Note: Descriptions are shown in the official language in which they were submitted.
- 23189-7514
The inven~ion relates to substituted (benzothiazolyl- and
quinoxalinyl methoxy)phenyl-acetic acid derivatives, to
processes for their prepara~ion and to their use in
medicaments.
It has already been disclosed that ~-substituted 4-(quin-
olin-2-yl-methoxy)phenylacetic acid derivatives have a
lipoxygenase inhibiting action [cf. EP 344,519 (US
4,970,215) and EP 339,416].
The present invention relate~ to substituted ~benzo-
thiazolyl- and quinoxalyl-methoxy)phenyl-acetic acid
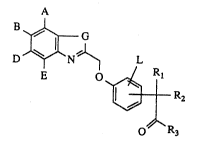
derivatives of the general formula (I)
A
D ~ N ~ L
in which R
~, B, D, E and L are identic~l or different and
represent hy~rogen, hydroxyl, halogent trifluoro-
methyl, trifluoromethoxy or carboxyl, or
repre~nt straight-chain or branched alkyl having up
to 10 carbon a~om~, which i8 optionally sub~ti~uted
Le A 29 140 - 1 -
- `~ 2 ~ ~ ~ 3 ~ ~
by hydroxyl or halogen, or
repres~nt straight-chain or branched alkoxy or
alkoxycarbonyl having up to 10 carbon atoms, or
represent aryl having 6 to 10 carbon atoms, which i.s
optionally su~stituted by halogen, nitro or cyano or
by straight-chain or branched alkyl or alkoxy each
having up to 8 carbon atoms,
G represents a sulphur atom or the group of the
formula -N=CH-,
R1 represents cycloal~yl having 3 to 12 carbon atom~,
or
represents ~kraight-chain or branehed alkyl having
up ~o 10 carbon atoms, which i8 optionally ~ub~tl-
tuted by aryl having 6 to lO carbon atoms,
R2 represents hydrogen, or
represents a group of the formula -oR4,
in which
R4 denote~ hydrogen, straight-chain or branched
alkyl having up to 8 carbon a~om~, ben~yl or
phenyl,
R3 repre3ants hydroxyl, ~traight-chaln or branched
alkoxy having up to 8 carbon atoms or phenoxy, or
repre~ents a group of the formula -NHSo2-R5J
Le A 29 140 - 2 -
`
~9~
in which
R5 ~denotes s~raight-chain or branched alkyl having
up to 8 carbon atoms, which is optionally
substituted by phenyl, which can in turn be
sub~tituted by straight-chain or branched alkyl
having up to 6 carbon atoms or halogen, ~r
d~notes phenyl which can optionally be ~ubsti-
tuted by halogen or trifluoromethyl or by
s~raight-chain or branched alkyl or alkoxy each
having up ~o 6 carbon a~o~s
and their salt~.
In the context of the present invention, physiologically
acceptable ~alts are preferred. Phy~iologically accep-
table 8alt9 of the substituted (benzothiazolyl- and
quinoxalyl-methoxy)phenyl-acetic acid derivatives c~n be
~alts of the substances according to the invention with
mineral acid~, carboxylic acids or sulphonic acids.
Particularly preferred ~alts are, for example, tho~e with
hydrochloric acid, hydrobromic acid, sulphuric acid,
~0 . phosphoric acid, methane~ulphonic acidr ethanesulphonic
acid, toluenesulphonic acid, benzene~ulphonic acid,
naphthalenedisulphonic acid, acetic acid, propionic acid,
lactic ~a~id, tartaric acid, ci~ric ac~d, f~maric acid,
maleic acid or benzoic acid.
:: :
Salt3 in the context of ~he present invention are addi-
tLon-lly metal aalt~, preferably of th- unlval-nt metals,
Le A 29 140 - 3 -
- ~2~9~3~
and the am~onium salts. Alkali metal salts such as, for
example, sodium, potassium and ammonium salts are
preferred;
The compounds according to the invention exist in stereo-
isomeric forms which behave either as image and mirror
.image (enantiomers), or which do not behave as image and
mirror image (dias~ereomers). The invention relates both
to the antipodes and to the racemic forms as well as to
the diastereomer mixtures. Like the diastereomer~, the
racemic forms can also be separated in a known manner
into the stereoisomerically uniform constituents
tcf. E.L. Eliel, Stereochemistry of Carbon Compounds,
McGr~w Hill, 1962].
Preferred compounds of the general formula (I) are those
in which
A, B, D, E and L are identical or different and
represent hydrogen, fluorine, chlorine, bromine,
trifluoromethoxy or carboxyl,
represent straight~chain or branched alkyl having up
to 8 carbon atom~, which is optionally ubsti~uted
by hydroxyl, fluorine, chlorine or bromine,
represent straight-chain or branched aI~oxy or
alkoxycarbonyl each having up to 8 carbon atomæ, or
repre~ent phenyl which is optionally ~ub~tituted by
fluorine, chlorine, bromine, nitro or cyano or by
straight-chain or branched alkyl or alkoxy each
Le A 29 140 - 4 -
3 ~ ~
having up to 6 carbon atom~,
G repr~sent~ a sulphur atom or the group of the
formula -N=CH-,
Rl represents cyclopropyl~ cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl or cyclooctyl, or
represent~ straight chain or branched alkyl having
up to 8 carbon atoms r which is optionally substi-
tuted by phenyl or naphthyl,
R2 represents hydrogen or a group of the formula -oR4,
in which
.
