Note: Descriptions are shown in the official language in which they were submitted.
_...
1 BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a process for
producing an aromatic compound represented by the
general formula (I):
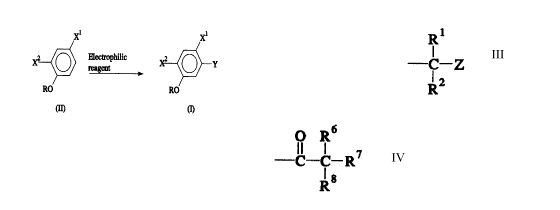
X1
X2 O Y (I)
RO
[wherein X1 and X2, which may be the same or different,
are halogen atoms; R is a group represented by the
formula
R1
-C-Z
R2
(wherein Rl and R2, which may be the same or different,
are hydrogen atoms or lower alkyl groups, Z is a cyano
group, -CO-OR3 (wherein R3 is a hydrogen atom or a lower
alkyl group) or -CO-N(R4)R5 (wherein R4 and R5, which
may be the same or different, are hydrogen atoms or
lower alkyl groups, R4 and R5 being able to be taken
together to represent an alkylene group)); and Y is a
- 1 -
1 nitro group, a halogen atom, a haloalkyl group or a
group represented by the formula:
O R6
-C -C R~
-
R8
(wherein R6, R~ and R8, which may be the same or
different, are hydrogen atoms, halogen atoms or cyano
groups)) which comprises reacting an electrophilic
reagent with a compound represented by the general
formula (II):
X1
X2 O (II)
RO
(wherein X1, X2 and R have the same meanings as those
defined above), and aromatic derivatives thus produced.
Related Art
Electrophilic substitution reaction on a
benzene ring has been known since early times, but there
has not been known any process by which a 1,2,4,5-
substituted benzene derivative of the general formula
(I) can be selectively obtained from the compound of the
general formula (II) used in the present invention.
- 2 -
~~98~~~
Rec. Trav. Chim., 75, 190 (1956) discloses the following
process:
C1 C1
C1 O A1C13/C1COCH2C1 C1
CH30 HO COCH2C1
When the above process is employed, a
substituent cannot be introduced at the desired position
of substitution and moreover the methoxy group is
converted to a hydroxyl group. Thus, there cannot be
obtained a compound formed by selective introduction of
a substituent into the position of substitution corre-
sponding to the general formula (I) which represents the
compound obtained in the present invention.
SUMMARY OF THE INVENTION
The present inventors earnestly investigated a
method for introducing a substituent into an aromatic
ring selectively, and have consequently accomplished the
present invention. The aromatic compound of the general
formula (I) obtained by the production process of the
present invention is useful as an intermediate of
medicines, pesticides, chemicals, etc, and some of them
are novel.
- 3 -
1 The term "lower" alkyl group or the like in
the present specification denotes a group having one to
six carbon atoms.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present inventive process for producing an
aromatic compound of the general formula (I) is
explained below in detail.
Nitration reaction
This reaction is such that an aromatic
compound of the general formula (I-I) can be produced by
selective nitration of a compound of the general formula
(II) with a nitrating agent in the presence of an inert
solvent.
Xi Xi
X2 ~ Nitrating X2 O NOZ
agent
RO CH30
(II)
(I-1)
wherein Xl, XZ and R have the same meanings as those
defined above.
As the inert solvent usable in this reaction,
any solvent can be used so long as it does not inhibit
the progress of the reaction greatly. There can be
exemplified nitric acid, sulfuric acid, acetic acid,
- 4 -
~,~~~3~
1 trifluoroacetic acid, and trifluoromethanesulfonic acid.
These inert solvents may be used singly or as a mixture
thereof .
As the nitrating agents, there can be used,
for example, nitric acid, nitric acid-sulfuric acid,
fuming nitric acid, fuming nitric acid-sulfuric acid,
nitric acid-acetic acid, nitric acid-acetic anhydride,
nitric acid-trifluoroacetic acid, and nitric acid-
trifluoromethanesulfonic acid.
