as the percent inhibition of binding relative
to the no-competitor control.
Alternatively, cells were incubated for 2.5 hours in 100
~1 of buffer (HBSS, 0.1% BSA, 20 mM HEPES, pH 7.4) at 4C with
700 pg of l2sI-EGF, washed three times with buffer and the
pellet counted in a gamma counter. Non-specific binding (that
obtai~ed in the presence of a 200-fold excess of cold EGF) was
subtracted.
5. CYtotoxicitY Assay
Cytotoxicity assays were carried out using 5lCr-labeled
tumor targets and TIL 660 cells as effectors. A fixed number
SUBSTiTUTr S'I~
, , ', ~, " ' - , ' . -,
- ,- ~ ,
, : :
092/08801 PCT/US91/0~21
C~ Q, ~ J (S '~ ~
of labeled targets ( 104 per well) in 50 ~1 and varying numbers
of effectors in 50 ~1 were mixed with 100 ~1 of diluted
antibody or conjugate in the wells of a microtiter plate. The
plates were centrifuged and assayed for released s1Cr following
a 4 hr incubation at 37C. Spontaneous release was subtracted
from experimental values and the percent of specific lysis was
determined by dividing the corrected release value by the
total released with detergent lysis.
The assay for activity of Ig/CD4 constructs may be
carried out in a manner analogous to that for Ig/EGF
constructs, with the modification tnat the target cells used
would be those expressing gpl20 on their surfaces, such as
HIV-infected cells or cells that have been transfected with a
gene for gpl20 and are expressing it on their surfaces.
EXAMPLE 2
Construct ~ h~LA Sin~le Chain Binding Site
Non-Immunoclobulin Bindinq Aaent
1. Plasmid Construction
The VL and VN regions from the mouse antibody 9.2.27
(described by Beavers et al. in the published European patent
application No. 411893, published February 6, 1991), specific
for a human melanoma-specific proteoglycan antigen, were
adapted using the polymerase chain reaction (PCR) technique to
form a single-chain binding site-encoding sequence. Native
9.2.27 sequences were modified by the addition of 5' and 3'
primers. Primers added to the 5' end of each V region were
identical to the "sense" strand of the DNA encoding the first
six amino acid residues of the mature H and L proteins.
Vpstream of these were provided sequences encoding a BglII
restriction site for subsequent joining steps, and an EcoRI
site for use in cloning the PCR products. Likewise, primers
derived from the 3' end of each V region (in this case anti-
,
:.
092/08801 PCT/US91/0~21
22Olnà'8~2
sense primers) were identical to the last six amino acids ofeach. Additional sequences were added for cloning purposes
and for either joining purposes (for the VL region) or to
introduce a stop codon and a convenient XhoI restriction site
(in the VH region). A carboxyl-terminal Lys was added to the
end of VH since all antibody H chains end with this amino acid.
The sequences of the sense and anti-sense primers were as
follows:
9.2.27 5' L chain sense primer:
5'-CGGAGAATTCAGATCT AAC ATT GTG CTG ACC CAA-3'
'----''----'Asn Ile Val Leu Thr Gln
EcoRI BglII
9.2.27 3' L chain anti-sense Primer:
5'--TTTGTCGA CTT TAT TTC CAA CTT TGT C-3'
'----'Lys Ile Glu Leu Lys Thr
SalI
9.2.27 5' H chain sense Primer: -
5'-CCCGAATTCAGATCT CAG GTC CAG CTG CAG CAG-3'
'----''----' Gln Val Gln Leu Gln Gln
EcoRI BglII
9.2.27 3' H chain anti-sense Primer:
5'-CGCCCTCGTG TCA CTT TGA GGA GAC GGT GAC TGA GG-3'
'----'STOP Lys Ser Ser Val Thr Val Ser
XhoI
Underlined portions of the above sequences are those
which are homologous to the original 9.2.27 V regions. The
coding of each codon in the above anti-sense primers is shown
in reverse and represents the non-coding strand; e.g., CTT in
the above anti-sense primer shown 5' to 3' corresponds to the
coding sequence AAG (Lys).
The V~ and VH PCR products were synthesized by mixing l ng
of template (a plasmid containing both V regions) with 50 ng
of each set of primers in l00 ~L standard PCR reactions
SUBST5~UTE S~
: . : . . - -
.. . . . .. .
- . . .
