Note: Descriptions are shown in the official language in which they were submitted.
33135CA
-
3 5 ~ ~
ALKYLATION CATALYST REGENERATION
The present invention relates to the regeneration of a catalyst
composition uti]ized in a hydrocarbon conversion process. More
particularly, the iDvention relates to the regeneration of a catalyst
mixture, comprising a sulfone compound and a hydrogen halide compound,
utilized in the alkylation of olefin hydrocarbons by isoparaffin
hydrocarbons.
Background of the Invention
It has recent]y been discovered that a mixture, comprising a
sulfone compound and a hydrogen halide compound, is an effective catalyst
for use in the alkylation of olefin hydrocarbons by isoparaffin
hydrocarbons to produce an alkyla-te reaction product, or alkylate. The
alky]ate reaction product generally contains hydrocarbons having seven or
more carbon atoms, and it is a high]y desirflble gasoline blending component
becau~e of its high octane value as a motor fuel.
2099359 33135Ch
_ 2
While a process which utilizes a catalyst composition comprising
a sulfone component and a hydrogen halide component produces an alkylate
product of very high quality, one side effect from using such a process in
the production of alkylate is the formation of certain polymeric reaction
by-products such as those referred to as acid-soluble oils, or ASO. These
polymeric reaction by-products are referred to as acid-soluble oils because
they are soluble in the catalyst utilized in the alkylation process and,
thus,remain in the catalyst phase when the alkylate product resulting from
the contact of a hydrocarbon mixture with an alkylation catalyst is
separated from the alkylation catalyst. In an alkylation process which
continuously separates the catalyst phase from the alkylation reaction
product for reuse in the process reaction zone, there is a buildup of ASO
in the catalyst. Over time the ASO concentration will reach unacceptable
concentration levels if not removed. A low concentration of ASO in the
alkylation catalyst comprising a sulfone component and a hydrogen halide
component is believed to have a beneficial effect upon the alkylation
process or its product. However, higher concentrations of ASO in the
alkylation catalyst have an adverse effect upon the catalyst activity and
the final alkylate end-product. An ASO concentration in the alkylation
catalyst that exceeds certain acceptable limits wi]l result in lowering the
octane of the alkylate end-product with incrementfll increases in the ASO
concentration causing incremental decreases in the alkylate octane.
In conventional alkylation processes that use hydrogen fluoride
(HF) as a catalyst, as opposed to the use of the aforementioned novel
catalyst comprising a sulfone component and fl hydrogen halide component,
there are certain known methods used to remove the ASO from the HF catalyst
used in a continuous alkylation process. Particularly, enough of a portion
20993~9 33135CA
~_ 3
of the HF catalyst that is utilized in the alkylation process is treated,
or regenerated, so as to remove an amount of ASO at a rate that
approximates the rate of accumulation of ASO in the alkylation catalyst.
This is done by passing a portion of the HF catalyst to a stripping vessel
whereby the HF is stripped from the ASO by means of a vaporous hydrocarbon
such as isobutane with the HF passing as a part of the vaporous overhead
stream from the stripping vessel and the ASO passing as a bottoms stream
from the stripping vessel for further processing.
While the conventional alkylation catalyst regeneration
techniques have worked well in the regeneration of the conventional HF
catalyst, conventional means cannot be used to regenerate an alkylation
catalyst mixture which includes a sulfone component. This is because the
boiling range of ASO overlaps the boiling temperatures of certain sulfones
such as sulfolane. Therefore, simple distillation techniques as are used
to separate HF from ASO cannot be used to effectively regenerate a
sulfone-containing alkylation catalyst. Additionally, it is necessary to
separate ASO from the sulfone in order to reclaim the sulfone for reuse as
a catalyst in the alkylation process.
Summary of the Invention
It is, therefore, an object of this invention to provide a novel
process for the regeneration of alkylation catalysts.
A further object of this invention is to provide a process for
the removal of ASO from alkylation catalysts containing a sulfone
component.
Thus, the process of the present invention relates to the
alkylation of olefin hydrocarbons by paraffin hydrocarbons by utilizing a
catalyst mixture that includes a sulfone component. A sulfone-containing
33135CA
4 2~
mixture comprising a sulfone and AS0 i9 contacted with a reversible base
under conditions suitable for the removal of at least a portion of the AS0
component of the sulfone-containing mixture to produce a treated
sulfone-containing mixture.
Brief Description of the Drawings
In the accompanying drawings:
FI~. 1 provides schematic representation of the process which is
one embodiment of the invention; and
FIG. 2 is a plot demonstrating the capacity of an activated
carbon to adsorb AS0 from a sulfone-containing mixture as a function of the
weight percent HF contained in such mixture.
Other objects and advantages of the invention will be apparent
from the foregoing detai].ed description of the invention and the appended
claims.
Detailed Description of the Invention
The acid soluble oil of the present invention is produced as a
reaction by-product in an alkylation process comprising the step of
contacting a hydrocarbon mixture, which comprises olefins and isoparaffins,
with an alkylation catalyst, which comprises, consists of, or consists
essentially of a hydrogen ha]ide component and a sulfone component. As
used within this descrip-tion and in the appended claims, the term "acid
so]uhle oil", or "AS0", means those conjunct polymers which are highly
olefinic oils produced by acid-cataly~ed reactions of hydrocarbons. An
extensive description and characteriza.tion of certain types of conjunct
po].ymer oils is provided in the Journal of Chemical and Engineering Data
article entit].ed "Molecular Structure of Conjunct Polymers", pages 150-160,
Volume 8, Number 1, by Miron and Lee. The physical properties of AS0
33135CA
2~3~
depend upon the particular hydrocarbon feed processed, the catalyst
utilized in the process, feed contaminants such as hydrogen sulfide,
butadiene, oxygenates and other compounds, and the alkylation process
reaction conditions. Thus, as the term is more narrowly defined, AS0 will
be those conjunct polymers produced as a by-product in the catalyzed
reaction of mono-olefins with i.soparaffins utilizing a catalyst mixture
comprising, consisting of, or consisting essentially of a sulfone component
and a hydrogen halide component. The preferred mono-olefins for use in the
catalyzed reaction are those having from three to five carbon atoms and the
preferred isoparaffins are those having from four to six carbon atoms. The
preferred sulfone component is sulfol.ane and the preferred hydrogen halide
component is hydrogen fluoride.
The AS0 by-product derived from the hydrocarbon reaction
catalyzed by a sulfone-containi.ng alkylation catalyst can be further
generally characterized as having a specific gravity, with water at 60~F as
the reference, in the range of from about 0.8 to about 1.0, an average
molecular weight in the range of from about 250 to about 350, and a bromine
number in the range of from about 40 to about 350.