The amount of the nitrating agent used may be
properly chosen in the range of 1 mole to excess moles
per mole of the compound of the general formula (II).
The reaction temperature may be chosen in the
range of -20°C to 150°C and is preferably 0°C to
50°C.
Although the reaction time is varied depending
on the reaction temperature, the degree of reaction,
etc., it may be chosen in the range of several minutes
to 100 hours.
After completion of the reaction, the desired
compound is isolated from the reaction mixture
containing the compound by a conventional method such as
solvent extraction, and if necessary, purified by
recrystallization, etc., whereby the desired compound
can be produced.
~ Halogenation reaction
This reaction is such that an aromatic
compound of the general formula (I-2) can be produced by
- 5 -
~~98~~9
1 selective halogenation of a compound of the general
formula (II) with a halogenating agent in the presence
of an inert solvent.
X1 X1
X2 O Halogenating X2 O y1
agent
RO RO
(II) (I-2)
wherein X1, X2 and R have the same meanings as those
defined above, and Yl is a halogen atom.
As the inert solvent usable in this reaction,
any solvent may be used so long as it does not inhibit
the progress of the reaction greatly. There can be
exemplified halogenated hydrocarbons (e. g. dichloro-
methane, chloroform, carbon tetrachloride, and dichloro-
ethane), sulfuric acid, acetic acid, trifluoroacetic
acid, trifluoromethanesulfonic acid, dimethylformamide,
1,3-dimethyl-2-imidazolidinone, and sulfolane. These
inert solvents may be used singly or as a mixture
thereof.
As the halogenating agent, there can be used,
for example, chlorine, bromine, chlorine-bromine,
bromine-aluminum chloride, bromine-iron, and bromine-
silver sulfate.
The amount of the halogenating agent used may
be properly chosen in the range of 1 mole to excess
- 6 -
~~9~~3~
1 moles per mole of the compound of the general formula
(II).
The reaction temperature may be chosen in the
range of 0°C to 150°C and is preferably 20°C to
100°C.
Although the reaction time is varied depending
on the reaction temperature, the degree of reaction,
etc., it may be chosen in the range of several minutes
to 100 hours.
After completion of the reaction, the desired
compound is isolated from the reaction mixture contain-
ing the compound by a conventional method such as
solvent extraction, and if necessary, purified by
recrystallization, etc., whereby the desired compound
can be produced.
~3 Friedel-Crafts reaction
This reaction is such that an aromatic
compound of the general formula (I-3) can be produced by
reacting a compound of the general formula (II) with a
Lewis acid and a compound of the general formula (III),
(IV) or (V) in the presence or absence of an inert
solvent and in the presence or absence of a salt.
X1 X1
Friedel-Crafts reaction
X2
X2 O Y1
0 R6 O R6
RO II ~ II I RO
X3-C-C-R~ O(-C-C-R~)2 C(X4)q
I
(II) R8 R8 (V) (I-3)
(III) (IV) or
",.
1 wherein Xl, X2 and R have the same meanings as those
defined above, and Y1 is a haloalkyl group or a group
represented by the formula:
O R6
-C-C-R~
R8
(wherein R6, R~ and R8, which may be the same or
different, are hydrogen atoms, halogen atoms or cyano
groups), X3 is a halogen atom, and X4's, which may be
the same or different, are halogen atoms.
