Note: Descriptions are shown in the official language in which they were submitted.
CA 02097824 2001-11-23
FUNCTIONALIZED VINYL AZOLES AND THEIR USE
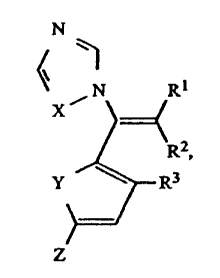
This invention relates to functionalized vinyl azoles of
general formula I
> >
v I R
~ CI),
\R2
\Y
/ / R3
Z
in which
X means an N atom or a CH group,
Y means an S atom or a CH=CH group,
Z means a cyano group, a fluorine, chlorine or bromine atom
and R~ or RZ means
an optionally est.erified carboxyl group,
an optionally substituted carboxylic acid amide group,
an aldehyde group,
an alkylketone or arylketone group,
an optionally substituted sulfonamide group or a nitrile
group
and the respective other group R' or Rz means
P~ l~~LwJ9I9
Aid ~ a.. 7 r4 le"y
a hydrogen atom,
a,low-aa.kyl group or cycloalkyl group,
an optionally substituted aryl group,
an aralkyl group,
an, optionally esterified carboxyl group,
an optionally substituted carboxylic acid amide group,
an aldehyde group,
an alkylketone or arylketone group as well as
a nitrite group
or
R7 and Rz together with the carbon atom, on which they are
bound, mean a 5-, 6- or 7-membered ring, which contains a ketone,
ester, lactone, tact«m or imide grouping placed so that at least
one carbonyl group is conjugated with a vinyl double bond, and
R3 means a hydrogen atom or R3 together with RZ means an
--O-C=d grouping or an -N-C=o group9.ng optionally subs~ta.tuted on
the N atom whose carbonyl group is conjugated with a vinyl double
bond,
as well as pharmaceutical preparations containing a
pharmaceutically compatible vehicle;
use of these vinyl azoles for the productian of
pharmaceutical agents, vinyl azoles themselves as well as process
for their production.
Substituent Z preferably stands for a fluorine atom or a
cyana group.
If R' andfor Rz stands for an esterified carboxyl group, the
Latter is-esterified first with a straight-chain or branched-
3
r?
acW ~r a,.n r, ~'9i,~~ ~
chain or cyclic O-alkyl radical with up to 10 carbon atoms, with
an O-aryl radical, and aryl is a phenyl or naph'thyl radical
optionally substituted by one or three low-alkyl groups (1-4
carbon atoms) or halogen atoms (F, C1, Br, I) or an o-aralkyl
radical, and in the latter, the aryl and the alkyl fragments have
the above-indicated meaning. In this case, the methoxy, ethoxy,
propoxy, isopropoxy, isobutoxy, teat.-butoxy, cyclohexyloxy,
cyclopentyloxy, phenyloxy or 2,6-dichlorophenoxy radical is
especially preferred.
If R~ and/or Rz is a substituted carboxylic acid amide
group, the latter above all a.s substituted with one or two, in
the latter case same or different, radicals. These radicals can
be straight-chain or branched-chain alkyl radicals with 1'to 10
carbon atoms, or aryl radicals with 6 to l0 carbon atoms
optionally substituted by one to three alkyl groups or halogen
atoms. Further, the amidic nitrogen atom can also be part of a
5- to ~-membered ring, which also can contain the grouping~N-R6
with R6 meaning a hydrogen atom or a straight-chain or branched-
chain alkyl group with 1 to 6 carbon atoms, an oxygen or sulfur
atom as a ring member.
Quite especially to be emphasized is the slabstitution of the
carboxylic acid amide group with a methyl, ethyl, propyl, phenyl,
benzyl radical,.two methyl, ethyl, propyl radicals, a phenyl and
a methyl, a phenyl anal an ethyl, and a benzyl and a methyl
radical or a pyrrolidine, piperidine, piperazine, N-
methylpiperazine, morpholine or thaomorpholine ring formed
together With the amidic nitrogen atom.
4
The preferred substituents suitable for the substituted
sulfonamide group are identical with the preferred N-substituents
for the carboxylic acid amide group. First of all, Rg and R~°
each mean an alkyl substituent with 1 to 10 carbon atoms.
As alkylketone group R' and/or R2, radical -CO-R7 is
preferred, and R7 means a straight-chain or branched-chain alkyl
radical with l to 10 carbon atoms or a cycloalkyl radical w9.th 3
to 12 carbon atoms,.and as an arylJcetone.group, radical -CO-Ra is
preferred, and R8 means a phenyl, naphthyl or heteroaryl radical,
optionally substituted by one or more alkyl, halogen, hydroxy or
alkoxy radicals, such as, e.g., a thiophene, furan, pyridine,
thiazole, oxazole or diazin,e ring,
In particular, R~ is a methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, pentyl, isopentyl, neopentyl, cyclopentyl or
cyclohexyl radical and Rg is a phenyl, hydroxyphenyl,
methoxyphenyl or chlorophenyl radical.
