Note: Descriptions are shown in the official language in which they were submitted.
2~3~'8
The present invention relates to a method for
preventing the formation of jarosite and ammonium- and alkali-
based double salts in the solvent extraction of acidic
leaching processes, whereby valuable metals are separated.
The organic leaching solution is neutralized by ammonium or
alkali salts prior to the separation of valuable metals in
order to improve the extraction recovery. In a pre-extraction
step after neutralization, the extractant is contacted with
an aqueous solution containing a metal which replaces the
ammonium or alkali ions contained in the extraction solution.
Thus the ions are removed from the solvent used for extraction
prior to the separation of valuable metals in the main solvent
extraction stages.
The treatment of iron is an important factor when
processing metallurgical concentrates and ores. In
particular, the behaviour of iron is an extremely important
consideration in leaching processes and cases where the
treatment is based on the combination of leaching and
smelting. One of the characteristics of iron is that, in its
trivalent state, iron forms alkali double salts with a
composition D[Fe3(SO4)2(OH)6]. In these so-called jarosite
compounds, D represents an alkali metal such as sodium,
potassium or ammonium.
Jarosite compounds are formed from acidic solutions
cont~;n;ng trivalent iron and ammonium, sodium or potassium.
In particular, jarosite is formed within the pH range of from
0.5 to 5Ø An increase in temperature supports this
formation. Jarosite is readily formed at a temperature in the
range of from 60 to 220~C. The higher the temperature, the
lower the pH at which jarosite is formed.
In leaching-based zinc processes iron is typically
removed from zinc-bearing solutions by formation of jarosite
compounds. The present invention in turn relates to processes
where the formation of jarosite and other ammonium- and
alkali-based double salts should be avoided. Such processes
include nickel, cobalt and copper production processes. The
raw material can be an ore, a concentrate or an intermediate
20~6~8
product obtained from the smelting of a concentrate or other
similar metal-bearing material.
The process in question includes processing steps
conducted at temperatures greater than ambient temperature.
These steps are atmospheric or pressurized leaching steps
conducted at temperatures greater than 60~C and containing
from 0.5 to 85% iron. The removal of iron from process
solutions obtained from these leaching steps is also within
the scope of the processing steps, when the applied method is
a normal hydrolysis conducted at a temperature in the range
of from 60 to 220~C.
In the above-mentioned cases, the formation of
jarosite cannot be avoided if the process requires an addition
of ammonium- or alkali-bearing materials. Such materials are,
for example, ammonium or sodium used to adjust the pH value
and to intensify solution cleaning which occurs at a higher
pH value than leaching and iron removal. For example, in a
nickel process, zinc, copper and cobalt are generally removed
from the raw material. However, ammonium double salts, such
as ammonium nickel sulfate, can be formed in the process
causing problems in crystallization.
Earlier, the creation of jarosite and double salts
during processing was not as problematic as it is today.
However, there is now an increased demand for environmental
protection which restricts these processes. When ammonia is
used for neutralization, the creation of ammonium jarosite
causes nitrogen emissions, in the form of NOX. Under these
process conditions used, the formation of jarosite cannot be
prevented in the leaching and iron removal steps. The
inevitable result is that the leach residue contains jarosite
in these cases.
The jarosite is decomposed in a subsequent
processing step, such as smelting, thereby consuming energy
and reducing the cost efficiency. Similarly, an iron
precipitate consumes energy in the smelting treatment.
However, the iron is bound to the inert slag of the smelting
treatment to an e~cellent degree, thereby eliminating the
209~638
problems connected to the storage of finely divided iron
precipitates. Alkali metals in turn are traditionally
non-desirable materials in smelting.
According to the present invention, there is
provided a method for preventing the formation of jarosite and
ammonium- and alkali-based double salts in a solvent
extraction process of an acidic leaching process, comprising
the steps of neutralizing an organic extraction solution with
an ammonium or alkali salt prior to separating a valuable
metal by solvent extraction, conducting the extractant
solution to pre-extraction, contacting an extractant with an
aqueous solution containing a metal as an exchange ion,
whereby the exchange ion replaces the ammonium or alkali ions
contained in the extraction solution, so that formation of - i
and alkali-based double salts in the leach process solution
is prevented, and the extraction solution can be conducted to
the main extraction contact with the aqueous solution
containing valuable metals.