This reaction proceeds in the presence or
absence of an inert solvent. As the inert solvent,
there can be used, for example, nitroalkanes such as
nitromethane, etc.; halogenated hydrocarbons such as
dichloromethane, carbon tetrachloride, tetrachloro-
ethane, dichloroethane, etc.; aromatic hydrocarbons such
as nitrobenzene, etc.; amides such as N-methyl-
pyrrolidone, N,N-dimethylformamide, etc.; urea
derivatives such as N,N,N',N'-tetramethylurea, N,N-
dimethylimidazolinone, etc.; organic bases such as
pyridine, triethylamine, etc.; organosulfur compounds
such as carbon disulfide, dimethyl sulfoxide, sulfolane,
etc.; alcohols such as ethanol, ethylene glycol, etc.;
nitriles such as acetonitrile, benzonitrile, etc.; and
organophosphorus compounds such as phosphorus
oxychloride, hexamethylphosphoramide, etc. These inert
solvents may be used singly or as a mixture thereof.
_ g _
2.p9~~~
1 Although not critical, the amount of the inert
solvent used is preferably 0.5 to 10 moles per mole of
the compound of the general formula (II).
As the salt usable in the present invention,
there can be exemplified sodium chloride, potassium
chloride, calcium chloride, magnesium chloride, lithium
chloride, sodium bromide, potassium bromide, lithium
bromide, ammonium salts (e. g. tetramethylammonium
chloride), and sulfonates (e. g. sodium trifluoromethane-
sulfonate). These salts may be used singly or as a
mixture thereof.
The amount of the salt used may be properly
chosen in the range of 0.5 to 10 moles per mole of the
compound of the general formula (II).
As the Lewis acid, there can be used Lewis
acids such as A1C13, AlHrg, AlIg, FeCl3, FeHr3, TiCl4,
SnCl4, ZnCl2, GaCl3, etc.
The amount of the Lewis acid used may be
properly chosen in the range of 1 mole to excess moles
per mole of the compound of the general formula (II) and
is preferably 3 to 8 moles per mole of this compound.
The amount of the compound of the general
formula (III), (IV) or (V) used may be properly chosen
in the range of 0.5 to 2 moles per mole of the compound
of the general formula (II).
The compound of the general formula (V) may be
used both as reactant and as inert solvent. In this
case, it may be used in large excess.
- 9 -
,..
1 The reaction temperature may be chosen in the
range of 0°C to 180°C and is preferably 60°C to
100°C.
Although the reaction time is varied depending
on the reaction temperature, the degree of reaction,
etc., it may be chosen in the range of several minutes
to 100 hours.
After completion of the reaction, the desired
compound is isolated from the reaction mixture contain-
ing the compound by a conventional method such as
solvent extraction, and if necessary, purified by
recrystallization, etc., whereby the desired compound
can be produced.
As mentioned before. some of the compounds
thus prepared are novel. That is, an aromatic compound
represented by the general formula (I):
X1
X2 ~ Y (I)
RO
[wherein Xl and X2, which may be the same or different,
are halogen atoms; R is a group represented by the
formula
R1
-C-Z
R2
- 10 -
2098239
(wherein R1 and R2, which may be the same or different, are
hydrogen atoms or lower alkyl groups, Z is a cyano group,
-CO-ORS (wherein R3 is a hydrogen atom or a lower alkyl
group) or -CO-NR4R5 (wherein R4 and R5, which may be the same
or different, are hydrogen atoms or lower alkyl groups, R4
and R5 being able to be taken together to represent an
alkylene group)); and Y is a nitro group, a halogen atom, a
haloalkyl group or a group represented by the formula:
O R6
-C-C-R~
Rg
(wherein R6, R7 and R8, which may be the same or different,
are hydrogen atoms, halogen atoms or cyano groups),
provided [1] that X1 is fluorine atom, X2 is chlorine atom
and Z is cyano group or -CONR4R5, when Y is nitro group, [2]
that X1 is fluorine atom, X2 is chlorine atom and Z is cyano
group, when Y is fluorine atom) [3] that X1 is fluorine atom,
X2 is chlorine atom and Z is -COORS (wherein R3 is other than
a hydrogen atom), -CONR4R5 or cyano group, when Y is chlorine
atom and [4] that Z is a cyano group, -COORS (wherein R3 is
other than a hydrogen atom) or -CONR4R5, when Y is an iodine
atom] is novel.