If R~ and Rz together with the carbon atom, on which they
are bound,~form a cyclic system, which should contain at least
one carbonyl group, the following ring systems are to be
5
~i ~:i';:a cn
emphasized:
0
v- -~~_\I oW.o
0 0 0 0~ o,
0
O~iCFI2~n , , ~-W .
0 in=2 30 0 0 0
__~
tN_W .~~~
N 0
~W ~
p 0 ~ ~w ~ 1'~N~'W
0 0
and W means a hydrogen atom or an alkyl group with 1-10 C atoms.
With the bridge formed from 1~2 and R3, the following partial
structures result together with the aromatic ring containing Y:
Z 0 0 Z ~ N 0
i
w
s ~~ z s
0 0 N 0
W
If R~ and RZ in formula I are different, Z- and E-isomeric
compounds are produced (except in the case of the cyclical
structures abovej. The invention therefore also comprises the
pure Z- or E-compounds as well as any mixtures of both.
The separation o~ the isomers takes place with standard
methods, such. as crystallization or chromatbgraphy.
6
°~ °~:"~~J ~a
The following compounds are preferred:
~-(4-Cyanophenyl)-3-(~.-imidazolyl)-acrylic acid methyl ester
3-(4-cyanophenyl)-3-(1-imidazolyl)-acrylic acid-tart-butyl
ester
E-3-(4-cyanophenyl)-~-(1-imidazolyl)-acrylic acid
E-3-(4-cyanophenyl)-3-(1-imidazolyl)-acrylic acid piperidide
E-3-(4-cyanophenyl)-3-(1.-imidazolyl)-acrylic acid
methylamide
3-(4-cyanophenyl)-3-(1-imidazolyl)-acrylonitrile
4-[1-(1-imidazolyl)-3-oxo-1-butenyl]-benzonitrile
3-[(4-cyanophenyl)-(1-imidaz~lyl)-methylene]-dihydro-2(3H)-
furanone
3-(5-cyano-2-thienyl)-3-(1-imidazolyl)-acrylic acid-tert-
butyl ester
3-(5-cyano-2-thienyl)-3-(1-imidazolyl)-acrylonitrile
3-(4-cyanophenyl)-3-(1,2,4-triazol-1-yl)-acrylic acid-tert-
butyl ester
3-(4-cyanophenyl)-3-(1,2,4-triazol-1-yl)-acrylonitrile
7-cyano-4-(1-imidazolyl)-coumarin
3-(4-fluorophenyl)-3-(1-imidazolyl,)-acrylic acid-tart-butyl
ester
3-(4-chlorophenyl)-3-(7.-imidazolyl)-acrylic acid-tart-butyl
ester
3-(4~-bromophenyl)-3-(1-imidaz~lyl)-acrylic acid-tart-butyl
ester.
3-(4-fluorophenyl)-3-(1,2,4-triazol-1-yl)-acrylic acid-tert-
butyl ester
7
~.e~ a ~ ~~
o..Ji p, ~ pL."
The compounds of general formula I are :inhibitors of
estrogen biosynthesis (aromatase inhibitors). Thus, they are
suitable for the treatment of diseases which are caused by
estrogens or are dependent on estroge:ns. Thus, they are suitable
for the treatment of estrogen-induced, or estrogen-stimulated
tumors, such as, for example, breast cancer, endometrial cancer,
melanoma or prostatic hyperplasia (The Lancet, 1984, 1237-1239).
Said compounds are also valuable for influencing fertility.
Thus, a male infertility, which results from increased estrogen
levels, can be overcome with the new active ingredients.
Further, the compounds can be used in females of child-bearing
age as a means of birth control, to inhibit ovulations by removal
of estrogen. Aromatase inhibitors probably are also suitable for
treatment of impending myocardial infarction, since increased
estrogen levels in the male can precede a myocardial infarction
(US Patent 4,289,762).
This invention therefore also relates to the use of
compounds of general formula I for the productian of
pharmaceutical agents for treating estrogen-induced and estrogen-
depenc~ent diseases.
Phenylalkenones of general formula RaC(O)CH=CR~RC, in which
R~ means an optionally substituted alkyl or cycloalkyl radical,
R~' means a 1,2,4-triazolyl or 1-imidazolyl radical and R~ means a
phenyl or naphthyl ring, which optionally can be substituted,
among-others, with a fluorine, chlorine or bromine atom or a
cyano group, can already be seen from FP-A-0 003 884. These
8
.~ ,~(:w'~ ._~~
~d~o_7~ 4i ~i4,rl~.
phenylalkenones are described as compounds with herbicidal
activity.
