Note: Descriptions are shown in the official language in which they were submitted.
WO9~/08691 PCrlEP~
2~96~3`~
1,3-BIS-r3-(MONO- OR POLY-~YDROXY)ACYLAM_~o-5 ~MONO~ OR
PO~Y-~YDROXYALRYL)AMINOCARBONYL-2,4,6-TR~IODo-B~RZOYL~
AMINO]-HYDROXY- OR HYDROXYALRYL-PROPANES _ n~IR ~r~C~s
OF P~:PARATION_AND X--RAY CONTR~ST M~DIA CONTAININ
~M.
__
This invention relates to symmetrical or asymme~
trlcal 1,3-bis-[3-(mono- or poly-hydroxy) acylamino~5~ :
(mono- or polyhydroxyalkyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-hydroxy- or hydroxyalkyl-propanes, use~
5, ful to constitute the opacifying component of X-ray
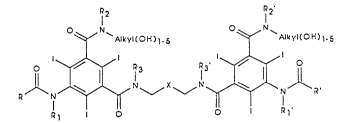
contrast media, of general formula (I)
2'
kyltoH)l-5 ~ Alkyl~OH~l~5
o D~ 3 73 1~ o (1)
R N ~ N ~ X ~ N ~ N R '
. I
A B
15~ wherein:
R, R', which are the same or different, are a straight
or branched mono- or poly-hydroxyalkyl Cl-C3 re-
sidue containing from 1 to 2 OH groups,
Rl, R2, R3, which are the same or dif ferent, are H or
CH3~
Rl" R2 " R3 " which are the same or different, are H
or CH3
Alkyl(oH)l-s is one of the groups of formula
-CH(CH20H)CH(OH)CH20H, -CH(CH20H)2, -cH2cH(oH)
CH2OH, -CH2~CHOH)4CH20H, or -CH2CH20H,
,
.
.,. ~ ., ,, ,, ... ~ . .. , . . .. .. :
WO92/08691 PCT/EP~ 2.~5D
2 ~ 3 ~ 2
X is one of the groups -CH~OH)-, -CH(CH2O~
-C(OH)(C~2OH)- or C(C~2OH)2-,
A and B, may be the same or different.
The invention also comprises the possible enan~lo--
5 mers, diastereoisomers and/or rotamers of compounds
(I). ;
The invention also relates to a process for ~h2preparation of these non-ionic compounds, as well as ~o
the X-ray contrast media which contain them as opa-
cifying components. ~ .
Preferred compounds of formula (I) are the ones inwhich R2, R2', R3, R3', are H. Particularly preferred
are the ones in which X represents the -CH(OH)- group.
From the structural point of view, the non-ionic X-ray
contrast agents which are more similar to the ones o
this invention are the a,~-bis-[3-hydroxyacylamino~5
(dihydroxyalkyl)aminocarbonyl-2,4,6-triiodo-benzoyl-
amino]-oxaalkanes described in patents DE 2805928 and
US 4,139,605. In these compounds the two ~riiodo-ben-
zcyl-amino residues are joined via an oxaalkylenic re-
sidue of formula ~CnH2n ( CnH2n)0-4 n 2n
ning from 4 to 12 atoms of C and from 1 to S atoms of
O. They can produce oversaturated solutions and after
some time, these ones may spontaneously crystallize,
therefore causing a limit~tion to the use of such com-
pounds. Patent US 4,062,934 describes N,N'-bis-(3-N-
methyl-N-acetylamino-5-N-gluconylamino-2,4,6-triiodo-
benzoyl)-diaminoalkanes. Due to an iodine content
comparatively low, they show a reduced opacifying capa-
city. In addition, their solutions are quite viscous.These features cause some problems when the product is
, , . , : .. .- .
W092/08691 P~CT/EP91~0f215
(~ ~ .;
3 2~9G13~
..~ .
used in procedures which require administration via
catheter.
N,N'-bis-(3-acylamino-5-N-methyl-carbamoyl-2ff4,ff~
triiodo-ben~oyl-amino)-carboxyalkanes are described in
patent application GB 1,488,904. The preparation of so~
lutions acceptable from the pharmacological point of
view requires that free carboxylic groups, where pre~
sent, are neutralized. Therefore, if compared to ~h~
non-ionic contrast agents, the solutions of the com~
pounds described in the above mentioned patent applica-
tion have a high osmolality. Slmilarly, their neuroto-
xicity is higher than the one of the non-ionic contrast
media.
Sova~ et Al. described in application WO 8501727
among other compounds, also N,N~-bis-[3,5-bis-(N-2,3
dihydroxy-propyl-N-acetyflamino)-2,4,6-triiodo-be~zo~ffffl~
ethylendiamines and the corresponding N'-h~ffdroxyal~yl~
derivatives. The synthesis of these compounds is quite
difficult. The four 2,3-dihydroxy-propyl groups are ob~
tained by treating the corresponding tetraalkenylderi-
vatives with l-butyl-hydroperoxide in the presence of
osmium tetroxide. The reaction occurs in a fragmented
and unaccomplished way. None of the compounds descri-
bed, mentioned or claimed by Sovak has as conjunction
bridge of the two 2,4,6-txiiodo-benzoyl-amino residues
th~ hydroxyalkylenic chain of formula -CH2-X-CH2-, with
X equivalent to C~(OH)-, -CH(CH2O~)~, -C(OH)(CH2OH)-
or -C(CH2OH)2-, which is characteristic of this inven-
tion.
A last reference concerns the European patent ap-
plications EP 74307 and EP 74309 abandoned in 1985.
: :
W0 92iO8691 P~EP9]/u2151 1~
2~13``3 4
, ~
The first one only describes aromatic bi- or
tricyclic compounds where the aromatic moiety contains
iodine and bromine atoms at the same t:ime and in the
same molecule.
S The second one does not describe new compounds~
but a process which increases the tolerability of opa~
cifying compositions by using a mixture of iodobenzenic
and bromobenzenic compounds. The general formulas de~
scribing these compounds are extremely wide, unclear
and indefinite and include billions of compounds basi-
cally different among them.
The compounds of formula (I) belong to the class
of non-ionic contrast agents for X-ray diagnosis, which
are more and more replacing the salts up to now used of
the derivatives of 2,4,6-triiodo-benzoic acid, due to
their better tolerability and the reduced side-effectsO
Thanks to the elimination of the ionic species, their
. .
solutions, if compared to the ones of the ionic agents9
have the same content o iodine, but a lower osmotic
pressurel that's to say a lower osmolality. Therefore,
for instance, in angiography, they cause less pain and
endothelial damage. In subarachnoidal administration
for myelography and cisternography, unlike the previou-
sly used contrast media, they rarely cause arachnoidi-
tis or epileptic disorders. Unfortunately non-ionic
contrast agents, consisting of iodinated monocyclic
aromatic nu~lei, are still too hypertonic, despite
their excellent physical and pharmacological proper-
ties, if compared with blood, at the high dosages and
high concentrations which many diagnoses require. This
fact led to the development of bicyclic hexaiodinated
,
WO92t08691 PCT/EP91/02~5~
1 . i :
`~ 2~61~ ~
compounds, whose osmolality is reducecl in comparison
with the total amount of iodine in the moleculeO
However, concentrated solutions of these compounds are
frequently too much viscous. In addition, a larye
number of products, even if they are expected to be
promising from the theore~ical point of view, are not
enough soluble.
For this reason, researchers investigated on new
hexaiodinated bicyclic derivatives characterized byo
high solubility, low viscosity and osmolality as well
as high intravenous, intracisternal and intracerebral
tolerability and minimum tendency to give side effects
(pain, temperature increase, nausea, decrease of
pressure and vessel damages).
In particular, ?hysicians need new contrast media
characterized by a wide range of possible uses, i.e. in
urography, angiography, cardioangiography, flebography~
myelography, cisternography, lymphography, isterosal~
pingography, bronchography and gastrography or the vi-
sualization of articular cavities.
The products of this invention are generally cha-
racterized by a good water solubility, which can exceed
100 g per 100 ml of solution at 20C, and a low toxi-
city.
If compared to the known hexaiodinated dimers,
they show low osmolality without causing a foreseeable
corresponding rise in the viscosity.
One of the preferred compounds of the invention is
for example 1,3-bis-[3-( L-2-hy~roxy-propionyl ) amino-5-
(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2-hydroxy-propane (compound A of Table
.
., ~ ,
W092/08691 PCT/EP91/0215~ ¦
~ 6
2). It gives stable aqueous solutions which ContaiRO at
2QC, more than 100 g of product in 100 ml of solutionO
As far as the physiological characteristics are
concerned, the study of plasma kinetics revealed half
life for the distribution and elimination phases of 2~9
and 22.9 min, after intravenous administration in rats
(200 mgI/kg).
The apparent volume of distribution is 12305 ml/kg
showing that the produot is distributed in plasma and
the extracellular spaces. The total clearance is 709 ml
min-l.. kg~l,
Elimination occurs mainly through urinary pathway
and in less extent through the bile. After 7 hours from
the administration, 76.7% of the administered dose was
found in the urine and 3 . 5% in the bile.