- 11 -
25711-668
2098239
Among them, those whose Y is
O R6
-C-C-R'
Rg
- lla -
25711-668
'~Q~~~3
1 wherein R6, R~ and R8, which may be the same or
different, are hydrogen atoms, halogen atoms or cyano
groups are useful as an intermediate for producing the
herbicides disclosed in Japanese Patent Kokai (Laid-
Open) No. 3-163063 (JP-A-3-163063).
Especially, compounds whose R is
R1
-C-Z
R2
wherein R1 and R2, which may be the same or different,
are hydrogen atoms or lower alkyl groups, Z is
-CON(R4)R5 (wherein R4 and R5, which may be the same or
different, are hydrogen atoms or lower alkyl groups, R4
and R5 being able to be taken together to represent an
alkylene group) is quite useful as an intermediate for
said herbicides.
EXAMPLES
Typical examples of the present invention are
given below but they should not be construed as limiting
the scope of the invention.
Example 1
Production of (2-chloro-4-fluoro-5-nitro-
phenoxy)acetamide (compound No. 1)
- 12 -
,.
F F
C1 O C1 O N02
NCCHZO HZNCCH20
0
1 In 8 ml of 97% sulfuric acid was dissolved
3.7 g (0.02 mole) of (2-chloro-4-fluorophenoxy)aceto-
nitrile, and a mixed acid of 2.5 ml of 60 - 62% nitric
acid and 5.8 ml of 97% sulfuric acid was added to the
resulting solution with stirring at 10°C or lower, after
which the reaction was carried out at room temperature
for 1.5 hours.
After completion of the reaction, the reaction
solution was poured into ice water and the crystals
1~ precipitated were collected by filtration, washed with
water and then dried to obtain 3.4 g of the desired
compound as yellow crude crystals (yield: 68%).
The crude crystals obtained were recrystal-
lined from ethyl acetate to obtain 2.5 g of the desired
compound as light-yellow crystals.
Physical properties: m.p. 182 - 182.5°C, yield
50.5%.
NMR [DMSO/TMS, d values (ppm)]
4.75 (s, 2H), 7.50 (bd, 2H, J=0.6Hz),
7.75 (d, 2H, J=7Hz), 7.97 (d, 2H, J=llHz).
- 13 -
1 Example 2
Production of (2-chloro-4-fluoro-5-nitro-
phenoxy)acetamide (compound No. 1)
F F
C1 O C1 O N02
HZNCCH20 H2NCCH20
O 0
Reaction was carried out for 5 hours in the
same manner as in Example 1, except that 4.1 g (0.02
mole) of (2-chloro-4-fluorophenoxy)acetamide was used in
place of (2-chloro-4-fluorophenoxy)acetonitrile, to
obtain 3.6 g of the desired compound.
Yield: 72.4.
Example 3
Production of (2-chloro-4-fluoro-5-nitro-
phenoxy)acetic acid (compound No. 2)
F F
C1 O C1 O NOZ
C2H50CCH20 HOCCHZO
O 0
In the same manner as in Example 1, 4.6 g
(0.02 mole) of ethyl (2-chloro-4-fluorophenoxy)acetate
14 was reacted, followed by overnight standing at room
temperature.
- 14 -
,~~~g~3g
1 After completion of the reaction, the reaction
solution containing the desired product was poured into
ice water, and the desired product was extracted with
ethyl acetate.
The extracted solution was washed with water
and dried over magnesium sulfate, after which the
solvent was distilled off under reduced pressure. The
resulting residue was purified by a silica gel column
chromatography (CHZC12-CHgOH) to obtain 1.3 g of the
l0 desired compound asocherous crystals.
Yield: 30.2$.