. - .. , ~ .. .. ~ .
- , - . .. . ~ . .- . -
: . . . . .. , . .. . ~ ~ , ,
.- :. .
~'092/08801 PC~/US91/OX421
~a9~3'~ 22
(Perkin Elmer/Cetus). These products were digested within
EcoRI and Sal I (for VL) or EcoRI and XhoI (for VH) . The v~
product was cloned as an EcoRI-to-XhoI fragment and verified
by DNA sequencing. The VL region was ligated to the 5' end of
a synthetic linker fragment encoding a 5' XhoI site, a
flexible peptide linker composed of Ser and Gly residues, and
a 3' BamHI site:
5'-(C)TCGAGCGGGGGCAGCGGGGGCGGAGGCAGCGGCGGGGGCG-3'
CGCCCCCGTCGCCCCCGCCTCCGTCGCCGCCCCCGCCTAG(G)
______ ______
XhoI BamHI
and cloned as an ~coRI-to-BamHI fragment (the XhoI and SalI
site having compatible ends). After verification of the VL-
linker sequence, the cloned VH fragment was digested with BglII
and XhoI and joined to the VL-linker fragment at the 3' BamHI
site (BglII and BamHI having compatible ends).
The joining of the VL and VH segments via their respective
Sal I and BglII sites with the XhoI-to-BamHI linker fragment
is illustrated in FIG. 6A. These restriction sites became
non-functional after they were ligated, the protein sequences
encoded by these restriction sites being composed of either
Gly or Ser.
The resulting 9.2.27 VL-linker-VH sequence, herein
referred to as 9.2.27sca, was joined to the CH3 exon of the
human C74 gene by first modifying the 3' end of the CH3 exon
to encode a BamHI site. A short oligonucleotide (GGGATCCC)
was ligated to the SmaI site near the end of the CH3, changing
the 3' end sequence from
BamHI
SmaI SmaI '-----'
________ ________
C CCG GGA AAA to C CCG GGA TCC
Pro Gly Lys Pro Gly
...,, . . .. .: , . . :
:
W092/08801 PCT/US91/OX421
~9S8~12
9.2.27sca was joined to this CH3 samHI site via its
unique 5' BglII site resulting in the addition of a single Ser
residue. The C74-9.2.27sca fusion protein coding sequence was
then inserted into a pdHL2 chimeric antibody expression vector
containing the V regions of the anti-CD3 antibody, as
described in Example l and shown in FIG. 6B. A poly-A
addition site (pA) was provided by the vector and, in the
completed vector, was located to the 3' side of the
translation stop signal in the 9.2.27 VH region.
2. Production of Construct and CytotoxicitY AssaY
Cell culture and transfection with the above vector,
protein purification of the resulting proteins, and
cytotoxicity assays using those proteins were carried out in
the same manner as with the fusion proteins of ~xample l.
Figure 7 shows the results of a killing assay using varying
concentrations of the anti-CD3/9.2.27sca bridging antibody and
varying effector-to-target ratios of TIL 660 (effector) and
M2l melanoma (target) cells. Significant killing of target
cells occurred at relatively low effector-to-target ratios;
this killing was seen to increase with the concentration of
bridging antibody.
The invention may be embodied in other specific forms
without departing from the spirit or essential characteristics
thereof. The present embodiments are therefore to be
considered in all respects as illustrative and not
restrictive, the scope of the invention being indicated by the
appended claims rather than by the foregoing description. All
changes which come within the meaning and range of equivalency
of the claims are therefore intended to be embraced therein.
SUBST~TUTE S~EEI
. . : -... .- . . . . ., ........ ~ ~ . ~ . - . ... -
. . .
.. . .. . .. . . . . .
.. . .
.
- ... ~ . . .. ..
., . . . .~ : . . .
WO92/08801 PCT/US91/0~21
2~-J~ ~ 24
SEQUENCE LISTING
(1) GENERAL INFORMATION
(i) APPLICANT: Gillies, Stephen D.
(ii) TITLE OF INVENTION: Bridging Antibody Fusion
Constructs
(iii) NUMBER OF SEQUENCES: 2
(iv) CORRESPONDENCE ADDRESS
(A) ADDRESSEE: Abbott Laboratories
(B) STREET: One Abbott Park Road, D-377,AP6D
(C) CITY: Abbott Park
(D) STATE: Illinois
(E) COUNTRY: U.S.A.