Qther herbicidal 1,2,4-triazolylvinyl and imidazolyl vinyl
compounds, which, in geminal position in the heterocycle, have a
phenyl ring chlorosvbsti~tuted in p-position, are known from DE-A--
27 38 640.
Finally, the objects of DE-A 28 26 760 are 3-(4-
chlorophenyl)-3-(1,2,4-~triazolyl)-acrylic acid a7.kyl esters for
use as Fungicides and plant-growth regulators.
The above-named compounds, whose medicinal applicability was
previously unknown, are comprised by general formula I. On the
other hand, the compounds of general formula Ia
~N~
Rta
~~N~~'
~R2a
(Za)r
Y 3
~~R
~Z
in which
X means an N atom or a CH group,
Y means an S atom or a CH=CH group,
Z° means a cyano group or a fluorine or bromine atom and
R~e or Rz~ means
an optionally esterified carboxyl group,
- an optionally substituted carboxylic acid amide group,
an aldehyde group,
ari aryl~~torie group,
9
e~~rn~i y.J94.:~.
an optiona:Lly substituted sulfonamide group or a nitrite
group
and the respective other group R1a or Rz~ means
a hydrogen atom,
a low-alkyl group or cycloalkyl group,
an optionally substituted aryl group,
an aralkyl group,
an optionally esterified carboxyl group,
an optionally substituted carboxylic acid amide group,
an aldehyde group,
an alkylketone group as well as
a nitrite group
or
Rya and Rza together with the carbon atom, on which they are
bound, mean a 5-, 6- or 7-membered ring, which contains a ketone,
ester, lactone, lactam or imide grouping placed so that at least
one carbonyl group is conjugated with a vinyl double bond, and
R3 means a hydrogen atom or R3 together with Rza means an
-O-C=O grouping or an -N-C=O grouping optionally substituted on
an N atom whose carbonyl group is conjugated with a vinyl double
bond,
are new.
The compounds of general formula Ia therefore also belong to
the object of this invention.
The radicals possibly or preferably standing for
substituents Rya, Rza and R3 in the compounds of general formula Ia
are the same as tho:~e of formula I mentioned as possibly or
10
i~'~~a' ,~~.m~
preferably standing for substituents R~, RZ and Rs, with the
difference that in the compounds of general formula Ia, no
chlorine atom can stand for. Za and no alkylketone group can stand
for Rya or R28.
Known substances exhibiting an aromatase-inhibiting effect
are in addition to steroids also non:~teroidal substances, for
example, the various nitrogen heteroc:ycles described in European
patent applications EP-A 0165777 to 01657.84, the substituted
glutaric acid imides described in J. Med. Chem. 1986, 29, pages
1362-1369, the substituted imidazobenzenes described in European
patent application EP 0165904, the substituted heterocyclically-
substituted tolueneni~triles described in European patent
application EP-A 0236940 and the imidazo- and 5,6,7,8-
tetrahydroimidazo[1,5a]pyridines having an optionally substituted
phenyl ring, seen from US Patent US-A-4,728,465, from which in
particular 5-(p-cyanophenyl)5,6,7,8-
tetrahydroimidazo[1,5a]pyridine, hydrochloride stands out as a
greatly effective aromatase inhibitor (Cancer Res. 48, pp. 834-
838, 1988).
The compounds of general formula I are distinguished
relative to the previously known compounds in that they inhibit
the enzyme system of the aromatase more strongly and at the same
time more selectively. The selective action is shown in that
other enzyme systems are affected to a smaller extent.
The concentrations, in which the aromatase activity is
inhibited in vitro by the compounds of general formula T, are in
the range ~of 10-7 to 1U-~° mol/l.
1.1
~~:~'ej~6~~.
In comparison with the compounds of FP-A 023690 that are
structurally 'very close, by the introduction of the double bond
the compounds of general formula I no longer have a chirali~ty
center on the carbon atom, on which both the cyanoaryl and the N°
heteroaryl radical are present. An enantioselective synthesis or
the expensive separation of the enantiomers is avoided by the
elimination of the chirality center.
The amount of the compounds to be administered fluctuates
within a wide range and can cover every effective amount. As a
function of the condition to be treated and the type of
administration, the amount of the administered compounds can be
0.0001-10 mg/kg of body weight, preferably 0.001-1 mg/kg of body
weight daily.
For oral administration, capsules, pills, tablets, coated
tablets, etc. are suitable. In addition to the active
ingredient, the dosage units can contain a pharmaceutically
compatible vehicle, such as, for example, starch, sugar,
sorbitol, gelatin, lubricants, silicic acids, talc, etc. The
individual dosage units for oral administration can contain, for
example, 0.05-50 mg of the active ingredient (aromatase
inhibitor).