NMR [DMSO/TMS, d values (ppm)]
4.57 (s, 2H), 7.50 (bd, 2H, J=0.6Hz),
7.75 (d, 2H, J=7Hz), 7.97 (d, 2H, J=llHz),
13.90 (bs, 1H).
Example 4
Production of (5-bromo-2-chloro-4-fluoro-
phenoxy)acetonitrile (compound No. 3)
F F
C1 O C1 O Br
NCCH20 NCCH20
In 10 ml of methylene chloride was suspended
1.0 g (7.5 mmoles) of anhydrous aluminum chloride, and
1.0 g (5.4 mmoles) of (2-chloro-4-fluorophenoxy)aceto-
- 15 -
.. ~~39~~~~
1 nitrile was added to the suspension, after which 0.95 g
(5.9 mmoles) of bromine was added dropwise with
refluxing. After completion of the dropwise addition,
the reaction was carried out with refluxing for 2 hours.
After completion of the reaction, the reaction
mixture was allowed to cool and then poured into ice
water, and the desired compound was extracted with
ether.
The extracted solution was washed successively
with water, a 10% aqueous sodium thiosulfate solution
and a saturated aqueous sodium chloride solution, and
dried over magnesium sulfate. Then, the solvent was
distilled off under reduced pressure and the resulting
residue was recrystallized from n-hexane to obtain 1.1 g
of the desired compound.
Physical properties: m.p. 72.3°C, yield 77%.
Example 5
Production of (2-chloro-5-chloroacetyl-4-
fluorophenoxy)acetamide (compound No. 6)
F F
C1 O C1 O COCHZC1
H2NCCH20 HyNCCH20
O O
With 2.0 g (15.0 mmoles) of anhydrous aluminum
chloride was mixed 0.85 g (7.5 mmoles) of chloroacetyl
- 16 -
z
1 chloride, and the resulting mixture was heated to 80°C.
Then, 1.0 g (4.9 mmoles) of (2-chloro-4-fluorophenoxy)-
acetamide was added and the reaction was carried out at
90°C for 9 hours.
After completion of the reaction, the reaction
mixture was cooled to 80°C and 5 ml of acetic acid was
added. The mixture thus obtained was poured into ice
water, and the crystals precipitated were collected by
filtration and recrystallized from ethanol to obtain 1.0
g of the desired compound.
Physical properties: m.p. 166.3°C, yield 73%.
Example 6
Production of (2-chloro-5-dichloroacetyl-4-
fluorophenoxy)acetamide (compound No. 7)
F F
'C 1
C1 O .---~ C1 O COC/H
-C 1
HZNCCHZO H2NCCH20
O O
With 2.0 g (15.0 mmoles) of anhydrous aluminum
chloride was mixed 0.93 g (6.3 mmoles) of dichloroacetyl
chloride, and the resulting mixture was heated to 50°C.
Then, 1.0 g (4.9 mmoles) of (2-chloro-4-fluorophenoxy)-
acetamide was added and the reaction was carried out at
7p - g0°C for 8 hours.
- 17 -
1 After completion of the reaction, the reaction
mixture was allowed to cool and ice water was added and
then stirred for 2 hours. The desired compound was
extracted with ethyl acetate and the extracted solution
was washed with water and dried over magnesium sulfate.
Then, the ethyl acetate was distilled off under reduced
pressure, and the resulting residue was purified by a
silica gel column chromatography to obtain 0.5 g of the
desired compound.
Physical properties: m.p. 132.3°C, yield 33%.
Example 7
Production of (2-chloro-5-chloroacetyl-4-
fluorophenoxy)acetonitrile (compound No. 11)
F F
C1 O C1 O COCHZC1
NCCH20 NCCH20
With 2.0 g (15.0 mmoles) of anhydrous aluminum
chloride was mixed 0.85 g (7.5 mmoles) of chloroacetyl
chloride, and the resulting mixture was heated to 60°C.