(F) ZIP: 60064-3500
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Diskette, 3.5 inch,
72Okb storage
(B) COMPUTER: IBM XT
(C) OPERATING SYSTEM: DOS 3.30
(D) SOFTWARE: Word Perfect 5.0
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE: herewith
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(2) INFORMATION FOR SEQ ID NO. l
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 175 nucleic acids
53 amino acids
(B) TYPE: nucleic acid, amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA, protein
S~JBSTITUTE S~EET
.. . . . .. - . .
. .. .. , .. - . .. ~ : - :
: - - - .,-,, . . . . , ` :: : -
.. .. j .~;. ~ .
-
.- - . . . .
. , ~ . .
W092/08801 PCT/US91/0~21
22(~9 ~
(iii) HYPOTHETICAL: DNA, yes; protein, no
(iv) ANTI-SENS~: no
(vi) ORIGINAL SOURCE
(A) ORGANISM:-human
(B) TISSUE TYPE: serum
(vii) IMMEDIATE SOURCE:
(A) LIBRARY:
(ix) SEQUENCE DESCRIPTION: SEQ ID No: l
C CCG GGT AAC TCC GAC TCT GAA TGT CCC CTG 3l
Pro Gly Asn Ser Asp Ser Glu Cys Pro Leu
+l 5
AGC CAC GAC GGC TAC TGC CT~ CAC GAC GGC 6l
Ser His Asp Gly Tyr Cys Leu His Asp Gly
l0 15
GTG TGC ATG TAC ATC GAG GCC CTG GAC AAG 9l
Val Cys Met Tyr Ile Glu Ala Leu Asp Lys
20 25
TAC GCATGC AAC TGC GTG GTC GGG TAC ATCl2l
Tyr Ala Cys Asn Cys Val Val Gly Tyr Ile
30 35
GGC GAG AGG TGC CAG TAC AGG GAC CTC AAG l5l
Gly Glu Arg Cys Gln Tyr Arg Asp Leu Lys
40 45
TGG TGG GAG CTC CGG TGACTCGAG l75
Trp Trp Glu Leu Arg
(2) INFORMATION FOR SEQ. ID NO: 2
(i) SEQUENCE CHARACTERISTICS:
SUBST~TUTC S~IEET
: - : -........ . .. : :
wos2/o8xol PCT/~S91/0~21
7. 26
(A) LENGTH: 1742 base pairs, 446 amino acids
(B) TYPE: nucleic acid, amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: CDNA, protein
(iii) HYPOTHETICAL: no
(iv) ANTI-SENSE: no
(vi) ORIGINAL SOURCE:
(A) ORGANISM: human/mouse
(B~ TISSUE SOURCE: blood
(vii) IMMEDIATE SOURCE: T cell
~A) LIBRARY: Charon 4 human genomic
(ix) SEQUENCE DESCRIPTION: SEQ ID No: 2
CAAGCCCAGA GCCCTGCCAT TTCTGTGGGC TCAGGTCCCT 40
ACTGCTCAGC CCCTTCCTCC CTCGGCAAGG CCACA ATG 78
met
AAC CGG GGA GTC CCT TTT AGG CAC TTG CTT CTG 111
Asn Arg Gly Val Pro Phe Arg His Leu Leu Leu
-20 -15
GTG CTG CAA CTG GCG CTC CTC CCA GCA GCC ACT 144
Val Leu Gln Leu Ala Leu Leu Pro Ala Ala Thr
-10 -5
CAG GGA AAC AAA GTG GTG CTG GGC AAA AAA GGG 177
Gln Gly Asn Lys Val Val Leu Gly Lys Lys Gly
Il 5 10
GAT ACA GTG GAA CTG ACC TGT ACA GCT TCC CAG 210
Asp Thr Val Glu Leu Thr Cys Thr Ala Ser Gln
15 20
AAG AAG AGC ATA CAA TTC CAC TGG AAA AAC TCC 243
. IJUTE S~EET
... .~ ` ~ ` . . . . .. ....
. .: .,
.
` . ` , , . . ~ ~ .
' .. ' . - ~ .
. . `
- : , ` .