For parenteral administration, the active ingredients can be
dissolved or suspended in a physiologically compatible diluent.
As a diluent, very often oils are used with or without adding a
solubilizer, a surfactant, a suspension mixture or an emulsifying
mixture. As examples for oils used, there can be mentioned:
CA 02097824 2002-07-03
12
olive oil, peanut oil., cottonseed oil, soybean oil, castor oil
and sesame oil.
The compounds can also be used in the form of a depot
injection or an implant~~tion preparation, which can be formulated
so that a delayed relea:~e of active ingredient is made possible.
Implantations can contain as inert materials, for example,
biodegradable polymers or synthetic silicones, such as, for
example, silicone rubber. The active ingredients can further be
worked into, for example, plasters for percutaneous
administration.
The tumor-inhibiting action of imidazole derivatives is
based on an inhibition of P-450-dependent enzyme systems (cf.,
e.g., J. P. Van Wanne and P. A. J. Janssen; J. Med. Chem. 32
(1989) 2231). Also, the action of antifungal therapeutic agents
of the series of imidazole and triazole derivatives is based on a
blocking of P-450-dependent. biochemical reactions.
Further, it is known from the patent literature that azole
derivatives have both antifungal and tumor-inhibiting action at
the same time (in this connection, see European Patent
Application 165,777 (Dec. ?7, 185)). The compounds according
to the invention should therefore also comprise an antifungal
action against human-, animal-- and plank.-pathogenic organisms.
The invention further relates to processes for the
production of compounds of general formula Ia as well as a
process for the production of certain compounds of general
formula I, identified below as compounds of general formula I'.
13
~~~~~~~~r~
For the production of the compounds of general formula Ta,
either
i) a compound of general formula II
1a
2a
UI) r
in which
. RZa. Y and Z° have the meaning indicated in formula za
and R~' means a hydrogen atom or 7R3' together with Rza forms a rang
of the above-indicated partial structures, reacts with a compound
of general formula V2I
N ~ (~zz),
p
in which
X means an N atom or a CH group and
A means a. hydrogen atom, an alkali metal. or a trialkylsilyl
radical with the same or different straight-chain or branched C~-
C8 alkyl groups,
in an inert solvent at a temperature between room temperature anol
boiling temperature of the soavent ox without solvent, optionally
~.-.N~
1~
~~D~.~~~f_'~~:~.
by adding a catalyst first to a compound of general formula IIi
x ~~~~
PI ~ ~H 1 a
H
2a
(IIi),
y
Z
in which R~aP RZa, R3~, X, Y and Za have the meaning already
indicated in farmula II or VII, and 'the latter is allowed to
further react by dehydration above 60°~, optionally in a solvent
and optionally by using a catalyst, to a compound of general
formula Ia, or
ii) a compound of general formula III
1a
2a
(III) ,
in which
R~, Rz.and Y have the meaning indicated in formula 1, R~'
means a hydrogen atom or, together with R2, foz~rns a ring of the
above-indicated partial structures, and Hal means halogen sterns,
in particular one bromine atom each,
15
v ~~fw~
~.,..~1 ~ p."
reacts with a compound a~ general Formula VI:L according to
conventional processes with or without adding a foreign base to a
compound of general formula I or
iii) to an acetylene compound of general formula V
Za Y
is
C_C ~- R
in which Y and Ze have the meaning indicated in formula I and g~a
means an esterified carboxyl group,
an optionally substituted carboxylic acid amide group,
an aldehyde group,
an arylketone group,
an optionally substituted sulfonamide group or
a nitrite group,
and the optionally possible substituents and the alkoxy radicals
of the esterified carboxyl group correspond to the definitions
already indicated in more detail,
is added a compound of general formula VII in a solvent
between room temperature and boiling temperature of this solvent
with forming a compound of general formula Iiii
~~
.. ~~~C= CH.~,,,d, R ) a
_ X~
(Iiii)
Y
Za_-----~._~
L l7
~Q.i~.,i~,.,~~~;~~.
The production of the compounds of gens:ral formula T
according 'to 'the invention takes place according to variant i)
starting from an epaxide of general formula II and an azole of
formula VII in a way known in the art.
The addition of the azole is performed in an inert solvent,
such as, for example, benzene, toluene, xylene, tetrahydrofuran,
dioxane, acetonitrile or dimethylformamide, preferably at a
temperature between 60°C and the boiling temperature of the
solvent or without solvent, preferably between 60°C and 150°C.
If necessary, a catalyst, e.g., a metal salt, such as lithium,
magnesium, sodium perchlarate, zinc chloride or calcium chloride,
can be added (Tetrahedron Letters 32 (1990) 4661).