The hydrogen ha]ide component of the catalyst composition or
catalyst mixture can be selected from the group of compounds consisting of
hydrogen fluoride (HF), hydrogen ch].oride (ITCl), hydrogen bromide (HBr),
a.nd mixtures of two or more thereof. The preferred hydrogen halide
component, however, i.s hydrogen f]uoride, which can be utilized in the
cata]yst composition in anhydrous formj bu-t, generally, the hydrogen
fluoride component u-tilized can have a sma.ll amount of water. In a
catalyst composition including hydrogen fluoride and sulfolane, the amount
of water present in no event can be more than about 30 weight percent of
.
2 0 9 9 3 5 9 33135CA
_ 6
the total weight of the hydrogen fluoride component, which includes the
water. Preferably, the amount of water present in the hydrogen fluoride
component is less than about lO weight percent. Most preferably, the
amount of water present in the hydrogen fluoride component is less than 7
weight percent. When referring herein to the hydrogen halide component, or
more specifically to the hydrogen fluoride component, of the catalyst
composition of the invention, it should be understood that these terms mean
that the hydrogen halide component is either an anhydrous mixture or a
non-anhydrous mixture. The references herein to weight percent water
contained in the hydrogen halide component means the ratio of the weight of
water to the sum weight of the water and hydrogen halide multiplied by a
factor of 100 to place the weight ratio in terms of percent.
The sulfones suitable for use in this invention are the sulfones
of the general formula
R-SO2-R '
wherein R and R' are monovalent hydrocarbon alkyl or aryl substituents,
each containing from 1 to 8 carbon atoms. Examples of such substituents
include dimethylsulfone, di-n-propylsulfone, diphenylsulfone, ethylmethyl-
sulfone and the alicyclic sulfones wherein the SO2 group is bonded to a
hydrocarbon ring. In such a case, R and R' are forming together a branched
or unbranched hydrocarbon divalent moiety preferably containing from 3 to
12 carbon atoms. Among the latter, tetramethylenesulfone or sulfolane,
3-methylsulfolane and 2,4-dimethylsulfolane are more particularly suitable
since they offer the advantage of being liquid at process operating
conditions of concern herein. These sulfones may also have substituents,
particularly one or more halogen atoms, such as for examp]e,
'~ -
2 0 9 9 3 ~ 9 33135CA
chloromethylethylsulfone. These sulfones may advantageously be used in the
form of mixtures.
The alkylation catalyst used in the alkylation process wherein an
AS0 reaction by-product is produced can comprise, consist of, or consist
essentially of a hydrogen halide component as described herein and a
sulfone component as described herein. Preferably, the AS0 by-product will
be produced in an alkylation process in which the hydrocarbon mixture is
contacted with an alkylation catalyst having sulfolane as its sulfone
component and hydrogen fluoride as its hydrogen halide component. In the
case where the alkylation catalyst comprises sulfolane and hydrogen
fluoride, good alkylation results can be achieved with weight ratio of
hydrogen fluoride to sulfolane in the alkylation catalyst in the range of
from about 1:1 to about 40:1. A preferred weight ratio of hydrogen
fluoride to sulfolane can range from about 2.3:1 to about 19:1 and, more
preferably, it can range from 3:1 to 9:1.
In order to improve selectivity of the alkylation reaction of the
present invention toward the production of the desirable highly branched
aliphatic hydrocarbons having seven or more carbon atoms, a substantial
stoichiometric excess of isoparaffin hydrocarbon is desirable in the
reaction zone. Molar ratios of isoparaffin hydrocarbon to olefin
hydrocarbon of from about 2:1 to about 25:l are contemplated in the present
invention. Preferably, the molar ratio of isoparaffin-to-olefin will range
from about 5 to about 20; and, most preferably, it shall range from 8 to
15. It is emphasized, however, that the above recited ranges for the molar
ratio of isoparaffin-to-olefin are those which have been found to be
commercially practical operating ranges; but, generally, the greater the
-
2~ 9 9 ~ 5~ 33135CA
_~ 8
isoparaffin-to-olefin ratio in an alkylation reaction, the better the
resultant alkylate quality.
Alkylation reaction temperatures within the contemplation of the
present invention are in the range of from about 0~F to about 150~F. Lower
temperatures favor alkylation reaction of isoparaffin with olefin over
competing olefin side reactions such as polymerization. However, overall
reaction rates decrease with decreasing temperatures. Temperatures within
the given range, and preferably in the range from about 30~F to about
130~F, provide good selectivity for alkylation of isoparaffin with olefin
at commercially attractive reaction rates. Most preferably, however, the
alkylation temperature should range from 50~F to 120~F.
Reaction pressures contemplated in the present invention may
range from pressures sufficient to maintain reactants in the liquid phase
to about fifteen (15) atmospheres of pressure. Reactant hydrocarbons may
be normally gaseous at alkylation reaction temperatures, thus reaction
pressures in the range of from about 40 pounds gauge pressure per square
inch (psig) to about 160 psig are preferred. With all reactants in the
liquid phase, increased pressure has no sign;ficant effect upon the
alkylation reaction.
Contact times for hydrocarbon reactants in an a]kylation reaction
zone, in the presence of the alkylation catalyst of the present invention
generally should be sufficient to provide for essentially complete
conversion of o]efin reactant in the alkylation zone. Preferably, the
contact time is in the range from about 0.05 minute to about 60 minutes.
In the alkylation process of the present invention, employing
isoparaffin-to-olefin mo]ar ratios in the range of about 2:1 to about 25:1,
wherein the alkylation reaction mixture comprises about 40-90 volume
~ 20~93~9 33135CA
percent catalyst phase and about 60-10 volume percent hydrocarbon phase,
and wherein good contact of olefin with isoparaffin is maintained in the
reaction zone, essentially complete conversion of olefin may be obtained at
olefin space velocities in the range of about 0.1 to about 200 volumes
olefin per hour per volume cata]yst (v/v/hr.). Optimum space velocities
will depend upon the type of isoparaffin and olefin reactants utilized, the
particular compositions of alkylation catalyst, and the alkylation reaction
conditions. Consequently, the preferred contact times are sufficient for
providing an olefin space velocity in the range of about 0.1 to about 200
(v/v/hr.) and allowing essentially complete conversion of olefin reactant
in the alkylation zone.
The alkylation process can be carried out either as a batch or
continuous type of operation, although it is preferred for economic reasons
to carry out the process continuously. It has been generally established
that in alkylation processes, the more intimate the contact between the
feedstock and the catalyst the better the quality of alkylate product
obtained. With this in mind, the present process, when operated as a batch
operation, is characterized by the use of vigorous mechanical stirring or
shak;ng of the reactants and catalyst.