W092/08801 PCT/US91/0~21
2 ~7~ 2
Lys Lys Ser Ile Gln Phe His Trp Lys Asn Ser
AAC CAG ATA AAG ATT CTG GGA AAT CAG GGC TCC 276
Asn Gln Ile Lys Ile Leu Gly Asn Gln Gly Ser
35 40
TTC TTA ACT AAA GGT CCA TCC AAG CTG AAT GAT 309
Phe Leu Thr Lys Gly Pro Ser Lys Leu Asn Asp
45 50 55
CGC GCT GAC TCA AGA AGA AGC CTT TGG GAC CAA 342
Arg Ala Asp Ser Arg Arg Ser Leu Trp Asp Gln
60 65
GGA AAC TTC CCC CTG ATC ATC AAG AAT CTT AAG 375
Gly Asn Phe Pro Leu Ile Ile Lys Asn Leu Lys
70 75
ATA GAA GAC TCA GAT ACT TAC ATC TGT GAA GTG 408
Ile Glu Asp Ser Asp Thr Tyr Ile Cys Glu Val
80 85
. .
GAG GAC GAG AAG GAG GAG GTG GAA TTG CTA GTG 44l
Glu Asp Gln Lys Glu Glu Val Gln Leu Leu Val
90 95
TTC GCA TTG ACT GCC AAC TCT GAC ACC CAC CTG 474
Phe Gly Leu Thr Ala Asn Ser Asp Thr His Leu
lO0 105 llO
CTT CAG GGG GAG AGC CTG ACC CTG ACC TTG GAG 507
Leu Gln Gly Gln Ser Leu Thr Leu Thr Leu Glu
ll5 120
SUE;~ITUT~ S~E~r
... . - . ~ .. .. . . .. .. ;.
.: ~ , - . :. ; ' ',
, ,
- :, ,~,.. -. : . . .. ; ,.. ~
; . ,
WO92/08801 ~ PCT/US91/0~21
~,~3~
28
AGC CCC CCT GGT AGT AGC CCC TCA GTG CAA TGT 540
Ser Pro Pro Gly Ser Ser Pro Ser Val Gln Cys
125 130
AGG AGT CCA AGG GGT AAA AAC ATA CAG GGG GGG 57 3
Arg Ser Pro Arg Gly Lys Asn ILe Gln Gly Gly
135 140
AAG ACC CTC TCC GTG TCT CAG CTG GAG CTC CAG 606
Lys Thr Leu Ser Val Ser Gln Leu Glu Leu Gln
145 150
GAT AGT GGC ACC TGG ACA TGC ACT GTC TTG CAG 639
Asp Ser Gly Thr Trp Thr Cys Thr Val Leu Gln
155 160 165
AAC CAG AAG AAG GTG GAG TTC AAA ATA GAC ATC 672
Asn Gln Lys Lys Val Glu Phe Lys Ile Asp Ile
170 175
GTG GTG CTA GCT TTC CAG AAG GCC TCC AGC ATA 705
Val Val Leu Ala Phe Gln Lys Ala Ser Ser Ile
; 180 185
GTC TAT AAG AAA GAG GGG GAA CAG GTG GAG TTC 738
Val Tyr Lys Lys Glu Gly Glu Gln Val Glu Phe
190 195
TCC TTC CCA CTC GCC TTT ACA GTT GAA AAG CTG 771
Ser Phe Pro Leu Ala Phe Thr Val Glu Lys Leu
200 205
ACG GGC AGT GGC GAG CTG TGG TGG CAG GCG GAG 804
Thr Gly Ser Gly Glu Leu Trp Trp Gln Ala Glu
210 215 220
-, :. ; . ... ,.... . . . . .: : : . -
- . -
.. . . .
- :, : - . , :
- . .
- . , ~ ~ :
.
., . .. ~ .
WO 92/OX801 , PCr/US9l/0X421
2~t~,3~,12
29
AGG GCT TCC TCC TCC AAG TCT TGG ATC ACC TTT 837
Arg Ala Ser Ser Ser Lys Ser Trp Ile Thr Phe
225 230
GAC CTG AAG AAC AAG GAA GTG TCT GTA AAA CGG 870
Asp Leu Lys Asn Lys Glu Val Ser Val Lys Arg
235 240
GTT ACC CAG GAC CCT AAG CTC CAG ATG GGC AAG 903
Val Thr Gln Asp Pro Lys Leu Gln Met Gly Lys
245 250
AAG CTC CCG CTC CAG CTC ACC CTG CCC CAG GCC 936
Lys Leu Pro Leu His Leu Thr Leu Pro Gln Ala
- 255 260
TTG CCT CAG TAT GCT GGC TCT GGA AAC CTC ACC 969
Leu Pro Gln Tyr Ala Gly Ser Gly Asn Leu Thr
265 270 275
.