The dehydration takes place thermally, preferably at
temperatures between 100°C and 200°C or the boiling temperature
of an optionally used solvent, such as, e.g:, toluene,
chlorobenzene or xylene. As catalysts, inorganic or organic
acids, such as, for example, sulfuric acid or p-taluenesulfonic
acid are suitable.
The dehydration can also be performed by using dehydrating
agents such as thionyl chloride ar phosphorus axychloride with or
without a solvent (e. g., dichloromethane, acetonitrile,
~tetrahydrofuran) at room temperature to boiling temperature of
the solvent, preferably between 2,0°C and 50°C.
In the reaction of the dihalide of general formula IIT with
an azole of general foxmu2a VII according to ii), methods
familiar to one skilled in the art are also involved. Possibly,
~.
o4s~~:i~E~~c~~M~
the additional use of a foreign base (Heterocycles 25 (19F31),
961) can be useful in facilitating the reaction.
First, the exchange of the halogen atom on the benzylic
carbon atom 'takes place and then the elimination of the hydrogen
halide. The intermediately formed monohalogen compound is not
isolated.
The necessary initial compounds of general formula II or
formula III are accessible by epoxidatio0 or halogenation,
preferably bromation, of the corresponding olefins in a way known
in the art; the olefins in 'turn can be produced, for example, by
Wittig or Knoevenagel reaction of the corresponding feedstocks.
In the addition of an azole of formula VII to an acetylene
compound of general formula V to be perfoz~med in a way known in
the art according to variant iii), compounds of general formula
Iiii are obtained with RZ = H as a Z-/E-isomeric mixture. The
production of compounds of formula V is known (e. g., Cliem. F3er.
94 (1961) 3005: J. Org. Chem. 30 (1965) 1915). The reaction of
compounds of formula V with the optionally substituted azoles VII
takes place preferably in solvents such as hydrocarbons (benzene,
toluene), ethers (ethyl ether, dioxane, tetrahydrofuran),
alcohols (tent-butanol) or halogenated hydrocarbons
(dichloromethane, chloroform, 1,2-dichloroethane) between room
temperature and boiling temperature of the solvent.
18
[ y ~~,P~g
~,:~7 a I~.r
Compounds o:C general farmula I'
R~~
N
~R~' cI°i,
Y'
z
in which R~, X, Y and Z have the meaning indicated in
formula x,
Ref means a hydrogen atom or
R~ and RZ' together with the methylene carbon atam mean a 5-,
6- or 7-membered ring, which contains a ketone, ester, lactone,
lactam or imide grouping placed so that at least one carbonyl
group is conjugated with a vinyl double bond,
are produced, by a compound of general formula IV
N
C=0
~I~3 .
in which X, Y and Z have the meaning indicated in formula I,
b~ing_reacted raith a phosphorane of farmula VIII
~ (VIII),
l3P _- C
~R Z
19
in which
L means an optionally substituted phenyl radical [with low-
alkyl(1-6C), low-alkoxy(1-6C), halogen] or a straight-chain or
branched low-alkyl radical with 1 to 6 carbon atoms and R~ and R2'
have the above-~indiaated meaning,
in an inert solvent between room temperature and boiling
temperature of the solvent used.
By reaction of an acylazole of general formula.IV with a
phosphorane of formula VIII, 'the last-mentioned process variant
according to the invention results in the compounds of general
formula x~ (wittig reaction). As inert solvents, for example,
benzene, toluene, xylene, chlorobenzene, tetrahydrofuran,
dioxane, acetonitrile, dimethylformamide or dimethylsulfoxide are
used. The reaction temperature is preferably selected above
60°C.
That acylazoles react with a phosphorane in the meaning of a
Wittig reaction was not previously known. Rather. it is known
that acylimidazoles (generally also acylazoles) are good
acylation agents (Comprehensive Heterocyclic Chemistry (Hds. A.
R. Katritzky, K. T. Potts) F~ergamon Press 1984, Volume 4A, page
451 ff). Accordingly, it was to be expected 'that a wittig
reagent (phosphorane) is acylated on the carbanion, as it is
observed, e.g., in the reaction with acid chloridesa
R R
B
ph3p--C\ ' ; R"-COCl ~ ph3p--~-R~ C1
R I ~0
R"
20
~w~r~~~r;~~r ;~~
Tn aliphatic acid imidazolides the acylation of
triphenylmethylenephosphorane is described (M. Miyano et al., J.