Its tolerability is very good as reported in Table
lo
In Table 2, there are reported the values of some
characteristic properties of preferred products of this
invention in comparison with IODIXANOL (Nycomed), which
is an hexaiodinated non-ionic dimer in advanced phase
o. development (EP 108538; CLINICA 413~ p 7~ 08.08.90)o
The process for the preparation of the 1,3-bis-[3-
(mono- or poly-hydroxy)acylamino-5-(mono- or poly-hy-
droxyalkyl~aminocarbonyl-2,4,6-triiodo-benzoyl-aminol~
hydroxy- or hydroxyalkyl-propanes of general formula
(I) is characterized in tha~ a 1,3-diamino-hydroxy- or
hydroxyalkyl-propane of general formula (II~
R3-HN-cH2-x-c~2-NH-R3l (II)
wherein R3 and R3' are H or CH3, X represents the
groups -CH(OH)-, -CHICH20H)-~ -C(OH)(CH20H)- or
WO92/08691 PCT/EP911021~ .
-
( 2~9~ 3~
-C(CH2OH~2- and one of the amino groups may be protec~
ted by a suitable protective group, is reacted, direc
tly or by a multi-step process, with a reactive deriva~-
tive of a 3-(mono- or poly-acyloxy)aclyamino-5-(mono~
or poly-hydroxyalkyl)aminocarbonyl-2,4,6-triiodo-ben~
zoic acid of general formula (III)
12
~ ~AIkYltOH)l S
R J~ N~ ( 111 ),
Rl I
wherein:
lS Rl, R2, are H or CH3,
R4, is a straight or branched mono- or poly-acy~
loxyalkyl Cl-Cl3 residue containing from 3 to l3
atoms of C and from l to 2 lower acyloxy groups
C2-C5,
Alkyl(OH~l 5, is one of the groups of formula
-CH2CH(OH)cH2oH, -cH2cH2oH, -CH(CH2OH)2'
-CH(CH2OH)CH(OH)CH2OH or -CH2(CHOH)4CH2OH, where
the hydroxy groups may be preferably protected by
acetalic or Ketalic groups,
CO-Y, is the residue of a mixed anhydride or, pre-
ferably, a halocarbonyl group,
to give a compound of general formula (IV)
:
. ~ ... . .. .
., : ,
.. - .... , .: . . . . .
WO92/08691 PCT/P91~02D51
?.
2 ~ 3 ~ ` `
1 2 1 2
~ ~iukyl(oH)l 5 q~ ,Alkyl(OH)1-5
\~ 13 R . 1~ o ~IV).
R 1 N /~ N ~ X ~ N ~
wherein Rl, R2, R3, Rl , R2 ' 3 4
and X have the meaning as previously defined and R5 may
be R or R4, preferably according to one of the two
following procedures: ;
a) a compound of formula (II) is reacted with a
compound of formula (III) in a molar ratio of 1-2
in a solvent and in the presence of a basic
condensation agent in order to obtain the
corresponding compound of formula (IV) where R5
corresponds to R~,
b) a compound of formula (II), where one of the two ~ .
amino groups is protected by a suitable protective
group, is reacted with a compound of formula (III)
in a molar ratio of 1:1 in a solvent and in the
presence of a basic condensation agent in order to
obtain, after hydrolysis of the protective groups,
the corresponding 1-[3-(mono- or poly-hydroxy~-
2S acylamino-5-(mono- or poly-hydroxyalkyl)aminocar~
bonyl-2,4,6-triiodo-benzoyl-amino]-3-aminohydroxy_
alkyl derivative of general formula (V)
R12
AlkYltOH))~5
(V)'.
~ N ~ X ~ N H
Rl I `.
,
WO92~08691 PCT/EP91/02151
f :'-'?
9 ~ 1 3 ~ ` `
wherein R~ Rl, R2~ R3~ R3'~ Alkyl(oH)l-5 and X ha
meaning as previously deined, and this compound (V) is
subsequently reacted with a derivative of formula (III)
in the presence of a basic condensation agent to obtain ¦
compound (IV) where R5 is R, then, the compound of
formula (IV), obtained by synthetic methods a) or b);
is txansformed by hydrolysis of the protective groups,
into the corresponding compound of formula (I)o
Finally, in case that Rl, Rl' are H, this last one rnay,
if desidered, be methylated in alkaline medium.
Methylation occurs through reaction with suitable
methylating agents, for instance methyl halides;
dimethylsulphate, methylsulphonate, dimethylcarbonate.
For instance, the di-sodium derivative of general
formula (VI)
72 72 ~ :
~ Alkyl~OH)1 5 q~ ~AIkYl(OH)l ~; ,
No O ~ N~
is prepared in a basic environment, preferably in the
presence of alcoholate or alkaline hydroxide, and is
then transformed into the corresponding dimethyl de-
rivative of general formula (VII)
72 72
qf Alkyl(oH)l-5 q~ Alkyl(oH)l-5
30 l ~ 73 73 ~ I ~ (Vll).
R J~ N ~ ~ ~\ C H 3
,` : , ::: ::' : ,.. . , , ,, , .. ~. :
WO92/08691 PCT/EP91/02~
~,'. `
~Q~13~ lo
by treatment with methyl halides, d:Lmethylsulphate~
dimethyl carbonate, methyl methanesulphonate, methyl
benzenesulphonate, methyl toluenesulphonate.
In case of direct reaction of the product of gene~
ral formula (II) with a compound of general formula
(III), according to synthesis a), symmetrical products
are obtained, where both residues A and B of general
formula (I) are identical.
Following the multistep reaction according to
synthesis b), asymmetrical products may be obtained, in
which the residues A and B of general formula (I) are
different. In fact, during the second step of the reac-
tion, a compound of formula (III), which is different
from the one used during the first step of the reac-
tion, may be used.
Anhydrous tertiary amines, potassium hydroxide;
sodium hydroxide, sodium bicarbonate, calcium caxbo~
nate, magnesium carbonate may be used as basic conden
sation agents.
In both a) and b) synthesis, anhydrldes with orga-
nic or inorganic acids~ azides, derivatives of phospho-
ric acids, alkylcarbonic acids may be used as compounds
of formula (III).
Therefore, the reactive residue Y in compounds of
formula (III) represents the residue of an inorganic or
organic acid such as: chlorine, bromine, iodine, pho-
sphite, azide, acyloxy or alkoxycarbonyloxy.
The preferred group C0-Y is the residue CO-Cl.
The hydroxy functions in the Alkyl(OH)l 5 group of
formula (III) may be preferably protected by tran-
sformation into the corresponding acetales or ketals.
: , . .............. ,.,., ,. , ,
: : . ~ .. :; : - .... '; ' . . ..
WO92/08691 PCT/EP91/02151
, -~
! ` - 2096~3 ~
11 :
Examples of such protective groups may be for instance
methylidene, ethylidene, l-methyl-ethylidene, l-ethyl~
ethylidene., l-t-butyl-ethylidene, l-ph~enyl-ethylidene,
2,2,2-trichloro-ethylidene, l-ethyl-propylidene. These
groups may be easily and fully submitted to hydrolysis
through a short treatment with a strong acid, for
instance diluted hydrochloric acid, aqueous
trifluoroacetic acid or through a strongly acidic ion
exchange resin, realeasing the corresponding ketonic
compounds, usually acetone, methylethylketone or
diethylketone.
The lower acyloxy protective C2-C5 groups, inclu- .
ded in the R4 residue of formula (III), may.be for in-
stance acetoxy, propionyloxy, butiroyloxy and may be
easily submitted to hydrolysis by treatment with alka-
line aqueous solutions, in order to obtain the desired
mono- or poly-hydroxyalkyl R groups of formula (I).
The reaction of a compound of general formula (II)
with one of formula (III) to give a compound (IV) takes
place preferably in an aprotic solvent, such as, for
instance, dimethylacetamlde (DMAC), dimethylformamide
(DMF), hexamethylphosphoramide (HMPT) or dioxane, but
also in acetone, ethyl alcohol and isopropyl alcohol.
The te~perature is not critical. The reaction takes
place when temperature ranges from -10C to + lS0aC~
preferably within 0C and 100C.
l,3-Diamino-hydroxy- or hydroxyalkyl-propanes of
general formula (II) are described in literature, for
instance in "Beilsteins Handbuch der Organischen Che-
mie":
l,3-diamino-2-hydroxy-propane, Beil.4 H 290, E II
.: ~: ......... . . .,. . :
.. ,: "
,;; ,': : : . : :'
WO92/08691 PCT/EP91/02151 ~
.
2 ~ 12
739, E III 766, E IV 1694;
1,3-bis-(me~hylamino)-2-hydroxy-propane, Beil. 4
IV 1695;
1,3-diamino-2,2-bis-hydroxymethyl-propane, BeilO 4
E III 850;
1,3-bis-(methylamino)-2,2-bis-hydroxymethyl-pro- '
pane, Beil. 4 E III 851. :~:
When a compound of formula (II) is reacted with
one of formula (III) in a molar ratio of 1:1 according
to synthesis b), then the 1,3-diaminoderivative of for-
mula (II) is monoprotected on one of the two amino
groups in order to avoid side-reactions. Protective
groups which meet this purpose may be for instance
acetyl, dichloroacetyl, trifluoroacetyl groups. Tri-
fluoroacetyl is particularly preferred. Afterwards~these groups may be easily and completely hydrolysed
through treatment with alkaline aqueous solutions~
The reactive derivatives of the 3-(mono- or poly~
acyloxy)acylamino-5-(mono- or poly-hydroxyalkyl)amino~
2,4,6-triiodo-benzoic acids of general formula (III)
are obtained by reaction of a reactive derivative of a
3-(mono- or poly-acyloxy)acylamino-2,4,6-triiodo-iso-
phthalic acid of general formula (VIII~
o~Y
2 5 ~ ~ (V l l l ~ .