In continuous operations reactants can be maintained at
sufficient pressures and temperatures to maintain them substantially in the
liquid phase and then continuously forced through dispersion devices into
the reaction zone. The dispersion devices can be jets, nozzles, porous
thimbles and the like. The reactants are subsequently mixed with the
catalyst by conventional mixing means such as mechanical agitators or
turbulence of the flow system. After a sufficient time, the product can
then be continuously separated from the catalyst and withdrawn from the
20!~9359
33135CA
,.. ..
reaction system while the partially spent catalyst is recycled to the
reactor. A portion of the catalyst can continuously be regenerated or
reactivated as descrihed herein, or by any other suitable treatment, and
returned to the alkylation reactor.
One embodiment of this invention includes a process for removing
ASO from a sulfone-containing mixture comprising the step of contacting a
sulfone-containing mixture, comprising a sulfone component and ASO, with a
contact material suitable for the removal of at least a portion of the ASO
component of said sulfone-containing mixture to produce a treated
sulfone-containing mixture. The sulfone-containing mixture of the
inventive process can also comprise a sulfone component, a hydrogen halide
component, and ASO. The contact material can be those materials described
herein and can include materials selected from the group consisting of
alumina, carbon, and mixtures thereof as well as the preferred reversible
bases as are defined herein. The preferred sulfone component of the
sulfone-containing mixture is sulfolane.
The ASO compoDent of the sulfone-containing mixture can be
present in an amount no more than about 20 weight percent of the sulfone
component. Preferably, the concentration of ASO is less than about 15
weight percent of the sulfone component, and most preferably, the ASO will
be present at a concentration of less than 10 weight percent. When a
hydrogen halide component is present in the sulfone-containlng mixture, its
concentration will be less than about ]0 weight percent of the mixture with
the weight percent determined by the weight fraction of the hydrogen halide
to total sum weight of hydrogen halide and sulfone multiplied by a factor
of 100 to yield a percent. But, generally, the concentration range of
hydrogen halide in the sulfone-containing mixture can range from about 0.1
2099359
33135CA
11
weight percent to about 10 weight percent. Preferably, however, the
concentration can range from about 0.l to about 7.5 weight percent, and
most preferably, it can range from 0.1 to 5.0 weight percent.
It is an important, if not critical, aspect of this invention for
the contacting of the sulfone-containing mixture with the contact material
to result in removal of at least a portion of the hydrogen halide component
of the sulfone-containing mixture to give the treated sulfone-containing
mixture having a reduced concentration of hydrogen halide. It is
preferred, however, to have a significant portion of the hydrogen halide
component removed from the treated sulfone-containing mixture to give a
concentration of the hydrogen halide component in the treated
sulfone-containing mixture of less than about 1.0 weight percent, but
preferably, the concentra-tion will be less than about 0.2 weight percent,
and most preferably, the concentration will be less than 0.1 weight
percent.
The treated sulfone-containing mixture can also have a reduced
concentration of AS0, generally being less than about 5 weight percent of
the treated sulfone-containing mixture. The preferred concentration of ASO
in the treated sulfone-containing mixture will be an amount less than about
2 weight percent, and most preferably, the ASO will be present in an amount
less than 1 weight percent.
Another embodiment of the process of this invention contemplates
the resolution of problems associated with the regeneration of
sulfone-containing alkylation catalyst mixtures, comprising a sulfone
component, a hydrogen halide component, and ASO, by the removal of at least
a portion of the ASO contained within such mixtures. The accumulation of
ASO in a sulfone-containing alkylation catalyst occurs when an alkylation
2099359
33135CA
12
process continuously reuses its catalyst. In a continuous alkylation
process, the ASO reaction by-product will build up in the catalyst until,
if not removed, it reaches unacceptable concentration levels that can have
negative effects upon the catalyst performance and, ultimately, the
alkylation product quality itself. It is generally desirable to maintain
the concentration of AS0 in the sulfone-containing alkylation catalyst at
no more than about 20 weight percent of the catalyst with the weight
percent ASO being based upon the total weight of the catalyst mixture
exclusive of the AS0 component. Preferably, the concentration of the ASO
in the sulfone-containing alkylation catalyst is less than about 15 weight
percent, and most preferably, the concentration of ASO is less than 10
weight percent.
While there may be some process advantages in maintaining a low
concentration of ASO in the sulfone-containing catalyst mixture, it is
believed that an ASO concen-tration exceeding about 10 weight percent of the
catalyst will have a detrimental effect upon the catalyst performance.
Thus, in order to maintain the catalytic activity of a sulfone-containing
alkylation catalyst mixture, at least a portion of the catalyst must be
processed to remove at least a portion of the ASO contained within such
catalyst. To achieve this, the sulfone-containing alkylation catalyst
mixture is contacted with a contact material followed by contacting with an
adsorbent material so as to remove at least a portion, and preferably a
substantial portion, of the ASO component of the sulfone-containing
alkylation catalyst mixture.
It is noted, however, that it is generally desirable to have at
least a portion, and preferably a substantia] portion, of the hydrogen
halide component of the sulfolane-containing alkylation catalyst mixture
2099359
33135CA
13
removed prior to contacting the resultant sulfone-containing mixture with
the contact material to thereby remove at least a portion of the ASO
component or remove at least a portion of the hydrogen halide component, or
both. Therefore, the sulfone-containing mixture will be the
sulfone-containing alkylation catalyst mixture having at least a portion,
and preferably a substantial portion, of the hydrogen halide component
removed. Any suitable method can be used to separate the hydrogen halide
component from the sulfone-containing alkylation catalyst mixture, such as,
for example, flash separation, distillation, extraction, stripping, and
other suitable separation methods. One preferred method is by stripping
means for separating the sulfone-containing alkylation catalyst mixture
into an overhead stream, comprising a major portion of the hydrogen halide
component of the sulfone-containing alkylation catalyst, and a bottoms
stream, comprising the sulfone-containing mixture, with the use of vaporous
isobutane as the stripping agent.
Generally, the concentration of the hydrogen halide component in
the sulfone-containing mixture will be less than about 10 weight percent of
the mixture with the weight percent determined by the weight fraction of
the hydrogen halide to the sum total weight of hydrogen halide and sulfone
multiplied by a factor of 100 to yield a percent. B~t, because it is very
difficult to remove the entire amount of hydrogen halide from the mixture,
the lower limit of hydrogen halide concentration from a commercially
practical standpoint can approach about 0.1 weight percent, but,
preferably, the concentration can be less than 0.1 weight percent. Thus,
the concentration range of hydrogen halide in the sulfone-containing
mixture can range from about 0.1 weight percent to about 10 weight percent.
