Note: Descriptions are shown in the official language in which they were submitted.
~y ~~
WO 92/08353 ~ ~ ~~1/02068
Description
Synergistic herbicidal compositions
The invention is in the field of the Grog protection
agents which can be used against monocotyledon and
dicotyledon weeds.
Glufosinate-ammonium (phosphinathricin-ammonium) is a
known herbicide which is taken up via the green parts of
the plant (foliar-acting herbicide); see "The Pesticide
Manual" 8th Edition, British Crop Protection Council
1987, p. 448. Glufosinate-ammonium is mainly used post
emergence for controlling weeds and grass weeds in
plantation crops and on areas which are not under culti
vation and also, by means of specific application tech
niques, for treatment between rows in agricultural field
crops such as corn, cotton etc.
Glyphosate is also a known herbicide for controlling
annual and perennial weeds and grass weeds. It also acts
via post-emergence application and foliar uptake; cf. the
abovementioned "The Pesticide Manual", p. 449.
Application is mainly carried out in plantation crops and
on areas which are net under cultivation. In the case of
commercially available products, the monoisopro~yl
ammonium salt of glyphosate is used.
Surprisingly, some herbicidal active substances have now
been found in biological. tests which, when applied
together with glufoeinate-ammonium or glyphosate, result
in pronounced synergistically increased effects.
The present invention relates to herbicidal compositions
with a herbicidally effecti~re content of a combination of
- 2~9~1:~~
A) one or more compounds of the formulae (Al) and (A2)
0
n
H3C - p - CH2CH2CH - CO - OH (A1)
OH ~2
O
~i
Ho ) 2 P - cH2 - NHZ - cH2 - co - off ( AZ ?
or salts thereof and
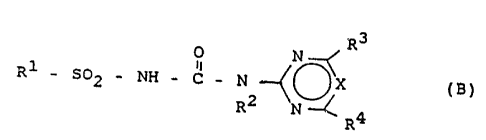
B) one or more campounds of the formula (B)
R3
O N
Rl - SO' - ~ - C - N ~ ~X (B)
R a
R_
where
R1 is a radical from the group comprising 2-ethoxy-
phenoxy, 2-propoxyphenoxy, 2-isoprapoxyphenoxy,
2-methoxycarbonylphenyl, 3-(dimethylaminocarbonyl)
pyrid-2-yl, 3-ethylsulfonylpyrid-2-yl, 3-[N-(C1-C~
alkyl)-N-(C~-C,-alkylsulfonyl)amino]pyrid-2-yl, (N
methyl-N-methylsulfonyl)aminosulfonyl, 2-(2-chloro
ethoxy)phenyl, 2-(methoxycarbonyl)phenyl, 2-(meth
oxycarbonyl)thien-3-yl,
R2 is H or methyl,
R3 and R~ independently of one another are C1-Cz-alkyl,
C1-CZ-alkoxy or Ci-C2-haloalkoxy and
X is CH or N,
or salts thereof, with the exception of the combinatiana
of a compound of the formula (A2) with one or more
compounds of the formula (B) in which
a) Rl is 2-methoxycarbonylphenyl, RZ is H or methyl and
R3 is methyl, R" is methoxy and X is N, and
CA 02096115 2001-10-05
28976-125
-3-
b) R1 is 2- (methoxycarbonyl) thien-3-yl, R2 is H, R3
is methyl, R4 is methoxy and X is N.
According to one aspect of the present invention,
there is provided a herbicidal composition with herbicidally
effective content of a combination of A) a compound of the
formula (A1)
O
Ii (Al )
I I
H3C -- P CH2CH2CH CO OH
i
OH NH2
or salts thereof and B) one or more compounds of the
formula (B)
R3
O ~ (B)
N
R 1 -- S O N H C --
N -~O
N-
R2 4
R
where R1 is a radical selected from the group consisting of
3-(dimethylaminocarbonyl)pyrid-2-yl, 3-ethylsulfonylpyrid-2-
yl and 3- [N- (C1-C4-alkyl) -N- (C1-C4-alkyl-sulfonyl) amino] pyrid-
2-yl, R2 is H or methyl, R3 and R4 independently of one
another are C1-C2-alkyl, Cl-C2-alkoxy or Cl-C2-haloalkoxy and
X is CH or N, or salts thereof.
