Note: Descriptions are shown in the official language in which they were submitted.
2096239
-2- 3420/00/
Use of 2H-1~2 4-benzothiadiazine-3(4~)-thione 1,1-
dioxides ac antiviral medicaments
The subject of the present invention iE the new
use of 2H-1,2,4-benzothiadiazine-3(4H)-thione 1,1-
dioxidec 8S antiviral medicamentc.
In the Application JP 60 97,970 is described,
inter alia, 2-propyl-2H-1,2,4-benzothiadiazine-
3(4H)-thione l,l-dioxide with antifungal action.
6-Chloro-2H-1,2,4-benzothiadiazine-3(4H)-thione
l,l-dioxide is used in U.S. 3,838,119 as starting
material for the preparation of dihydrofurano-
~2't3':4,57thiazolo/2,2-c7-/I,2,47benzothiadiazine
derivatives.
Furthermore, 2-(2-methylphenyl)-2H-1,2,4-benzo-
thiadiazine-3(4H)-thione l,l-dioxide is known from
the Application Fr. M4279 with tranqillising,
h~pnotic and hyp~ten~ive action.
In further publications, for this substance
clAss could be demonstrated a cardiovascular action
((Phsrmazie, 43, 37, 1988), sn anti-inflammatory
activity (Pharmazie, 44, 601, 1989, Boll. Chim.
Farm., 126, 239, 1987), an anti-hypertensive and
possible diabetogenic action (Arzneimittelforschung
31, 279, 1981) and anti-microbial properties
(Farmaco, Ed. Sci., 34, 189, 1979; ditto, 34, 81,
1979; ditto, 411, 1973; ditto, 27, 990, 1972;
ditto, 38, 366, 1983).
2096239
-3-
~ he s~nthesis of this substance cl~ss i~
described inter eli~ in Fsrmaco, Ed. Sci , 38, 466,
1983 snd in J. Med. Chem. ~, 524, 1976.
The present invention concerns the new use of
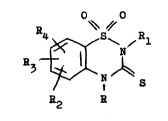
compounds of the genersl formuls I,
O~ ~O
_ Z~ ,Rl
in which Rl signif~es a h~drogen stom, 8 Cl-C6-
slk~l rsdicsl or a phen~l radicsl possibl~ substit-
uted b~ Cl-C6-alkgl, R2 ~ R4 sre the ssme or different
snd, independentl~ of one ~nother, signif~ a h~drogen
or hslogen stom, e Cl-C6-alk~l, Cl-C6-slkox~, -
trifluorometh~l or sminosulphon~l radicel snd R ~
h~drogen etom, 8 C~-C6-alk~l or phen~l-Cl-C6-slk~l
group, or of their t~utomers for the prepsration
of medicaments for the treatment of virel or
retrovirsl infections~
The "Cl-C6-alk~l parts" in the definition of
the slk~l or slkox~ groups can be straight-cheined
or brsnched, such 89 e.g. the methyl, eth~l,
isoprop~l, n-butJl, isobut~l or hex~l group.
Halogen signifies fluorine, chlorine, bromine or
iodine, prefersbl7 chlorine or bromine Phen~l-
C}-C6-elk~l groups sre, for exsmple, the benz~l
or phen~leth~l group~
2096239
-4-
In psrticulsr, for the prepsrstion of medi-
csments with sntiviral or anti-retrovirsl action,
those compounds sre u~ed of the formuls II
0~ 0
~4~ ~ ~N~ ~1
3 ~ N ~ S (II)
X2 R
in which Rl signifies 8 h~drogen stom, 8 Cl-C3-
slk~l radical or a 2-meth~lphen~l rsdical, R2 8
h~drogen, chlorine or bromine atom, R3 8 h~drogen,
chlorine or bromine atom or 8 methox~, meth~l or
trifluorometh~l radicsl and R4 8 h~drogen,. chlorine
or bromine atom or sn sminosulphonJl rsdicsl,
Surprisingl~, it hss now been found that these
2~ 2,4-benzothiszine-3(4H.)_thione l,l-dioxide~
displs~ sn out~tsnding sntiviral sction snd sret
therefore, especiall~ well suited for the trestment
o~ virsl or retroviral infections. ~irsl infections
of msmmals, especisllJ of humsns, are ver~ wide
spresd. In spite of intensive endeavours, hitherto
it hss not been possible to mske svsilable chemo-
therspeutics which interfere csusativel~ or
sJmptomsticsllJ with occursnces of disea~es caused
virsll~ or retrovirsllJ with recognisable succes~.
