Note: Descriptions are shown in the official language in which they were submitted.
~~~~ i :~
Dosageform for micro bubble echo contrast agents
Field of the invention
The invention relates to a dosage form for micro
bubble echo contrast agents.
Prior art
Numerous preparations which are ~~uitable as echo
contrast media and contain surface-act:.ive substances
which assist with the formation of micro bubbles and
stabilise them have been disclosed (for example EP-A-0
07? 752). The ultrasound reflected by the micro bubbles
is used to improve the ultrasonic images of cavities or
vessels filled with fluid in the human and animal body.
The micro bubbles are produced only just before
administration of the contrast medium. For example, the
contrast medium is drawn together with air or a
physiologically tolerated gas into a syringe, and
expelled again into a receiver vessel, several times. It
is self-evident that it is not passible in this way to
prepare contrast media which can be administered in a
reproducible manner and which contain a defined amount of
gas in micro bubbles of maximally uniform size. Attempts
have therefore been made to provide the contrast medium
in a syringe. Before use, the required amount of air is
drawn up. Then the syringe is connected by a connector to
a second, empty syringe. Vigorous pumping of the contrast
medium backwards and forwards between the two syringes
produces micro bubbles. However, it has emerged that the
care required to obtain reasonably utilisable suspensions
of micro bubbles is unacceptable in practice. 2n
addition, a very great amount of force needs to be
expended. Standardisation can scarcely be achieved in
practice.
D~-A 3 838 530 describes a package for a two
component composition, which consists of two flexible
containers for the two components. Before use, the two
containers are connected together by a tubular adaptor
which is provided in its interior with deflectors in a
mutually offset arrangement. To mix the components, the
2fl~~'"~~~.~
- 2 -
contents of the containers are forced backwards and
forwards through the adaptor several times.
EP-~A 0 148 116 discloses a connector with two
female connecting pieces for two syringe s to be inserted.
The channel through the connector has a constriction
which is intended to assist turbulent flow of the fluid
which is pumped backwards and forwards between the two
syringes.
US-C 4,049,241 discloses a mixing chamber for
fluid materials, in which a plurality of rod-like mixing
elements are provided in a tubular housing and are
inclined with respect to the axis of the tube.
Summary of the invention
The invention has the object of providing a
dosageform for echo contrast media which permits the
user to administer the micro bubble echo contrast media
in a standardised and optimised manner without
unacceptable effort. Another aim of the invention is to
improve the quality of the images of body structures
obtainable with a micro bubble echo contrast medium and
to improve the reproducibility of the images.
Another aim of the invention is to increase the
safety of use by avoiding the production of gas bubbles
which, because their diameter is too large, expose 'the
patient to the risk of embolism.
Another aim of the invention is to avoid a risk
of infection.
These objects are achieved according to the
invention by the dosage form for echo contrast media
comprising a syringe and a mixing chamber which is
unreleasably connected thereto and contains a pre-
determined amount of gas, plus a second syringe.
The invention ~tlxerefore relates to a dosage form_
for micro bubble echo contrast media comprising a first
syringe and a mixing chamber which is unreleasably
connected thereto and contains a predetermined amount of
gas, plus a second syringe. Further subject-matter is
evident from the patent claims. The invention further
relates to a syringe for echo contrast media, which is
2~~~'~~.
- 3 -
characterised in that it is unreleasably connected to a
tubular mixing chamber which has mixing elements in its
inner lumen.
The mixing chamber is preferably tubular and has
S mixing elements in its inner lumen.
In a preferred embodiment, the mixing elements
are designed in the form of spikes, that is to say the
mixing elements preferably stand at right angles to the
inner wall of the mixing chamber and thus point in the
direction of the long axis of the mixing chamber tube. It
is expedient to design the mixing elements with sharp
edges, that is to say to produce separation edges. There
is preferably a mutually offset helical arrangement of
the mixing elements. Additional separation edges can be
achieved by one or more perforated diaphragms.
Echo contrast media which are in the form of a
homogeneous solution are, according to the invention,
provided in a syringe or drawn up air-free into a syringe
from a storage container such as, for example, an ampoule
before use. The syringe is then connected to the free end
of the mixing chamber which is firmly connected to a
syringe and whose interior volume contains the predeter-
mined amount of gas. The echo contrast medium is pumped
through the mixing chamber into the second, empty syringe
and subsequently back again into the first syringe: A
relatively stable micro bubble suspension has been formed
after only a few repetitions of the pumping process. The
echo contrast medium is now ready for administration. The
user now replaces the empty syringe by an injection
needle and injects the contrast medium. It is expedient
to inject from the syringe with mixing chamber because in
this case injection directly entails passage through the
mixing chamber once more.