Rl l O
wherein Rl, R4, and Y are as previously defined, or by
reaction of a derivative belonging to the ones already
W092/0869] PCT/EP91fO295~ ~
~- 2~?~1 3f;)
described in patents ~S 4,001,323 and EP 26281 with the
hydroxyalkyamines 2-hydroxy-ethylamine, 1,3-dihydroxy~
lsopropylamine (serinol), 2,3-dihydroxy-propylam.ine
(isoserinol), 3-amino-1,2,4-butantriol, or with the
corresponding N-methylamines, or with N-methyl~
glucamine or with a corresponding derivative thereof
wherein the hydroxy sroups are preferably protected by
chelatization, for instance with 5-amino-2,2-dimethyl-
1,3-dioxane, 5-~mino-2-methyl-2-ethyl-1,3-dioxane, 5
amino-1,3-dioxane, 5-amino-2,2-diethyl-1,3-dioxane, 4-
aminomethyl-2,2-dimethyl-1,3-dioxolane, 4-aminomethyl-
2,2-diethyl-1,3-dioxolane, 5-amino-6-hydroxy-2,2-di-
methyl-1,3-dioxepane or 5-amino-6-hydroxy-2-ethyl-2-
methyl-1,3-dioxepane.
lS The reactive derivatives of 3-(mono- or poly-acy
loxy)acylamino-2,4,6-triiodo-isophthalic acid (VII~
are generally reacted with one of the above mentioned
amines in a molar ratio of about 1:2 without using a
basic condensation agent, or in a ratio of 1:1 by using
a basic condensation agent in an inert organic solvent.
For instance, dioxane or tetrahydrofuran (THF) are pre
~erred solvents. The HY acid, released during the reac-
tion of (VIII) with the amine, is.neutralized by the
second mole of the amine, giving the corresponding am-
monium salt, or by the basic condensation agent used.
Some of t~e preferred reactive derivatives of the
3-(mono- or poly-acyloxy)acylamino~5-(mono- or poly-hy-
droxyalkyl)aminocarbonyl-2,4,6-triiodo-ben7.oic acids of
general formula ~III) have already been des~ribed in
patents DE 2805928 or US 4,139,605.
The compounds of this invention may be used as
W092/08691 PCT/EP91/02~51
3 ~
1~
opacifying agents in non-ionic contrast me!dia for X~ray
diagnosis.
They may be formulated in a suitable carrier agent
which is acceptable from the pharmacological point of
view. Suitable carrier agents include, for instanceO
the ones which can be used for enteral or parenteral
administration, such as buffered sterile aqueous
solutions containing tromethamine, phosphate, ci~ra~e
and/or bicarbonate ions, ionically balanced solutions
containing physiologically acceptable anions and
cations such as: Cl( ), HC03( ), Ca(2~), Na(+), K(+)
and Mg(2~. Solutions of the contrast agents of this
invention may also contain a small amount of a
physiologically tolerable chelating agent, such as the
sodium and calcium salts of EDTA, in concentrations
from 0,05 to 2 mM/l, or even heparin, at a dosage
ranging from 5 to 500 units per 100 ml of solution, vr
another suitable anticoagulant agent.
~ypotonic solutions of the contrast media of this
invention may be isotonically balanced by adding the
correct amount of Merlis liquid (The Am. J. of Phy~
Vol. 131, 1940 p. 67-72). Useful concentrations of io-
dine for such contrast media vary from 140 to 500
m~I/ml at a dosage of 10-300 ml, according to the desi~
red diagnostic use.
The following experimen~al examples show the basic
principles of this invention.
EXA~PLE 1
1,3-bis-~3-(L-2-hydroxy-propionyl)amino-5-(1,3-dihyclro-
xy-isopropyl)-aminocarbonyl-2,4,6-triiodo-benzoyl~
no]-2-hydroxy propane.
WO92/08691 PCT/EP91/02l5~
~.`' .
2~6~3~
a) 124 g of 3-(L-2-acetoxy-propionyl)amino-5~ 3-
dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo~
benzoyl-chloride (0.162 mol), obtained according
to the procedure described in DE 2805928/Example
1, are solubilized in 490 ml of dimethylformamide
(DMF). The resulting solution is cooled to 0-S~C~
added of 32.4 g of anhydrous tributylamine (0O175
mol) and then dropwise mixed under stirring with a
solution of 7.66 g of 1,3-diamino-2-hydroxy-
propane (0.085 mol) in 200 ml of D~iF. The reaction
mixture is kept under stirring at 0-5C fcr 1 h,
then is left at room temperature for about 20 ho
Hence the solvent is evaporated under vacuum. The
residue is crystallized with ethyl acetate. The
crystalline precipitate is filtered and dried~
then suspendend in 500 ml of water at 50C. pR is
adjusted at l0.3 with NaOH 2N and the mixture is
stirred until the acetoxy groups of the propionyl
residues are totally hydrolysed. The resulting
solution is made free from salts by passage
through ion exchange resins t360 ml of AmberliteR
IR 120 and 400 ml of DuoliteR A 30B). The eluate
is evaporated to dryness and crystallized from 600
ml of ethyl alcohol. After filtration and drying
75.8 g of 1,3-bis-[3-(L-2-hydroxy-propionyl)amino~
5- ( 1, 3-dihydroxy-isopropyl )aminocarbonyl-2,4,6-
triiodo-benzoyl-amlno~-2-hydroxy-propane are
obtained.
Yield: ~4~ m.p.: 280C (dec.)
Elemental Analysis (%)
, -......... . ~: , .:
,......... . . .
:,: ;,, . ,. . , .. ~, ,
- ~: ;: . , i. . . , ., . :
:, .' ' :," : ' ' . , ::
.. ;: :.~
WO92/08691 PCT/EP91/02~5D
~;
2 0 ~
16
C H I N
Calculated: 25.47 2.48 52.08 5.76
Found: 25.47 2.35 52012 5O53
[~]20 = ~6.03 (c = 9-6% H2O)
S Solubility in H2O at 20C : > 100% w/v.
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/CH30H/NH40H 25% = 6/3/1 v/v/v. Rf = 0.14~
HPLC: Rt = 12.4 min. Titre = 98.5% (on the area)
Chromatographic conditions:
10 Column: LICHROSORB RP 18 (Merck), 5 ~ (250 x 4 mm)
Eluent: A) H2O B) CH3CN
Gradient (flow 1 ml/min; Detector UV 254 nm):
time (min) = 0 - 3; 3 - 25; 25 - 30
%B = 0; 0 - 40; 40
15 b) the above disclosed compound can be obtained also
by reacting, according to the procedure of the
previous Example la), 65.2 g of 3-(L-2-acetox~
propionyl)amino-5-(4,4-dimethyl-3,5-dioxa-cyclo-
hexyl)aminocarbonyl-2,4,6-triiodo-benzoylchloride
(0.081 mol); described in DE 2805928/Example
lB(b), with 3.83 g of 1,3-diamino-2-hydroxy-
propane (0.042 mol) in 350 ml of DMF in the
presence of 16.2 g o tributylamine (0.087 mol).
The obtained product is transformed into 1,3-bis~
[3-(L-2-acetoxy-propionyl)amino-5-(1,3-dihydroxy-
isopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-ami-
no]-2-hy~roxy-propane by treatment with HCl 2N and
corresponding elimination of acetone. This product
i~ suspended in water at 50C, the pH is adjusted
to 10.3 with ~aOH 2N and the basic treatment
continues until the acetoxy groups are completely
., ~ , ,, . .
: . . :: . . : .
WO~2/0B691 PCT/EP9~/02~1
~, ;:i .
17 ~ ~ 3 6~3 1~
hydrolysed. After elimination of the ionic
species, due to the passage through ion exchange
resins,. 41.4 g of 1,3-bis-[3--(L-2-hydroxy-
propionyl)amino-5-(1,3-dihydroxy-isop:ropyl)amino~
carbonyl-2,4,6-triiodo-benzoyl-amino]-2-hydroxy-
propane are obtained with a yield of 70%.
c) This reaction may also be performed in ethyl
alcohol (EtOH) instead of DMF: 588 g of 3-(L-2--
acetoxy-propionyl)-amino-S-(1,3-dihydroxy-isopro-
pyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chloride
(0.67 mol) in 5.8 1 of EtOH are reacted at room
temperature with 36.4 g of 1,3-diamino-2-hydroxy-
propane (0.404 mol) dissolved in 2.9 1 of ~tOH, in
the presence of 144 g of tributylamine (0.777
mol). The obtained precipitate is filtered,
suspended in water and treated with NaOH 2N up to
pH 10.3 as previously described in a). 350 g of
1,3-bis-[3-(L-2-hydroxy-propionyl)-amino-5-(1,3-
dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2-hydroxy-propane are obtained with
a yield of 6~.2~.
EXAMPL~ 2
1,3-bis-[3-hydroxyacetylamino-5-(1,3-dihydroxy-isopro-
pyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-2,2-bis-
hydroxymethyl-propane.
a) 144 g of 3-ace~oxyacetylamino-5-(1,3-dihydroxy-
isopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-
chloride ~0.192 mol), described in DE
2805928/Example 10, dissolved in 600 ml of DMF,
are dropwise added under stirring and between 5 to
10C to a solution of 13.4 g of 1,3-diamino 2,2-
WO92/08691 PCT/EP9~02~5D
~ - . .