prg. Chem. 40 (1975) 2840). It was there;~ore unexpected and
surprising that acylimidazoles or acylazoles enter into a normal
Wittig reaction:
Ri 0
N
L3P C/ + I ~ N°4C-R ' _" ] X Ny~ R, 2~ 13P0
~RZ' CR R
R = Y~I
z
The following examples are used to explain the invention in
more detail:
21
~,r ,~,~ y. ~~
!4r ~ ~_,~ s; ~,n' ~
EXAMPLE 1
3-(4--Cyanophenyl)-3-(1-imidazolyl)-acrylic acid methyl ester
2.85 g of 4-cyanobenzoyl chloride, dissolved in 20 ml of
ether, is instilled in a solution of 2.4 g of imidazole in 25 ml
of tetrahydrofuran and 50 ml of ether. After 10 minutes at room
temperature, it is filtered with es:clusion of air and the
filtrate is concentrated by evaporation in a vacuum. The residue
is dissolved in 20 ml of tetrahydrofuran.and refluxed with 5.7 g
of methoxycarbonylmethylenetriphenylphosphorane for.5 hours. 2t
is added to water, extracted with ethyl acetate and the ethyl
acetate phase is washed with water. Then, the ethyl acetate
phase is extracted three times with 2 M hydrochloric acid. The
hydrochloric acid phase is alkalized with potassium carbonate and
extracted with ethyl acetate. After drying the ethyl acetate
phase with sodium sulfate.and concentration by evaporation, an
oil remains, which thoroughly crystallizes. After suctioning ofg
the crystals with ether, 3.5 g (81m) of a Z-/E-mixture of the
title compound is obtained. By recrystallization from ethanol,
the E-compound is obtained pure. MP: 144-147°C
EXAMPLE 2
3-(4-Cyanophenyl)-3-(1-imidazolyl)-acrylic acid-teat-butyl ester
Analogously to example 1, with use of tert-
butoxycarbonylmethylenetriphenylphosphorane. Yield 37p. After
chromatography ow silica gel (eluant ethyl acetate) and
crystallization from ether, the E-isomer melts at 151-153°C.
2, 2
~i~~~ i~;~(~"~~~~
EX~3MP~E 3
E-3-(4-Cyanophenyl)-3-(1-imidazolyl)-acrylic acid
100 mg of the E-compound of example 1 is dissolved in 1.5 ml
of 10% methanolic potassium hydroxide and left for 15 minutes at
room temperature.
Then, it is concentrated by evaporation in a vacuum at 30°C
and the residue is dissolved in 2 ml of water. By adding 1 M
hydrochloric acid and later 10% acetic acid, it is adjusted to a
pH of 5 and extracted with ethyl acetate. After concentration by
evaporation of the solvent, 38 mg of the title compound is
obtained.
Mp: 230-246°C
The hydrochloride of the title compound is obtained as
follows:
200 mg of the E-tent-butyl ester of example 2 is stirred
with 20 ml of 6 M hydrochloric acid for 2 hours at room
temperature. It is concentrated by evaporation in a vacuum,
distilled twice more each with toluene and methylene chloride and
195 mg (100%) of E-3-(4-cyanopheny.l)-3-(1-imidazolyl)-acrylic
acid, hydrochloride, is obtained.
E~CAMPhE 4
E-3-(4-Cyanophenyl)-3-(1-imidazolyl)-acrylic acid piperidide
190 mg of the hydrochloride of example 3, 195 mg of 2-
chloro-1-methylpyridinium iodide, 57 mg of piperidine, 290 mg of
tributylamine and 12 ml of methylene chloride are refluxed for 20
hours. The acid phase is separated and saturated with potassium
23
~~.s~.,i'u,a.~~
carbonate. :Ct is extracted with ethyl acetate, the ethyl acetate
phase is concentrated by evaporation and the low-boiling material
is distilled off from the residue at 100°C and 0,01 mbar. The
residue is chromatographed on silica gel. With methylene
chloride/isopropanol (0.5 - 4% isopropanol), 52 mg of crystalline
product is obtained with a melting point of 150-165°C.
EX1~MQLE 5
E-3-(4-Cyanophenyl)-3-(1-imidazolyl)~-acrylic acid methylamide
1.35 g of methylammonium chloride in 10 ml, of toluene is
mixed under argon with 7.5 ml of trimethylaluminum in 10 ml of
toluene by instillation and stirred for 2 hours at room
temperature. 50 mg of 'the E-methyl ester of example 1 is mixed
in 2 m1 of toluene with 2 ml of the above-produced reagent. It
is stirred for 6 hours at 80°C, then mixed at room temperature
with 1 M hydrochlaric acid and extracted with ether. The acid
phase is alkalized with potassium carbonate and extracted with
ethyl acetate. After washing with water and drying, the ethyl
acetate phase is concentrated by evaporation. 30 mg of the title
compound (500) remains; melting range: 172-180°C.