R4 denote~ hydrogen, straight-chain or branched
alkyl having up to 6 carbon atoms, benzyl or
phenyl,
R3 represents hydroxyl or s~raight-chain or branched
alkoxy having up to 6 carbon atoms, or
: represents a.group of the formula ~NHSo2-~5,
in which
R5 denote~ straight-chain or branched alXyl having
up to 6 carbon atom~, which 1 optionally
sub~tituted by phenyl which can in turn be
sub~tituted by straight-chain or branched alkyl
having up to 6 carbon atom~, fluorine, chlorine
Le A 29 140 - 5 -
;~983~
or bromine,
or
denotes phenyl which can optionally be substi-
tuted by fluorine, chlorine or bromine or by
straight-chain or branched alkyl having up to
4 carbon atoms
and their ~alts.
Particularly preferred co~pounds of the general formula
(I) are those
in which
A, B, D, E and L are identical or different and
represent hydrosen, fluorine, ~hlorine, bromine or
traight-chain or branched alXyl or alkoxy each
having up to 6 carbon atom~,
15 G represents a sulphur atom or the group of the
formula -N=CH-,
:: Rl represents cyclopentyl, cyclohexyl or cycloheptyl r
or
repre~ents:straight-chain or branched al~yl having
: 20 up t~ 6 carbon atoms, which is optionally ~ubsti-
tuted by phe~yl,
R2: represents hydrogen or a group of the foxmula -OR'j
Le A 29 140 - 6 -
. ' '
,
,
.
æ~983~
in which
R4 denotes hydrogen, straight-chain or branched
alkyl having up to 4 carbon atoms or phenyl,
R3 represents hydroxyl or straight-chain or branched
alkoxy having up to 4 carbon atoms, or
represents a group of the formula -NHSoz-R5r
in which
Rs denokes ætraight-chain or branched alkyl having
up to 4 carbon atoms, which i6 optionally
subs~ituted by phanyl which can in turn be
~u~stituted by straight chain or branched alXyl
having up ~o 4 carbon atoms, fluorine or
chlorine~
or
denotas phenyl which can optionally be substi-
tuted by fluorine, chlorine or methyl
and their salts.
Very particularly preferred compounds are those in which
the radical -CRlR2-~oR3 is in th~ 3- or 4-position
relative to the heterocyclylmethoxy radical.
Moreover, a process for the preparation of the compounds
of the formula (I) ha~ been found, which is characterised
in that
Le A 29 140 - 7 -
c~9~3~
[A] compounds of the general foxmula (Ia~
A
D ~ N ~ L Rl (Ia)
~ CH- C - R3
in which
A, B, D, ~, G, L, Rl and R3 have the meaning mentioned,
are prepared by etherifying
c~mpounds of the general formula (II~
A
G : ~ (IIj
~: D ~ N l CH2-M
.
:: : :
in which
:
: : A,~ B, D, E and G have the meaning mentioned
:
10~ ~ and
~ represents halogen, preferably bromine,
:
Le A 29 140 - 8 -
" ~ ~
either directly with compounds of the general formula
(III)
R6O L Rl
CH-C - R7 (III)
o
in which
~6 repreæents a typical hydroxyl protective group such
as, for example, benzyl or tert-butyl, preferably
benzyl,
and
R7 ropresents Cl-C8-alkoxy,
in inert so1vents, i~ appropriate in the pre~ence of
ba~e, after removal of the protective group,
or
initially etherifying in a firs~ step with compounds of
the general formula (IIIa)
lS ~ CH2~ R7 (IIIa)
O
in which
Le A 29 140- 9 -
2~3~$
R5 and R7 have the meaning mentioned,
in inert solvents, if appropriate in the presence of a
base, after removal of the protective group and then
alkylating in a second step with compounds of the general
S formula (IV)
Rl-T (IV)
in which
Rl has th~ meaning mentioned,
and
: T represents halogen, pre~erably chlorine or bromine,
in inert solvents, if appropriate in the presenc~ of a
base
and
in ~he case of the acids (R3 = O~)j hydrolysing the esters
by customary methods,
and in the case of the sulphonamides (R3 = NHSo2R5),
adding an amidation~ starting from th~ corresponding
acids, if appropriate after priox activation thereof, by
reaction with the re~pective sulphonamines of ~he general
formula (VIII)
Le A 29 140 - 10 -
3 ~ ~
HzN--So2R5 (VIII ~
in inert solvents, if appropriate in the presence of a
base and/or auxiliary,
. . and in the case of the various esters, optionall~ carry-
ing out a transesterification, likewise starting from the
activated carboxylic acids,
and in the case of the pure enantiomers s~parating the
corresponding acida,
and introducing or varying the aub~ti~uents A, B, D,
and ~, if appropriate, according to cu~tomary methods,
lB] :co~pounds of the genoral formula (IIb)
~ G
D ~ N ~ L Rl ~ (IIb)
E O ~
~ C ~ 3
OR4 o
in which
A, B, D, E, G, L, R1, R3 and R4 have the meaning
mentioned,
Le A 29 140
~9~3~
are prepared by converting
compounds of the general formula (II) initially by
reaction with compounds of ~he general formula (V)
(V3
O
in which
~, R5 and R7 have the abovementioned meaning
to the compounds of the general formula (VI~
A
D ~ N ~ ~ ~ (VI)
n which 7
:
A, B, D,:E, G, L and R7 have the abovemen~ionsd meaning,
by~theri~ication
and in a second step by reducing the product by reaction
with Grignard compound3 of the general formula (VII)
Le A 29 140- 12 -
L~ 3 ~
R1-V (VII)
in which
Rl has the abovementioned meaning
and
S V represents the typical Grignard radical W-Z,
in which
W denotes magnesium or ~inc
and
Z denotes chlorine, bromine or iodine,
or
represents lithium, sodium, magnesium~ aluminium or
zincl:
: i n inert . olvents, if appropriate in the presence of a
base, with removal of the group V,
and in the case in which R4 ~ H, etherifying according to
cus~o~ary methods
.