Then, 0.9 g (4.0 mmoles) of (2-chloro-4-fluorophenoxy)-
acetonitrile was added and the reaction was carried out
at 70°C for 3 hours.
After completion of the reaction, the reaction
mixture was poured into ice water and stirred for 1
- 18 -
.. ..
1 hour. The crystals precipitated were collected by
filtration and recrystallized from ethanol to obtain
0.93 g of the desired compound.
Physical properties: m.p. 122.1°C, yield 73%.
Example 8
Production of (2-chloro-5-dichloroacetyl-4-
fluorophenoxy)acetonitrile (compound No. 13)
C1
C1 O ~. Cl O LOCH
'C 1
NCCH20 NCCH20
With 2.0 g (15.0 mmoles) of anhydrous aluminum
chloride were mixed 0.93 g (6.3 mmoles) of
dichloroacetyl chloride and 0.9 g (4.9 mmoles) of (2-
chloro-4-fluorophenoxy)acetonitrile, and the reaction
was carried out at 60°C for 2 hours.
After completion of the reaction, the reaction
mixture was allowed to cool and 5 ml of nitromethane was
added) The resulting mixture was poured into ice water,
after which the desired compound was extracted with
ethyl acetate and the extracted solution was washed with
water and dried over magnesium sulfate. Then, the
solvent was distilled off under reduced pressure, and
the resulting residue was purified by a silica gel
column chromatography to obtain 0.97 g of the desired
compound.
Physical properties: m.p. 98.7°C, yield 67%.
- 19 -
1 Example 9
Production of (2-chloro-4-fluoro-5-trichloro-
methylphenoxy)acetonitrile (compound No. 14)
F F
C1 O C1 O CC13
NCCHZO NCCH20
In 10 ml of carbon tetrachloride was suspended
1.5 g (11.2 mmoles) of anhydrous aluminum chloride, and
1.0 g (5.4 mmoles) of (2-chloro-4-fluorophenoxy)aceto-
nitrile was added dropwise. After completion of the
addition, the reaction was carried out at 60°C for 1
hour.
After completion of the reaction, the reaction
mixture was allowed to cool and ice water was added and
then stirred for 1 hour. The desired compound was
extracted with ethyl acetate and the extracted solution
was washed with water and dried over magnesium sulfate.
Then, the solvent was distilled off under reduced
pressure, and the resulting residue was purified by a
silica gel column chromatography to obtain 1.2 g of the
desired compound as an oil.
Physical properties: oil, yield 72~.
NMR [CDC13/TMS, d values (ppm)]
4.88 (s, 2H), 7.09 (d, 1H, J=10.4Hz),
7.79 (d, 1H, J=7.lHz).
- 20 -
1 Example 10
Production of (2-chloro-5-cyanoacetyl-4-
fluorophenoxy)acetonitrile (compound No. 17)
F F
C1 O C1 O COCH2CN
NCCHZO NCCH20
To 4.5 g (33.6 mmols) of anhydrous aluminum
chloride was added 0.57 g (7.8 mmols) of dimethyl-
formamide (DMF), and 1.0 g (5.6 mmoles) of (2-chloro-4-
fluorophenoxy)acetonitrile was added to the suspension
at room temperature. Then, 2.9 g (28.0 mmoles) of
cyanoacetyl chloride was slowly dropped into the
resulting mixture. After completion of the dropping,
the reaction was carried out at 55°C for 3 hours.
After completion of the reaction, the reaction
mixture was analyzed by a thin layer chromatography and
a gas chromatography (area percentage: 7.0%). The
analysis results obtained were in agreement with those
obtained for a standard substance, whereby the
production of the desired compound was confirmed.
Compounds of the general formula (I) are
listed in Table 1.
X1
X2 ~ Y (
RO
- 21 -
2098239
Table 1
No R X1 X2 Y Physical properties
1 H2NCOCH2 F C1 N02 m.p. 182.0-182.5C
2 HOOCCH2 F C1 N02 d (DMSO)=4.57 (s, 2H),
7.57 (d, 2H, J=7.OEz),
7.89 (d, 2H, J=1l.OHz),
13.9 (bs, lE).