The present invention relates to a method for
avoiding the formation of jarosite or ammonium- and alkali-
based double salts in solvent extraction by preventing the
access of ammonium, sodium or potassium to solution
circulation, even in cases where ammonium or sodium is used
to boost the solution cleaning. The extraction solution
obtained from the circulation of the extraction process is
neutralized by an ammonium or alkali salt, but the access of
these ions into the main extraction circuit, where the
valuable metals are separated, is prevented by using a
pre-extraction step, wherein the ammonium or alkali ions are
transferred into an aqueous solution, and are replaced in the
extraction solution by a so-called exchange ion. Preferably,
of the valuable metals to be separated in the solvent
extraction step, at least one is extracted more intensively
than the exchange ion. when the ammonium and alkali ions are
removed from the solution, a higher temperature can be used
for intensifying the leaching, and smelting can be applied as
a natural further processing step for recovering one of the
CA 02098638 1997-10-14
metals to be separated and for improving environmental
protection.
A preferred aspect of the invention provides a
method for preventing the formation of jarosite, or ammonium
or alkali metal double salts during leaching and solvent
extraction of nickel and cobalt, comprising:
1) neutralizing an organic extraction solution of a di-
(2,4,4,trimethylpentyl)-phosphinic acid with an ammonium,
sodium or a potassium base;
2) conducting the neutralized organic extraction
solution to a pre-extraction step, where the organic
extraction solution is contacted with an aqueous solution
containing magnesium exchange ions, wherein the magnesium
exchange ions replace the ammonium or alkali ions in the
organic extraction solution;
3) crystallizing the aqueous solution containing the
ammonium or alkali ions to recover ammonium or alkali salt;
4) contacting the organic extraction solution with an
aqueous solution containing nickel and cobalt to selectively
extract cobalt into the organic extraction solution, leaving
nickel in the aqueous solution;
5) recovering cobalt from the organic extraction
solution; and
6) recovering nickel from the aqueous solution.
In drawings which illustrate an embodiment of the
present invention,
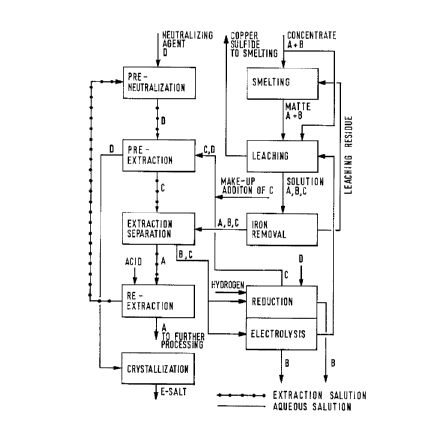
Figure 1 is a flowchart of a preferred embodiment
of the invention; and
Figure 2 illustrates the extraction of some metals
as a function of the pH value with a fixed extractant.
Certain process technical advantages are also
achieved in accordance with the method of the present
invention. When a neutral salt, such as ammonium, sodium or
potassium sulfate, is not accumulated in the solution
circulation, the solubility of nickel sulfate, for example,
is increased. This can be utilized to raise the capacity of
the reduction and electrolysis steps included in the process.
CA 02098638 1997-10-14
Furthermore, thickening, filtering and electrolysis become
easier. A high metal content also improves the quality of the
metal produced. Another specific advantage is that it is not
necessary to introduce a separate removal of neutral salt from
the main process solutions by crystallization, for example.
Referring now to Figure 1, according to the present
invention, a process including smelting steps, leaching and
recoveries of two valuable metals, A and B, by means of
reduction and/or electrolysis is further complemented by
extraction process steps complemented with crystallization.