According to another aspect of the present
invention, there is provided a herbicidal composition with
herbicidally effective content of a combination of A) a
compound of the formula (A2)
CA 02096115 2001-10-05
28976-125
-3a-
0
HO ) ., -- P -CHZ - NH CHZ CO - OH
(A2)
or salts thereof and B) one or more compounds of the
formula (B)
O R
N~/ (B)
R 1 -_- S O 2 --- N H -C -- N ~~ ~X
N---
2 \~ q
R R
where R1 is a radical selected from the group consisting of
2-ethoxyphenoxy, 2-propoxyphenoxy, 2-isopropoxyphenoxy,
3- (dimethylaminocarbonyl) pyrid-2-yl, 3- [N- (C1-C4-alkyl) -N-
(C1-C4-alkylsulfonyl)amino]pyrid-2-yl and (N-methyl-N-
methylsulfonyl)aminosulfonyl, Rz is H or methyl, R3 and R4
independently of one another are C1-C2-alkyl, C1-Cz-alkoxy or
C1-C2-haloalkoxy and X is CH or N, or salts thereof.
Preferred salts of the compounds of the formula A1
and A2 are ammonium salts, mono-, di- and trialkylammonium
salts, alkali metal salts and alkaline earth metal salts.
The monoammonium salt of glufosinate (A1-1) and the
monoisopropylammonium salt of glyphosate (A2-1) are
particularly preferred. Glufosinate exists in the D- and L-
form and mixtures of these, for example in the form of a
racemate. Formula A1 embraces all abovementioned spatial
configurations and their mixtures, preference being given to
the racemate and to the L-form and their mixtures.
Compounds of the formula (B) can form salts with
bases in which the hydrogen of the SO2NH group is replaced by
a can on which is suitable for agriculture, for example
metal salts such as alkali metal salts or alkaline earth
CA 02096115 2001-10-05
28976-125
-3b-
metal salts, or ammonium salts or salts with organic amines.
Acid addition salts with, for example, HCl, HBr, H2S04 and
HN03 are also possible.
Examples of suitable compounds of the formula (B)
are 1-[(2-ethoxyphenoxy)sulfonyl]-3-(4,6-dimethoxypyrimid-2-
yl) urea (B1) , 1- [ (2-n-propoxyphenoxy) sulfonyl] -3- (4, 6-
dimethoxypyrimid-2-yl)urea (B2), 1-[(2-
isopropoxyphenoxy)sulfonyl]-3-(4,6-dimethoxy-pyrimid-2-
yl) urea (B3) , 1- [ (2-methoxycarbonylphenyl) sulfonyl] -3- [4, 6-
bis-(difluoromethoxy)pyrimid-2-yl]urea (B4; primisulfuron-
methyl, CGA 136872), 1-[(3-dimethylaminocarbonylpyridin-2-
yl ) sul f onyl ] -
- 4 -
3-(4,6-dimethoxypyrimidin-2-yl)urea (B5; nicosulfuron,
SL-950),
1-[(3-ethylsulfonylpyridin-2-yl)sulfonyl]-
3-(4,6-dimethoxypyrimid-2-yl)uxea (B6; DPX-E 9636),
1-[3-(N-methyl-N-methylsulfonylamino)pyrid-2-ylsulfonyl]-
3-(4,6-dimathoxypyrimid-2-yl)uo,aa (B7),
1-[3-(N-ethyl-N-methylsulfonylamino)pyrid-2-ylsulfonyl]-
3-(4,6-dimethoxypyrimid-2-yl)urea (B8),
1-[3-(N-methyl-N-ethylsulfonylamino)gyrid-2-ylsulfonyl]-
ZO 3-(4,6-dimethoxypyrimid-2-yl)urea (B9),
1-[3-(N-methyl-N-methylsulfonylamino)pyrid-2-ylsulfonyl]-
3-(4,6-dimethylpyrimid-2-yl)urea (B10),
1-(3-(N-methyl-N-methylsulfonylamino)pyrid-2-ylsulfonyl]-
3-(4-methoxy-6-methylpyrimid-2-yl)urea (B11),
1-(N-methyl-N-methylsulfonylaminosulfonyl)-
3-(4,6-dimethoxypyrimid-2-yl)urea (812; amidosulfuron),
1-(2-methoxycarbonylthien-3-ylsulfonyl)-3-(4-mathoxy-
6-methyl-1,3,5-triazin-2-yl)urea(Bl3;thiameturonmethyl,
BPX-M 6316),
1-[2-(2-chloroethoxy)phenylsulfonyl]-3-(4-methrsxy~
6-methyl-1,3,5-triazin-2-yl)urea (B14; triasulfuron)
i-[(2-methoxycarbonylphenyl)sulfonyl]-3-(4-methoxy-
6-methyl-1,3,5-triazin-2-yl)urea (B15; metsulfuron-
methyl, DPX 6376),
and
1[(2-methoxycarbonylphenyl)sulfonyl]-3-methyl-
3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl)usea (H16;
- 5 -
tribenuron-methyl, DPX-L 5300).