Le A 29 140 - 13 -
2~$3~
and
in the case of the acids (R3 = OH) hydrolysing the esters
by customary methods,
and in the case of the sulphonamides (R3 = NH-So2-R5)
adding an amidation, starting from the corresponding
acids, if appropriate after prisr activation thereof, by
reaction with the respective sulphonamines of the general
formula (VIII)
H2N-So2-R5 (VIII)
in which
R5 has the abovementioned meaning,
in inert ~olvents, if appropriate in the pre~ence of a
ba~e and/or auxiliary,
and in the case of the various e~t~rsl optionally carry-
ing out a transestarification, likewise starting from the
aotivated carboxylic acids,
and in the case of the pure enantiomers separating the
corresponding enantiomerically pure acids,
and introducing or varying the substituent A, B, D, E
and L, if appropriate, according to cuJtomary methods.
: The processes according to the invention can be
Le A 29 140 - 14 ~
~9~3~
illustrated by way of example by th~ following reaction
scheme:
~A]
~S~CH2 8~
CO2CH~
~ N
Co2H
I,e A 29 140 - 15 -
2~9~3~
[A]
0~S~CH2~r HO~0~CH2-CO2CHI
~S~ H3C-(CH2)3-Br
0~0,CH2-C02CH3
~N CO2H ~ H2N-sO2-cH3
o~~CH3
~S ~ CO-NH~$02~CH~
(CH2)3-c113
Le A 29 140 - 16 -
2~9~3~
[B]
~NlCH2-E1r ~ 1
C02CH~
M~
0~ , ,.. _. ~
CO2CH3
~N ~
~
. HO CO2CH3
' ~
HO CO~H
~he protective groups are removed ~ro~ the corresponding
ethers by a customary method, for example by hydrogenoly-
tio cleavage of the benzyl ethers in the abovemeneioned
::
Le A 29 140 - 17 -
3 ~ ~
inert solvents in the presence of a catalyst using
hydrogen gas [cf. additionally Th. Greene: ~Protective
Groups in Organic Synthesis~, J. Wiley/Sons, 1981, New
York].
Solvents for the etherification can be inert organic
solvent which do not change under the reaction condi-
tions. These preferably include ethers such as, for
ex~mple, dioxane, tetrahydrofuran or diethyl ether,
halogenohydrocarbons such as dichloromethane, trichloro-
methane, tetrachloromethana, 1,2-dichloroethane or
trichloroethylene, hydrocarbons such as benzene, xylene,
toluene, hexane, cyclohexane or mineral oil fractions,
nitromethane, dimethyl~ormamide, ace~onitrile, acetone or
hexamethylpho6phoramide. It is al80 possible to employ
mixtures of the solvents.
Bases which can be employed for the etherification are
inorganic or organic bases. These preferably include
alkali metal hydroxides such as, for example, sodium
hydroxide or potassium hydroxide, alkaline earth metal
hydroxide~ such as, for example, barium hydroxide, alkali
metal carbonates such as sodium carbonate or potassium
carbonate, alkaline earth metal carbonates such as
calcium carbonate, or organic amines ~trialkyl(C1-C6~-
amines) such as triethylamins t or heterocycles such as
pyridine, meth~lpiperidine, piperidine or morpholine.
I~ iB also possible to employ alkali metals such as
sodium and their hydrides, such as ~odium hydride, as
Le A 29 140 - 18 -
3 ~ ~
bases;
The ethe~ification is in general carried out in a
temperature range from 0C to l150C, preferably from
~10C to +100C.
.The etherification is in general carried out at normal
pressure. Howevert it is also possible to carry out the
process at reduced pressure ox elevated pressure (for
example in a range from 0.5 to 5 bar).
In general, 0.5 to 5, preferably 1 to 2 mol, of halide,
relative to 1 mol of the reaction component, are
employed. The base is in general employed in an amount
~rom 0.5 to 5 mol, preferably from 1 to 3 mol, relative
to the halide.