Another metal C is also utilized in the process. Metal C does
not necessarily have to be a valuable metal. A C-bearing
solution is conducted from a recovery step of the valuable
metal B, for example from the reduction step, to a
pre-extraction step. The amount of metal C in the solution
is continuously increased to a degree that compensates the
losses. Substance D, for example, ammonium, sodium or
potassium salt, is used as a neutralizing agent. In the
pre-extraction step, the extraction solution is neutralized
by mixing with the neutralizing agent D. Generally the
extraction solution is kerosene-based and contains an
extractant which, according to its extraction equilibriums,
has a preference to extracting metal A in the subsequent
extraction separation step, whereto a solution containing A,
B and C is conducted from the iron removal step. The aqueous
solution is advantageously a sulfate solution.
In the pre-extraction step, the extraction solution
containing substance D contacts a C- and D-bearing solution
from the reduction step. An ion exchange reaction takes place
and C is extracted into the extraction solution. At the same
time, substantially all of D is exchanged out of the
extraction solution to an aqueous phase which is conducted to
the separation of D-salt, such as a crystallization step. The
extraction solution containing metal C is conducted to the
main extraction separation together with an aqueous solution
containing the valuable metals A and B, so that an ion
exchange takes place between C and A. C is transferred back
CA 02098638 1997-10-14
to the aqueous phase and returns, via the recovery of B, to
pre-extraction for a new cycle of solvent extraction. Thus
C serves as a type of exchange ion. First, C displaces D from
the extraction solution in the pre-extraction step, and then
returns to the aqueous solution in the extraction separation
itself. Thus C is not consumed in the process, apart from
small quantities along with D and A. This consumption of C
can be replenished with an addition of a small amount of C
prior to the pre-extraction step. Metal A is recovered from
the extraction solution by extraction with acid, and subjected
to further processing steps.
The method of the present invention does not require
the use of any special metals or extraction solutions. In
accordance with the present invention, the extraction
equilibrium favours the extraction of metal A over metals B
and C. It is advantageous but not necessary that C is
extracted more intensively than B, thereby enabling the
selective extraction of A relative to B. The metals are
mainly extracted by cation exchange with extractants requiring
neutralizer addition in order to boost the extraction
reaction. Such extractants are di-(alkyl)-phosphoric acids,
monoalkyl esters of alkyl phosphonic acid and di-(alkyl)-
phosphinic acids and organic carboxylic acids, for example
C-10 acids, as well as a large number of other acidic organic
extractant compounds.
The following Example illustrates the invention for
the production of pure nickel and the recovery of cobalt.
Example
In this example, metal A is cobalt and metal B is
nickel. Metal C is advantageously magnesium, and D ammonia.
The nickel separation process is advantageously arranged in
connection with nickel and copper smelting processes, wherein
the leaching object may be a sulfidic nickel concentrate
and/or matte produced in a smelting process. The copper
sulfide-bearing material formed as leaching residue is
CA 02098638 1997-10-14
advantageously further processed in copper smelting for
recovering copper and possible precious metals.
According to the method of the invention, the
magnesium-bearing solution, separated in hydrogen reduction,
is conducted to the pre-extraction step to which is also
supplied an extraction solution preneutralized with ammonia.
This is advantageously composed of di-(alkyl)-phosphinic acid
dissolved in kerosene. It is also advantageous that the
phosphinic acid is a di-(2,4,4-trimethyl-pentyl)-phosphinic
acid, which according to its extraction properties is capable
of separating cobalt and nickel.
In the pre-extraction step, nearly all magnesium is
transferred to the extraction solution, which returns an
equivalent quantity of ammonium to the aqueous solution. Next
the aqueous solution is conducted to the ammonia/sulfate
crystallization step. This procedure ensures that ammonium,
which is beneficial to the extraction, is not emitted to the
nickel solution circulation. Also the crystallization of the
so-called neutral salt is avoided in the main process flow.
Next the extraction solution, in magnesium form, is
conducted to the extraction separation, where it is contacted
with an aqueous solution of cobalt-bearing nickel. As is seen
from the extraction curves of Figure 2, cobalt is extracted
more intensively than magnesium with Cyanex 2720. The
extraction solution is a technical di-(2,4,4-trimethyl-
pentyl)-phosphinic acid product. Thus cobalt displaces
magnesium from the extraction solution. As a result, a nickel
solution free of cobalt and a cobalt-concentrated extraction
solution are produced. To recover cobalt, the next step is
the treatment of the extraction solution with acid and further
treatment of the re-extraction solution.