~ 18
bis-hydroxymethyl-propane (0.10 mol) and 39 g o
tributylamine ~0.21 mol~ in 80 ml of D~F. The
reaction mixture is kept under stirring for some
hours at 10-20C. Hence it is poured under heavy
stirring into 5.5 1 of ethyl acetate from which
the final product crystallizes. After filtration
and drying, 140 g of raw 1,3-bis [3-acetoxyacetyl-
amino-5-(1,3-dihydroxy-isopropyl)aminocarbonyl~
2,4,6-triiodo-benzoyl-amino]-2,2-bis
hydroxymethyl-propane are obtained.
A sample crystallized from methyl alcohol has the
following characteristics:
m.p.: 284-287C ;~
Elemental Analysis (%)
C H
Calculated: 26.91 2.58 48.74 5O37
Found: 26.82 2.70 48.45 5O37
b) 105 g of raw 1,3-bis-[3-acetoxyacetylamino-5~ 3-
dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2,2-bis-hydroxymethyl-propane are
diluted in 1. 9 1 of water and 0.8 1 of methyl
alcohol tCH3OH) and slowly mixed with 170 ml of ~:
~aOH lN until a constant pH value of 10.3 is
o~tained. The mixture is kept under stirring for
about 10 h at 20C. Then, the methyl alcohol is
evaporated under vacuum. The resulting aqueous
solution is made free from salts by passage
through ion exchange resins (160 ml of AmberliteR
IR 120 and 150 ml of DuoliteR A 30B). The eluate
is evaporated to dryness under vacuum, diluted
again in 1.2 1 of water, bleached on active carbon
. .: . , . .: , . ., " , - . .:: .
:. , , . :: .. . . . : ::. ... :
::. -:::: :~: .: ; : :. :
, " ~ . , ,., ', , . , . ' : ' , :
W092/08691 PCr/EP91/02151
19 2$~3~
and percolated on 1200 ml of AmberliteR XAD-2. The
fractions containing the product are gathered and
dried. 66 g of 1,3-bls-[3 hydroxyacetylamino-5
(1,3-dihydroxy-isopropyl~aminocarbonyl-2,4,6-tri- !
iodo-benzoyl-amino]-2,2-bis-hydroxymethyl-propane
are obtained.
The last impurities are eliminated by dissolving
the product in aqueous ethyl alcohol 80% and filtering
the turbid solution.
Yield: 62% m.p.: 285C
~lemental Analysis ~%)
C H I N
Calculated: 25.19 2.45 51.51 5.68
Found: 25.26 2.60 51.36 5.57
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/CH30H/NH40H 25% = 3/2/1 v/v/v. Rf = 0.230
HPLC Rt - 9.6 min. Titre = 99% ~on the area)
Chromatographic conditions:
Column: as reported in Example 1
Eluent: A) KH2PO4 0.01 M B) H20 75%, CH3CN 25%
Gradient (flow 1 ml/min; Detector UV 254 nm):
time (min) = 0 - 5; 5 - 15; 15 - 25
%B = 20; 20 - 80; 80
In the same way the following compounds are obtained:
- 1,3-bis-[3-hydroxyacetylamino-5-(2,3-dihydroxypro~
pyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-
2,2-bis-hydroxymethyl-propane.
- 1,3-bis-[3-hydroxyacetylamino-5-(1,3,4-trihydroxy-
2-butyl)aminocarbonyl-2,4,6-triiodo-benzoylamino]-
2,2-bis-hydroxymethyl-propane.
W092/OB691 PCT/EPgl/02~51
~ 20
EXAMPLE 3
1,3-bis-l3-(L-2-hydroxy-propionyl)amino-5-(2,3-di
hydroxy-propyl)aminocarbonyl-2,4,6-triiodo-benzoyl~ .
amino]-2-hydroxy-propane.
197 g of 3-(L-2-acetoxy-propionyl)amino-5~(2~3-
dihydroxy-propyl)aminocarbonyl-2,4,6-triiodo-benzoyl~
chloride (0.25 mol), described in DE 2805928/Example 6,
are reacted in 500 ml of DMF and in the presence o
55.6 g of tributyl.amine (0.30 mol) with 11.3 g of 1,3-
diamino-2-hydroxy-propane ~0.125 mol) according to the
procedure described in the Example 2a). The condensa-
tion product is submitted to hydrolysis, as described
in the Example 2b). Similarly, the isolation and the
purification of the final product are performed.
94.2 g of 1,3-bis-~3-tL-2-hydroxy-propionyl)amino-5~
(2,3-dihydroxy-propyl)aminocarbonyl-2,4,6-triiodo-ben~
zoyl-amino]-2-hydroxy-propane are obtained.
Yield: 50% m.p.: 278-.282C
Elemental Analysis (%)
C H I N
Calculated: 25.47 2.48 52.09 5~75
Found: 25.21 2.53 51.89 5059
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/CH30H/NH40H 25% = 6/3/1 v/v/v. Rf = 0~24,
HPLC: Rt = 15.4 min. Titre = 99.4% (on the area~
Chromatographic conditionso
Column: LICHROSPHERRRP18 (Merck), 5 ~ (250 x 4 mm)
Eluent: A) H3P04 0.1 M B) H20 50%, CH3CN 50 ~
Gradient (flow 1 ml/min; Detector UV 254 nm):
time (min) = O - 5;5 - 20; 20 - 30; 30 - 35
hB = 10;10 - 28; 28 - 70; 70
,.. . .
,; ! `,'. . " ', ; ~ , : , ,,
" ' ` ' `"' '' i ' ''` "' " ' ' : ' ` .
WO92/OB69l PCT/EI91/02i5~ ¦
~ !
21 2Q3~
EXAMPL~ 4
1,3-bis [3-(L-2-hydroxy-propionyl)amino-5-(1,3,4-tri~ 3
hydro~y-2-butyl)zminocarbonyl-2,4,6-triiodo-benzoyl- ¦
amino]-2-hydroxy-propane~
24 g of 3-(L-2-acetoxy-propionyl)amino-5-(1,3~4-
trihydroxy-2-butyl)aminocarbonyl-2,4,6-triiodo-benzoyl~
chloride (0.03 mol), obtained by known methods descri--
bed in DE 2805928, in 30 ml of DMF and in presence of
3.8 g of triethylamine (O.037 mol1 are reacted with ,a
solution of 1.45 g of 1,3-diamino-2-hydroxy-propane
(0.016 mol) in 30 ml of DMF at 5 to 8C, according to
the procedure described in Example 3.
7.2 g of the title compound are obtained.
~ield: 30% m.p.: 250-256C
Elemental Analysis (~)
C ~ I N
Calculated: 26.05 2.65 50.02 SO52
Found: 26~04 2.95 49.15 5~33
[~]20 = _5.01 (c = 10.13% H2O)
Solubility in H20 at 20C: 100% w/v
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13¦CH3OH/NH4OH 25% = 5/4/1 v/v/v- Rf = 00240
lH-and 13C-NMR spectra are in accordance with the
proposed structure~
EXAMoeLE 5
1,3-bis-[3-(h-2-hydroxy-propionyl)amino-5-[N-methyl-N-
(D-l-deoxy-glucitol)]aminocarbonyl-2,4,6-triiodo-ben-
zoyl-amino]-2-hydroxy-propane.
20.6 g of 3-(L-2-acetoxy-propionyl~amino-5-[N-me-
thyl-N-(D-l-deoxy-glucitol)]aminocarbonyl-2,4,6-tri-
iodo-benzoyl-chloride (0.024 mol), obtained by known
-~ , ~.,: -
. : . . , . ~.. .: : . :. ~
W092iO8691 PCT/EP9~dO~5~ ~
~"
2~ 22
methods described in DE 2805928, in 100 ml of DMF are
reacted with a solution of 1.124 g of 1,3-diamino-2-hy~
droxy-propane (O.012 mol) and 2.53 g of triethylamine
~0~025 mol) in 30 ml of DMF, according to the procedure
described in Example 3.
9.26 g of the title compound are obtained.
Yield: 47% m.p.: 266C (decO)
El2mental Analysis (%)
C H
Calculated: 28.04 3.14 45.58 5003
Found: 28.19 3.29 45.48 5.03
~] 436 = -19 (c = 7.04% H20)
13C-NMR spectrum is in accordance with the proposed
structure.
In the same way the following compounds are obtainedo
- 1,3-bis-[3-tL-2-hydroxy-propionyl)amino-5- E N-me-
~hyl-N-(1,3-dihydroxy-isopropyl)3aminocarbonyl
2,4,6-triiodo-benzoyl-amino]-2-hydroxy-propaneO
- 1,3-bis-[3~(L-2-hydroxy-propionyl)amino-5-[N-me~
thyl-~-(2,3-dihydroxy-propyl)]aminocarbonyl-2,4,6-
triiodo-benzoyl-amino]-2-hydroxy-propane.
~XAMPLE 6
1,3-bis-[N-methyl-N-[3-(L-2-hydroxy-propionyl)amino-5-
(2-hydroxy-ethyl)aminocarbonyl-2,4,6-triiodo-benzoyl]-
amino]-2-hydroxy-propane.