Preference is given to herbicidal compositions according
to the invention with combinations of the compound (Al-1)
with one or more compounds from the group (B1) to 816),
in particular (B1), (82), (B3), (H5), (B7), (B8), (B9),
(B10), (811), (B13) and (B16).
Preference is furthermore given to herbicidal composi-
tions according to the invention with combinations of the
compound (A2-1) with one or more compounds fram the group
(H1) to (H12), in particular (H1)~, (82), (H3), (85) and
(B12).
The compounds of the formulae (B1) to (H3) are
1-[(2-alkoxyphenoxy)sulfonyl]-3-(4,6-dimethoxypyrimid-
2-yl)ureas and disclosed in EP-A-0,342,569. Applied pre-
and post-emergence, they are tolerated by the annual crop
plant species such as cereals, rice and corn. The effect
covers a broad spectrum of annual and perennial weed
species, grass weed species and Cyperaceae species.
The compound of the formula (H4) (pirimisulfuron) is
known from Hrighton Crop Protection Conference-Weeds
1987, p. 41-48.
The compound of the formula (B5) is known under the name
of nicosulfuron or SL-950 (see F. Kimura et al., Hrighton
Crog Protection Conference-Weeds-1989, pages 29°34).
Nicosulfuron (SL-950), i.e. 3-(4,6-dimethoxypyrimidin-
2-yl)-1-(3-dimethylaminocarbonylpyridin-2-ylsulfonyl)-
urea, is a sulfonylurea herbicide which has been employed
to date mainly for controlling grasses and broad-leaved
weeds in corn. Applied post-emergence, a large number of
annual and perennial weeds and grass weeds are con-
trolled.
The compound of the formula (B6) (DPX-8 9636) ie known
from Brighton Crop Pratection Conf.-Weeds-1989, p. 23 et
6
seq.
The compounds of the formula (87) to (B11) have been
disclosed in German Patent Application P 4,000,503.8.
Amidosulfuron (B12) has been disclosed in EP-A-0,131,258
and is known from Z. Pfl. Krankk~. Pfl. Schutz, Supplement
XII, p. 489'497 (1990).
Compounds (B13) to (H16) are described in Farm Chemicals
Handbook '90, Meister Publishing Company, Willoughby,
Ohio, USA (1990).
All the abovementioned herbicides have in common that
they are taken up post-emergence via the leaves (partly
or completely) and act in this manner.
Some combinations of compounds of the formula (A2) and
sulfonylureas are already known; see S. B. Eorsley, Proc.
Northeast. Weed Sci. Soc. 42, 84 ( 1988) ; H. R. Mashadi
and J. O. Evens, Res. Prog. Rep. West. Soc. Weed Sci.
1987 Meet., 348-50; K. E. Howren, G. S. Noble, Res. Rep.
Expert Comm. Weeds West. Can. (33 Meet.) Vol. 2, 240
(1986); D. G. Pchajek, J. D. Gingerich, Res. Rep. Expert
Comm. Weeds West. Can. (34 Meet.) Vol. 2, 524-26 (1987).
The herbicidal compositions according to the invention
have an excellent herbicidal activity against a broad
spectrum of economically important monocotyledon and
dicotyledon noxious plants.
If the active substance combinations are applied post-
emergence to the green parts of the plant, growth stops
dramatically very soon after the treatment, and the weed
plants remain in the growth stage of the time of applica-
tion, or die more or less rapidly after a certain period
of time. The weeds are controlled'very effectively in
this manner. When used for controlling weeds in planta-
tions, competition by the w~eds, which is harmful to the
-
crop plants, is eliminated in a sustained manner by using
the novel compositions according to the invention.