Preferably, however, the concentration can range from about 0.1 to about
2 0 9 9 3 5 9 33135CA
~ .._
14
7.5 weight percent, and most preferably, it can range from 0.1 to 5.0
weight percent.
The treated sulfone-containing mixture as earlier described
herein can further be contacted in a second contacting step with an
adsorbent materia] preferably selected from the group consisting of carbon,
alumina and mixtures thereof under conditions suitable for removing at
least a portion of the ASO contained therein. It is preferred, however,
for a substantial portion of the ASO in the treated sulfone-containing
mixture to be removed by contacting it with the adsorbent material to give
a substantially ASO free sulfone-containing mixture which can have a
concentration of ASO generally less than about 2 weight percent of the
substantially ASO free sulfone-containing mixture. The preferred ASO
concentration is less than about 1 weight percent and most preferably the
ASO will be present in an amount less than 0.1 weight percent.
Generally, both the contact materials and the adsorbent materials
contemplated for use by this invention can be contained within a vessel
defining a contacting zone in which the sulfone-containing fluids can be
contacted with either the contact materials or the adsorbent materials.
However, this invention is not confined to the use of standard vessels for
defining a contacting zone, but any suitable means known in the art can be
utilized for contacting the sulfone-containing fluids with either the
contact materials or the adsorbent materials.
The adsorbent material utilized to remove ASO from the ~reated
sulfone-containing mixture can be any adsorbent that can either suitably or
effectively remove at least a portion of the ASO component contained in
such mixture. Preferably, the adsorbent material is selected from the
group consisting of alumina, carbon and mixtures thereof.
33135C~
~0!~3~
The carbon adsorbent material can be any activated carbon
material that is suitable for use as contemplated by this invention and for
the selective removal of at least a portion of the ASO component contained
in the treated sulfone-containing mixture. The activated carbon adsorbent
can be characterized by its large specific surface area which can range
from about 300 m2/g to about 2500 m2/g as determined by the American
Society for Testing Materials (ASTM) Standard Test Nethod D3663-84 entitled
"Standard Test Method for Surface Area of Catalysts". Also, the activated
carbon adsorbent can further be characterized by its pore diameter which
can range from about 10 ~m to about 50 ~m as determined by the method of
mercury intrusion porosimetry described by ASTM Standard Test D4284-8$
entitled "Standard Test Method for Determining Pore Volume Distribution of
Catalysts by Mercury Intrusion Porosimetry". It is generally desirable to
use commercially available activated carbon. One such suitable
commercially available activated carbon, for example, is the product known
by its tradename as Calgon Filtrasorb 400*, which is manufactured and
marketed by Calgon Carbon Corporation.
The alumina adsorbent material can be any alumina suitable for
use as contemplated by this invention and for the selective removal of at
least a portion of the ASO component contained in the treated
sulfone-containing mixture or for use as a neutralizing agent for the
removal of at least a portion of the hydrogen halide component of a
sulfone-containing fluid. Such suitable aluminas include, for example, a
variety of the commercially available activated aluminas and calcined
aluminas. Generally, the alumina material will have a surface area in the
range of from about 150 m2/g to about 500 m2/g as determined by ASTM
* trademark
2 ~ 33135C~
~_ ]6
D3663-84. Also, the pore diameter of the a].umina material can range from
about 25 ~m to about 125 ~m as determined by ASTM D4284-88. It is
generally desirable to use commercially available a.luminas. One such
sultable commercially available a].umina is the product known by its
tradename IIF-200 manufactured and marketed by Alcoa. The most preferred
alumina for use i.n this invelltion is a calcined alumina having a gamma
crystalline structure, also known as gamma-alumina, and other aluminas,
such as chi-alumina having surface areas greater than about 50 m2/g.
The process conditions under which the treated sulfone-containing
mixture is contacted with an adsorbent composition can be any conditions
that flre suitable or effective for removing at least a portion of the
concentration of ASO from the treated sulfone-containing mixture. The
removal efficiency of the adsorbent material is not believed to be highly
dependent upon the contact pressure because the adsorption phenomenon is
thought to be the result of a liqui.d-solid interaction; however, the
process pressure should exceed about 0.5 atmospheres of absolute pressure
and can range upwardly to about 30 atmospheres, or more, of absolute
pressure. The more common operating pressure will generally range from
about a.tmospheric pressure to about 200 pounds per square inch of gauge
pressure (psig).
As for the contacting temperature, any suitable temperature can
be uti]ized that provides for an effective removal of at least a portion of
the ~SO from the treated sulfone-containing mixture. Generally, the upper
and lower temperature limits are set by the physical characteristics of the
mixture being treated and the physical characteristics of the ASO contained
in such mixture. Considering the lower temperature limit, pure sulfolane
has a melting point of about 81.3-82.0~F, but when sulfolane is in the form
33135CA
17 ~ 3 ~ ~
_
of a mixture w;th water and hydrogen fluoride, the melting point is
s;gnificant].y lower. Therefore, the lower limit for the contacting
temperature approximates ~~F. As for the upper temperature limit, it is
determined by such factors as the initial boiling temperature of the ASO
and the temperature at which the sulfone component of the mixture begins to
thermally decompose. Thus, the upper contacting temperature approximates
400~F. Therefore, the contact temperature generally will range from about
0~F to about 400~F. Preferably, the contacting temperature will range from
about 50~F to about 350~F, and most preferably, it will range from 60~F to
325~F.
It has been determined that, in the process of removing AS0 from
a treated sulfone-containing mixture by contacting it with an adsorbent
material, the presence of even a small concentration of a hydrogen halide
compound, particularly hydrogen fluoride, in the m;xture has the effect of
reducing the ability of an activated carbon adsorbent to selective].y remove
ASO from the mixture. As illustrated by the data presented in FIG. 2, a
smflll. concentration of hydrogen fluoride in a sulfone-containing fluid
being contacted with an activated carbon material can have the effect of
essentially rendering the carbon ineffective for aso removal. Thus, one
important, and potentially critica:l, aspect of this invention is for an AS0
contaminated sulfone-containing mixture to be substantially free of a
concentration of hydrogen halide or, more general].y, for the ASO
contamlnated sulfone-containing mixture to be neutralized prior to, or
concurrently with, contacting the mi.xture with a carbon material. Any
means suitable for the removal of at least a portion, and preferably, a
significant portion, of a concentration of hydrogen halide from an ASO
contaminated sulfonQ-containing mixture or composition cfln be used.
33135C~
. _ 18
Alternatively, any neutrallzing agent suitable for the removal of at least
a portion of the hydrogen halide contained in an ASO contaminated
sulfone-con-taining mixture can be used. Examples of such suitable
neutralizing agents can include, but are not limited to, basic hydroxides,
such as those of alkali and alkaline earth metals, e.g., KOH, Ca(OH)2, and
NaOH; basic oxides, such as zinc oxide and tin oxide; amphoteric oxides,
such as aluminum oxide; and reversible bases. Preferred neutralizing
agents can include the various types of aluminas, hydroxide compounds and
reversible bases with the most preferred neutralizing material being either
gamma-alumina or reversible bases.