~ he hydrolysis of the carboxylic acid esters is carried
out by customary method~, by treating the esters with
customary bases in inert solvents.
. Suitable bases .for the hy.drolysis. are the customary
inorganic bases. These preferably include alkali metal
hydroxides or alkaline earth metal hydroxides such as,
for example, sodium hydroxide, potassium hydroxide or
barium hydroxide, ox alkali ~etal carbonates ~uch as
sodium carbonate or potassium carbonate or sodium
hydrogencarbonate. Sodium hydroxide or pota~ium
hydroxide are particularly preferably employed.
Le A 29 140 - 19 -
2~9~3~
Suitable solvents for the hydrolysis are water or the
organic solvents customary for hydrolysis~ These prefer
ably include alcohols such as methanol, ethanol, pro-
panol, isopropanol or butanol, or ethers such as tetra-
S hydrofuran or dioxane, or dimethylformamide or dLmethylsulphoxide. Alcohols such as methanol, ethanol, propanol
or isopropanol are.particularly preferabl~ used. It is
also possible to employ mixtures of the solvents
mentioned.
The hydrolysis i5 in general carried out in a temperature
range from 0C to +100C~ preferably from +20C to ~80C.
In general, the hydrolysis is carried out at normal
pres3ure. However, it i8 al80 possible to work at reduced
pressure or at elevated pressure (for example from 0.5 to
S bar).
When carrying out the hydrolysis, the base is in general
employed in an amount from 1 to.3 mol, preferably from 1
to 1.5 mol, rela~ive to 1 mol of the ester. Molar amounts
. o..the reactants are particularly.preferably used.
Suitable ~olvents for the reduction are ~he customary
organic solvents which do not change under the reaction
condition~. The~e preferably include ethers such as
diethyl ether, dioxane, tetrahydrofuran, glycol dLmethyl
ether, or hydrocarbon3 such as benzene, toluene, xylene,
hexane, cyclohexane, or mineral oil fractions or dimeth-
ylformamide. I~ is also possible to use mixtures of the
Le A 29 140 - 20 -
` 2~3~
~olvents mentioned. Tetrahydrofuran and diethyl ether are
preferred.
The reduction is in general carried out in a temperature
range from -80C to +30C, preferably at -40C to +25C.
The reduction is in general carried out at normal
pressure. However, it is also possible to carry out the
process at elevated pressure or at reduced pressure ~or
example in a range from 0.5 to 5 bar).
The removal of the group V i5 carried out by the method
cu~tomary for Grignard reaction~ using aqueous ammonium
chloride ~olu~ion ~cf~ J. March, Advanced Organic
Ch~mistry, Second Edition p. 836].
In general, 1 to 3 mol, preferably 1.1 mol, of the
Grignard compound~ or of the organometallic compounds of
the general formula (VI) are employed, relative ~o 1 mol
of the glyo~yl e~ter of the ~eneral formula (V).
: The ~ulphamidation i8 in general carried out in inert
solvent~ in the presence of a base and of a dehydrating
agent.
Suitable solvent~ in this case are inert oxganic ~o~vents
which do not change und~r ~he reaction conditions. The~e
include halo~enohydrocarbons ~uch as dichloromethane,
trichloromethane,tetrachloromethane,l,2-dichloroethane,
trichloroethane, tetrachloroethane, 1,2-dichloroethylene
Le A 29 140 - 21 -
or trichloroethylene, hydrocarbons such as benzene,
xylene, toluene, hexane, cyclohexane, or mineral oil
fraction ; nitromethane, dLmethylformamide, acetonitrile
or hexamethylphosphoramide. It is also possible to employ
mixtures of the solvents. Dichloromethane is particularly
pre~erred.
Suitable ba~e~ for the sulphamidation are the customary
ba3ic compounds. These preferably include alkali metal
and alkaline earth metal hydro~ides such as lithium
hydro~ide, ~odium hydroxide, potassium hydroxide or
baxium hydroxide, alkali me~al hydrides such as sodium
hydride, alkali metal or alkaline aarth metal carbo~ates
such as ~odium carbonate, pota~sium carbonate, or alkali
metal alkoxide~ such as, for exampl~, ~odium methoxide or
ethoxide, potassium methoxide or ethoxide or potas~Lum
tert-butoxide, or organic EmineS such as benzyltrimethyl-
ammonium hydroxide, tetrabutyla~monium hydroxide, pyrid-
ine, triethylamine or N-methylpiperidlne.
The sulphamidation i8 in general carried out in a
temperature range from 0~ to 150C, pre~erably at 25C
to 40C.
The sulphamidation i~ in general carried out at normal
pres~ure~ However, i~ i~ al80 possible to carry out the
process at reduced pressure or at elevated pre~sure ~for
example in a range from 0.5 to 5 bar).
When carrying out the sulphamidation, the ba~e is in
Le A 29 140 22 -
2~
general employed in an amount from 1 ~o 3 mol, preferably
from 1 to 1.5 mol, relative to 1 mol of the carboxylic
acid.