20.85 g of 3-(L-2-acetoxy-propionyl)amino-5-(2-hy- ;;~
droxy~ethyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chlori-
de (0.025 mol), obtained by known methods described in
DE 2805928, in 80 ml of DMF are reacted with a solution
of 1.47 g of 1,3-bis-(methylamino1-2-hydroxy-propane
(0~012 mol) and 2.63 g of triethylamine (0.026 mol~ in
W092iO8691 PCTtEP91/02~5D
.._
23 2 ~
30 ml of DMF at 5C to room temperature, according to
~he procedure described in Example 3.
9.6 g of the title compound are obtained.
Yield: 56% m.p.: 285C (decO)
5 Elemental Analysis (%)
C H
Calculated: 26.03 2.54 53.24 5088
Found: 25.86 2.43 52.81 5~78
~X]20 _3.85 (c = 9.95% H20)
lH-and 13C-NMR spectra are in accordance with the
proposed structure.
EXAMPLE 7
1,3-bis-[N-methyl-N-[3-(L-2-hydroxy-propionyl)amino-5-
(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl]amino]-2-hydroxy-propane.
21.4 g of 3-(L-2-acetoxy-propionyl)amino-5-(103~-
dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-ben-
zoylchloride (0.025 mol), obtained by known methods
described in DE 2805928, in 85 ml of DMF are reacted
with a solution of 1.~7 g of 1,3 bis-(methylamino)-2-
hydroxy-propane (00012 mol) and 2.63 g of triethylamine
(0.026 mol~ in 30 ml of DMF at 5C to room temperature,
according to the procedure described in Example 3.
11.1 g of ~he title compound are o~tained.
Yield: 62% m.p.: 295C (dec
El~mental Analysis (%)
C H I N
Calculated: 26.60 2.70 51 09 5.64
Found: 26.31 2.95 50.68 5.48
~] 436 = -3.540 (c = 10-44% H20)
Solubility in H20 at 22DC: > 100% w/v
,, ,, . : . .
WO 92/08691 PCI/EP91/02~5~
2 a ~ 24
'.
lH and 13C-NMR spectra are in accordance with the
proposed structure.
EXAMPLE 8
l,3-bis-[N-methyl-N-[3-(L-2-hydroxy-propionyl)amino~5
5 (2,3-dihydroxy-propyl)aminocarbonyl-2,4,6-triiodoben-
zoyl]aminoJ-2-hydroxy-propane.
Title compound is obtained by reacting the desired
reagents in the same quantities disclosed in Example 7
and following the same procedure. 7.9 g of final com-
pound are obtained.
Yield: 44% m.p.: 280~C (dec.)
Elemental Analysis (%)
C H I N
Calc.: 26.60 2.70 51.09 5.64
Found: 26.32 2.81 50.24 5.44 (H2O=1OS4~
[o~J2~36 = -3.2 (c= 9-8% H2O~ ;
Solubility in H20 at 20C: >100% w/v
13C-NMR spectrum is in accordance with the proposed
structure.
~ XA~E?LE
1,3-bis-[N-methyl-N-[3-(L-2-hydroxy-propionyl)amino-5-
(1,3,4-trihydroxy-2-butyl)aminocarbonyl-2,4,6-triiodo- ~`?
benzoyl]amino]-2-hydroxy-propane.
19.9 g of 3-(L-2-acetoxy-propionyl)amino-5-(1,3,4-
trihydroxy-2-butyl)aminocarbonyl-2,4,6-triiodo-be~zoyl~
chloride (0.025 mol), obtained by known methods dPscri-
bed in DE 2805928, in 30 ml of DMF are mixed with 3.8 g
of triethylamine (0.037 mol), then are reacted with a
solution of 1.5 g of 1,3-bis-(methylarnino)-2-hydroxy-
propane (O.013 mol) in 25 ml of DMF at 5 C to room
temperature, according to the procedure described in
- , . . , . ~ .:
, . : . ~ , -. . ., ' . ,
, , , , ... : :
WO92/08691 PCT/EP9l/02151
. .
, . .. .
2 0 9 ~
Example 3.
5.68 g of the title compound are obtained.
Yield: 29.5~ m.pO 256C
Elemental Analysis (%)
C H I N
Calculated: 27.12 2.86 49~12 50~2
Found: 27.46 3.15 48.55 So24
[~]20 _3.16 (c = 9.93% H2O)
lH-and 13C-NMR spectra are in accordance with the
proposed structure.
EXAMPLE 10
1-[3-(L-2-hydroxy-propionyl)amino-5-(1,3-dihydroxyiso
propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino] 3-[3-
hydroxyacetylamino-5-(2,3-dihydroxy-propyl)aminocarbo~
15 nyl-2,4,6-triiodo-benzoyl-amino]-2-hydroxy-propane.
a) 125 g of 3-(L-2~acetoxy-propionyl)amino-5-(1,3~di~
hydroxy-isopropyl)aminocarbonyl-2,4,6-triiodoben~-
zoyl-chloride (0.163 mol), diluted in 150 ml of
DM~, are dropwise added, while keeping ~he
temperature between 0 and 10C, to a solution of
45.6 g of 1-trifluoroacetylamino-2-hydroxy-3-ami-
nopropane trifluoroacetate (0.152 mol) and 3508 g
of triethylamine (0.35 mol) in 280 ml of DMF. The
resulting mixture is stirred at 10C for 8 hG
~fter filtration, the solution is evaporated to
dryness, then is diluted with 3 1 of methylene
chloride (CH2C12). The condensation product preci-
pitates, then, after filtration, the protective
groups are removed by hydrolysis, according to the
procedure described in Example 2b). The resulting
product is purified by means of fixation on a
,, ,,;. :-, - ., ;; , - :.
` l ~
WO92/08691 PCT/EWl/0~15~ I
~' ''.
9 ~ 26
strongly acidic resin ~950 ml of AmberliteR XR
120) and subsequent elution with about 6 1 o~
NH40H 2M. After evaporation of the solvent, 76~5 g
of l-[3-(L-2-hydroxy-propionyl)amillO- 5- ( 1, 3-dihy~-
droxyisopropyl)aminocarbonyl-2,4,6-t:riiodo-ben-
zoylamino]-2-hydroxy-3-amino-propane are obtained
with a yield of 65%.
Acidimetric titre = 99.4~ m.p.: 211C
b) 73.5 g of 3-acetoxyacetylamino-5-(2,3-dihydroxy-
propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chlori-
de (0.098 mol) in 500 ml of DMF are slowly added
dropwise, at a temperature between 5 and 10C, to
a solution of 76 g of the product obtained at
point a) (0.098 mol) and 11.1 g of triethylamine
(0.11 mol) in 500 ml of DMF. The mixture is kep~
under stirring for 20 hl then filtered and driedO
The residue is dissolved in 1.9 1 of water and 0~8
1 of methyl alcohol, then it is submitted to
hydrolysis and purification according to the
procedure described in Example 2b). 92 g of 1-[3-
(L-2-hydroxy-propionyl)amino-5-(1,3-dihydroxy-iso-
propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-
3-~3-hydroxyacetylamino-5-(2,3-dihydroxypropyl)~
aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-2-hy-
droxypropane are obtainedO
Yield: 64.8% m.p.: 300C
Elemental Analysis (%)
C H I N
Calculated: 24.88 2.37 52.58 5.80
Found: 24.70 2.29 52.15 5.74
[~]2~36 = -2.9 (c = 10% ~I~O)
.... .
r
,',: . ; ; ` ' : `'
WO92/08691 PCT/EP9lJ021~D
!,'"`~
27 2~
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/CH30H/NH40H 25% = 6/3/1 v/v/v- Rf = 00180
HPLC: Rt - 13.6 min. Titre = 97.5% (on the a~ea)
Chromatographic conditions:
Column: as reported in Example 1
Eluents: A) K2P04 0.01 M B) KH2P04 0.01M 50%, CH3CN 50
Gradient (flow 1 ml/min; Detector UV 254 nm):
time (min) = 0 - 6; 6 - 25; 25 - 30
%B = 2; 2 - 80; 80
EXAMPLE 11
1-[3-hydroxyacetylamino-5-(1,3,4-trihydroxy-2-butyl)-
aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-3-[3-(L-2-
hydroxy-propionyl)amino-5-(1,3-dihydroxy-isopropyl)ami-
nocarbonyl-2,4,6-triiodo-ben2Oyl-amino]-2-hydroxypropa-
ne.
Title compound is obtained following the procedure
described in Example 10.
So 8.58 g of 3-acetoxyacetylamino-5-tl,3,4-trihy-
droxy-2-butyl)aminocarbonyl-2,4,6-triiodo-benzoyl~chlo~
ride (0.011 mol) in 60 ml of DMF are reacted with a so-
lution of 8.54 g 1-[3-(L-2-hydroxy-propionyl)amino~5-
(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
ben~oyl-amino]-2-hydroxy-3-amino-propane (0.011 mol),
obtained according to Example 10 a)~ and 2.22 g of
triethylamine (0.022 mol) in 50 ml of DMF to give the
de~ired compound.
8.75 g of the title compound are obtained.
Yield: 51% m.p.: 283~C (dec.)
~lemental Analysis (%)
C H I N
CalcO: 25.19 2.45 51.51 5.68
.. : . . :: . .
, ~ , :: .. . .: : , . :
. ~, : :, .,: ... , , . :. ..
.~ ~ .. .