The term "reversible base" as used herein refers to an aromatic
compound, which has one or more nitrogen atoms in an aromatic ring, or a
polymer with pendant aromatic compounds having one or more nitrogen atoms
in an aromatic ring, wherein the nitrogen atom is sterically hindered in a
manner such that the salt formed by the nitrogen atom of the aromatic
compound and a strong protic base wil] undergo dissocia.tion to the aromatic
compolmd and the protic acid at a temperature below the decomposition
temperature of the aromatic compound.
Preferred reversible bases are compounds which correspond to one
of the fol].owing formulas:
2099359
33 135CA
-
19
(R2 )a
~<~
~Nl Rl
(R2 )b (R2 )b
~,
(R2 )b (R2 )c ~R2 )a
"JX
(R2 )b (R2 )c ~R2 )a
or
) a (R2 ) a
~/~ ( R 2 ) b
2099359 33135CA
or is a polymer containing units corresponding to one of the following
formulas
CH-CH2
(R2)a ~
CH-CH2--
,~
(R2)a ~ )
N
CH-CH2
Rl ~ (R2)a
or
CH-CII
(R2)a
R
wherein
Rl is separately in each occurrence C2 20 alkyl, C6_20 aryl,
C7_20 alkaryl~ C7 20 aralkyl or C3 20 cycloalkyl, wherein the C2 20 alkyl,
C6_20 aryl~ C7_20 aralkyl, C7_20 alkaryl or C3 20 cycloalkyl is
2~993~9
~ 3135CA
21
unsubstituted or substituted with a halo, nitro, cyano, Cl 20 alkoxy, C6 20
aryloxy, C7_20 alkaryloxy or C7 20 aralkoxy; R2 and R3 are separately in
each occurrence Cl 20 alkyl~ C6_20 aryl, C7_20 alkaryl, C7_20 aralkyl,
C3 20 cycloalkyl, nitro, cyano, halo, Cl_20 alkoxy~ C6_20 aryloxy~ C7_20
alkaryloxy or C7 20 aralkoxy wherein the Cl_20 alkyl, C6_20 aryl~ C7_20
alkaryl C 20 aralkyl, Cl_20 alkoxy~ C6_20 Y 7-20
C7 20 aralkoxy or C3 20 cycloalkyl group is unsubstituted or substituted
ith a halo nitr~, cyano, Cl_20 alkoxy, C6_20 Y 7-20
~7_20 aralkoxy;
a is separately in each occurrence an integer of from 0 to 4;
b is separately in each occurrence an integer of from 0 to 3;
c is separately in each occurrence the integer 0 or 1; and
d is separately in each occurrence the integer of from 0 to 2.
Examples of preferred reversible bases include 2,4,6-tri-t-butylpyridine,
2,6-di-t-butyl-4-methylpyridine, 2,6-di-t-butylpyridine, 2-t-butylpyridine,
2-benzylpyridine, 2,6-diphenylpyridine, 2-phenylpyridine, 2,6-di.methoxy-
pyridine, 2-phenoxypyridine, 2,6-diphenoxpyridine, 2-methylquinoline,
6-methylquinoline, 7,8-benzoquinoline, and the like. More preferred
reversible bases include 2,4,6-tri-t-butylpyridi.ne, 2,6-di-t-butyl-4-
methylpyridine, 2,6-di-t-butylpyridine, 2-t-butylpyridine, 2-benzyl-
pyridine, 2,6-diphenylpyridine, 2-phenylpyridine, 2-phenoxypyri.dine,
2,6-diphenoxypyridine and 2,6-dimethoxypyridine.
The polyvinyl pyridine resins useful in this invention include
homopolymers of vinyl pyridine compounds, which are appropriately
sterically hindered, and copolymers of vinyl pyridine compounds with
1,2-ethylenically unsaturated compounds, for example, styrene,
divinylbenzene, ethylene, viny] ch:loride, and the like. Furthermore, the
33135CA
22 2~
vinyl pyridines mAy be polymerized with 2 or more of such 1,2-ethylenically
unsaturated compounds. Such polymerization processes are well-known in the
art. See for example, D'Aelio, U.S. Patent 2,623,013; Kirk-Othmer
Encyclopedia of Chemical Technolo~y, 3rd Ed., Vol. 21, p. 816 et seq. and
Vol. 19, pp. 475-76.
In the hereinbefore presented formulas Rl is preferably C3 10
slkyl~ C6_10 aryl~ C7_10 alkaryl, C7_10 aralkyl, C5 10 cycloalkyl, C6 10
aryloxy and C7 10 alkaryloxy. More preferably Rl is C3 10 alkyl, C7 10
alkaryloxy, C6 10 aryloxy or C6 10 aryl; Rl is most preferably isopropyl,
isobutyl, t-butyl, phenoxy, or phenyl. R2 is preferably halo or Cl 10
alkyl. R2 is more preferably Cl 3 alkyl. R3 is preferably C2 10~ C6 10
C6 10 aryloxy or C7 10 alkaryloxy. R3 is more preferably C3 10 alkyl,
phenoxy or phenyl. R3 is most preferably isopropyl, isobutyl, t-butyl,
phenoxy or phenyl. Preferably, a is an integer of from O to 2, and most
preferably O or 1. Preferably, b is an integer of O or 1. Preferably, d
is an integer of O or 1.
The most preferred reversible base for use in the invention is
the aforementioned polymers wherein both a and b are zero (O). Of these,
the most preferred reversible base polymers are those selected from the
group consisting of poly-(2-vinylpyridine), poly-(4-vinylpyridine), and
mixtures thereof.
It is desir~ble that the reversible bases used for this invention
have a purity of above 90 percent, preferably above 95 percent and most
preferably above 99 percent by weight. The boiling temperatures of the
bases should preferably be at least 20 degrees above the thermal
dissociation temperature under dissociation conditions.
-
2099359
33135CA
~_ 23
One problem associated with the use of many types of neutralizingagents is their non-regenerability. Many neutralizing agents, when they
are used to remove a hydrogen halide component from a liquid medium,
particularly a sulfone-containing mixture comprising a sulfone component
and ASO, eventually become spent; therefore, requiring costly replacement.
Because of the many known disadvantages associated with the use of
non-regenerable neutralizing materials, it is desirable to use a material
that can be regenerated and repeatedly reused. Thus, the reversible base
described herein provides a preferred neutralizing material, or contact
material, for removing hydrogen halide from an ASO contaminated
sulfone-containing mixture or a sulfone-containing mixture comprising a
sulfone component and ASO.