Suitable dehydrating reagents are carbodiLmide~, ~uch as,
for example, dii~opropyl carbodiLmide, dicyclohexyl
carbodiimide or N-t3-dimethylaminopropyl)-N'-ethylcarbo-
diimide hydrochloride or carbonyl compounds such as
carbonyldiimidazole or 1,2-oxazolium compounds such as
2-ethyl-5-phenyl-1,2-oxazolium-3-sulphonate or propane-
phosphonic anhydride or i~obutyl chloroformate or benzo~tria~olyloxy-tris-~dimethyl~mino)phosphonium hexafluoro-
phosphate or diphenyl phosphor2midate or methanesulphonyl
chloride, if appropriate in the pre~ence of bases such as
triethylamine or N-ethyl~orpholine or N-methylpiperidine
or dicyclohexylcarbodiimide and ~-hydroxysuccinimide [cf.
J.C. Sheehan, 8.L. Ledîs, J. Am. Chem. Soc. 95, 875
(1973); F.E. Freeman et al., J. Biol. Chem. 225, 507
(1982) and ~.B. Benoton, K. Kluroda, Int. Pept. Prot.
Res. 13, 403 (1979), 17, 187 (1981)].
. -The compounds of: the general formula (II) are mainly
known or can be prepared, for example, by halogenating
the corresponding methylene function in one of the
above~entioned ~ol~en~s, preferably carbon tetrachloride,
if appropriate in the pre~ence of a catAlyst, pre~erably
AIBN, using halogenating agents, preferably using
N-bromosuccinLmide, in a te~perature range from 20C ~o
100C, preferably from 60C to 80C and at normal
pressure.
Le A 29 140 - 23 -
~9836~
The compounds of the general formulae (III), (IIIa) and
(V) are mainly known or can be prepared by a customary
method (cf. for this Chem. Commun. 1~72, (11), 668].
The compounds of the general ~ormula (IV) are also known
[cf. Beilstein 5.19 / 5.24 / 5.29] or can be prepared by
customary methods from the corresponding alcohols or
cycloalkanes.
The compound~ of the general formula ~VI) are new and can
be prepared by the abov mentioned process.
The compounds of the general formula (VII) are known per
se or can be prepared ~y a customary method [cf.
R. Nutzel, ~ouben-Weyl, Methoden der organischen Chemie
(Methods of Organic Ch~mistry), 4th ed. vol. 13/2a, 53
ff. (Thieme ~erlag, S~ut~gart~ 1973, M.S. Rharash,
O. Reinmuthl Grignard Reactions o~ No~me~allic Compounds,
Prentice Hall, New York, 1974; Uhlman ~II, 370; ~ouben-
Weyl XIII/2a, 289-302; R.I. Trust, R.E. Ireland, Org.
Synth. 53, 116, (1973); O. &rummitt, E.I. Becker, Ors.
Synth. Coll. Vol. IV, 771 (1963); H. ~dkins, W. Zartman,
Org. Synth. Coll. Vol. II, 606 (1943)].
The sulphonsmine~ of the general formula ~VIII) are known
or can be prepared by a customary method.
The pure enantiomers of the compounds of the general
formula (I) according to the invention ~an he prepared,
Le A 29 140 - 24 -
2~3~
for example, by separating the corresponding enantio-
merically pure acids by a customary method and then
reacting ~hem further as mentioned above.
The compounds of the general formula (I) surprisingly
S exhibit a high activity as leukotriene synthesis inhi-
bitors, in particular after oral administration.
~he compounds according to the invention can be employed
as active compounds in medicaments. The substances can
act as inhibitors o~ enz~matic reactions in the context
of arachidonic acid metabolism, in particular as inhi-
bitor~ of 5-lipo~ygenase.
They are thus preferred for the treatment and pre~ention
of diseases of the airway~, such a~ allergies/asthma,
bronchitis, emphysema, ~hock lung, pulmonary hyper-
tension, inflammations/rheumatism and oedema~, gout,thrombo~e and thromboemboli~m3, ischaemias (peripheral,
cardiac or cerebral circulatory disorders), cardiac and
cerebral infarcts~ cardiac arrhythmia~, angina pectoris,
arteriosclerosis, in tissue transplants, dermatoses such
as psoriasis, inflammatory dermatoses, for example
eczema, dermatophyte infection, infections of the skin by
bacteria, metas~a~e and for cytoprotection in the
gastrointestinal tract.
p
The sub~tituted (benzothiazolyl- and quinoxalin-2-yl-
me~hoxy)phen~l acetic acid derivatives according to the
invention can be used both in human medicine and in
Le A 29 140 - 25 -
- ' 2 ~ ~ ~ s~
veterinary medicine.
The pharmacological activity data of the substances
according to the invention are determined by the follow-
ing method:
As a measure of the S-lipoxygenase inhibition (LOI), the
release of the leukotriene B4 (LTB4) from pol~morpho-
nuclear human leukocytes (PNN~, was determined after
addition of sub~tances and Ca~ ionophore A 23187 by means
of "revers~ phase HPLC~ according to Borgeat, P. et al.,
Proc. Nat. Acad. Sci. 76, 2148-2152 (1979)~
Ex. No. LOI
IC50
tmol/l]
9 5.4 x 10-6
2.8 x 1o-7
11 3.1 x 10-7
1.8 x 1o-7
. 17 1.2 x 10-7
18 2.8 x 1o-7
19 1.3 x 10-7
4.4 x 1o-7
23 3.~ x 10-7
24 2.4 x 1o-7
-
The present invention also includes pharmaceutical
preparations which, in addition to inert, non-toxic,
Le A 29 140 - 26 -
~ 3 ~ ~23189-7514
pharmaceutically suitable auxiliaries and excipientsl contain one
or more compounds of the ~eneral formula (I), or which consist of
one or more active compounds of the formula (I), and processes
for the production of these preparations.