The syringe with mixing chamber is produced by
processes known to the person skilled in the art out of
materials customary for such medical articles. For
example, the mixing chamber is produced by the injection
moulding process and connected unreleasably tn the
connecting piece of a conventional syringe, for example
_
~~~~~a~~?
by adhesive or ultrasonic welding or is produced from the
outset in one piece together with the syringe barrel. The
free end of the mixing chamber can have the form of a
male or female connecting piece. In the case where the
free end is designed as male connecting piece, 'the second
syringe has a female connecting piece, or an adaptor with
two female connectors is fitted on the :male connecting
piECe of the second syringe. In the case where the free
end is designed as female connecting piece, the injection
needle is fitted via an adaptor with two male connecting
pieces.
It has emerged that the second syringe can be
dispensed with when the formation of stable micro bubbles
of suitable size is possible with relatively low energy
expenditure with the echo contrast medium formulations
used. In favourable cases, even the single passage
through the mixing chamber on injection suffices to form
micro bubbles whose quality and amount are adequate. This
procedure is expedient, for example, in investigations of
the right ventricle.
The dosage form according to the invention for
micro bubble echo contrast media is preferably provided
in a set ready for use. If the echo contrast medium
consists of a single liquid component, the latter can be,
for example, contained in a normal syringe or present in
a vial from which. it is drawn up air-free into the
syringe. The preset amount of gas to be dispersed :is
located in the mixing chamber. It has proved to be
expedient to choose the free interior volume of the
mixing chamber such that it corresponds to the volume of
the predetermined amount of gas which yields an optimal
micro bubble echo contrast medium with the contrast
medium components. The connecting pieces of the two
syringes are closed with appropriate caps or stoppers. It
is expedient to provide the said parts together with an
injection needle and, if required, with further aids
customary for i.v. use, such as alcohol swab and plaster
dressing, sterile in a pack, for example a conventional
blister pack.
- 5 -
Suitable contrast medium components are those
compositions which yield sufficiently stable micro
bubbles on foaming with gases for ultrasonic
investigations, The components contain surface-active
substances and, if required, further substances promoting
the stabilisation of micro bubbles, such as, for example,
substances which increase the viscosity. Suitable
components are described, for example in EP 0 077 752 and
EP 0 212 568.
The dosage form according to the invention for
micro bubble echo contrast media contains 1 to 20 ml,
preferably 2 to 8 ml, and particularly preferably 5 ml,
of liquid contrast medium component. The dosage form
according to the invention contains 0.01 to O.I, prefer-
ably 0.04 to 0.06 ml of gas par 1 ml of liquid contrast
medium component.
A preferred embodiment of the dosage form
according to the invention comprises two syringes with an
inner volume of 5 ml, of which one is unreleasably
connected to a mixing chamber which has an inner volume
of 0.18 ml. The syringe which is unreleasably connected
to the mixing chamber is empty. The mixing chamber
contains 0.18 ml of gas, preferably sterile air, and is
closed by either a stopper or a cap. The second syringe
contains 3 ml of a liquid contrast medium component. This
syringe is also closed by a stopper or cap.
The invention is to be explained in more detail
hereinafter by means of Figures 1 to 4.
Fig. 1 shows a longitudinal section through a mixing
chamber.
Fig. 2 shows a cross-section through a mixing chamber
along the line 1I in Fig. 1.
Fig. 3 shows a side view of a syringe with mixing
chamber.
Fig. 4 shows a side view of a syringe with mixing
chamber with second syringe attached>
Fig. 1 depicts an embodiment of the mixing
chamber 1 in longitudinal section. The cylindrical
tubular sleeve 2 has mixing elements 3 which stand at
- 6 -
right angles to its interior wall and have a mutually
offset helical or spiral arrangement. A connecting piece
4 is shaped at the left-hand end and is designed as male
Luer connection. A connecting piece 5 is shaped on the
right-hand end and is designed as female Luer connection.
It appears expedient, for reasons of production tech-
nique, to fabricate the mixing chamber from three parts,
that is to say from the 'tubular sleeve 2 with the mixing
elements 3 and the two connecting pieces 4, 5 and to
connect in a suitable manner. The connection lines are
designated 8 and 9 in Fig. 1. The connecting piece 5 can
be directly replaced by a corresponding connecting part
of a piston syringe. As mentioned hereinbefore, the
connecting piece 4 can also be in the form of a female
Luer connection. If required, a perforated diaphragm 12
is provided in the connecting piece 4. An intensification
of the dispersion can be achieved by this optional
perforated diaphragm 12.
Fig. 2 shows a cross-section through a mixing
chamber along the line II in Fig. 1. The mixing elements
3 in the form of spikes standing at right angles on the
inner wall of the tubular sleeve 2 are seen.