While an important aspect of the invention described herein
includes taking advantage of the physical properties of reversible bases so
as to use them as a neutralizing material to remove hydrogen halide from
sulfone-containing mixtures described herein, it has been discovered that
such reversible bases also have certain adsorption properties. Therefore,
when a liquid medium containing ASO, such as an ASO contaminated
sulfone-containing mixture, is contacted with a reversible base such as
poly(vinylpyridine), at least a portion of the ASO component is removed
from the sulfone-containing mixture by adsorption onto the reversible base.
Over time, the combination of the hydrogen halide removed from a
sulfone-containing mixture and ASO adsorption will cause the reversible
base to become spent thereby necessitating its regeneration.
One traditional method known for regenerating a spent reversible
base, which has previously been exposed to a liquid medium containing a
strong protic acid, is to expose the resultant salt to thermal energy to
2099359
33135CA
_.
24
thereby cause the salt to dissociate and liberate the protic acid. This
traditional means for regenerating a spent reversible base is not suitable,
however, when the reversible base has been exposed to a sulfone-containing
mixture having a concentration of ASO. It is believed that one possible
reason for the inadequate thermal regeneration of a spent reversible base
that has been contacted with a sulfone-containing mixture having a
concentration of ASO is due to the aforementioned adsorption
characteristics of the reversible base. When a reversible base is utilized
as an agent for removing hydrogen halide from an ASO contaminated
sulfone-containing mixture, at least a portion of the ASO concentration of
the sulfone-containing mixture is adsorbed by the reversible base thus
contaminating the reversible base and rendering it less efficient as an
agent for removing hydrogen halide from a liquid medium or a neutralizing
agent.
It has been discovered that the ASO adsorbed by the reversible
base cannot substantially be removed exclusively by thermal means,
therefore, requiring the use of other means for the removal of the adsorbed
ASO. One such means is exposing the spent reversible base to a solvent
under conditions such that at least a portion of the ASO adsorbed by the
reversible base is removed. It is preferred that a significant portion of
the ASO adsorbed by the reversible base be removed by the exposure of the
reversible base to the solvent.
Any solvent can be used to expose or contact the spent reversible
base provided the solvent suitably removes at least a portion of the
adsorbed ASO or, alternatively, removes a significant portion of the
adsorbed ASO. Such suitable solvents can be those solvents in which ASO is
soluble and can include organic solvents selected from the group consisting
20~9359
33135CA
_ 25
of alcohols, aliphatic hydrocarbons, alkyl halides, amines, aromatic
hydrocarbons, esters, glycols, glycol ethers, aromatic halides and mixtures
of two or more thereof.
The spent reversible base, after having been exposed to the
solvent, is then exposed to a stripping fluid under conditions suitable for
removing a substantial portion of the remaining adsorbed ASO not removed by
the solvent and to remove at least a portion, preferably, a substantial
portion, of the hydrogen halide removed from the sulfone-containing
mixture. The stripping fluid can be any fluid which suitably performs the
stripping function described herein including, for example, water,
hydrocarbons and inert gases. It is desirable for the stripping fluid to
be used in the gaseous phase. The hydrocarbons which can suitably be used
as a stripping fluid include methane, ethane, propane, butane, pentane,
hexane, heptane, octane and mixtures of two or more thereof, but the most
preferred stripping hydrocarbon is isobutane. Nitrogen is the preferred
inert stripping gas.
The conditions under which the reversible base is stripped or
exposed to a stripping fluid are such that a regeneration of the reversible
base is effected, and it is generally a thermal process whereby the spent
reversible base is regenerated by use of thermal energy. Therefore, the
stripping temperature is preferably in the range of from about 100~F to
about 600~F. When isobutane is used as the stripping fluid, it is
preferred for it to be in the supercritical state in order to achieve the
best regeneration results. The stripping pressure is not an important
aspect of the invention and can range from about 0.1 to about 140
atmospheres.
~099359
33135CA
~,_
26
As earlier described herein, it is desirable for the hydrogen
halide component of the ASO contaminated sulfone-containing alkylation
catalyst mixture to be minimized before contacting the resultant
sulfone-containing mixture with a neutralizing agent such as a reversible
base. In particular, when a significant portion of the sulfone-containing
alkylation catalyst mixture comprises hydrogen halide; for instance, when
the weight ratio of hydrogen halide to sulfolane is in the range of from
about 1:1 to about 40:1, it is preferable for a major portion of the
hydrogen halide to be removed from the catalyst mixture to give a
sulfone-containing mixture or a recovered catalyst mixture. This
sulfone-containing mixture or recovered catalyst mixture can comprise,
consist of, or consist essentially of a sulfone component, a hydrogen
halide component, and ASO. Generally, the concentration of the hydrogen
halide component in the recovered catalyst mixture will be less than about
10 weight percent of the catalyst mixture with the weight percent
determined by the weight fraction of the hydrogen halide to total weight of
hydrogen halide and sulfone multiplied by a factor of 100 to yield a
percent. Because it is very difficult to remove the entire amount of
hydrogen halide from the catalyst mixture, the lower limit of hydrogen
halide concentration can approach about 0.1 weight percent, but~
preferably, the lower concentration limit of hydrogen halide can be less
than 0.1 weight percent. Thus, the concentration range of hydrogen halide
in the recovered catalyst mixture can range from about 0.1 weight percent
to about 10 weight percent. Preferably, however, the concentration can
range from about 0.1 to about 7.5 weight percent, and most preferably, it
can range from 0.1 to 5.0 weight percent.
2099359
33135CA
_ 27
As for the use of the neutralizing agent or neutralizing
material, the recovered catalyst mixture, having a concentration of
hydrogen halide, is contacted with the neutralizing material to thereby
remove a significant portion of the hydrogen halide component of the
recovered catalyst mixture to produce a neutralized sulfone-containing
mixture. The neutralized sulfone-containing mixture will be significantly
free of hydrogen halide; and, generally, it will have a concentration of
less than about 1.0 weight percent. Preferably, the neutralized
sulfone-containing catalyst mixture will have a concentration of less than
about 0.2 weight percent, and most preferably, it will have less than 0.1
weight percent hydrogen halide.