The active compounds of the formula (I) should be
present in these preparations in a concentration from 0.1 to
99.5% by weight, preferably from 0.5 to 95% by weight, of the
total mixture.
In addition to the active compounds of the formula (I),
the pharmaceutical preparations can also contain other pharma-
ceutical active compounds.
The above-mentioned pharmaceutical preparations can be
prepared in a customary manner by known methods, for example
using the auxiliary(ies) or excipient(s).
In general, it has prouen advantageous to administer
the active compound of the formula (I) in total amounts from
about 0.01 to about 100 mg~kg, preferably in total amounts from
about 1 mg/kg to 50 mg/kg of body weight every 24 hours, if
appropriate in the form of several individual doses, to achieve
the desired result.
The invention also extends to a commercial package
containing, as active pharmaceutical ingredient, a compound of
the invention, together with instructions for its use for
treatment of any of the above-mentioned indications.
However, it may possibly be advantageous to depart from
the amounts mentioned, namely depending on the type and on the
body weight of the subject treated, on individual behaviour
towards the medicament, the nature and severity
- 27 -
2Q~33~
of the diseasel the type of prepa~ation and admini-
stration, and the time or i~terval at which admini-
stration ~akes place.
Startinq compounds
Example I
Methyl 4-benzylox~phenylacetate
¢ ~
co2CH3
397 g (2.39 mol) of methyl 4-hydroxyphenylacetate and
330 g (2.39 mol) of potassium carbonate are stirred at
25C for 1 h in 2 1 of dimethylformamide. 302 g
(2.39 mol) of henzyl chloride are then added and the
mixture is heated at 50C for 15 h. Af~er concentra~ing
in vacuo, the re~idue is partitioned between water and
ethyl acetate, and the organic phase is dried over sodium
sulphate and concentrated. The product is recrystallised
from methanol.
Yield: 511 g (83% of theory)
Melting point: 60C.
The compound~ ~hown in Table 1 are prepared in analogy to
the procedure of Example I:
Le A 29 140 - 28 -
2~98~6~
Table I:
0` CH2-~ CH2-C02CH3
Ex. No. L m.p.C Yield (~ of
theory)
II -Cl 72-74 98
III -Br 40 91
Example IV
~ethyl 2-(4-benzyloxyphenyl)-2~cyclopentylacetate
~
co2CH3
: 10~ 256.3 g (1 mol) of the compound rom Example I are
dissolved in 1 1 of DMF and the ~olution is added drop-
: wise under pro~ective gas at 0C to a ~uspension of 24 g
~1 mQl) of sodium hydride in 100 ml of DMF. ~fter evolu-
tio~ of H2 i8 complete, the mixture is stirred at 0C for
152 h. 149 g (1 mol) of cyclopPntyl bromide, disso1ved in
400 ml of DMFj are then added dropwise at the same
;
Le A. 29 140 - 29 -
,
2~9~3~
temperature. After addition is complete, the mixture is
stirred at room temperature for 15 h. The solvent is
removed on ~ rotary evaporator and the residue is treated
with hot water (80C). After stirring, the mixture is
slowly cooled. The crystallised product is filtered off
with suction, washel well with water, dried and recrys-
tallised from methanol.
Yield^ 276 g (85% of theory)
Melting point: 77-78C (methanol)
The compounds shown in Table II are prepared in analogy
to the procedure of Example IV:
Table II: ~ L
W` CH2~ R2
~02CH3
Ex. No- ~ Rl R2 m.p.C/ Yield
b.p.(~ of
th~y)
V ~-(cH2)3-cH3 H 145-15087
0.2 mm
VI H ~ H 59 76
VII Cl ~ H 165-17074
0.2 mm
Le A 29 140 - 30 -
3 ~ ~
Example VIII
Methyl 2-cyclopentyl-2-(4-hydroxyphenyl)acetate
HO
co2CH3
65 g (0.2 mol) of the compound from Example IY are
dissolved in 100 ml of THF, 200 ml of ethanol and 100 ml
of triethylamine. After addition of 1.5 g of palladium
catalyst (100% stxength on carbon), ~he mixture is
hydrogenated at 3 bar of hydrogen for 2 h. The catalyst
is filtered off, tha fil~rate i9 concentrated and the
residue is chromatographed on silica gel (eluent: methy-
lene chloride).
Yield: 43.7 g ~93% o~ theory)
b.p.: 150-153/0.2 mm
The compound~ ~hown in Table III are prepared in analogy
to th- procedure of Ex~m~le VIII:
Le A 29 140 - 31 -
) 3 ~ 6
.