24
'°J~,~ ~e~:l~"~q~".~/~
~.W :) :l I~ A
EXAMPLE 6
3-(4-Cyanophenyl)-3-(1-imidazolyl)-acrylonitr:ile
Analogously to example 1, the title compound is obtained
with use of cyanomethylenetriphenylphosphorane. The E-isomer is
obtained pure from ethanol; mpa 188-193°C.
EXAMPLE ?
4- [ 1- ( 1-Tmidazolyl) -3-oxo-1--bwt~enyl ~ -benzonitr:ile
a) Analogously to example 1, with use of acetonylidine--
triphenylphosphorane. A Z-/E-mixture of the title compound is
obtained.
b) By stirring 3..02 g of 4-cyanobenzaldehyde and 7.32 g of
acetonylidene-triphenylphosphorane in 50 ml of dichloromethane
and then recrystallizing from isopropanol, 3.15 g (80~) of 4-(3-
oxobutenyl)-benzonitrile is obtained. 1.71 g of it is mixed in
20 ml of dichloromethane with 20 m1 of a 0.5 M solution of
bromine in dichloromethane. After decolorization, the solvent is
drawn off in a vacuum. The remaining oil is refluxed with 0.95 c~
of imidazole and 7 ml of triethylamine in 40 ml of -toluene fox 2
hours.. A precipitate is filtered off, the filtrate is
distributed in ethyl acetate-2 M hydrochloric acid, the
hydrochloric acid phase is alkalized with potassium carbonate and
extracted with ethyl acetate. 1.2 g of a crude product is
obtained, which, after recrystalliza~tion from isopropanol, yields
the ~-isomer with mp. 131-135°C. After chromatography of the
mother liquor on silica gel (eluant ethyl acetate), the E-isomer
25
" ~~ 0.:W" ~°"~r
~~A.:J ~ p,~~
is obtained from ethyl acetate/ether as crystals of mp: 123-
126°C.
EXAMPLE 8
3-((4-Cyanophenyl)-(1-imidazolyl)-methylene)-dihydro-2(3H)-
furanone
Analogously to example 1, with use of 3-
(triphenylph:osphoranylidine)-dihydro-2(3~I)-furanone in the
solvent toluene under reflux (20 hours). Chromatography of 'the
crude product on silica gel (eluant dichloromethane/methanol
99:1) and recrystallization from isopropanol produces the title
compound in E-form with mp: 194-197°C.
EXAMPLE 9
3-(5-Cyano-2-thienyl)-3-(1-imidazolyl)-acrylic acid-tert-
butyl ester
The acid chloride, is produced from 2 g of 5-cyanothiophene-
2-carboxylic acid by boilzng with thionyl chloride and distilling
off excess thionyl chloride. The acid chloride is dissolved in
ml.of ether and mixed with 1.9 ml of N-
trimethylsilylxmidazole. It is concentrated by evaporation in a
vacuum, dissolved in a mixture of 150 ml of tetrahydrofuran and
50 ml of acetonitrile and mixed with 4.9 g of tart-
butoxycarbonylmethylene-~.riphenylphosphorane. It is refluxed for
haurs and worked up as in example'1. The crude product is
chromatoc~raphed on silica gel using dichlaromethane with 1°°2%
isopropanol> 2:4 g (63%) of the title compound is obtained as a
26
~r ~ d
i~s~.r"::, r, w~,~
Z-/E-mixture. The E-isomer is obtained pure by recrystallizatian
from isopropanol; mp: 90-91°C
EX1'lMPhE 10
3-- ( 5--Cyano-2-thienyl ) -3- ( 1-imide~z olyl ) -acrylonitrile
Analogously to example 9, with use of
cyanomethylenetriphenylphosphorane, a~ Z-/E-mixture of the title
compound is obtained. The E-isomer is obtained pure from
ethanol; mp: 129-133°C.
FX7MPhl~ 11
3-(4-Cyanophenyl)-3-(1,2,4-tr:iazol-1-yl)-acrylic acid-tert-
butyl ester
Analogously to example 9, with use of 1-trimethylsilyl-
1,2,4-triazole and tert-
butoxycarbonylmethylenetriphenylphosphorane.
Z-isomer: mp: 132-133°C
E-isomer: mp: 83- g5°C
EXAPSPhE 12
3--(4-Cyanophenyl)-3--(1,2,4-triazol-2-yl)-acrylonitrile
Analogously to example 9, with use of 1-trimethylsilyl-
1,2,4-triazole and cyanomethylene-triphenylphosphorane:
2%
~u va,;~~ ~
~~.,.al ~r i4f'~
EXAP3P:LE ~.3
7-Cyano-4-(1-.imidazolyl)-coumarin
g of 7-hydroxycoumarin in 25 ml of pyridine is mixed by
instillation under argon at 0°C with a mixture of 8.1 m:1 of
trifluoromethanesulfonic acid anhydride and 65 ml of pyridine.