The neutralization of the recovered catalyst mixture or the
sulfone-containing mixture will permit further processing or treatment of
the neutralized sulfone-containing mixture or the resultant treated
sulfone-containing mixture to remove at least a portion of the ASO
component not removed during the neutralization step. A significant
portion of the ASO component of the neutralized catalyst is removed by
contacting it with an adsorbent material suitable for removing a
significant portion of the ASO component contained therein to produce a
regenerated catalyst mixture or a treated sulfone-containing mixture. The
ASO component of the regenerated catalyst mixture or the treated
sulfone-containing mixture will, in most instances, be present in a
concentration of less than about 2 weight percent of the total weight of
the sulfone component. Preferably, the weight percent of ASO present in
the treated sulfone-containing mixture can be less than about 1.0, and most
preferably, the AS0 will be present in an amount less than 0.1 weight
percent. The regenerated catalyst mixture or treated sulfone-containing
20993~9 33135CA
-
28
mixture can be reused as a portion of a sulfone-containing alkylation
catalyst mixture comprising, consisting of, or consisting essentially of a
sulfone and a hydrogen halide.
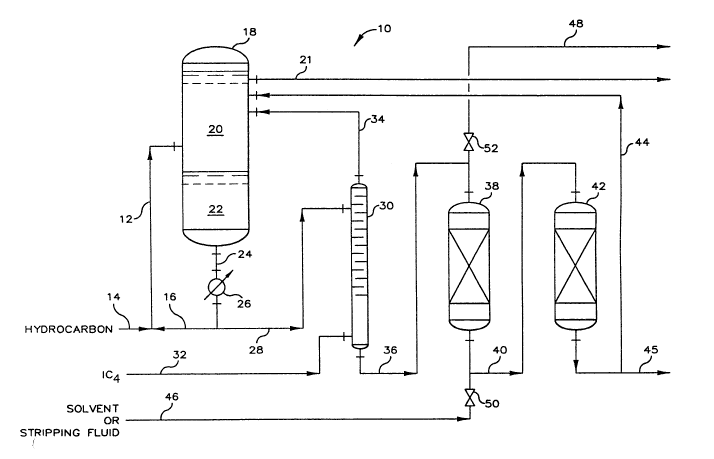
Now referring to FIG. 1, there is depicted by schematic
representation an alkylation process 10. A hydrocarbon feed mixture,
comprising olefins and isoparaffins, is introduced into reactor-riser 12
through conduit 14. Reactor-riser 12 defines a reaction zone wherein the
hydrocarbon mixture is contacted, or admixed, with a catalyst mixture,
comprising sulfolane and hydrogen fluoride, in order to produce a reaction
product and a reaction by-product. The olefins of the hydrocarbon feed
mixture generally comprise one or more olefins having from three to five
carbon atoms, and the isoparaffins of the hydrocarbon feed mixture
generally will have from four to six carbon atoms. The catalyst mixture is
introduced into reactor-riser 12 via conduit 16. The admixture of
hydrocarbon feed mixture and catalyst mixture passes through the reaction
zone defined by reactor-riser 12 wherein a reaction takes place in which
the olefins of the hydrocarbon feed mixture react with isoparaffins of the
hydrocarbon feed mixture to produce an alkylate reaction product. Also,
within the reaction zone, the reaction by-product, AS0, is formed. The
reaction effluent from reactor-riser 12 passes to settler vessel 18, which
defines a separation zone for separating the alkylate reaction product from
the catalyst mixture to produce a separated reaction product 20 and a
separated catalyst mixture 22. The separated catalyst mixture 22 will
contain a substantial amount of the alkylation reaction by-product, AS0.
The separated reaction product 20 passes to downstream processing via
conduit 21. The separated cata]yst mixture 22 can be recycled via conduits
24 and 16 to reactor-riser 12 for reuse as the alkylation catalyst mixture.
20~9359 33135CA
29
Interposed in conduit 24 is catalyst cooler 26, which defines a heat
transfer zone for exchanging heat from separated catalyst mixture 22 to a
heat transfer fluid such as water.
At least a portion, sometimes referred to as a slip stream or a
drag stream, of the separated catalyst mixture 22 passes by way of conduit
28 to stripping column 30, which defines a separation zone for separating
the slip stream of separated catalyst mixture 22 into an overhead stream,
comprising a major portion of the hydrogen fluoride contained in the slip
stream, and a bottoms stream, comprising a major portion of the sulfolane
component of the slip stream. The bottoms stream will also contain a major
portion of the reaction by-product, ASO, contained in the slip stream.
Introduced into stripping column 30 by way of conduit 32 is vaporous
isobutane for stripping the hydrogen fluoride from the slip stream. The
overhead stream passes by way of conduit 34 to settler vessel 18 wherein
the hydrogen fluoride is recombined with the separated catalyst mixture 22
for reuse, and the stripping isobutane is combined with the separated
reaction product 20.
The bottoms stream from stripping column 30 passes by way of
conduit 36 to first contacting vessel 38, which contains a contact
material, said contact material is preferably a reversible base and most
preferably a polyvinylpyridine compound. First contacting vessel 38
defines a separation zone for removing by adsorption or by neutralization,
or both, of a substantial portion of the hydrogen fluoride contained in the
bottoms stream to produce a neutralized bottoms stream or a treated
sulfone-containing mixture. Also, at least a portion of the AS0 contained
in the bottoms stream is adsorbed by the contact material and thereby
removed therefrom.
2099359
33135CA
The neutralized bottoms stream then passes through conduit 40 to
second contacting vessel 42, which contains an adsorbent material and
defines a separation zone for removing a substantial portion of the ASO
contained in the neutralized bottoms stream to produce a regenerated
catalyst, or sulfolane stream, that is substantially free of ASO and
hydrogen fluoride. This sulfolane stream passes through conduit 44 to
settler vessel 18 wherein it is remixed with separated catalyst mixture 22
for reuse as the sulfolane component of the alkylation catalyst mixture.
The sulfolane stream can optionally pass by way of conduit 45 to downstream
processing.
To regenerate the contact material contained within first
contacting vessel 38, conduits 46 and 48 each respectively having valves 50
and 52 are provided to permit the periodic regeneration of the spent
contact material. Periodically, the contact material in contacting vessel
38 is exposed to a solvent which passes by way of conduit 46 into first
contacting vessel 38 to thereby expose the contact material under
conditions such that at least a portion of the ASO adsorbed by the contact
material is removed by the solvent. The so]vent containing the ASO which
has been removed from the contact materifll leaves first contacting vessel
38 by way of conduit 48 to downstream processing. Following the exposure
of the contact material with a solvent suitable for the removal of at least
a portion of the ASO contained upon the contact material, the previously
exposed contact material is exposed to a stripping fluid. The stripping
fluid can pass by way of conduit 46 into first contacting vessel 38 to
expose the contact material contained therein under conditions so as to
regenerate the contact material. The stripping fluid is conveyed from
first contacting vessel 38 by way of conduit 48.
20993S9
33135CA
_ 31
The following examples demonstrate the advantages of the present
invention. These examples are by way of illustration only, and are not
intended as limitations upon the invention as set out in the appended
claims.