Table III- HO ~ R2
CO2CH3
Ex. No. L R1 R2 m.p.C/ Yield
b.p. (~ of
~ Y)
IX ~(CHz)3-cH3 H 134-138 81
0.2 mm
X H: ~ H 80 79
: XI Cl ~ H 155-~60 7
0.~ mm
Example XII
~ethyl 2-[3-(benzothiazol~2-yl-methoxy)phenyl]acetate
: ~ N ~ CO2C~3
: ~ ~ O~
~: 14.~9 g (0.1 mol) of 2-methylbenzothiazole are brominated
in 250 ml of C14 using 19.6 g (0.11 mmol~ of ~S and
0.5 g of AIBN. ~fter reaction : i~ complete,; ~he
Le A 29 140 - 32 -
~33~
precipitated succinimide is filtered off, the filtrate is
concentrated, the residue is taken up in 100 ml of DME
and the ~solution is added to a solution of 16.6 g
(O.1 mol) o~ methyl 3-hydroxyphenylacetate, and 13.8 g
(0.1 mol) of KzC03, in 250 ml of DMF. The mixture is
heated at 50C for 10 h. After cooling, the solvent is
evaporated, ~he residue is -partitioned between ethyl
acetate/water, the organic phase is concentrated and the
residue is chromatographed on silica gel 60 (eluent:
CH2Cl2)-
Yield: 11.7 g (37.3% of theory)
Melting point: 58-61C (diisopropyl ether)
Example ~III
Methyl 2-~3-bromo-4-(quinoxalin-2-yl-methoxy)phenyl]~
aceta~e
~ N ~ Br
.~ .
co2CH3
The title compound is prepared in analogy to the
procedure of Example XII.
Yield: 4.7 g (94.2% of theory)
Melting point: 120 122C (ethyl aceta~e)
Le A 29 140 - 33 -
3~3
E~ample XIV
Methyl 4-(quinoxalin-2-yl-methoxy)phenylglyoxylate
~XNN~
O~
~0
CO~H3
The ~itle compound i5 prepared in analogy to the proce-
dure of Example I.
Yield: 8.0 g (37.7% of theory)
~elting poine: 125C ~methanol)
=~e :w
Meth~1 4-tbenzothiazol-2-yl-methoxy~phenylglyoxylate
,
; 10 ; ~
CO2CH3
The title compound i8 prepared in analogy to the proce-
dure of Example XIV.
Le A 29 140 - 34 -
.
- ~iO9~36~
Yield: 10.3 g (31.4~ of theory)
Melting point: 114C (methanol)
Preparation Examples
Example 1
Methyl 2-t4~(benzothiazol-2-yl-methoxy~phenyl]-2-cyclo-
pentylacetate
~S
~ N ~
o~
CO2CH3
3.0 g (20 mmol) of 2-mathylbenzothiazole are brominated
using 4.0 g (22.5 mmol) of N-bromo-succinimide and 0.3 g
of AIBN in 50 ml of CC14. ~fter reaction, the precipitated
~uccinimide is filtered off, the filtrate is concentrated,
the re idue i9 taken up in 50 ml of DME and the solution
is added to a solution of 4.7 g (20 mmol) of methyl 2-
cyclopentyl-2-(4-hydroxyphenyl)acetatel and 2.8 g
(20 mmol) of K2CO3, in 100 ml of DMF. The mixture is
heated at 50C for 10 h. ~fter cooling, the solvent is
evaporated, the residue is partitioned between ethyl
acetate/water, the organic phase is concentra~ed, and the
Le A 2g 140 - 35 -
-
residue is chromatographed on silica gel 60 (eluent-
cyclohexa~e/ethyl acetate 3:1).
Yield: 1.7 g (22.3% of theory)
Melting point: 97-98C (diisopropyl ether)
S The compounds shown in Table 1 are prepared in analogy to
the procedure of Example 1:
Table 1:
~G
~NJ~ L
~C/-~
Ex . No . G L R2 ~.1 R3 o C Y eld g
2 S H H -(CH2)3CH3 -OCH3 65-66 2.6 (14,1)
(Diisopropyl
ether)
3 -N=CH- H H ~~ -OCH3 9s-g7 5,7(68)
(Diisopropyl
~ ether)
4 -N=CI 1- Cl H ~/ -OCH3 98-100 2,3 (90)
(Diisopropyl
ether)
-N=CH- H H ~ -OCH3 108-110 1,7 (93)
e~her)
.
~ Le A 29 140 ~ 36 -
. .
2~9~
~B~
Methyl 2-~3~(benzothiazol-2-yl-methoxy)phenyl]caproate
~ S
"~ co2CH3
O ~ CH3
The title ~ompoun~ is prepared from 10.9 g (0.035 mol) of
the compound from Example IX in analogy to ~he procedure
of Exa~ple IV.
Yield: 6.4 g (50~ of theory)
Melting poin~: 108C (me~hanol)
Example 7
Nethyl 2-[3-bromo-4-(guinoxalin-2-yl-methoxy)phenyl]-2-
cycloheptylacetate
~ B
~
CO2CH3
Le A 29 140 - 37 -
2~983~
The title compound i5 prepared in analogy to thë pro-
cedure of Example 6.