For example, using the active substance combinations
according to the invention, a herbicidal action is
achieved which exceeds what would have been expected as
an additive action of the individual components. Such
increased effects permit the dosage rates of the indivi-
dual active substances to be reduced substantially. While
_ the dosage rates are comparabl~, the weed-grass weed
spectrum controlled is much broader by virtue of the
synergistic effects. At the same dime, properties which
are of the utmost importance in practical use are con
siderably improved in the ease of most combinations.
These include, for example, the speed of action, the
long-term action, the flexibility upon use, etc. This
permits comprehensive, rapid, sustained and economical
control of weeds and grass weeds. Such properties are
therefore economically progressive because they offer
considerable advantages to the user in practical weed
control by allowing him to control weeds more
economically or more rapidly or in a more sustained
manner, therefore obtaining higher yields in a stand of
crop plants.
The selectian of the ratios by weight and dosage rates
depend, for example, on the components in the mixture,
the development stage of the weeds or grass weeds, the
weed spectrum, environmental factors and climatic condi-
tions.
The ratios by weight A : H can therefore vary within wide
limits and are generally at 1500a1 to 1:10, based on
weight.
Ratios by weight of 200:1 to 1:2, in particular 50:1 to
5:1, are preferably used.
The dosage rates of the herbicides A in the active
g ..
substance combinations are preferably between 10 and
2500 g/ha, based on active ingredient. Glufosinate is
preferably applied in amounts of 10 to 1200 g/ha, and
glyphosate is preferably applied in amounts of 500 to
2000 g/ha. The dosage rates of compounds of type H are
generally from 2 to 200 g/ha, preferably from 2 to
120 g/ha, in particular from 2 to 100 g/ha, based on
active ingredient.
The active substance combinations according to the
invention can exist either as mixed formulations of the
two components, which are then applied in the customary
manner after dilution with water, or can be prepared in
the form of so-called tank mixes by conjointly diluting
the components, formulated separately, with water.
Compounds A and B or their combinations can be formulated
in many ways, depending on the biological and/or chemico-
physical parameters. The following possibilities are
therefore suitable for formulation: wettable powders
(WP), emulsifiable concentrates (EC), aqueous solutions
(SL), emulsions (EW) such as oil-in-water or water-in-oil
emulsions, sprayable solutions or emulsions, dispersions
on an oil or water base, suspo-emulsions, dusting agents
(DP), seed-dressing agents, granules for soil application
or for broadcasting, water-dispersible granules (WG), ULV
formulations, microcapsules or waxes.
These individual formulation types are known in principle
and are described, for example, in: Winnacker-Rtichler,
"Chemische Technologie [Chemical Technology]", Volume 7,
C. Hauser Verlag Munich, 4th Ed. 1986; van Valkenburg,
"Pesticides Formulations", Marcel Dekker N.Y., 2nd Ed.
1972-73; K. Martens, "Spray Drying Handbook", 3rd Ed.
1979, G. Goodwin Ltd. London.
The formulation auxiliaries required, such as inert
materials, surfactants, solvents and other additives, are
also known and are described, for example, in: Watkins,
-
"Handbook of Insecticide Duat Diluents and Carriers",
2nd Ed., Darland Hooks, Caldwall N.J.; H.v.Olphsn,
"Introduction to Clay ColloLd Chemistry", 2nd Ed.,
J. Wiley & Sons, N.Y.; Marsden, "Solvents Guide", 2nd
Ed., Interscience, N.Y. 1950; McCutcheon's "Detergents
and Emulsifiers Annual", MC Publ. Corp., Ridgewood N.J.;
Sisley and Wood, "Encyclopedia of Surface Active Agents",
Chem. Publ. Co.~ Inc., N.Y. 1964; SchBnfeldt,
"GrenzflHchenaktive l~ithylenaxidaddukte [Surface-active
Ethylene Oxide Adducts]", Wiss. Verlagsgesell., Stuttgart
1976; Winnacker-Kiichler, "Chemische Technologie [Chemical
Technology]", Volume 7, C. Hauser~Verlag Munich, 4th Ed.
1996.
Combinations with other pesticidally active substances,
such as other herbicides, fungicides or insecticides, and
also safeners, fertilizers and/or growth regulators may
also be prepared on the basis of these formulations, for
example in the form of a readymix or as a tank mix.