Fig. 3 depicts a syringe 6 with mixing chamber 1
with male connecting piece, where the mixing chamber 1 is
directly shaped on the base of the syringe barrel.
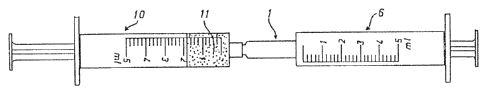
Fig. 4 shows the syringe 6 with mixing chamber 1
which is shaped on and onto whose male connecting pieces
is fitted a second syringe 10 which contains the echo
contrast medium 11. The amount of gas required for 'the
micro bubbles is contained in mixing chamber 1. The micro
bubble suspension ready for administration is prepared by
pumping the echo contrast medium 11 backwards and
forwards between syringe 10 and syringe 6 through mixing
chamber 1.
The dosage ~orm according to tl-Ae invention
achieves increased safety of use. It should be particu-
larly emphasised in this connection that, owing to the
predetermined amount of gas, exactly the amount of gas
which is optimally suited to the composition and amount
f
~~~~a~~
_~_
of the liquid component is used, and thus leads 'to a
maximum information content of the obtainable images.
Errors which may arise through conventional drawing up of
too much or too little gas are reliably avoided thereby.
In particular, the drawing up of too much gas might
result in a risk of embolism for the patient. However,
even the drawing up of too little gas in the conventional
method may make repetition of the investigation necessary
because of deficient imaging. In addition, the risk of
infection for the patient is considerably reduced by the
dosage form according to the invention because, in
contrast to the drawing up of ambient air which has been
hitherto customary, sterile gas is used and no ambient
air which may be contaminated with resistant hospital
organisms enters the blood circulation of the patient via
the micro bubbles. An additional factor is that connec-
tion procedures are avoided, owing to the unreleasable
connection between mixing chamber and syringe, with
regard to possible intrusion of organisms.
The safety of use is also greatly improved by the
possibility now of considerably reducing the number of
backward and forward pumping procedures, while there is
a simultaneous increase in the quality of the micro
bubble suspension. Since, in addition, the force
expenditure necessary for the pumping backwards and
forwards is considerably reduced, there is an increased
readiness of the user to carry out the foaming procedure
in accordance with the instructions until the state of
the micro bubble echo contrast medium is optimal for the
3a intended purpose.
The dosage form according to the invention
results in micro bubble suspensions which are distin-
guished by a surprising improved quality of contrast.
In addition, it has been observed that, after
disappearance of the contrast Pram the lumen of the body
cavities investigated, the inner surfaces of the body
cavities surprisingly remain readily visible for a
substantial period. For example, 'this marking of
surfaces, which has to date been observed only with.
'~ ~~1~
_ g _
dosage forms according to the invention, is
outstandingly suitable for diagnosis of the heart. It
appears that the presentation according to the invention
favours the formation of micro bubbles adhering to
interior body surfaces.
In a comparative test, 3 ml samples of an infu-
sion solution containing crosslinked polypeptides
(Haemaccel~ supplied by Behringwerke), which is suitable
for the preparation of micro bubble contrast media, were
pumped backwards and forwards together with 0.18 ml of
air, on the one hand five times between two syringes
connected by a three-way tap (test A), and on 'the other
hand five times in a ~osa~e form according to the
invention between the syringe connected to a mixing
chamber and a syringe attached to the mixing chamber
(test B). Subsequently formulations A and B were
administered i.v. to conscious dogs. Echocardiography of
the right ventricle was carried out with a Sonoscope 4 at
3.5 MHz. The resulting video printouts were evaluated by
densitometry. The densitometer (Gretag D182) measures
changes in the brightness in the range from 0.00 to 2.50
DU (density units) in 100 steps. The calibration is based
on the calibration card which is provided by the
manufacturer and complies with DIN 16536 (calibration
reference), where the brightest white is assigned the
value 1.64 and the darkest black is assigned the value
0.00. The value for each animal is determined from the
mean of four individual measurements within a square
centimetre.
The following results were obtained:
Contrast maximum 10 sec 20 sec
~ '~
quality intensity after after
admini.s--adminis-
tration tration
Test poor - 1.06 0.39 0.19
A
Test goad 1.56 1.09 0.58
B
It is evident, that in addition to the easier and
_ g
safer use, the dosage form according to the invention
results not on:Ly in a considerably improved contrast
quality but also in a surprising increase in intensity,
and the qualitatively and guantitatively superior con-
s trast is in fact observable for very much longer too.
The novel dosage form for macro bubble echo
contrast media not only simplifies the manipulation of
echo contrast media and increases safety on use but, in
particular, achieves a considerable additional gain in
diagnostic information.