, .: : : ~
WO92/08691 PCT/EP91/02151
r~
2~ 3~ 28
Found: 24.13 2.72 48.88 5.32 (H2O = 2071%)
[~]2436 = -2.6 (c = 10.44% H2O)
Solubility in H20 at 25DC: >100% w/v
13C-NMR spectrum is in accordance with the proposed
structure.
EXAMPLE 12
1-[3-(L-2-hydroxy-propionyl)amino-5-(1,3-dihydroxyiso-
propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-3-[3-
(L-2-hydroxy-propionyl)amino-5-(2,3-dihydroxypropyl)-
aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-2-hydroxy-
propane.
Following the procedure described in Example 10,
8.75 g of 3-(L-2-acetoxy-propion~l)amino-5-(2,3-dihy-
droxy-propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chlo-
ride (0.01 mol) in 60 ml of D~F are reacted with a so~
lution of 7.76 g 1-[3-(L-2-hydroxy-propionyl)amino-5-
(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2-hydro~y-3-amino-propane (0.01 mol),
obtained according to Example 10 a)j and 2.02 g of
triethylamine (0.02 mol) in 60 ml of DMF to give the
desired compound. 8.15 g of the title compound are ob~-
taine~.
Yield: 55% m.p.: 287C (dec.)
Elemental Analysis (%)
C H I N
Calculated: 25.46 2.48 52.08 5O75
Found: 25.49 2.34 51.67 5.73
[&~ 436 = -5.67 (c = 10.01% H20)
Solubility in H20 at 20C: >100% w/v
3C-NMR spectrum is in accordance with the proposed
struc~ure.
.
. . , -- . . . ~:
, :, , : .. , . . :. .,
: .. : , :
WO 92/08691 PCI`/EP9~1/02151
. --. .
! ;
29 2~
MPLE 13
1-[3-(L-2-hydroxy-propionyl)amino-5-(1,3-dihydroxyiso~
propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-3~3-
(L-2-hydroxy-propionyl)amino-5-[N-methyl-N-(D-l-deoxy~
S glucitol)]aminocarbonyl~2,4,6-triiodo-benzoyl-amino~-2
hydroxy-propane.
Following the procedure described in Example 10,
6.9 g of 3-(L-2-acetoxy-propionyl)-5-[N-methyl-N-(D 1-
deoxy-glucitol)]aminocarbonyl-2,4,6-triiodo-benzoyl-
chloride (0.008 mol) in 50 ml of DMF are reacted with a
solution of 6.13 g 1-[(L-2-hydroxy-propionyl)-5-(1,3-
dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-ben-
zoyl-æmino]-2-hydroxy-3-amino-propane (0.008 mol), ob-
tained according to E:xample 10 a), and 1.6 g of
15 triethylamine (0.016 mol) in 50 ml of DMF to give the
desired compound.
4.2 g of the title compound are obtained.
Yield: 34% m.p.: 285C (deeO 3
Elemental Analysis (%)
C H I N
Calculated: 26O84 2.83 48.62 5O37
Found: 27.29 2.92 48.62 5022
[o~]20 = -11.9 (c =5.0% H20)
Solubility in H20 at 20C: >100,~ w/v
25 13C-NMR spectrum is in accordance with the proposed
structure.
EXAMPLE 14
1,3-bis-~3-hydroxyacetylamino-5-(1,3-dihydroxy-isopro-
pyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-2-
30 hydroxy-propane.
llZ.5 g of 3-acetoxyacetylar~ino-5-(1,3-dihydroxy-
;.: . ,: . , , ;:
: . . . :
~,: . : :
W092~08691 PCT/EPg1/0~5~
~ ,. ~.,,
2 0 ~ a ~ 3 ~ 30
isopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chloride
(0.15 mol), described in DE 2805928/Example 10, in 400
ml of DMF are reacted with 16.7 g of triethylamine
(0.165 mol) and with 7.13 g of 1,3-diamino-2-hydroxy~
propane (0.075 mol), according to the procedure descri-
bed in Exam?le 2.
63 g of 1,3-bis-~3~hydroxyacetylamino-5-(1,3-dihy-
droxy-isopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-
amino]-2-hydroxy propane are obtained.
Yield: 56% m.p.: > 280C ;~
Elemental Analysis (%)
C H I N
Calculated: 24.49 2.25 53.10 5086
Found: 24.19 2.28 53.26 5083
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/C~30H/NH40H 25% = 3/2/1 v/v. Rf = 0060o
HPLC: Rt - 7.1 min. Titre = 99% (on the area)
Chromatographic conditions: as reported in Example 20
EXAMPLE 15
1,3-bis-[3-hydroxyacetylamino-5-(1,3,4-trihydroxy-2-bu-
tyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-2-hydro-
xy-propane.
23.4 g of 3-acetoxyacetylamino-5-(1,3,4-trihydro-
xy-2-butyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chloride
(0.03 mol), obtained by known methods described in DE
2805928, in 25 ml of DMF are re~cted with 3.8 g of
triethylamine (0.037 mol) and with 1.45 g of 1,3-dia-
mino-2-hydroxy-propane (0.016 mol~, according to the
procedure described in Example 2.
4.7 g of the title compound are obtained.
Yield: 21% m.p~: 275C
, . , . : , ~
WO92/08691 PCT/EP91/02~51
r, . .
t ~
31 2~ 3~
Elemental Analysis (%)
C H ]:
Calculated:. 24.92 2.43 50~96 5O62
Found: 24.g3 2.50 49~,98 5~53
Solubility in H20 at 20C: 100% w/v
In the same way the following compounds are obtainedn
- 1,3-bis-[3-hydroxyacetylamino-5-(2,3-dihydroxypro--
pyl)aminocarbonyl-2,4,6-triiodo-benzoyl-amino~2
hydroxy-propane.
- 1,3-bis-[3-hydroxyacetylamino-5-~N-methyl-N~ 3-
dihydroxy-isopropyl)]aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2-hydroxy-propane.
- 1,3-bis-[3-hydroxyacetylamino-5-[N-methyl-N-(2,3- .`
dihydroxy-propyl)~aminocarbonyl-2~4~6-triiodo-ben-
zoyl-amino]-2-hydroxy-propane.
EXAMPLE 16
1,3-bis-~N-methyl-N-[3-hydroxyacetylamino-5-(1,3j4~tr.i~
hydroxy-2-butyl)aminocarbonyl-2,4,6-triiodo-benzoyl~
amino]-2~hydroxy-propane.
23.4 g of 3-acetoxyacetylamino-5-(1,3,4-trihydro-
xy-2-butyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chloride
(0.03 mol), in 25 ml of DMF are reacted with 3.8 g o
triethyl~mine (0.037 mol) and with l.9 g of 1,3-bis-
(methylamino)-2-hydroxy-propane (0.016 mol), according
to the procedure described in Example 2
7 g of the title compound are obtained.
Yield: 30% mOp. 263C
Elemental Analysis (%)
C H I N
Calculated: 26.042.65 50.02 S~52
Found: 26~1~ 2.69 50.86 S.57
~ . ., .', ; ' ~ " :
WO92/08691 PCT/EP91/02l51
29~3~ 32
Solubility in H20 at 20C: 100% w/v
EXAMPL~ 17
1,3-bis-[N-methyl-N-[3-hydroxyacetylamino-5-[N-methyl-
N-(D-l-deoxy-glucitol)]aminocarbonyl-2,4,6~triiodo~
benzoyl]amino]-2-hydroxy-propane.
24 g of 3-acetoxyacetylamino-5-[N-methyl-N~(D~
deoxy-glucitol)]aminocarbonyl-2,4,6-triiodo-benzoyl-
chloride (0.028 mol), obtained by known methods descri
bed in DE 2805928, in 100 ml of DMF are reacted with a
10 solution of 1.65 g of 1,3-bis-(methylamino)-2-hydroxy-
propane (0.014 mol) and 2.93 g of triethylamine (0~029
mol) in 30 ml of DMF, according to the procedure de-
scribed in Example 2.
10.14 g of the title compound are ob~ained.
15 Yield: 41~ m.p.: 276~C (decO)
Elemental Analysis (~)
C H
Calculated: 28.04 3.14 45.58 5O03
Found: 27.95 3.21 45.48 4O97
20 ~20 = -12.6 (c = 10.6% H2O)
Solubility in H20 at 25~C: >100~ w/v
13C-NMR spectxum is in accordance with the propo~ed
structure.
In the same way the following compounds are obtained
25 _ 1,3-bis-~N-methyl-N-[3-hydroxyacetylamino-5~ 3
dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo~
benzoyl~amino]-2-hydroxypropane.
- 1,3-bis-~N-methyl-N-[3-hydroxyacetylamino-5-(2,3-
dihydroxy-propyl)aminocarbonyl-2,4,6-triiodo-ben-
zoyl]amino]-2-hydroxy-propane.
'' ~ :
WO92/08691 PCT/~P9~0~2~
33 209~3~
~XAMPLE 18
1,3-bis-[N-methyl-N-[3-hydroxyaCetylamino-5-[N-methyl~
N-(1,3-dihydroxy-isopropyl)]aminocarbonyl-2,4,6-triio~
do-benzoyl]amino]-2-hydroxy-propane.