Wettable powders are preparations which are uniformly
dispersible in water and which, besides the active
substance, also contain wetting agents, for example
polyoxethylated alkylphenols, polyoxethylated fatty
alcohols or fatty amines, fatty alcohol polyglycol ether
sulfates " alkanesulfonates or alkylbenzenesulfonates,
and dispersing agents, for example sodium lignin-
sulfonate, sodium 2,2'-dinaphthylmethane-6,6'-disul-
fonate, sodium dibutylnaphthalenesulfonate, or alterna-
tively sodium oleylmethyltaurinate, in addition to a
diluent or inert substance.
Emulsifiable concentrates are prepared by dissolving the
active substance in an organic solvent, for example
butanol, cyclohexanone, dimethylformamide, xylene or else
higher-boiling aromatic compounds or hydrocarbons, with
the addition of one or more emulsifiers. Examples of
emulsifiers which can be used are: calcium salts of an
alkylarylsulfonic acid, such as Ca dodecylbenzene-
to - ~~9~~~.
sulfonate, or non-ionic emulsifiers, such as fatty acid
polyglycol esters, alkylaryl polyglycol ethers, fatty
alcohol polyglycol ethers, propylene oxide/ethylene oxide
condensation products, alkyl polyethers, sorbitan fatty
acid esters, polyoxyethylene sorbitan fatty acid esters
or polyoxyethylene sorbitol esters.
Dusting agents can be obtained by grinding the active
substance with finely divided solid substances, for
example talc or natural clays, such as kaolin, bentonit~,
pyrophyllite or diatomaceous earth.
Granules can be produced either by spraying the active
substance onto adsorptive, granulated inert material or
by applying active substance concentrates onto the '
surface of carriers, such as sand, kaolinites or granu-
lated inert material, by means of binders, for example
polyvinyl alcohol, sodium polyacrylate or, alternatively,
mineral oils. Suitable active substances can also be
granulated in the manner which is conventional for the
production of fertilizer granules, if desired in a
mixture with fertilizers.
As a rule, the agrochemical preparations contain 0.1 to
99 percent by weight, in particular 2 to 95% by weight,
of active substances A + H. In the formulations, the
active substances A + B can exist in various concentra
tions.
The concentration of active substance in wettable powders
is, for example, approx. 10 to 95% by weight, the remain-
der to 100% is composed of customary formulation auxilia-
ries. In the case of emulsifiable concentrates, the
active substance concentration can be approx. 1 to 85% by
weight, preferably 5 to 80% by weight.
Formulations in the form of dusts contain approx. 1 to
25% by weight, usually 5 to 20% by weight, of activ~ sub-
stance, eprayable solutions approx. 0.2 to 25% by weight,
- 11 -
preferably 2 to 20% by weight, of active substance. In
the case of granules such as water-dispersible gxanules,
the active substance content depends partly on whether
the activs compound is liquid or solid, and which granu-
lation auxiliaries and fillers era used. As a rule, the
water-dispersible granules have a content of between 10
and 90% by weight.
In addition, the active substance formulations mentioned
contain, if appropriate, the adhesives, wetting agents,
dispersing agents, emulsifiers, penetrants, solvents,
fillers or carriers which are conventional in each case.
For use, the formulations, present in commercially
available form, are diluted, if appropriate, ire a cus-
tomary manner, for example using water in the case of
wettable powders, emulsifiable concentrates, dispersions
and water-dispersible granules. Preparations in the form
of dusts, soil granules or granules for broadcasting, and
also sprayable solutions are usually not further diluted
with other inert substances before use.
The joint agplication of the active substances in the
form of tank mixes is preferred, in which case the
concentrated formulations of the individual active
substances which are formulated optimally are conjointly
mixed with water in the tank, and the resulting slurry is
applied.
The examples which follow are intended to illustrate the
invention:
A. Formulation Examples
a) A dusting agent is obtained by mixing ZO parts by
weight of an active substance combination according
to the invention and 90 parts by weight of talc as
inert substance and comminuti,ng the mixture in a
hammer mill.