3 NCCH F C1 Br m.p. 72.3C
2
4 HZNCOCH2 F Cl COCH3 d (CDC13)=2.64 (d, 3H,
J=3.3Hz), 4.51 (s, 2H),
5.70 (bs, 1H), 6.60
(bs, 1H), 7.28 (d, 1H,
J=7.9Hz), 7.4. (d, 1H,
J=5.9Hz).
H2NCOCH2 C1 C1 COCH2C1 m.p. 171.7C
6 HZNCOCfi2 r~ Ci COCH2C1 m.p. 166.3C
7 H2NCOCH2 F C1 COCHC12 m.p. 132.3C
8 H2NCOCH2 F C1 CC13 m.p. 214.7C
9 NCCH2 F C1 COCH3 d (CDC13)=2.63 (d, 3H,
J=3.3Hz), 4.35 (s, 2H),
7.31 (d, 1H, J=7.6Hz),
7.54 (d, 13, J=6.lHz).
i0 NCCH2 C. C1 COCH2C1 m.p. 110.9C
11 NCCHZ r~ C1 COCfi2C1 m.p. 122.1C
12 NCCH2 F C1 COCH2Br d (CDC13)=4.49 (d, 2fi,
J=2.4Hz), 4.90 (s, 2H),
7.33 (d, 1H, J=9.9Hz),
7.60 (d, 1H, J=6.OHz).
13 NCCH2 F C1 COC3C12 m.p. 98.7C
14 NCCH2 F C1 CC13 d (CDC13)=4.88 (s, 2H),
7.09 (d, 13, J=10.4Hz),
7.79 (d, lE, J=7.lHz).
(to be continued)
- 22 -
25711-668
~~9~~~9
Table 1 (Cont'd)
No R X1 XZ Y Physical properties
15 H2NCOCH F C1 COCH2C1 8 (CDC13)=1.63 (d, 3H,
J=6.6Hz), 4.65 (q, 2H,
CH3 J=6.6Hz), 4.63 (d, 2H,
J=3Hz), 6.00 (bs, 1H),
6.67 (bs, 1H), 7.26 (d,
1H, J=9.9Hz), 7.45 (d,
1H, J=5.7Hz).
16 NCCH F C1 COCHZC1 8 (CDC13)=1.67 (d, 3H,
J=6.8Hz), 4.98 (q, 2H,
CH3 J=6.98Hz), 4.70 (d, 2H,
J=3.lHz), 6.00 (bs,
1H), 7.30 (d, 1H,
J=lOHz), 7.59 (d, 1H,
J=5.8Hz).
17 NCCH2 F C1 COCH2CN
Aromatic compound derivatives represented by
the formula (I') are important especially as inter-
mediates in preparation of the herbicides disclosed in
Japanese Patent Kokai (Laid-open) No. 3-163063. The
typical herbicides which are final products can be
prepared, for example, by the process as illustrated
below.
- 23 -
.. ~~9~~~9
Y R1 0 y Rl
I ~~ I
X - O O-C-R R3"O-C-OR3" X O 0-C-R
Base
CH3C R2 R3"0-C-CH2C R2
O 0 O
(I)
Cyclyzation X ~ alkylation
Rl N
I
R-C-O ~ OH
R2 R4 n
Halogena-
y tion y Hal
R1 R~
I 5" I ORSn
R. ~ -0 al R- ~ -0 al
R4 n R4 n
R2 R2
1 (wherein R, R1, R2, X and Y are as defined above, R3"
denotes a lower alkoxyl group, R4" denotes a lower alkyl
group or a lower haloalkyl group, R5" denotes a lower
alkyl group or a lower haloalkyl group and Hal denotes a
halogen atom.)
- 24 -