21.31 g of 3-acetoxyacetylamino-5-[N-methyl-N~
(1,3-dihydroxy-isopropyl)]aminocarbonyl-2,4,6-triiodo~
benzoyl-chloride (0.025 mol), obtained by known methods
described in DE 2805928, in 95 ml o~ DMF are reacted
with a solution of 1.47 g of 1,3-bis-(methylamino)-2~
hydroxy-propane (0.12 mol) and 2.63 g of triethylamine
(0.026 mol) in 30 ml of DMF, according to the procedure
described in Example 2.
8.1 g of the title compound are obtained.
Yield: ~5% m.pO: 290C
Elemental ~nalysis (%)
C H I N
Calc.: 26~98 2.86 50.33 5.55
Found: 26.29 3.01 50.21 5.24(H2O = 1~02~3
Solubility in H20 at 25C: 100% w/v
13C-NMR spectrum is in accordance with the proposed
structure.
EXAMPLE 19
1,3-bis-[3-(N-methyl-N-hydroxyacetyl)amino-5-(1,3-dihy-
droxy-isopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-
amino]-2-hydroxy-propane.
a) 46 g of 3-(N-methyl-N-acetoxyacetyl)amino-2,4,6
triiodo-isophthaloyl-dichloride (0.065 mol), de
scribed in EP 26281/Example 11, in 250 ml of
anhydrous tetrahydro'uran (THF) are added dropwise
to 12.6 g of 1,3-dihydroxy-isopropylamine (0.138
mol ) in 100 ml of THF. The mixture is kept for 3 h
, , ,. , ~ .... . . . . , , ~ .
, . . . ,,, ~ ,
WO9~/0869] PCT/EP9t/0215D
x~ , :
~;,,
~ Q ~ 34
at room temperature under stirring. Then it is
filtered and evaporated to dryness under vac-uumO
The residue is constituted by 50,4 g of 3-(N~
methyl-N-acetoxyacetyl)amino-5-(1,3-dihydroxy-iso-
propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chlori~
de.
b) 50 g of 3-(N-methyl-N-acetoxyacetyl)amino-5-(1,3-
dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo~
benzoyl-chloride (about 0.065 mol), obtained
accordi~g to a), diluted in 200 ml of DMF, are
reacted with 3 g of 1,3-diamino-2-hydroxy-propane
(0.034 mol) in 100 ml of DMF, according to the
procedure described in Example 1.
21 g of 1,3-bis-[3-(N-methyl-N-hydroxyacetyl)ami~
no-5-(1,3 dihydroxy-isopropyl)aminocarbonyl-2,4,6-tri-
iodo-benzoyl-amino]-2-hydroxy-propane are obtainedO
Yield: 44.2% m.p.~ ~ 250~C
Elemental Analysis ~%)
C H
Calculated: 25.47 2.48 52.08 5u75
Found: 25.82 2.60 52.37 5o62
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/CH30H/NH40H 25% = 6/3/1 v/v/v. Rf = 0.23
HPLC: Rt = 14.6 min. Titre = 96.7% (on the area)
Chromatographic conditions: as reported in Example 2O
c) This product may be also obtain d by N-methylation
of the compound already described in Example 14.
14.3 g of 1,3-bis-[3-hydroxyacetylamino-5-(1,3-di-
hydroxy-isopropyl)aminocarbonyl;~2,4,6-triiodo-ben-
~oyl-amino]-2-hydroxy-propane (0.01 mol) in 500 ml
of anhydrous DMF 2re mixed under stirring with a
: .
.. . ~ .'': .
W092/0869t PCTtEP91/0215~
2 0 ~ ~ A ~ O
solution of 1.08 g of sodium methylate (0.02 mol)
in 30 ml of methyl alcohol. After 3 h, methyl
alcohol is removed by distillation. An excess o
methyl iodide is added and the solution is stirred
for one day at room temperature. Then the reaction
mixture is poured under stirring in 600 ml of
ethyl acetate and the raw product precipitatesO
This one is filtered, diluted in water and made
free from salts by passage through ion exchange
resins (Amberlite IR 120 and IRA 400). The eluate
is tréa,ed with active carbon and evaporated to
dryness.
7 g of 1,3-bis-[3-(N-methyl-N-hydroxyacetyl)amino-
5-(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2-hydroxy-propane are obtained with a
yield of 47.9%. This compound is identical to the one
obtained through ~rocess b).
In the same way the following compound is obtai~
ned:
20 - 1,3-bis-[3-~N-methyl-N-(L-2-hydroxy-propionyl)]-
amino-5-(1,3,4-trihydroxy-2-butyl)aminocarbonyl-
2,4,6-triiodo-benzoyl-amino]-2-hydroxy-propane.
EXAMPLE 20
1,3-bis-[3-[N-methyl-N-(L-2-hydroxy-propionyl)]amino-5-
(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2-hydroxy-propane.
80.4 g of 1,3-bis-[3-(L-2-hydroxy-propionyl)amino-
5-(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2-hydroxy-propane ~0.055 mol), described
in Example 1, are dissolved in 900 ml of DMF and mixed
under stirring with 132 ml of sodium methylate 1 M in
WO92/08691 PCT/EP91/02151
209~ 3'o
methyl alcohol (0.132 mol). Methyl alcohol is removed
by distillation. Hence, 23.7 g of dimethylsulphate
(0.188 mol)iare dropwise added at a temperature of 5Co
Stirring is maintained for about 1 h, then the solvent
is removed by vacuum distillation. The raw material is
diluted with ethyl acetate and forms a solid powder~
which is filtered, diluted in water and made free from
salts bv percolation on ion exchange resins (AmberliteR
IR 120 and Duolite~ A 30B). ~rhe solution is evaporated
to dryness and then dissolved in boiling ethyl alcoholO
After some days, 62 g cf 1,3-bis-[3-[N-methyl-N-(L-2-
hydroxy-propionyl)]amino-5-(1,3-dihydroxy-isopropyl)-
aminocarbonyl-2,4,6-triiodo-benzoyl-amino]-2-hydroxy-
propane crystallize.
Yield: 75.6% m.p.: > 300C (dec
Elemental Analysis (%)
C H I N
Calculated: 26.60 2.70 51.10 5o64
Found: 26.58 2.75 50.95 5.60
] 436 = +29-7 (c = 10% H20)
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/CH30H/NH40H 25% = 6/3/1 v/v/v. Rf = 0.16.
~XaMPLE 21
1,3-bis-[3-[N-methyl-N-(L-2-hydroxy-propionyl)]amino-5-
(2,3-dihydroxy-propyl)aminocarbonyl-2,4,6-triiodoben-
zoyl-amino]-2-hydroxy-propane.
In 250 ml of DMF, 102 g of the product described
in Example 3 (0.07 mol) are reacted with 150 ml of so-
dium methylate lM in methyl alcohol (0~15 mol) and with
30 g of metansulphonate methyl ester (0.27 mol), accor-
ding to the procedure described in Example 20.
.:. ,, ~, ., " : , .: ,:. :
.: . :, , , , , . , : ,,.
W092/08691 PCT/EP91/0215~
, . .
I;, ,, '
37 2~
42~6 g of l,3-bis-[3-[N-methyl-N-(L-2-hydroxy-pro-
pionyl)]amino-5-(2,3-dihydroxy-propyl)aminocarbonyl-
2,4,6-triiodo-benzoyl-amino]-2-hydroxy-propane are ob~
tained.
Yield: 41% m.p.: 284C
Elemental Analysis (%)
C H I N
Calculated: 26.50 2.70 5l.lO 5064
Found: 26.24 2.80 50.88 5.47
EXAMPL~ 22 ~
1,3-bis-[3-tN-methyl-N-hydroxyacetyl)amino-5-(2 ,3-dihy ~ ~ .
droxy-propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-ami-
no]-2-hydroxy-propane.
23 g of 3-(N-methyl-N-acetoxyacetyl)amino-2,4,6-
triiodo-isophthaloyl-dichloride (0.032 mol), described
in EP 2628l!Example ll, are reacted with 5.8 g of 2,3-
dihydroxy-propylamine (0.064 mol) in THF according to
the procedure descxibed in Example l9a). The resulting
product is 3-(N-methyl-N-acetoxyacetyl)amino-5-(2,3-di~
hydroxy-propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-
chloride, which is reacted in DMF with 1.5 g of l,3-
diamino-2-hydroxy-propane tO.016 mol). The resulting
product is isolated and purified according to the pro-
cedure of Example l. ll g of 1,3-bis-[3-(N-methyl-N-hy-
droxyacetyl)amino-5-(2,3-dihydroxy-propyl)aminocarbo-
nyl-2,4,6-triiodo-benzoyl-amino]-2-hydroxy-propane are
obtained.
Yield: 47% m.p.: 295C (dec.)
Elemental Analysis (%)
C H I N
Calculated: 25.47 2.~8 52.08 5 75
W092/08691 PCT/EP91/02151
~o~3$ 38 ;
Found: 25.97 2.50 51 71 5077
In the same wa~ the following compound is obtained~
- 1,3-bis-[3-(N-methyl-N-hydroxyacetyl)amino-5-
(1,3,4-trihydroxy-2-butyl)]a~inocarbonyl-2,4,6
triiodo-benzoyl-amino]-2-hydroxy-propane~
EXAMPL~ 23
1,3-bis-[3-(L-2-hydroxy-propionyl)amino-5-(1,3-dihydro-
xyisopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-aminoJ
2,2-bis-hydroxymethyl-propane.