It is stirred far 20 more hours at room temperature, poured on
400 ml of ice-cold, semiconcentrated hydrochloric acid and
extracted with ethyl acetate. After washing the ethyl acetate
phase with semiconcentrated hydrochloric acid, water and
potassium bicarbonate solution in succession, it is dried and
concentrated by evaporation. 6.9 g of 7-hyc~roxycoumarin--
trifluoromethanesulfonic acid ester is obtained; mp: 75-76°C.
3 g of it is refluxed with 960 mg of potassium cyanide, 2.4
g of tetrakis-(triphenylphosphine)-palladium(O) and 30 mg of
1,4,7,10,13,16-hexaoxacyclooctadecane (28-crown-6) in 180 ml of
tetrahydrofuran for 5 hours. It is diluted with water, extracted
with ethyl acetate and the ethyl acetate phase is washed in
succession with 1 M sodium hydroxide solution, water and common
salt solution. After concentration by evaporation in a vacuum,
it is.recrystallized from ethanol and 975 mg of 7-cyanocoumarin
is obtained: mp: 217-225°C.
This material is dissolved in 95 ml of dichloromethane,
mixed with 10 ml of bromine and stirred under action of light
(100 W) far 4 days. Then, it is concentrated by evaporation in a
vacuum. The crystals are suctioned off with ether. 1.2 g of
3,4-dibromo-7-cyano-2-chromanone is obtained: mp: 220-230°C.
28
~~'~a:~~ a~
~~l',..J s, ~H
The dibromo compound is refluxed with 372 mg of imidazole
and 2.52 ml of triethylamine in 20 ml of toluene for 7 hours.
The solution is distributed between 1 M hydrochloric acid and
ether, the acid water phase is separated and it is alkalized with
potassium carbonate. After extraction with ethyl acetate and
concentration of the solvent by evaporation, the remaining
crystals are suctioned off with ether, dried at 50°C in a vacuum
and 140 mg of the title compound of melt~.ng point 260-268°C is
thus obtained.
EXAMPLE ~4
3-(4-Cyanophenyl)-3-(1-imidazolyl)-acrylic acid methyl ester
4-Cyanophenyl-propiolic acid methyl ester is obtained from
g of 4-cyanobenzoyl chloride and 40 g of
methoxycarbonylmethylene-triphenylphosphorane (yield 5.5 g; mp:
103-106°C) analogously to Chem. Ber. 9'4 (1961) 3005 and further
reacted as follows:
a) 185 mg of the ester and 75 mg of imidazole are refluxed
in 5 ml of tetrahydrofuran for 20 hours. It is distributed
between 1 M hydrochloric acid and ether, the acid water phase is
separated, it is alkalized with potassium carbonate and then
extracted with ethyl acetate. After drying and concentration by
evaporation, 150 mg of crystals, which represent a Z:E mixture in
the ratio 5.2:1 of the product produced in example 1, is
obtained.
2. 9
e~~r~;i' ~~;n~~~
b) 185 mg of the ester, 75 mg of imidazole and 0.07 mg of
triethylamine in 5 m7, of tetrahydrofuran are reacted and worked
up as in a). 150 mg of product is obtained: Z:E = 6.5:1.
c) 185 mg of ester and 155 mg of N-trimethylsilylimidazole
in 5 ml of. tetrahydrofuran are treated as in a). 100 mg of
product is obtained; Z:E = 4:1.
EXAt~tPLE 9.5
3-(4-Fluorophenyl)-3-(1-imidazolyl)-acrylic acid-tart-butyl
ester
Analogously to example 9, with use of 4-fluorobenzoic acid,
a Z-/E-mixture of the title compound is obtained. After
chromatography and recrystallization, the E-isomer is obtained
pure.
Mp: 90-93°C
EXAMPLE 1.6
3-(4-Chlorophenyl)-3-(1-imidazolyl)-acrylic acid-tart-butyl
ester
.Analogously to example 9, with use of 4-chlorobenzoic acid.
The E-isomer melts at 114-122°C (from cyclohexane).
3~
~1 1~(~S~~~ qtr
~~I:.:1 el ~Pnl~
E~~AMMPE~~ 17
3-(4-Bromophenyl)-3-(1-imidazolyl)-acrylic acid-tert-butyl
ester
Analogously to example 9, with use of 4-bromobenzoic acid.
The E-isomer melts at 131-132°C (from isopropanol).
~XAMPl,E ~.8
3-(4-Fluorophenyl)-3-(1,2,4-triazol-1-yl)-acrylic acid-tert-
butyl ester
Analogously to example 9, with use of 4-fluorobenzoic acid
and 1-trimethylsilyl-1,2,4-triazole. The E-isomer melts at 72--
74°C.