Example I
An AS0 by-product derived from the hydrocarbon reaction catalyzed
by a catalyst mixture of sulfolane and HF was obtained to determine some of
its physical properties. The catalyst mixture used in the hydrocarbon
reaction contained a weight ratio of HF to sulfolane of about 1.5, and the
hydrocarbon charge included isobutane and 2-butenes (60% trans, 40% cis
isomers) with a molar ratio of isobutane to 2-butenes of about 11. The
reaction temperature was about 90~F, and the reaction pressure was about 90
psig. Table I presents certain physical properties, including a
distillation, of the resultant ASO obtained from the hydrocarbon reaction.
Table I
Distillation of the ASO derived from hydrocarbon reaction
catalyzed by a sulfolane/HF catalyst mixture and other
physical properties of the AS0.
Bromine Number of
Temperature ~F Volume % of Sample Fraction
70 - 200 19 51
200 - 210 8 45
210 - 225 18 56
225 - 250 15 58
>250 40 59
Bromine Number of AS0 32
API Gravity (60~F) 37.1
Specific Gravity (60~F) 0.8391
2099359
33135CA
32
Example II
This Example II describes generally the experimental method used
to obtain data relating to the adsorption properties of carbon, alumina,
and mixtures thereof and the neutralization properties of alumina.
The general experimental procedure for testing the use of the
materials of carbon or alumina, or both, in the recovery of ASO from a
sulfolane-containing mixture of sulfolane and ASO included the use of a
glass cylinder of approximately one inch in diameter and from 12 inches to
24 inches in length. Placed in the bottom of the cylinder was either glass
wool or glass beads to provide support for the active material, and on top
of the active material was placed either glass beads or glass wool to
assist in providing an even distribution of the sulfolane-containing
mixture over the active material. Heat was optionally provided to the
glass cylinder to induce the flow of the sulfolane-containing mixture
through the bed of active material. The sulfolane-containing mixture had a
weight ratio of sulfolane-to-ASO of approximately 9 to 1. The color of the
resultant filtrate provided an indication as to when the adsorption
capacity of the active material was spent and thus was monitored to
determine when the experiment was complete.
Example III
This Example III illustrates the unexpected relationship between
the capacity of activated carbon to adsorb ASO from a sulfolane-containing
mixture of sulfolane and ASO as a function of the concentration of hydrogen
fluoride in the sulfolane-containing mixture.
The experimental method used to obtain the data presented in
Table II is substantially similar to that described in Example II. Various
concentrations of hydrogen fluoride in the sulfolane-containing mixture
2 0 9 9 3 5 9 33135CA
33
were established before contacting the mixture with an activated carbon
material. The data obtained are presented in Table II, which unexpectedly
demonstrates that the level of acid concentration in the
sulfolane-containing mixture has a large impact upon the ASO adsorption
capacity of activated carbon. These data are also plotted in FIG. Z.
Table II
The capacity of activated carbon to adsorb ASO from a sulfolane-
containing mixture, having a ratio of sulfolane to ASO of 9 to 1,
as a function of HF concentration.
Concentration of
HF in sulfolane- Adsorption Capacity
containing Mixture of Carbon
Weight % HF Weight % AS0 on Carbon
0.02 50
O. 10 19
0.50 4
1.00 Nil
Example IV
This Example IV demonstrates that various commercially available
aluminas can suitably be used to remove HF from a mixture of sulfolane and
AS0, either by adsorption or by neutralization. Also, this example
demonstrates that alumina can also adsorb a portion of the ASO contained in
the sulfolane-containing mixture as well as perform a neutralization
function.
The experimental method used to obtain the data presented in
Table III is substantially similar to that described in Example II with the
exceptions that the pH of effluent from the cylinder was monitored to
determine when the neutralization capacity of the alumina was reached. The
2099359
33135CA
34
sulfolane-containing mixture was provided with a 5 weight percent
concentration of HF. The data presented in Table III demonstrate that
various commercially available aluminas can suitably be used to neutralize
a sulfolane-containing mixture with some adsorption of ASO prior to
contacting the thus-neutralized mixture with an activated carbon material.
Table III
The capacity of various aluminas to neutralize and remove AS0
from a sulfolane-containing mixture having a weight ratio of
sulfolane to AS0 of 9 to 1.
Neutralization AS0 Adsorption
Capacity Capacity
Alumina Type (meq* HF/g) (mg/g)
LaRoche Alumina A-202 1.8 50
Alcoa Alumina HF-200 5.0 150
Engelhard Activated Bauxite "Sure cat" 1.3 35
LaRoshe SAS Alumina 4.1 120
*meq represents milequivalents
Example V
This Example V demonstrates that polyvinylpyridine reversibly
adsorbs and desorbs HF from a mixture of sulfolane and AS0. Also, this
example demonstrates that the polyvinylpyridine can also adsorb a portion
of the AS0 contained in the sulfone-containing mixture as well as
performing a neutralization function.
The experimental method used in this example is substantially
similar to that described in Examples II and IV, except that a metal
reactor was used instead of a glass cylinder in order to withstand
pressures of up to 600 psig. In addition, the process and regeneration
fluids were pumped across the absorbent bed rather than allowed to flow by
force of gravity.
2099359
33135CA
A mixture of 90/10 ~by volume) sulfolane/AS0 + 5% HF was pumped
over a bed of 50 mL Reillex~ 425 polyvinylpyridine maintained at 100~F and
ambient pressure. The mixture was pumped over the polymer at a rate of 20
mL/hour. About 108 mL of eff]uent was collected before the pH of the
effluent dropped below 4Ø The feeding of the mixture was discontinued
followed by raising the bed temperature to about 300~F and the pressure to
550 psig. Isobutane was passed over the polymer at a rate of 30 mL/hour
and under the aforementioned conditions. The isobutane effluent was
initially quite dark and very acidic. Pumping of the isobutane was
continued until the pH of the effluent was greater than 6Ø At that
point, the temperature and pressure were lowered to their initial
conditions, and the sulfolane/AS0/HF mixture was again pumped over the
polymer. In the second contacting of the polyvinylpyridine bed with the
mixture, 100 mL of effluent was collected before its pH dropped to below
4.0, thus showing that regeneration with isobutane was fairly efficient.
Calculations of the amounts of HF and AS0 removed from the
polyvinylpyridine bed and obtained during the regeneration step show that
the polymer adsorbed about 5 mmole HF/gram po]ymer and about 80% of the AS0
in the original sample mixture, measured cumulatively over the entire
absorption part of the cycle.
While this invention has been described in terms of the presently
preferred embodiment, reasonable variations and modifications are possible
by those skilled in the art. Such variations and modifications are within
the scope of the described invention and the appended claims.