12 - ~~~6:~:~~
b) A wettable powder which is readily dispersible in
water is obtained by mixing 25 parts by weight of
active substance A +1 B, 64 parts by weight of
kaolin-containing quartz as the inert substance, 10
parts by weight of potassium ligninsulfonate and 1
part by weight of sodium oleoylmethyltaurinate as
the wetting and dispersing agent, and grinding the
mixture in a pinned disk-mill.
c) A dispersion concentrate which is readily dispare-
ible in water is obtained by mixing 20 parts by
weight of active substance A + H with s parts by
weight of alkylphenol polyglycol ether (°Triton X
207), 3 parts by weight of ieot~cidecanol palyglycol
ether (8 E0) and 71 parts by weight of paraffinic
mineral oil (boiling range, for example, about 255
to above 277°C), and grinding the mixture in a ball
mill to a fineness of below 5 microns.
d) An emulsifiable concentrate is obtained from 15
parts by weight of active substance A+B, 75 parts by
weight of cyclohexanone as the solvent and 10 parts
by weight of oxathylated nonylphenol as th~
emulsifier.
e) Water-dispersible gianules are obtained by mixing
75 parts by weight of active substances A + H,
10 " of calcium ligninsulfonate,
5 " - of sodium lauryl sulfate,
3 °' of polyvinyl alcohol and
7 " of kaolin,
grinding the mixture 3n a pinned disc mill, and
granulating the powder in a fluidized bed by
spraying on water as the granulation 7Liquid.
f) Water-dispersible granules are also obtained by
homogenizing and precomminuting
- 13 -
25 parts by weight of active substance A + B,
" of sod3.um 2,2'-dinaphthylmethane-
6,6'-daaulfonate,
2 " of eod~'Lum oleoylmethyltaurinate,
5 1 part by weight of pol;Yvinyl alcohol,
17 parts by weight of calcium carbonate and
50 " of water
on a colloid mill, subsequently gainding the mixture
on a bead m3.11, and atomizing and drying the reaul-
tang suspension in a spray tower by means of a
single-substance nozzle.
B. Biological examples
A range of economically important weeds and grass weeds
were grown under natural field conditions. After they had
reached certain development stages (expressed by the
number of unfolded leaves or by the plant height), the
herbicide mixtures~wera applied by means of specific plot
sprayers. As rule, 300-400 liters of water were used per
hectare, and the slurry was applied at a pressure of
2.5 bar.
Over a period of 3-8 weeks after the application, the
herbicidal activity of the treated part-plots were
assessed by comparing them with untreated control plot
by visual scoring. This included assessment of the damage
and the development of all aerial parts of tlae plant.
In the combinationa,'a distinction was made between the
calculated~and the found degree of. effectiveness. The
calculated degree of effectiveness, which i~ to be
expected theoretically, of a combination is determined by
S. R. Colby'a formula: Calculation of synergistic and
antagonistic responses of herbicide combinations, Weeds
15 (1967) 2D-22:
- 14 - ~~'~~~1~
This formula reads:
X
E = X + Y -
100
where
X ~ % damage by herbicide A at a dosage rate of x kglhao
Y - % damage by herbicide B at a~dosage rate of y kg/ha;
E ' damage to be expected by herbicides A + B at dosage
rates of x + y kg/ha.
If the actual damage is greater than the damage to be
expected from the calculations, then the action of the
combination is superadditive, i.e. a synergistic effect
of action is present.
In most cases, however, the synergistic total increase in
action is so high that Colby's criterion can be dispene~d
with; in this case, the reaction of the combination
noticeably exceeds the formal total (total in figures) of
the actions of the individual substances.
It moat be expressly stated that an assessment of the
synergism in the active substances used here must take
into account. the dosage rates of the individual actitre
substances, which vary greatly. It would therefore not
make sense to compare the actian of the active substance
combinations and the individual active substance$ at ixa
each case identical application rates. The quantities of
active substance which can be saved according to the
invention are only noticeable in the case of superaddi-
tive increased action when the combined dosage sates are
applied, or when the application rates of both individual
active substances in the combination are reduced oompar~d
with the individ'aal active substances, while having the
- 15 -
same effect in each case.
Example 1
Table 1~ Combination (A1-1) + (B1) on Cyperua rotundas
Herbical active DoE~ag~ Action in %
substance rage in
g a~i/ha
( A1- 1 ) 400 ' 0
800 30
1200 S3
1500 55
(51) 30 5
60 ' 5
120 25
(A1-1) T (5?) 400+ 30 35
400+ 60 55
400+120 68
800+ 30 90
800+ 60 90
800+120 96
.200+ 30 g2
.200+ 60 . 96
'200y120 97
Table 1 abbreviations
ai = active ingredient (= based on pure active
substance)
(A1-1) = monoammonium salt of glufosinate, added to the
tankmix in the form of a 20% aqueous formula-
tion (SL 20).