In accordance with the present invention, the
magnesium does not adversely affect the nickel recovery in the
successive electrolysis and/or reduction steps, but remains
in the solution and can again be extracted therefrom in the
pre-extraction during the next process cycle. Magnesium is
used as an exchange ion for cobalt extraction thereby
CA 02098638 1997-10-14
preventing the access of magnesium to the process circulation.
However, the use of an exchange ion such as magnesium is
necessary, because the cobalt extraction would be seriously
incomplete without the use of ammonia to boost the extraction
step.
In the above-described example, metal A can also be
some other metal than cobalt. A general requirement is that
A is extracted more intensively than magnesium. In the method
of the invention, it is thus possible also to remove such
metals as zinc, manganese, cadmium, copper, iron, vanadium,
molybdenum and uranium. Furthermore it is possible to
simultaneously remove several of these metals.
In all of the above-described cases, the valuable
metal B could be cobalt instead of nickel, or B could be a
solution mixture of nickel and cobalt. Then the amount of
ammonia used and, therefore, the amount of the extraction
solution in magnesium form are reduced, so that the pH value
of the extraction separation is adjusted substantially with
the quantity of the extraction solution in magnesium form.
Then the extraction separation succeeds with a pH where the
metal or metals to be extracted are capable of taking the ion
exchange places of the extraction solution, whereas cobalt is
not. As with nickel, cobalt can be electrolyzed or reduced
from magnesium-bearing solutions.
The method of the present invention can be applied
in a corresponding fashion for cleaning a number of solutions
containing metals B and C. This is technically efficient and
economical in cases where B and C represent metals which can
be separated with known methods. Metal A, to be removed in
extraction separation, must be extracted more intensively,
i.e. at a lower pH, than the metals B and C. B can also be
extracted more intensively than C. In order to achieve the
desired extraction separation, the extent of the extraction
solution from which C is extracted, is adjusted, so that the
pH of the extraction separation is suitable for the metal
separation.
CA 02098638 1997-10-14
When treating zinc-, copper- and cobalt-bearing raw
materials, it is also difficult to combine acidic leaching and
the use of ammonia as the neutralizing agent, owing to the
limited solubility of double salts containing ammonia and
cobalt. For example, metal A is zinc, metal B is copper, and
metal C is also copper. The decisive factors are the relative
proportions of the metals and other further processing steps.
As above, metal A can also represent other metals that are
extracted more intensively than metals B and C, such as iron
and indium.
Other metal groups, such as zinc, manganese and
copper, can be separated in a corresponding fashion. In this
case metal A is zinc and/or iron. Manganese is either B or
C, and C can also be copper.
While extractants of the di-(alkyl)-phosphine type
are advantageous when separating cobalt from nickel because
of their separation sharpness, the method of the present
invention is not restricted to these extractants. For
example, monoalkyl esters of alkyl phosphonic acid and
di-(alkyl)-phosphoric acids can be used to extract metals in
the following order: UO22 > Fe3 > zn2 > Mn2 > Cu2 > Cd2 >
Co2 > Mg2 > Ni2. Calcium does not show consistent behaviour,
as it is dependent on the type of extractant used. This must
be taken into account when grouping metals into the categories
A, B and C according to their extraction behaviour in order
to apply the method of the invention.
Carboxylic acids, generally C-10 acids, form a group
of extractants that is used in extraction. When arranging
metals into groups according to the invention, the following
extraction order must be taken into account: Fe3 > UO22 >
> Hg > Cu > zn2 > pb2~ > Cd2' > N2+ > C 2~ 2- z
Ca > Mg .
Other extractants behaving on the ion exchange basis
can also be used in the metal separation based on the method
of the invention. The most important of these are kelatine-
formers such as oximes, as far as they need neutralization.
CA 02098638 1997-10-14
-- 10 --
Usually neutralization is applied when the metal to be
extracted has a high content in the leach solution.