At present, it i9 not possible to cure or chemo-
therspeuticsll~ to influence fsvourabl~ the s~mptoms
2096239
Or certsin viral diseases, such 0~ for ~xsmple the
acquired immune deficiency s~ndrome (AIDS), the
AIDS-related complex (ARC) and their preliminar~
steges, herpes, c~tomegslovirus (CMV), influenza
snd other viral infections. At pre~ent, for exsmple,
for the treatment of AIDS, there is almost exclusivel~
svailable 3'-ezido-3'-deox~th~midine (AZ~), known as
zidovudine or Retrovir R~ ~owever, AZT i8 charscter-
ised b~ e ver~ narrow therspeutic index or bg ver~
severe toxicities slread~ occurring in the thera-
peutic rsnge (Hirsch, M.D (1988) J. Infect~ Dis.
157, 427-431). The compounds of the genersl formuls I
do not possess these disadvantages The~ act snti-
virsll~ without being c~totoxic in phsrmscologicsll~
relevant doses.
~ he compounds of the formula I and II displs~
vsluable pharmacological properties In psrticular,
the~ are suitable for the ther~p~ and proph~laxis
of infections which sre csused b~ DNA viruses, such
89 e,g. the herpes simplex virus, the c~tomegslo-
virus, papillomsviruses, the vsricells zoster virus
or Epstein-Bsrr virus, or RNA viruses, such ss
togaviruses, or especiallg retroviruses, such ss
the oncoviruses HTLV-L snd II, as well ~8 the lenti-
viruses visns and humsn immune deficiency virusHIV-l snd -2.
The compound~ of the formula I snd II appear
to be especisll~ suitsble for the trestment of the
2~96239
-6-
clinical manifeststions of the retrovirsl ~IV
infection in humans~ ~uch as the persistent,
teneralised l~mphadenopath~ (PG~), the ~dvanc~d
stage of the AIDS_related complex (ARC) and the
clinicall~ complete manifestation of AIDS.
It could now be demonstrated that compounds of
the general formula I and II inhibit the multi-
plication of DNA or RNA viruses, respectivel~, st
the stage of the virus-specific D~A or RNA
transcription, respectivel~. Via the inhibition of
the enz~me reverse transcriptase, the substances
can influence the multiplicstion of ~etroviru~e~
(cf. Proc. Natl. Acad. Sci. USA 83, l911t 1986 and
Nature t- 325, 773, 1987).
Since a very great need exists for chemothera-
peutics with interfere as specifically as possible
with retrovirall~-cau~ed diseases or their s~mptoms
without influencing tbe normall~ occurring n0tural
bod~ function, the said compounds could be sdvant-
ageousl~ uEed proph~lscticall~ or therspeuticall~ in
the treatment of diseases in which a retroviral
infection is of pathoph~siologica~ s~mptomatic or
clinical relevance.
~he medicsments conta^ining compound~ of the
formula I or II for the treatment of viral infections
can be admini~tered enterall~ or parenterall~ in
liquid or solid form. There hereb~ come into question
the usual forms of administration, such a8 for
2096239
~7~
example tablets, capsules, coated tablets, ~rups,
solutions Qr suspensions. As injection medium, wster
is preferabl~ used which contains the sdditives
usual in the case of injection solutions, such as
stabilising agents, solubilising agents and buffers.
Such additives sre e.g. tartrate and citrate buffer,
ethanol, complex formers, such as eth~lenediamine-
tetraacetic acid and its non-toxic salt~ high
molecular pol~mers, such as li~uid polgeth~lene oxide,
for viscosit~ regulstion Liquid carrier materials
for in~ection Eolutions must be sterile and are
preferabl~ filled into ampoules. Solid carrier
materials are, for example, starch, lactose, mannitol,
meth~l cellulose, talc, highlg-dispersed silicic
scids, high molecular fatt~ acids, such as stearic
ecid, gelatine, agar-agar, calcium phosphate,
magnesium stearate, animal and vegetable fats, solid
high molecular pol~mers, such as pol~eth~lene gl~col~,
etc. COm~ositions suitable for oral administration
can, if desired, contain flavo~ring and sweetening
materials.
~ he dosaging can depend upon variouE factors,
such a8 mode of administration, species, age or
individual Etate of health. The compoundE according
to the invention are usuall~ administered in amounts
of 0.1 - 100 mg, preferabl~ 0.2 - 8~ mg per dag and
per kg of bod~ weight. It is preferred to divide up
the daily dose into 2 - 5 admini~trations, whereb~
2096239
-8-
in the case of each administration 1 - 2 teblets
with an active materisl content of 0.5 - 500 mg
are given. The tablets can also be ret~rded, whereb~
the number of administrations per dag i9 reduced
to 1 - ~. The active material content of the
retsrded tablets can amount to 2 - 1000 mg, The
active msterial csn also be given by continuous
infusion, whereb~ the amounts of 5 - 1000 mg per
da~ normallg suffice~
~or the production of the final forms of
administration, the medicements produced according
to per se known methods are individuall~ confectioned
snd provided with the instruction (as 8 rule in the
form of a package leaflet) that the~e medicsments
are suitable for the treatment of viral or retro-
viral infections.
~ he compounds of the general formuls I sccording
to the invention csn be prepared according to
instructions of the patent applications and ~itèr-
ature references mentioned in the prior art.