Following the procedure described in Example 2,
the 3-(L-2-acetoxy-propionyl)amino-5-(1,3-dihydroxy-
isopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-chloride
is reacted in DMF with 1,3-diamino-2,2-bis-hydroxyme
thyl-propane to give the desired product.
Yield: 65% m.p.: 285DC (dec
Elemental Analysis (%)
C H I N
Calculated: 26.32 2.68 50.55 5058
Found: 26.21 2.72 50.31 5053
TLC: (Silica Gel 60 F2S4, Merck)
Eluent: CHC13/CH30H/NH40~ 25% = 6/3/1 v/v/v. Rf = Onl2
HPLC: Rt = 17.7 min. Titre = 98% (on the area)
Chromatographic conditions: as reported in Example 3.
EXA~P~E 24
1,3-bis-[3-(L-2-hydroxy-propionyl)amino-5-~N-methyl-N~
(D-l-deoxy-glucitol)~aminocarbonyl-2,4,6-triiodo-ben-
zoyl-amino]-2,2-bis-hydroxymethyl-propane.
Following the procedure described in ~xampl~ 2, 10
g of 3-(L-2-acetoxy-propionyl)amino-5-[N-methyl-N-(D-l-
deoxy-glucitol)]aminocarbonyl-2,4,6-triiodo-benzoyl-
chloride (0.011 mol) in 50 ml of D~5F are reacted wi~h
.. . . , . .. :
WO92/08691 PCT/EP91~02~5D
~' .'
39 ~ 3~
1.19 g of 1,3-diamino-2,2-bis-hydroxymethyl-propane
(0.006 mol) and 4.94 g of tributylamine (0.027 mol~ in
20 ml o DMF.
11.5 g of the title compound are obtained,.
Yield: 58%
Elemental Analysis (%)
C H I N
Calculated: 28.72 3.29 44.41 4 90
Found: 28.60 3.33 44.37 4085
13C-NMR spectrum is in accordance wlth the proposed
structure.
In the same way the following compounds are obtainedo
- 1,3-bis-[3-(L-2-hydroxy-propionyl)amino-5-(2,3~dl-
hydroxy-propyl)aminocarbonyl-2,4,6-triiodo-benzo~
yl-amino]-2,2-bis-hydroxymethyl-propane.
- 1,3-bis[3-(L-2-hydroxy-propionyl1amino-5-(1,3,4
trihydroxy-2-butyl)aminocarbonyl-2,4,6-triiodo~
benzoyl-amino]-2,2-~is-hydroxymethyl-propaneO
EXAMPLE 25
1,3-bis-[3-[N-methyl-N-(L-2-hydroxy-propionyl)Jamino 5-
(1,3-dihydroxy-isopropyl)aminocarbonyl-2,4,6-triiodo-
benzoyl-amino]-2,2-bis-hydroxymethyl-propane.
The product described in Example 23 is submitted
to N-methylation with sodium methylate and CH3I foI
lowing the procedure of Example l9c).
Yield: 65.5~ m.p.: > 300C
Elemental Analysis (%)
C H I N
Calculated: 27.40 2.89 49.63 5.48
Found: 27.38 2.91 49.47 5.45
436 +26.7 (c - 10% H2O)
", , "" " , , ,, ~ ~ " : ~,, ", ,.,, , " ",
W092/08691 PCT/EP9~/02~5~ ~
3~ 40
In the same way the following compounds are o~tainedD
- 1,3-bis-[3-~N-methyl-N-(L-2-hydroxy-E)ropionyl)3
amino-5-(2,3-dihydroxy-propyl)aminocarbonyl-2~4~6-
triiodo-benzoyl-amino]-2,2-bis-hydroxymethyl-pro-
pane.
- 1,3-bis-[3-~N-methyl-N-~L-2~hydroxy-propionyl)~-
amino-5-(1,3,4-trihydroxy-propyl)aminocarbonyl
2,4,6-triiodo-benzoyl-amino]-2,2-bis-hydroxyme-
thyl-propane.
EXAMPLE 26
1,3-bis-[N-methyl-N-[3-[N-methyl-N-(L-2-hydroxy-propio-
nyl)]amino-5-(2-hydroxy-ethyl)aminocarbonyl-2,4,6-tri-
iodo-benzoyl]amino]-2-hydroxy-propane.
The product described in Example 6 is submitted ko
N-methylation with sodium methylate and CH3I following
the procedure of Example 19 c).
Yield: 58% mOpoo 28~e
Elemental ~nalysis (%)
C H I N
Calculated: 27.18 2.76 52.22 5076
Found: 27025 2.77 52.14 5D9
3C NMR spectrum is in accordance with the proposed
structure.
EX~KPLE 27
1,3-bis-[N-methyl-N-[3-[N-methyl-N-(L-2-hydroxy-propio~
nyl)]amino-5-tl,3-dihydroxy-isopropyl)aminocarbonyl-
2,4,6-triiodo-benzoyl3amino]-2-hydroxy-propane.
The product described in Example 7 is submitted to
N-methylation with sodium methylate and CH3I following
the procedure of Example 19 c).
Yield: 5~% m.p.: 285C (dec.)
W092~08691 PCT/EPgl/02l~8
~l 2~9~13~
Elemental Analysis ~%)
C H I N
Calc.: 27.69 2.92 50.15 5.53
Found: 25.25 3.23 48.7~ 5.27 (~20 = 207%)
Solubility in H20 at 20C: 100% w/v
13C-NMR spectrum is in accordance with the proposed
structure.
EXAMPLE 28
1,3-bis-[3-(N-methyl-N-hydroxyacetyl)amino-5-(1,3-dihy~
droxy-isopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl-
amino]-2,2-bis-hydroxymethyl-propane.
The product described in Example 2 is reacted with
2 equivalent of NaOH, hence with an excess of CH3Br in
DMF for one day under stirring in a sealed system. The
subsequent work follows the procedure described in
~xample 19 c).
Yield: 54% m.p~: > 285-288C
Elemental Analysis (%)
C H I N
Calculated: 26.32 2.68 50.55 5.53
Found: 25.90 2.80 50.12 5.53
TLC: (Silica Gel 60 F254, Merck)
Eluent: CHC13/CH30H/NH40H 25% = 3/2/1 v/v/v. Rf = 0.26
HPLC: Rt = 15.5 min. Titre = 97.7% (on the area)
Chromatographic conditions: as reported in Example 2
In the same way the following compounds are obtainedo
- 1,3-bis-[3-(N-methyl-N-hydroxyacetyl)amino-5-(2,3-
dihydroxy-propyl)aminocarbonyI 2,4,6-triiodo-ben-
zoyl-amino3-2,2-bis-hydroxymethyl-propane.
~ 1,3-bis-[3-(N-methyl-N-hydroxyacetyl)amino-5-
(1,3,4-trihydroxy-2-butyl)aminocarbonyl-2,4,6-
,: . ; . .,. ~ . ;~,, ., ,. ., ,.. ,.. , . , - , . . , .: .
.
WO92/08691 PCT/EF91/OZ~SI
2 ~ 3
triiodo-benzoyl-amino~-2,2-bis-hydroxymethylp~o~
pane.
:: : , .. .. : . ,.; . .. .. : .: .. :. , ~ .
WO92/08691 PCTtEP91/0215n
.,.~ . . .
. . . ~
2 ~ 3 :3 ~
Table 1 - Acute toxicity of an aqueous solution of 1,3- ' ~
__ !
bis[3-(L-2-hydroxy-propionyl)amino-5-~1,3-dihydro~
xyisopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl~
amino]-2-hydroxy-propane.
Toxici~yDoses ConcentTation ¦ Administered I DeadlTreated LDSo
mgl/kg mgVml Volume Ra~o C.L. 95~G
mV3;g ~
900 300 30.0 ~ 0/10 .
a) 1 1600 300 38.7 0/10 ~15000
15000 _ 300 50.0 0/10 _
b) 900 300 _~3.0 l0/10 _ > 900_
a): Intravenous administration (14 days of observation)
in mice.
b): Intracisternal administration (7 days of observa~
tion) in rats.
Table 2 - Comparison among some preferred compounds of
the inven~ion and IODIXANOL.
Compound Osmolality Viscoslty
37C. 300 mgI/ml37C. 300 mgUml
mosmlkg H20 mPa-s
. .
A _ _14l _ 85
A:1,3-bis-[3-(L-2-hydroxy-propionyl~amino-5-(1,3-di-
hydroxy-isopropyl)aminocarbonyl-2,4,6~triiodoben-
zoyl-amino]-2-hydroxy-propane.
B:1-[3-~L-2-hydroxy-propionyl)amino-5-(1,3-dihydro-
xyisopropyl)aminocarbonyl-2,4,6-triiodo-benzoyl- ;~
. . :..... ,. . .. . :................. ~
:: ~ . :- : : : : :, :: ~ " ;.,.,: :, : :, : , :
W092/08691 PCT/EP91/0215a
~ ~. .
2~6~ 4
amino]-3-[3-hydroxyacetylamino-5-(2,3-dihydroxy-
propyl)aminocarbonyl-2,4,6-triiodo-benzoyl-aminGJ-
2-hydroxy-propane.
C: 1,3-bis-[N-[3,5-bis-(2,3-dihydroxy-propyl)amino~
S carbonyl-2,4,6-triiodo-phenyl]-acetylamino]-2-hy-
droxy-propane [IODIXAN01: EP 108638, Example 3J
i