H1 ~ 1-((2-ethoxyphenoxy)sulfonyl]-3-(4,6
dimethoxypyrimid-2-yl)urea, added to the
tankmix in the form of a 20% water-dispersible
powder.
- 16 - ~~3~~.
Example 2:
Cyperus rotundas was treated shortly before anthesis
(stage 51) and the damage after application was assessed
(see Table 2).
Table 2: Combination (A2-1) + (H1) on Cyperus rotundas
Herbical active Dosage Action in ~
substance raise in
~ ~~.rh~
(A2) 1080 g0
2160 g3
(B1) 60 5
fA2-11f(H1) 1080 60 95
Table Z abbreviations:
(A2-1) = monoisopropylammonium salt of glyphosate in th~
form of an aqueous formulation, added to the
tankmix at a dosage rate of 4130 g/1
(B1) ~ see Table 1
Example 3
The results shown in Table 3 were obtain~ad analogously to
Examples 1 and 2.
- 17 - ~~'~~:~.:~t~
Table 3: Combination (A1-1) + (B5) against perennial and
annual species
Herbicides Dosage CYPR() AMASp 4'AGMICOMBE
rate in
~t si/ha
(A1) 1000 85 100 100 97
400 35 49~ 55 42
(85) 30 0 0 0 2
20 0 0 ~ 0 0
10 0 0 0 7
(A1-1)+(B5) 400+10 50 ' 100 100 75
400+20 60 100 100 B5
400+30 80 100 100 87
Notes to Table 3:
Treatment: post-emergence, 4-6-leaf stage to
anthesis/25-50 cm height
Evalution: 28-57 days after application
The following were tested under natural conditions:
- CYPRO a Cyperus rotundus
° AMASp ~ Amaranthus spinosus
- TAGMI a Tagmites minor
- COMBE g Commelina benghalensis
(A1-1) = see Table 1
(B5) = nicosulfuron added to the tankmix in the form
of a 20% water dispersible powder.
Example 4
The results shown in Table 4 were obtained analogously to
Examples l to 3.
- 18 - ~~9~~~.
Table 4: Combination (Al-1) + (B5) against annual
species.
Herbicides Dosage BRSNN MEUIN VICVI GALAP POLCU URTDI
rate in
a ail~a
(A1-1) 600 ?2 98 92 0 92 50
300 20 25 42 0 57 10
(HS) 40 87 0 0 25 52 85
(A_-1)t(r5) 300+40 95 , 85 62 ~75 100 96
(E=79) (E=80) (~=86)
Notes to Table 4:
Treatment: In the 3-8-leaf stage
Evaluation: 30 days after assessment
Abbreviations: BRSNN, = Brassica napus napus
MEUIN = Melilotus indicus.
VICVI = Vicia villosa
GALAP = Galiwn aparine
POLCU = Polygonum cuspidatum
URTDI = Urtica dioica
E = expected value using COLBY formula
(A1-1) = see Table 1
(B5) _ .. 3
al = see Table 1
Example 5
The results :in Table 5 mere obtained analogously to
Examples 1 to 4.
-i9-
Table 5: Combination (A1-1) + (B7)
HerbicidesDosage rate GALA.P VERPE VIOAR ECRCG
in
cL ai/ha
(F,?-? loo0 80 9o es 95
~
600 70 80 35 92
400 25 50 0 50
200 0 0 0 0
100 0 0 0 0
(B7} 25 50 . 0 65 85
12, 5 0 0 40 75
(R1-1)+(&7)200+12,5 95 50 65 98
200+25 90 70 80 95
400+12,5 98 90 89 90 (E=88)
400+25 80 93 97 98 (E=92)
Abbreviations: GALAP = Galium aparine
VERPE = Veronica pereicaria
VIOAR = Viola arvense
ECHCG = Echinochloa crus galli
(A1-1) = see Table 1
(B7) $ 1- [ 3- ( N-anethyl-N-
methylsulfonylamino)-
pyrid-2-yl-sulfonyl]-3-(4,6-
dimethoxy pyrimid-2-yl)urea
Application: In the 1-8-leaf stage;
Evaluation: 6 weeks after application