Note: Descriptions are shown in the official language in which they were submitted.
W~92/1296') ~ Q 9 9117 PCT/EP92tO0200
Salts of a 4-amino-3-acyl quinoline derivative and their
use as inhibitors of gastric acid secretion.
The present invention relates to certain salts of a
quinoline compound, pharmaceutical compositions
containing them and their use in therapy as inhibitors of
gastric acid secretion.
Quinoline compounds which have activity as gastric
acid secretion inhibitors are known in the art, for
example, EP-330485-A discloses a series of 4-amino-3-
acylquinoline derivatives in which the quinoline is
substituted in the 8-position by, for example,
hydroxyalkyl and hydroxyalkoxy groups.
The compounds of EP 33~485-A have been found to have
poor dissolution rates in water and, as a consequence,
could potentially exhibit poor bioavailability in vivo
and hence low and poorly reproducible levels of
therapeutic activity. It has now been found that the
problem of poor dissolution can be overcome by producing
the compounds in the form of a particular class of
salts. Furthermore, in selecting compounds for use in
therapy it is important to take a number or criteria into
account, for example, in addition to physical qualities
such as good dissolution (and hence good
bioavailability), the desired level of intrinsic potency
and duration of action of the chosen compounds has to be
at the desired level. It has been found ~hat a
particular compound of EP-330485-A when produced in the
form of a salt as described herein, in addition to having
the desired physical qualities such as a high dissolution
rate, also has the desired levels of potency and duration
of action and, as such, form the subject matter of the
present invention.
.. . . . .
..
: . .
"~
.~
WO 92/12969 PCT/EP~2/00200
2099~ 2- ~
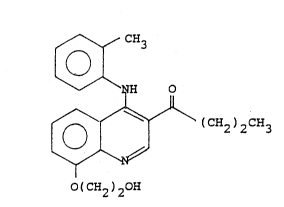
The present invention therefore provides in a first
aspect a compound of structure (I):
~ CH3
~ NH 0
~ (CH2)2c~3 (I)
(CH2 ) 20H
in the form of a salt characterised in that the salt is
that formed by reaction of said compound of structure (I) ~:-
with a strong acid.
As used herein, the term strong acid shall be taken
to mean an acid with a pka of less than about 4Ø The
nature of such acids will be apparent to those skilled in
the art and include, for example, mineral acids such as .
hydrochloric acid, and sulphonic acids such as alkyl
sulphonic acids, in particular methane sulphonic acid.
Particularly preferred salts of the present
invention are those formed by reaction with hydrochloric
acid or methane sulphonic acid, that is to say,
3-butyryl-4-(2-methylphenylamino)-8-(2-hydroxyethoxy)-
quinoline hydrochloride, and 3-butyryl-4-(2-methylphenyl-
amino)-8-(2-hydroxyethoxy)quinoline mesylate.
The salts of the present invention, in particular
the hydrochloride and mesylate salts referred to above,
have been found to exhibit exceptionally fast intrinsic
dissolution rates when compared to the free base compound
of structure (I) disclosed in EP-330485-A. Thus,
-., : :.. .-~ ., , : . , , -
- ~ : ~ . . . , -.
:-
: , ~ - : .. ~ .
,. ,,:
W092/l2969 PCT/EP92/00200
~-, 2~93117
,
whereas the free base has a poor dissolution rate and, as
such, may be expected in vivo to exhibit poorly
reproducible bioavailability (and so be less effective
therapeutically), the salts of the present invention are
expected to exhibit a much more consistent
bioavailability (since their dissolution rates are far
more favourable) and to prove more effective per given
dose and more reliably effective per given dose on
administration to patients.
The salts described herein can be used in therapy in
the treatment of gastrointestinal diseases in mammals, in
particular humans. Such diseases include, for example,
gastric and duodenal ulcers, aspiration pneumonitis and
Zollinger-Ellison syndrome. Further, the salts can be
used in the treatment of other disorders where an
anti-secretory effect is desirable, for example in
patients with a history of chronic and excessive alcohol
consumption, and in patients with gastrooesophageal
reflux disease (GERD).
In therapeutic use, the salts can be administered in
a standard pharmaceutical composition comprising the salt
and a pharmaceutically acceptable carrier. The present
invention provides in a further aspect therefore a
pharmaceutical composition comprising a salt as described
herein in association with a pharmaceutically acceptable
carrier.
Suitable pharmaceutical compositions are as
described in EP-330485-A.
Suitable daily dosage regimens for an adult patient
may be, for example, an oral dose of between 1 and
lOOO mg, preferably between 1 and 500 mg, or an
. .
:, . .
- -
. .. . .. - ".:
:. :: ::. . - . ,: ... , . :
' , . ' : '
, . : , .
Wo 92/12969 P~lEP92tO~200
2~99~
intravenous, subcutaneous or intramuscular dose of
between 0.1 and 100 mg, preferably between 0.1 and 25 mg
of the salts described herein, the salt being administered
in a unit dosage 1 to 4 times a day.
In addition, the salts can be co-administered with
further active ingredients such as antacids (for example,
magnesium carbonate or hydroxide and aluminium hydroxide),
non-steroidal anti-inflammatory drugs, steroids or nitrite
scavengers or other drugs used for treating gastric
ulcers (for example, prostanoids or H2-antagonists such
as cimetidine).
... . . . . . .. .. . . . .
. ~ . . -~
. . .
W092/l2969 2 0 9 9 1 1 7 PCTtEP92/0020~
EXAMPLE '
3 -Butyryl-4 - ( 2-methylphenylamino)-8-(2-hydroxyethoxy)-
quinoline can be prepared according to the procedures
described in EP-330485-A.
Preparation of 3-butvryl-4-(2-methyl~henylamino)-8-(2-
hYdroxvethoxy!quinoline hydrochloride
3-Butyryl-4-(2-methylphenylamino)-8-(2-hydroxy-
ethoxy)quinoline (10 g) was suspended in methanol (100 ml)
at room temperature, conc. hydrochloric acid added slowly
to give a clear solution, then the solvent evaporated.
The residue was twice taken up in 2-propanol and
re-evaporated, and was then recrystallised from
2-propanol/ether to obtain the desired salt (9.7 g),
m.p. 214-215C.
CZ2H24N2o3 HCl 0 2H2
Found C 65.50, H 6.21, N 6.88
Requires C 65.32, H 6.33, N 6.93. -
EXAMPLE 2
Preparation of 3-butvryl-4-(2-methvl~henvlamino)-8-(2-
hvdroxvethoxy)auinoline mesylate
3-Butyryl-4-(2-methylphenylamino)-8-(2-hydroxy-
ethoxy)quinoline (60 g) was suspended in ethyl acetate
(400 ml), warmed to 50OC, and methanesulphonic acid
(16.3 g) added with vigorous stirring. The desired salt
crystallised on cooling, and was filtered off and washed
with ethyl acetate; yield 50.1 g, m.p. 83-85C.
C22H24N203-CH403s~H20
Found C 57.78, H 6.28, N 5.84
Requires C 57.73, H 6.32, N 5.85.
. .