Y.ield: 2.1 g (40% of theory)
Melting point: 132C (methanol)
Example 8
2-[~-(Benzothiazol-2-yl-methoxy)phenyl]-2-cyclopentyl-
acetate
~S
~ N ~
~
CO2H
,~
. 1.5 g (3.9 mmol) of methyl 2-t4-~benzothiazol-2-yl-meth-
o~y)phenyl]-2-cyclopen~ylacetate are hydrolysed under
reflux in 50 ml of methanol and 20 ml of NaOH (1 normal).
After cooling, 20 ml of HCl (1 normal) are added. The
product precipitates. After filtering off with suction,
it is washed with water, dried and recrystallised ~rom
methanol.
Yield: 1.3 g (97% of theory)
Melting point: 152-154C (methanol)
The compound~ shown in Table 2 are prepared in analogy to
the procedure of Example 8:
Le A 29 140 - 38 -
209~3~
Table 2:
~G
N J~ L
0~
CH-R2
o~R3
Ex. No. G L R E~.3 m-p- C Yield g
( solvent ) ( % f theory
9 -N=CH- H ~ OH 173-175 3,~ (60)
(Diethyl
~ ether~
-N=CH- Cl ,~J OH 101 -t 03 1,7 (78.4)
(Di2thyl
~ ether)
11 -N~CH- H JJ OH 163-16~ 1,2 t87)
(CH2C12)
12 S H -(CH2)3CH3 OH 117-118 2,1 (67.8)
(Methanol)
13 -N=CH- Br - ,~ OH 149 1.3 (51,5)
(CH3oH)
14 :S ~ H -(CH2)3-CH3 OH 99-102 ~.9 (95)
(CH30H)
Le A 29 140 - 39 -
.. æo~g~$
Example 15
N-Methylsulphonyl-2-[3-(benzothiazol-2-yl-methoxy)-
phenyl]caproamide
S
N~ CONHSO2CH3
o~,L ~, ,CH3
1 g ~2.8 mmol) of 2-t3-benzothiazol-2-yl-methoxy)phenyl]-
caproic acid, 0.267 g (2.8 mmol) of methanesulphonamide,
0.54 g (2.8 mmolj of N'~ethyl-N~-(3-dimethylaminopropyl)-
carbodiimide and 0.45 g (3.7 ~mol) of DMAP are dissol~ed
in 30 ml of CH2Cl2 and the solution is stirred at 25C for
15 h. The solvent is evaporated off and the residue is
chromatographed on silica gel 60 leluent: CH2Cl2/MeOH
100s5).
Yield: 0.42 g (34.7% of theory)
Melting point: 99-101C ~methanol)
The compounds shown in Ta~le 3 are prepared in analo~y to
the proc dure of Example 15:
Le A 29 140 - 40 -
~9~3~ ~
Table 3:
~¢N ~ L
0~
5H-R,
Ex. No. L ~l R3 : m.p. ~C Yield g
(solvent) (% of theory)
~ `
16 Cl ~/ -NHSO2CH3 163-165 0.3 (34)
(Diethyl
~ ethar)
17 Cl ,~/ -NHSO2-CH2-C6Hs 150-152 0,7 (53,6)
~Diethyl
~ ether)
1~ H l~J -NHSO2CH~ 160-162 Q,5 (51,4)
(Die~hyl
elher)
I r~
19 H ~ -NHSO2-CH2-c~Hs 148-150 0,6 (57)
(Di~thyl
~ether~
:
~ H : ~ H-so2-(c6H4)-~cH3 137 : 1,3 (92.5)
(Die~hyl
ethar)
~; ` : :
Le A 29 140 - 41 -
~9~3~
Example 21
Methyl 2-t4-(quinoxalin-2-yl-methoxy)phenyl]-2-cyclo-
heptylhydroxyacetate
~ N
~ .
H0 CO2C~3
A freshly prepared eth0real Grignard solution from 5.49 g
(3.1 mmol) of cycloheptyl bromide and 0.75 g (3.1 mmol)
of magnesium is added dropwi~e under protective gas to a
cooled solution (0C) of S g (1.55 mmol~ of methyl 2-~4-
(quinoxalin-2-yl-m thoxy)phenyl]glyoxylate in 50 ml of
THF. After warming to 25C, the reaction mixture is
~tirred overnight and poured into ice-water, acidified
with HCl and extracted with eth~1 acetate. ~fter evapora
tion, the residue is chromatographed on silica gel 60
(eluent: cyclohexane/ethyl acetate 3:1).
Yield: 2.26 g (34.7~ of theory~
Melting point: 121C (CH30H)
The compound~ shown in Table 4 are prepared in analogy to
the procedure of Example 21:
.
Le A 29 140 - 42 -
~2~9~3~
Table 4:
,~G
~NJ~
~,~,R1
HO CO-R3
Ex. No. G Rl R3 m.p. C Yield g
( sol~rent ~ ~ % of theory
22 ~ 5 ~J -OC~3 (CH30H) 2.56~ (39.4)
,/~
23 -N=CH-~ ~J ~H 1(CH30H) 1.t (50,7)
24 5 J / -OH 190 1,8 (86,5)
:
,
:: : ` ::
`:
Le A 29 140 - 43 -
.
,