CTG GCC CTT GAA GCG AAA ACA GGA AAG TTG CAT 1002
Leu Ala Leu Glu Ala Lys Thr Gly Lys Leu His
280 285
CAG GAA GTG AAC CTG GTG GTG ATG AGA GCC ACT 1035
Gln Gln Val Asn Leu Val Val Met Arg Ala Thr
290 295
CAG CTC CAG AAA AAT TTG ACC TGT GAG GTG TGG 1068
Gln I,eu Gln Lys Asn Leu Thr Cys Glu Val Trp
300 305
GGA CCC ACC TCC CCT AAG CTG ATG CTG AGC TTG 1101
Gly Pro Thr Ser Pro Lys Leu Met Leu Ser Leu
310 315
SlJB~ IUT--Sff'Et
,. . . ' . ` :: ~
. . :. ` . . ~ ,: - ' . . ,
~, .. . . .` .
,. . . ... '
.. . ..
... . , ;,. ..
-:.; . . -
; . . .. ; , ;. `., :.; . . , .-.. , ,, . . :
,..
WO 92/08801 PCr/US91/08421
2~3~ ~ ~ 30
AAA CTG GAG AAC AAG GAG GCA AAC GTC TCG AAG 1134
Lys Leu Glu Asn Lys Glu Ala Lys Val Ser Lys
320 325 330
CGG GAG AAG GCG GTG TGG GTG CTG AAC CCT GAG 1167
Arg Glu Lys Ala Val Trp Val Leu Asn Pro Glu
335 340
GCG GGG ATG TGG CAG TGT CTG CTG AGT GAC TCG 1200
Ala Gly Met Trp Gln Cys Leu Leu Ser Asp Ser
345 350
GGA CAG GTC CTG CTG GAA TCC AAC ATC AAG GTT 1233
Gly Gln Val Leu Leu Glu Ser Asn Ile Lys Val
355 360
CTG CCC ACA TGG TCC ACC CCG GTG CAG CCA ATG 1266
Leu Pro Thr Trp Ser Thr Pro Val Gln Pro Met
365 370
GCC CTG ATT GTG CTG GGG GGC GTC GCC GGC CTC 1299
Ala Leu Ile Val Leu Gly Gly Val Ala Gly Leu
375 380 385
CTG CTT TTC ATT GGG CTA GGC ATC TTC TTC TGT 1332
Leu Leu Phe Ile Gly Leu Gly Ile Phe Phe Cys
390 395
GTC AGG TGC CGG CAC CGA AGG CGC CAA GCA GAG 1365
Val Arg Cys Arg His Arg Arg Arg Gln Ala Glu
400 405
CGG ATG TCT CAG ATC AAG AGA CTC CTC AGT GAG 1398
Arg Met Ser Gln Ile Lys Arg Leu Leu Ser Glu
410 415
SUBSTITU~ S~ T
.. . .
.
W092/08801 2 ~ J ~ ~ ~ PCT/US91/0~21
AAG AAG ACC TGC CAG TGC CCT CAC CGG TTT CAG 143l
Lys Lys Thr Cys Gln Cys Pro His Arg Phe Gln
420 425
AAG ACA TGT AGC CCC ATT TGA GGCACGAGGC CAGG 1466
Lys Thr Cys Ser Pro Ile ---
430 435
CAGATCCCAC TTGCAGCCTC CCCAGGTGTC TGCCCCGCGT l506
TTCCTGCCTG CGGACCAGAT GAATGTAGCA GATCCCACGC 1546
TCTGGCCTCC TGTTCGTCCT CCCTACAATT TGCCATTGTT l586
TCTCCTGGGT TAGGCCCCGG CTTCACTGGT TGAGTGTTGC l626
TCTCTAGTTT CCAGAGGCTT AATCACACCG TCCTCCACGC 1666
CATTTCCTTT TCCTTCAAGC CTAGCCCTTC TCTCATTATT 1706
TCTCTCTGAC CCTCTCCCCA CTGCTCATTT GGATCC l742
SUBSTITUTE ~EET
. . .- ~ . , ~, ,
~i ~ . .. .
.. . ~ ., .
.
.