In the mesning of the present invention, spartfrom the compounds possible b~ combination of all
claims, the following mentioned derivatives
especiall~ come into question.
1. 2-eth~1-2H-1,2-4-benzothiadiazine-3(4~)-thione
l,l-dioxide, m.p. 143C
~9~.,23:g
_9_
2. 5-chloro-2-eth~1-2H-1,2-4-benzothiadiazine-
3(4H)-thione l,l-dioxide, m.p. 125-126C
3. 6-meth~1-2-eth~1-2H-1,2-4-benzothiadiazine-
3(4H)-thione l,l-dioxide, m.p~ 176-177C
4. 6-chloro-2-eth~1-2H-1,2-4-benzothiadiazine-
3(4H)-thione l,l-dioxide, m.p. 176-177C
5. 6-methox~-2-eth~1-2H-1,2-4-benzothiadiazine-
3(4H)-thione l,l-dioxide, m.p. 172-173C
6, 5,7-dichlore-2-eth~1-2H-1,2-4-benzothiadiazine-
3(4H)-thione l,l-dioxide
7. 5,7-dibromo-2-eth~1-2H-1,2-4-benzothiadiazine-
3(4H)-thione l,l-dioxide
8, 7-chloro-2-eth~1-2H-1,2-4-benzothisdiazine-
3(4H)-thione l,l-dioxide, m.p. 205-209C
9, 7-bromo-2-eth~1-2H-1,2-4-benzothiadiazine-
3(4H)-thione l,l-dioxide
10. 6-chloro-7-sulphonsmido-2-eth~1-2H-1,2-4-
benzothiadiazine-3(4H)-thione l,l-dioxide
11 6-trifluorometh~1-7-~ulphonamido-2-eth~1-2H-
1,2-4-benzothiadiazine-3(4H)-thione l,l-dioxide
12. 2H-1,2,4-benzothiadiazine-3(4H)-thione 1,1-
dioxide, m.p. 220C.
13. 2-meth~l-2H-1,2,4-benzothiadiazine-3(4H)-thione
l,l-dioxide, m.p. 194C
14. 2-prop~1-2e-1,2,4-benzothiadiazine-3(4H)-thione
l,l-dioxide
15. 7-chloro-2-prop~1-2H-1,2,4-benzothiadiazine-
3(4H)-thione l,l-dioxide
- 2096239
--10--
16. 2,6-dimethyl-2H-1,2,4-benzothisdiazine-3(4H)-
thione l,l-dioxide
17. 5-chloro-2-prop~1-2H-1,2,4-benzothiadiazine-
3(4~)-thione l,l-dioxide
18. 7-meth~l-2-eth~l-2~-1,2,4-benzothiadiazine-
3(4H)-thione l,l-dioxide, m.p. 172-177C
19. 5-meth~1-2-eth~1-2H-1,2,4-benzothiadiszine-
3(4H)-thione l,l-dioxide, m.p. 167-171C
20~ 5-methox~-2-eth~1-2H-1,2,4-benzothisdiazine-
3(4H)-thione l,l-dioxide, m.p. 173-174C
21~ 2-isoprop~1-2H-1,2,4-benzothiadiazine-3(4H)-
thione l~l-dioxide, m.p; 161C
22, 2-phen~1-2H-1,2,4-benzothiadiazine-3(4H)-thione
l,l-dioxide, m.p. 210-211C
15 23. 2-isobut~1-2H-1,2,4-benzothiadiazine-3(4H)-
thione l,l-dioxide, m.p. 145-146C
24. 8-methox~-2-eth~1-2H-1,2,4-benzothisdiazine-
3(4H)-thione l,l-dioxide, m.p. 198-200C
25. 2-ethyl-4-benzyl-ZH-1,2,4-benzothiadiazine-
3(4H)-thione l,l-dioxide, m.p. 100-103C.
RT test
The screening teett system contain~ the purified
R~ (rever~ transcriptass) from HIV-l, which W8~
expressed by gene technological methods in E. coli,
as well a9 the components of the initiation complex,
such a~ the in vitro trsnscripts of the HIV-LTR's
with the neighbouring primer binding site a9
templste and an 18mer oligonucleotide ss primer
--` 2096~
--11--
complementar~ to the primer binding ~ite. The
3H7-th~midine-5'-triphoFphate incorporstion ws~
meaRu~ed b~ counting 1~ e.~-counter.
Results
inhibition of the HIV-RT
sub~tance IC50 ~ M~
2-eth~1-2H-1,2,4-
benzothiadiazine- 8.3 x 10 6
3(4H)-thione 1,1-
dioxide
10 7-chloro-2-eth~1-2H_
1,2,4-benzothis-1.6 x 10 6
diazine-3(4H)-thione
l,l-dioxide
_
2-isoprop~l-2H-1,2,4-
15 benzothiadiazine- -5
3(4H)-thione 1,1- 1.6 x 10
dioxide